Abstract
Background
Neostigmine is a widely used muscle relaxant antagonist that requires combination with anticholinergic agents like atropine to mitigate its muscarinic effects. Glycopyrrolate exerts less influence on cardiac conduction than atropine, making it a preferable option. However, clinical research on its pediatric use is limited. This study aims to compare the effects of glycopyrrolate and atropine, in combination with neostigmine, on heart rate and adverse events in pediatric patients.
Methods
Children undergoing penile flap surgery under general anesthesia combined with a caudal block were randomly assigned to either the glycopyrrolate group (Group G, n = 100) or the atropine group (Group A, n = 100). Postoperatively, patients in Group G received glycopyrrolate (8 µg/kg) and neostigmine (40 µg/kg), while those in Group A received atropine (20 µg/kg) and neostigmine (40 µg/kg) to reverse neuromuscular blockade. The baseline heart rate was defined as the heart rate measured before administering the trial drug, with heart rates recorded every minute thereafter, along with any adverse events noted within 24 h post-operation.
Results
There were no statistically significant differences in age, surgery duration, or baseline heart rates between the two groups (p > 0.05). Heart rate changes within 15 min post-administration were less pronounced in the glycopyrrolate group compared to the atropine group (p < 0.05), indicating reduced fluctuation from baseline. Additionally, the area under the curve (AUC) for heart rate changes in the first 15 min post-administration was lower in the glycopyrrolate group (p < 0.05). No statistically significant differences in adverse events were observed between the two groups (p > 0.05).
Conclusions
Compared to atropine, the combination of glycopyrrolate and neostigmine results in less fluctuation in heart rate but a higher incidence of dry mouth, with no significant differences in other complications. Therefore, glycopyrrolate may be preferred in scenarios where hemodynamic stability is prioritized, considering its higher risk of dry mouth.
Keywords: Glycopyrrolate, Heart rate, Atropine, Neostigmine, Pediatric, Anesthesia
Background
Muscle relaxants are commonly used anesthetic agents that are often administered alongside sedatives and analgesics to facilitate tracheal intubation and meet surgical needs. However, residual muscle relaxants following anesthesia can lead to complications such as respiratory failure and aspiration. In pediatric practice, neostigmine, a cholinesterase inhibitor, is frequently used to counteract the effects of muscle relaxants. However, it can lead to muscarinic side effects, such as bradycardia and increased secretions, which require the use of anticholinergic agents.
Atropine is commonly used in pediatric anesthesia, but its rapid onset and short duration may lead to significant fluctuations in heart rate when administered in combination with neostigmine. Glycopyrrolate does not have central effects, exerts a lesser impact on cardiac conduction, and provides stronger inhibition of salivary secretion, making it a more suitable alternative.
We conducted a randomized, double-blind controlled trial to compare the effects of glycopyrrolate and atropine on heart rate and postoperative complications in pediatric patients undergoing muscle relaxant antagonism.
Materials and methods
Study design and ethics
This randomized double-blind controlled study was conducted at Shanghai Children’s Hospital from October 2023 to June 2024. The trial was approved by Clinical Trial Ethics Committee of Shanghai Children’s Hospital (2023R065-E02) and registered in Chinese Clinical Trial Registry (clinical trial registration No. ChiCTR2300076270, 28-09-2023). All participating anesthesiologists completed pre-training prior to the study, and the trial was conducted in accordance with the principles of the Helsinki Declaration. The trial report complies with the Consolidated Standards of Reporting Trials (CONSORT) checklist.
Participants
We recruited pediatric patients scheduled for elective penile flap surgery under general anesthesia with laryngeal mask combined with sacral block. Inclusion Criteria were as follows: (1) Males aged 3–12; (2) American Society of Anesthesiologists (ASA) class I or II; (3) The children and their guardians voluntarily participated in this study and signed informed consent forms.
Exclusion Criteria were as follows: (1) Children with obesity (> 95th percentile for age and sex); (2) allergies to anticholinergic medications and their components; (3) comorbidities affecting heart rate in the respiratory, circulatory, or digestive systems, abnormal liver or kidney function, glaucoma; (4) currently taking medications affecting the cholinergic system.
During the pre-anesthesia visit, written informed consent will be obtained from legal guardians and children aged 8–12, while detailed oral explanations related to the study will be provided to children under 8. Any adverse events related to the study that occur during the research period, serious protocol violations, or withdrawal of informed consent will result in the termination of the study.
Randomization and blinding
Patients were randomly assigned to the glycopyrrolate group (Group G) or the atropine group (Group A) in a 1:1 ratio using computer-generated random numbers. Group information was placed in sequentially numbered, sealed opaque envelopes and then given to a nurse who was not involved in the study. The nurse used the same 5 mL syringe to draw the trial drug. Anesthesiologists and nurses involved in anesthetic management and postoperative follow-up were blinded to the group assignments.
Anesthetic and surgical management
Patients fasted for at least six hours prior to surgery, and a peripheral intravenous line was established for fluid infusion. Thirty minutes before the procedure, patients entered the waiting area and received oral midazolam (0.5 mg/kg) (maximum dose 15 mg) for sedation. Once in the operating room, patients were randomly assigned to either the Group G or the Group A. Continuous monitoring included electrocardiogram (ECG), pulse oxygen saturation (SpO2), non-invasive blood pressure (NIBP), and end-tidal carbon dioxide (ETCO2). The heart rate values are derived from electrocardiogram (ECG) readings by the anesthesia monitor, which is routinely tested and calibrated.
The medications used for intravenous anesthesia induction include propofol (3 mg/kg), sufentanil (0.1 µg/kg), Cisatracurium besylate (0.1 mg/kg) and atropine (0.01 mg/kg). Following the induction, a properly sized laryngeal mask airway (LMA) is inserted. The anesthesia machine was set to volume control mode, and respiratory parameters were adjusