Abstract
P-glycoprotein (P-gp) is a plasma membrane ATP-binding cassette transporter, responsible for multidrug resistance in tumor cells. P-gp catalyzes the ATP hydrolysis-dependent efflux of numerous amphiphilic compounds of unrelated chemical structures. In the absence of any identified substrate, P-gp exhibits an apparently futile, basal ATPase activity. By using native membrane vesicles containing high amounts of P-gp, we show here that (i) this basal ATPase activity is tightly dependent on the presence of cholesterol in the membrane; (ii) the stimulation of P-gp ATPase activity by drugs transported by P-gp is higher in the absence than in the presence of cholesterol and, conversely, the stimulation of P-gp ATPase activity by cholesterol is higher in the absence than in the presence of known P-gp substrates; (iii) P-gp mediates the ATP-dependent relocation of cholesterol from the cytosolic leaflet to the exoplasmic leaflet of the plasma membrane; and (iv) the decrease of the cholesterol dependence of P-gp ATPase activity induced by known P-gp substrates is correlated with the inhibition of the ATP-dependent cholesterol redistribution within the membrane. These data are highly evocative of a coupling between the basal ATPase activity of P-gp and its intramembrane cholesterol-redistribution function, and they are fully consistent with the possibility that P-gp may actively translocate cholesterol in the membrane. Finally, this P-gp-mediated cholesterol redistribution in the cell membrane makes it likely that P-gp contributes in stabilizing the cholesterol-rich microdomains, rafts and caveolae, and that it is involved in the regulation of cholesterol trafficking in cells.
P-glycoprotein (P-gp) is an ATP-binding cassette (ABC) transporter responsible for the multidrug resistance (MDR) phenotype when overproduced in the plasma membrane of tumor cells (1). P-gp exhibits an ATP hydrolysis activity, coupled to the efflux of a variety of hydrophobic molecules (2, 3). Different physiological roles have been proposed for P-gp, including cellular detoxification (4), lipid flip-floppase activity (5), and the secretion of steroid metabolites (6). In the absence of any known transport substrate, P-gp exhibits a basal ATPase activity, which is unusual for an active transporter (3). It has been suggested that this P-gp basal activity reflects the presence of an unknown endogenous substrate (5, 7). Some relationships have been reported between P-gp and cellular cholesterol: (i) P-gp expression is regulated by cholesterol levels (8); (ii) P-gp is predominantly localized in cholesterol-rich membrane microdomains, referred to as “lipid rafts” (9); and (iii) when P-gp is reconstituted in proteoliposomes with cholesterol (7), it exhibits a higher basal ATPase activity than in the absence of cholesterol (10), as recently confirmed by a direct comparison (11). These observations prompted us to investigate whether a functional link exists between P-gp and cholesterol.
Materials and Methods
Membrane Vesicles.
Human P-gp-containing membranes (purchased from Interchim, Montluçon, France) were prepared according to Sarkadi et al. (12) from baculovirus-infected Sf9 insect cells transfected with the human MDR1 gene; control membranes were prepared from insect cells infected by wild baculovirus. The MDR cell line DC-3F/ADX was originally selected from spontaneously transformed Chinese hamster lung fibroblasts DC-3F (13). Overexpression of the pgp1 gene confers to these cells a high resistance to various drugs. In these cells, P-gp represents 15% (w/w) of total membrane proteins, and it is the only membrane protein so highly overexpressed with respect to the parental sensitive cells (DC-3F line) used to prepare the control vesicles, in which P-gp was undetectable (13). DC-3F/ADX and DC-3F cell lines are grown to confluence in roller bottles and harvested by scraping in the presence of protease inhibitors. Membrane vesicles are prepared from DC-3F or DC-3F/ADX cells by sonication as described (13). Membrane protein concentration was determined by the Bradford method by using a protein assay kit (Bio-Rad) with BSA as the standard.
Control of the Membrane Cholesterol Content.
Membrane vesicles were incubated in buffer A (30 mM Tris⋅HCl, pH 7.5/100 mM NaCl/10 mM KCl/2 mM MgCl2) for different periods at 37°C with various concentrations of methyl-β-cyclodextrin (from a 100 mM stock solution in water). The vesicles were then sedimented by centrifugation at 95,000 × g for 15 min (4°C), and membrane cholesterol concentration was measured in the resuspended pellet. The cholesterol contents of membrane vesicles solubilized by 10 mM SDS was assayed by using an assay based on cholesterol oxidation (“Cholesterol 20 Total” kit, Sigma). The production of a quinoneimine was measured by spectrophotometric detection at 500 nm. Cholesterol concentration in the medium was determined by comparison with a standard cholesterol solution (Sigma), and expressed as a ratio to the protein content as assayed by the Bradford method.
ATPase Activity Measurements.
ATPase activity of membrane vesicles (14–22 μg of membrane protein/ml) was measured at 37°C in buffer A supplemented with 1 mM MgATP/1 mM phosphoenolpyruvate/0.1 mg/ml of pyruvate kinase/0.1 mg/ml of lactate dehydrogenase by continuous monitoring of NADH absorbance at 340 nm with a coupled enzyme assay as described (13). Measurement accuracy was estimated by the error on the determination of the rate of NADH absorption decrease (±1 mAb unit/min). Ion transport ATPases were specifically inhibited by 10 mM sodium azide/0.5 mM ouabain/1 mM EGTA (13). The stock solutions were prepared in DMSO for verapamil and vinblastine and in ethanol for cholesterol, progesterone, cyclosporin A, and PSC833. PSC833 was a kind gift from B. Willi (Novartis Pharma AG, Basel, Switzerland). The maximal amount of solvent added to the assay medium was 2%, and it did not alter P-gp ATPase activity. The difference between the ATPase activity of the P-gp-containing membrane vesicles (190 ± 25 nmol⋅min−1⋅mg protein−1) and the ATPase activity of control vesicles (40 ± 5 nmol⋅min−1⋅mg protein−1) was assigned to P-gp (13).
Radioactivity Measurements.
Membrane vesicles (0.03–0.1 mg of protein/ml) in buffer A supplemented with 1 mg/ml of BSA/5 mM phosphoenolpyruvate/0.1 mg/ml of pyruvate kinase were incubated at 37°C for various times in the presence of radiolabeled cholesterol {[1α,2α(n)-3H]cholesterol, Amersham Pharmacia} and various cholesterol concentrations with or without 1 mM MgATP. Vesicles (1 ml) were then perfused on a Gelman A/E glass-fiber filter soaked in distilled water. The filter was rinsed with buffer A, and the total radioactivity associated with the filter was counted by liquid scintillation. The amount of nonspecific cholesterol binding to the filters was unaffected by the presence of ATP in the medium. In control experiments, the binding to the filter and the passive [3H]cholesterol association to P-gp-free vesicles were unchanged in the presence of 5 μM vinblastine, 25 μM verapamil, or 75 μM progesterone.
Determination of Cholesterol Accessibility to Cholesterol Oxidase.
Membrane vesicles (0.15–0.3 mg protein/ml) were incubated for 15 min at 37°C in the presence or in the absence of 1 mM ATP in buffer A supplemented with 5 mM phosphoenolpyruvate and 0.1 mg/ml pyruvate kinase. Cholesterol oxidase (Sigma) was then added at a final concentration of 5–7.5 units/ml to the reaction medium and was incubated at 37°C for various times. The reaction was stopped by centrifugation (95,000 × g for 10 min at 4°C). The pellet was resuspended in buffer A, and cholesterol and protein concentrations were quantitated.
Results and Discussion
Influence of Cholesterol on P-gp ATPase Activity.
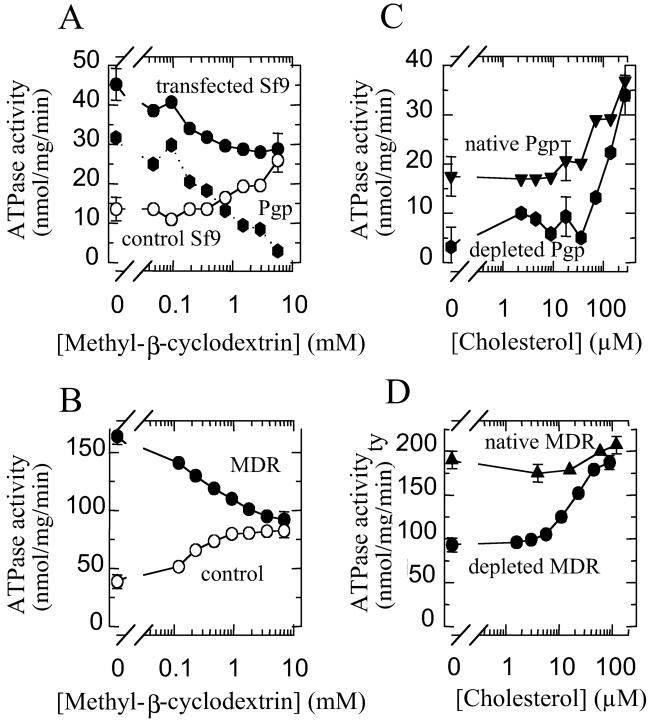
To determine whether cholesterol affects P-gp function, we used methyl-β-cyclodextrin (MβCD) to extract cholesterol from the lipid phase of native membrane vesicles containing P-gp or not. The P-gp-containing membrane vesicles were prepared either from Sf9 cells that had been transfected with the human MDR1 gene (“P-gp-transfected membranes”) or from MDR cells (DC-3F/ADX line) which highly overproduce P-gp (“MDR vesicles”) (13). MβCD is a highly hydrophilic cyclic oligosaccharide that specifically traps cholesterol, rather than other membrane lipids (14), without causing further membrane perturbation by insertion (15). Cholesterol content (90 ± 4%; n = 12) was removed from membrane vesicles by incubating the vesicles with 10 mM MβCD for 1 h at 37°C. When P-gp-transfected membranes or MDR vesicles were treated with increasing concentrations of MβCD (up to 5–10 mM), the membrane-associated ATPase activity decreased (Fig. 1 A and B). In contrast, the ATPase activity of the control membranes prepared from nontransfected Sf9 cells increased under the same conditions. This enhancement is not due to vesicle permeabilization because the activity of the membrane ion ATPases, measured under the standard conditions but omitting the inhibitors azide, ouabain, and EGTA, was unchanged. After subtraction of the control membrane ATPase activity from the activity of the P-gp-transfected membranes, it seems that the specific ATPase activity due to P-gp was totally inhibited in the presence of 5–10 mM MβCD (Fig. 1A). In MDR vesicles, the ATPase activity that remained after subtracting the ATPase activity of control vesicles (prepared from parental drug-sensitive DC-3F cells) can be attributed to P-gp, because P-gp is the only highly overproduced protein but it cannot be detected in the control vesicles (13). This subtraction gave the same result as when the P-gp-transfected membranes are used—i.e., the P-gp basal ATPase activity was largely inhibited after cholesterol depletion by MβCD.
Fig 1.
Effect of cholesterol on basal P-gp ATPase activity. (A and B) ATPase activity was measured in the presence of increasing concentrations of methyl-β-cyclodextrin in P-gp-containing vesicles prepared from Sf9-transfected cells (•, A), MDR cells (•, B), or from the corresponding P-gp-free vesicles (○, A and B). In the vesicles from transfected cells, the P-gp-specific ATPase activity was obtained by subtracting the activity of the control vesicles from the activity of the P-gp-containing vesicles ( , A). (C and D) ATPase activity was measured in the presence of increasing concentrations of cholesterol on untreated (triangles) or cholesterol-depleted vesicles (10 mM MβCD, 1 h, 37°C) prepared from Sf9-transfected cells (
, A). (C and D) ATPase activity was measured in the presence of increasing concentrations of cholesterol on untreated (triangles) or cholesterol-depleted vesicles (10 mM MβCD, 1 h, 37°C) prepared from Sf9-transfected cells ( , C) or MDR cells (•, D). In vesicles from transfected cells (C), the P-gp-specific ATPase activity obtained by subtracting the activity of the control vesicles from the activity of the P-gp-containing vesicles was plotted directly. Error bars represent measurement accuracy.
, C) or MDR cells (•, D). In vesicles from transfected cells (C), the P-gp-specific ATPase activity obtained by subtracting the activity of the control vesicles from the activity of the P-gp-containing vesicles was plotted directly. Error bars represent measurement accuracy.
We also used digitonin, a surfactant known to chelate cholesterol within the membrane phase (16). Digitonin (up to 250 μM) also induced a large decrease in the ATPase activity of the MDR vesicles such that it reached the level of the control vesicles, whose ATPase activity was partially inhibited (data not shown) in contrast to the MβCD treatment. Thus, the chelation of membrane cholesterol has a specific inhibitory effect on the basal ATPase activity of P-gp, and this inhibitory effect is independent of the way in which cholesterol is removed. At this point, the cholesterol dependence of P-gp ATPase could be due to a structural requirement of P-gp for a specific lipid environment, as for many membrane proteins (17). Alternatively, it may reveal a specific functional role for cholesterol, and further experiments were designed to investigate the action of cholesterol.
Addition of exogenous cholesterol (from a stock solution in ethanol) to cholesterol-depleted P-gp-transfected membranes or MDR vesicles (obtained after MβCD treatment: 10 mM, 1-h incubation, 37°C) led to the complete recovery of P-gp ATPase activity. The ATPase activity of cholesterol-depleted P-gp-transfected membranes rose when cholesterol was added between 10 and 200 μM (Fig. 1C). These concentrations also induced a more moderate increase in the activity of untreated P-gp-transfected membranes (Fig. 1C). The addition of exogenous cholesterol also stimulated P-gp ATPase activity in MβCD-treated MDR vesicles, with a half-activating cholesterol concentration of about 20–30 μM (Fig. 1D). This addition of exogenous cholesterol also restored the activity level to that of the untreated MDR vesicles, which, in this case, was not significantly modified by the addition of cholesterol (Fig. 1D). The different responses of the two types of native, untreated membranes from insect and mammalian cells to the addition of cholesterol may be related to their very different cholesterol contents: the Sf9 membranes contain 2 ± 1% (wt/wt membrane proteins) cholesterol and the MDR membranes contain 16 ± 6% (wt/wt). The full reactivation of P-gp basal ATPase activity showed that cholesterol depletion does not irreversibly denature the enzyme and confirms that cholesterol is directly involved in basal ATPase activity. The similar cholesterol dependence of basal ATPase activity in P-gp-transfected membranes and in MDR vesicles, the cholesterol composition and the high amount of P-gp in MDR vesicles validated the further use of the MDR vesicles.
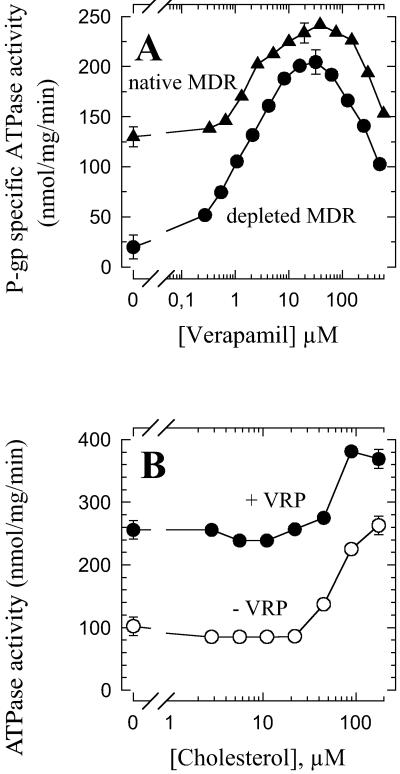
In cholesterol-depleted MDR vesicles, P-gp exhibited an ATPase activity in the presence of various P-gp substrates, such as verapamil or progesterone, which stimulate P-gp ATPase activity in native membranes. Increasing verapamil concentrations increased P-gp ATPase activity, with a bell-shaped curve characterized by a similar half-activating concentration and a slightly lower maximal activity value (at about 25 μM verapamil) than in native MDR vesicles (Fig. 2A). After cholesterol depletion, progesterone also stimulated P-gp ATPase with a bell-shaped curve close to that determined with native MDR vesicles (data not shown). Therefore, P-gp is fully functional in cholesterol-depleted MDR vesicles and is able to interact with its substrates. Furthermore, the verapamil- and progesterone-dependent ATPase activation is clearly higher in cholesterol-depleted vesicles than in native vesicles (180 vs. 110 nmol⋅mg−1⋅min−1 for verapamil; see Fig. 2A). Conversely, in the presence of saturating concentrations of verapamil (≈25 μM) or progesterone (≈75 μM), the ATPase activation induced by adding exogenous cholesterol is clearly less marked than in the absence of drug (Fig. 2B for verapamil, and not shown for progesterone). Thus, in the presence of these drugs P-gp ATPase activity is only moderately modulated by cholesterol, whereas P-gp basal ATPase activity is strongly and reversibly dependent on the presence of cholesterol in the membrane. This result shows that the activating role of cholesterol is influenced by the saturation state of P-gp transport sites, and is thus probably due to a direct interaction with P-gp rather than to a less specific effect mediated by the lipid phase. In addition, the ATPase activating effect induced by either verapamil (or progesterone) or cholesterol alone is more marked than in the presence of the other, independently of their order of addition. This observation suggests similar roles of drugs known as P-gp substrates (such as verapamil and progesterone) and of cholesterol for stimulating P-gp ATPase activity.
Fig 2.
Effect of cholesterol on drug-stimulated P-gp ATPase activity. (A) ATPase activity was measured in the presence of increasing concentrations of verapamil on native MDR vesicles (▴) or on cholesterol-depleted MDR vesicles (treated with 10 mM MβCD for 1 h at 37°C) (•). P-gp-specific ATPase activity was plotted after subtraction of the activity of the control vesicles from the activity of the P-gp-containing vesicles. (B) ATPase activity was measured in the presence of increasing concentrations of cholesterol on cholesterol-depleted MDR vesicles (10 mM MβCD, 1 h, 37°C) in the absence (○) or presence (•) of 25 μM verapamil. Error bars represent measurement accuracy.
Role of P-gp on Cholesterol Distribution Within the Membrane.
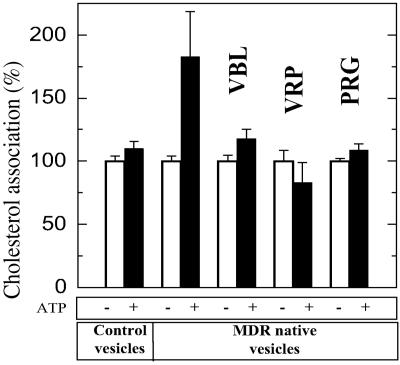
To test directly whether P-gp can use cholesterol as a substrate, we performed uptake experiments by using radiolabeled cholesterol. When MDR vesicles were incubated for 15 min at 37°C with 0.3 μM [3H]cholesterol, an ATP-dependent retention of radioactivity was observed in MDR vesicles (Fig. 3). The association of radioactivity was 1.49 ± 0.18 (SEM, n = 8) fold higher in the presence of ATP than in its absence (P = 0.028 for the paired Student's t test). In contrast, [3H]cholesterol association with the control vesicles devoid of P-gp did not depend on ATP (Fig. 3). When MDR vesicles were incubated for shorter periods, up to 1 min at 37°C, [3H]cholesterol association was the same regardless of whether ATP was present (data not shown), which indicates that the presence of ATP alone is not sufficient to induce the ATP-dependent association of [3H]cholesterol and that a significant hydrolysis of MgATP is required. The time dependence of [3H]cholesterol association with the MDR vesicles gives a crude determination of the rate at which P-gp incorporates cholesterol into the vesicles, ranging from 1 to 3 nmol⋅mg−1⋅min−1 at 10 μM exogenous cholesterol. The reactivation curve in Fig. 1D shows an increase of ATP hydrolysis rate of about 20 nmol⋅mg−1⋅min−1 at 10 μM cholesterol compared with the rate in the absence of cholesterol. A value of about 0.1 for the stoichiometry between cholesterol uptake and cholesterol-induced ATPase activity makes reasonable the possibility of a functional link between ATP hydrolysis and ATP-dependent cholesterol association to vesicles. The ATP-dependent association of [3H]cholesterol to MDR vesicles was inhibited by 5 μM vinblastine, 25 μM verapamil, or 75 μM progesterone (Fig. 3), whereas smaller, nonsaturating concentrations [1 μM vinblastine and 5 μM progesterone (18)] had no effect (data not shown). The inhibition of ATP-dependent cholesterol association by three different P-gp substrates further suggests that P-gp is directly involved in the uptake of exogenous cholesterol by MDR vesicles, even though no one of these substrates is highly specific with respect to P-gp.
Fig 3.
ATP-dependent association of [3H]cholesterol to MDR vesicles. The amount of [3H]cholesterol (0.3 μM) associated with the membrane vesicles after 15 min at 37°C in the presence or absence of 1 mM MgATP was measured on MDR or P-gp-free vesicles in a representative experiment performed in the presence and in the absence of P-gp transport substrates: vinblastine (VBL, 5 μM), verapamil (VRP, 25 μM), or progesterone (PRG, 75 μM). Cholesterol association is expressed as a percentage of the passive cholesterol binding in the absence of ATP, which was not significantly different for the five conditions, with a mean value of 2.24 ± 0.28 nmol/mg membrane proteins (SD, n = 5) and set at 100%. Error bars indicate the standard error of the mean.
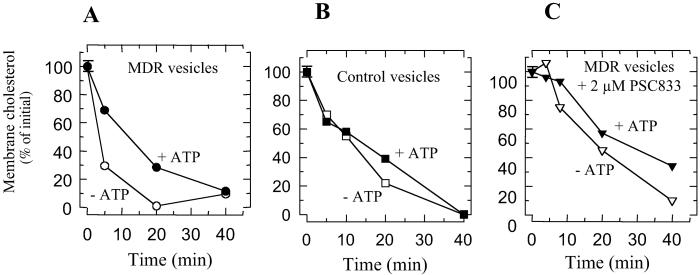
Because of its marked hydrophobicity, cholesterol preferentially accumulates within the membrane rather than within the vesicle lumen. To determine whether the ATP- and P-gp-dependent uptake of cholesterol by MDR vesicles was caused by cholesterol incorporation into the membrane from the aqueous medium or by its translocation from one leaflet of the membrane to the other, we performed accessibility experiments with cholesterol oxidase. Cholesterol oxidase is generally used to distinguish between membrane cholesterol pools because it reacts with the cholesterol molecules located on the external side of the vesicle membrane (19). When MDR or control vesicles were incubated with cholesterol oxidase in the absence of ATP, all of the membrane cholesterol was oxidized with fairly monophasic kinetics (Fig. 4), indicating a rather rapid spontaneous flip-flop of cholesterol. When MDR vesicles were preincubated in the presence of ATP, the cholesterol oxidation rate clearly decreased compared with the rate measured in the absence of ATP. The period necessary for the oxidation of 50% of the cholesterol (t1/2) increased by a factor of 2.9 ± 0.4 (SEM, n = 8) in the presence of ATP (Fig. 4A), indicating that cholesterol is preferentially located on the inner side of the vesicles hydrolyzing MgATP.† In contrast, no significant difference could be observed between the accessibility of cholesterol to cholesterol oxidase in control vesicles with or without ATP (Fig. 4B).‡ The difference between t1/2 measured in the presence or absence of ATP in MDR vesicles was almost abolished after incubation with 2 μM PSC833, which is a specific P-gp inhibitor (20); t1/2 only increased by a factor of 1.4 in the presence of ATP (Fig. 4C). A similar effect was observed in the presence of 2 μM cyclosporin A or 20 μM vinblastine (data not shown). The inhibitory effect of the three different transport modulators of P-gp on the ATP-dependent decrease of cholesterol accessibility to cholesterol oxidase supports a direct involvement of P-gp in membrane cholesterol redistribution. Because no exogenous cholesterol was added to the vesicles in these experiments, P-gp is responsible for intramembrane cholesterol translocation between the leaflets instead of mediating cholesterol exchange between aqueous bulk medium and membrane.
Fig 4.
ATP-dependent decrease in the accessibility of cholesterol to cholesterol oxidase in MDR vesicles. MDR vesicles (A and C) or control P-gp-free vesicles (B) were incubated for 15 min at 37°C in the presence (filled symbols) or absence (open symbols) of 1 mM MgATP and in the absence (A and B) or in the presence of 2 μM PSC833 (C). Cholesterol oxidase was added to the reaction medium (at 37°C) for various times before the reaction was stopped by centrifugation (at 4°C) and the amount of remaining membrane cholesterol was determined. The total amount of membrane cholesterol was considered to be 100%. The amount of cholesterol was measured to within 5%; the corresponding error bars are shown unless they are smaller than the symbol size.
To summarize, we presented evidence that (i) the basal ATPase activity of P-gp reversibly vanishes on cholesterol depletion of P-gp-containing membranes, whereas ATPase activity in the presence of various drugs known to be P-gp substrates is preserved; (ii) cholesterol is specifically accumulated in MDR vesicles when P-gp hydrolyzes ATP, whereas P-gp modifies the membrane cholesterol distribution between the two leaflets by an ATP-dependent mechanism; (iii) the decrease of the cholesterol dependence of P-gp ATPase activity induced by known P-gp ligands is correlated with the inhibition of the ATP-dependent cholesterol redistribution within the membrane. These results allowed us to conclude that P-gp actively mediates a membrane cholesterol redistribution, according to a coupling between stimulation of cholesterol-induced ATPase activity and cholesterol translocation. However, this coupling may be only apparent, resulting in indirect molecular mechanisms: (i) intramembrane cholesterol redistribution could be a secondary passive flux accompanying the active translocation of another lipid mediated by P-gp, coupled to its basal ATPase activity; (ii) simultaneously, cholesterol could be an allosteric activator, obligatory for P-gp basal ATPase activity, but optional for the drug-stimulated activity, thus mimicking known P-gp ligands for ATPase stimulation without being necessarily transported as a consequence of its binding to P-gp. Alternatively, the cholesterol dependence of the ATP hydrolysis required for this process can reflect the fact that P-gp catalyzes the transport of cholesterol within the membrane, possibly by a cotransport with other lipids. Accordingly, earlier observations on living cells reporting the P-gp-mediated transport of sphingomyelin (21) and of phospholipids (22, 23) might be explained by the transport of these lipids together with cholesterol, either by the specific cotransport of both substrates or by a passive flux of lipids accompanying an active P-gp-mediated cholesterol translocation. No phospholipid translocase activity was detected for P-gp in yeast secretory vesicles, which are devoid of cholesterol (24), and a very low phospholipid translocase activity has been recently reported for P-gp reconstituted into liposomes of egg phosphatidylcholine (25). Finally, a direct cholesterol floppase mechanism for P-gp is fully consistent with our observations that: (i) P-gp ATPase stimulation induced by P-gp substrates is enhanced after cholesterol depletion, whereas cholesterol-induced ATPase activation is higher in the absence of known P-gp substrates (Fig. 2). (ii) P-gp substrates are all hydrophobic and handled within the membrane phase (3). (iii) Various steroids are transported by P-gp (26, 27) and (iv) other mammalian ABC transporters are involved in lipid transport (28), such as the phosphatidylcholine translocase MDR3 present in the canalicular membrane of hepatocytes (29), the recently cloned ABCA1 transporter, which is responsible for the translocation of phosphatidylserine in macrophages (30), and BSEP (“bile salt export pump,” also called “sister of P-gp,” Spgp, because of its high sequence homology), which transports various cholate derivatives—i.e., surfactants presenting a cholestane structure (31).
Consequence of the P-gp-Induced Cholesterol Redistribution on Plasma Membrane Structure and Function.
Whatever the exact molecular mechanism involved, our results showed that P-gp mediates the flux of cholesterol from the cytosolic leaflet to the exoplasmic leaflet.§ These results suggest that P-gp can be involved in the cholesterol enrichment of the exoplasmic leaflet of plasma membrane at the level of rafts and caveolae (32, 33). This role is consistent with the finding that P-gp can be coimmunoprecipitated with caveolin-1 (34), a cholesterol-binding protein that is a main component of caveolae (35). Accordingly, the amount of caveolin-1 in MDR cells is increased compared with their parental sensitive cells (36). In the MDR DC-3F/ADX cells, the amount of caveolin-1 is much higher in the membrane fraction than in the whole-cell extracts (unpublished data). Such relocation of caveolin-1 from the cytosol to the plasma membrane in the MDR cells, which may raise the question as to whether caveolin is required for the floppase activity of P-gp, is consistent with the cholesterol enrichment of the exoplasmic leaflet leading to stabilization and increased number of caveolae by P-gp. This proposal is in line with the close correlation observed between expression of P-gp and regulation of cell cholesterol homeostasis at the level of efflux involving caveolae (37), even if the precise role of caveolae in cholesterol efflux and transport is still poorly understood (38). More generally, the cholesterol redistribution within plasma membrane induced by P-gp may affect cellular cholesterol trafficking, which involves both the endogenous biosynthesis and esterification of cholesterol in the endoplasmic reticulum, the import of exogenous cholesterol from the low-density lipoprotein by endocytosis, and the export of cholesterol to the high-density lipoprotein (39). This possibility is consistent with the reported decreased flux of cholesterol from the plasma membrane to the endoplasmic reticulum in the presence of P-gp inhibitors (40) and with the increase in the uptake of exogenous cholesterol in transfected cells producing P-gp (41). In addition, the enrichment of cholesterol in the exoplasmic leaflet of the plasma membrane may facilitate the efflux of cholesterol out of the cell to the high-density lipoprotein, possibly in cooperation with ABCA1.¶ Therefore, the cholesterol translocase activity of P-gp may well be implicated in the regulation of the physiological functions of rafts and caveolae (signal transduction, potocytosis) and in general membrane trafficking. This new function assigned to P-gp deserves additional experiments by using cultured cells, and confirmatory data will also require studies on mouse deficient in the mdr1a/1b genes.
Acknowledgments
We thank A. Sentenac, M. Lutz, M. le Maire, and P. Champeil for very fruitful discussions.
Abbreviations
P-gp, P-glycoprotein
ABC, ATP-binding cassette
MDR, multidrug resistance
MβCD, methyl-β-cyclodextrin
ABCA1 is necessary to transfer cholesterol to high-density lipoprotein, probably in association with other lipids because ABCA1 is now described first to coat apoA1 with phospholipids and then to transfer cholesterol from the plasma membrane (42), which can explain that the genetic disruption of ABCA1 induces more severe clinical disorders than disruption of P-gp does. Indeed, P-gp should not be considered as the major regulation mechanism of cellular cholesterol in the body because P-gp expression is not ubiquitous.
In the accessibility experiments, it is not necessary to use vesicles presenting a 100% inside-out orientation because what is measured is the slowing down of cholesterol oxidation induced by ATP. Indeed, the minor right-side-out vesicle population, which represents 20–25% of the vesicle suspension, is not sensitive to the presence of ATP because the vesicles are well closed, as shown by electron microscopy data (not shown). Hence, the ATP-dependent effect observed on cholesterol accessibility to cholesterol oxidase only involves the inside-out vesicles in the suspension.
Either in the presence or absence of ATP, cholesterol oxidation follows a fairly monoexponential kinetics, which can be described by its t1/2. This finding is consistent with a kinetic scheme where the two pools of membrane cholesterol present in the inner and the outer leaflets are in rapid equilibrium (with rate constants kout and kin for outward and inward flux, respectively) compared with the oxidation rate (constant kC). In this case, the observed rate for cholesterol decrease is: kobs = kout kC/(kout + kin). In the absence of ATP, cholesterol is only submitted to spontaneous flip-flop, with: kout = k+1,kin = k−1, and k+1 = k−1, thus kobs = kC/2. In the presence of ATP, cholesterol is also translocated from the outer to the inner leaflet of the vesicle membrane because of the action of P-gp, with a rate constant kP such as kout= k+1 and kin= k−1 + kP, thus leading to a decreased kobs. The observation of a 3-fold increase of the t1/2 of the reaction thus gives kin = 5 kout and kP = 4 k−1 .
A preferential handling by P-gp of cholesterol present in the cytosolic leaflet is consistent with the observation that P-gp ATPase activity depends mainly on the cholesterol pool which is the most easily chelated by MβCD, as shown by the fact that P-gp ATPase is nearly completely inhibited when one half of total cholesterol is extracted from the membrane, that is after 1-h incubation with 1–2 mM MβCD. Also, ATPase inhibition upon addition of MβCD occurs faster (within 1 min) than the time required for total cholesterol depletion (reached in more than 15 min).
References
- 1.Higgins C. F. (1992) Annu. Rev. Cell Biol. 8, 67-113. [DOI] [PubMed] [Google Scholar]
- 2.Gottesman M. M. & Pastan, I. (1993) Annu. Rev. Biochem. 62, 385-427. [DOI] [PubMed] [Google Scholar]
- 3.Sharom F. J. (1997) J. Membr. Biol. 160, 161-175. [DOI] [PubMed] [Google Scholar]
- 4.Schinkel A. H. (1997) Semin. Cancer Biol. 8, 161-170. [DOI] [PubMed] [Google Scholar]
- 5.Higgins C. F. & Gottesman, M. M. (1992) Trends Biochem. Sci. 17, 18-21. [DOI] [PubMed] [Google Scholar]
- 6.Leveille-Webster C. R. & Arias, I. M. (1995) J. Membr. Biol. 143, 89-102. [DOI] [PubMed] [Google Scholar]
- 7.Borgnia M. J., Eytan, G. D. & Assaraf, Y. G. (1996) J. Biol. Chem. 271, 3163-3171. [DOI] [PubMed] [Google Scholar]
- 8.Klucken J., Buchler, C., Orso, E., Kaminski, W. E., Porsch-Ozcurumez, M., Liebisch, G., Kapinsky, M., Diederich, W., Drobnik, W., Dean, M., et al. (2000) Proc. Natl. Acad. Sci. USA 97, 817-822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luker G. D., Pica, C. M., Kumar, A. S., Covey, D. F. & Piwnica-Worms, D. (2000) Biochemistry 39, 7651-7661. [DOI] [PubMed] [Google Scholar]
- 10.Shapiro A. B. & Ling, V. (1994) J. Biol. Chem. 269, 3745-3754. [PubMed] [Google Scholar]
- 11.Rothnie A., Theron, D., Soceneantu, L., Martin, C., Traikia, M., Berridge, G., Higgins, C. F., Devaux, P. F. & Callaghan, R. (2001) Eur. Biophys. J. 30, 430-442. [DOI] [PubMed] [Google Scholar]
- 12.Sarkadi B., Price, E. M., Boucher, R. C., Germann, U. A. & Scarborough, G. A. (1992) J. Biol. Chem. 267, 4854-4858. [PubMed] [Google Scholar]
- 13.Garrigos M., Belehradek, J., Jr., Mir, L. M. & Orlowski, S. (1993) Biochem. Biophys. Res. Commun. 196, 1034-1041. [DOI] [PubMed] [Google Scholar]
- 14.Ohvo H. & Slotte, J. P. (1996) Biochemistry 35, 8018-8024. [DOI] [PubMed] [Google Scholar]
- 15.Gimpl G., Burger, K. & Fahrenholz, F. (1997) Biochemistry 36, 10959-10974. [DOI] [PubMed] [Google Scholar]
- 16.Nishikawa M., Nojima, S., Akiyama, T., Sankawa, U. & Inoue, K. (1984) J. Biochem. 96, 1231-1239. [DOI] [PubMed] [Google Scholar]
- 17.Yeagle P. L. (1991) Biochimie 73, 1303-1310. [DOI] [PubMed] [Google Scholar]
- 18.Garrigos M., Mir, L. M. & Orlowski, S. (1997) Eur. J. Biochem. 244, 664-673. [DOI] [PubMed] [Google Scholar]
- 19.Boesze-Battaglia K., Clayton, S. T. & Schimmel, R. J. (1996) Biochemistry 35, 6664-6673. [DOI] [PubMed] [Google Scholar]
- 20.Böhme M., Büchler, M., Müller, M. & Keppler, D. (1993) FEBS Lett. 333, 193-196. [DOI] [PubMed] [Google Scholar]
- 21.van Helvoort A., Giudici, M. L., Thielemans, M. & van Meer, G. (1997) J. Cell Sci. 110, 75-83. [DOI] [PubMed] [Google Scholar]
- 22.van Helvoort A., Smith, A. J., Sprong, H., Fritzsche, I., Schinkel, A. H., Borst, P. & van Meer, G. (1996) Cell 87, 507-517. [DOI] [PubMed] [Google Scholar]
- 23.Bosch I., Dunussi-Joannopoulos, K., Wu, R.-L., Furlong, S. T. & Croop, J. (1997) Biochemistry 36, 5685-5694. [DOI] [PubMed] [Google Scholar]
- 24.Ruetz S. & Gros, P. (1994) Cell 77, 1071-1081. [DOI] [PubMed] [Google Scholar]
- 25.Romsicki Y. & Sharom, F. J. (2001) Biochemistry 40, 6937-6947. [DOI] [PubMed] [Google Scholar]
- 26.Ueda K., Okamura, N., Hirai, M., Tanigawara, Y., Saeki, T., Kioka, N., Komano, T. & Hori, R. (1992) J. Biol. Chem. 267, 24248-24252. [PubMed] [Google Scholar]
- 27.Barnes K. M., Dickstein, B., Cutler, G. B., Fojo, T. & Bates, S. E. (1996) Biochemistry 35, 4820-4827. [DOI] [PubMed] [Google Scholar]
- 28.Borst P., Zelcer, N. & van Helvoort, A. (2000) Biochim. Biophys. Acta 1486, 128-144. [DOI] [PubMed] [Google Scholar]
- 29.Smit J. J., Schinkel, A. H., Oude Elferink, R. P., Groen, A. K., Wagenaar, E., van Deemter, L., Mol, C. A., Ottenhoff, R., van der Lugt, N. M., van Roon, M. A., et al. (1993) Cell 75, 451-462. [DOI] [PubMed] [Google Scholar]
- 30.Hamon Y., Broccardo, C., Chambenoit, O., Luciani, M. F., Toti, F., Chaslin, S., Freyssinet, J. M., Devaux, P. F., McNeish, J., Marguet, D., et al. (2000) Nat. Cell Biol. 2, 399-406. [DOI] [PubMed] [Google Scholar]
- 31.Gerloff T., Stieger, B., Hagenbuch, B., Madon, J., Landmann, L., Roth, J., Hofmann, A. F. & Meier, P. J. (1998) J. Biol. Chem. 273, 10046-10050. [DOI] [PubMed] [Google Scholar]
- 32.Simons K. & Ikonen, E. (1997) Nature (London) 387, 569-572. [DOI] [PubMed] [Google Scholar]
- 33.Hooper N. M. (1999) Mol. Membr. Biol. 16, 145-156. [DOI] [PubMed] [Google Scholar]
- 34.Demeule M., Jodoin, J., Gingras, D. & Béliveau, R. (2000) FEBS Lett. 466, 219-224. [DOI] [PubMed] [Google Scholar]
- 35.Murata M., Peränen, J., Schreiner, R., Wieland, F., Kurzchalia, T. V. & Simons, K. (1995) Proc. Natl. Acad. Sci. USA 92, 10339-10343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lavie Y., Fiucci, G. & Liscovitch, M. (1998) J. Biol. Chem. 273, 32380-32383. [DOI] [PubMed] [Google Scholar]
- 37.Liscovitch M. & Lavie, Y. (2000) Trends Biochem. Sci. 25, 530-534. [DOI] [PubMed] [Google Scholar]
- 38.Fielding C. J. & Fielding, P. E. (2000) Biochim. Biophys. Acta 1529, 210-222. [DOI] [PubMed] [Google Scholar]
- 39.Fielding C. J. & Fielding, P. E. (1997) J. Lipid Res. 38, 1503-1521. [PubMed] [Google Scholar]
- 40.Metherall J. E., Li, H. & Waugh, K. (1996) J. Biol. Chem. 271, 2634-2640. [DOI] [PubMed] [Google Scholar]
- 41.Tessner T. G. & Stenson, W. F. (2000) Biochem. Biophys. Res. Commun. 267, 565-571. [DOI] [PubMed] [Google Scholar]
- 42.Fielding P. E., Nagao, K., Hakamata, H., Chimini, G. & Fielding, C. J. (2000) Biochemistry 39, 14113-14120. [DOI] [PubMed] [Google Scholar]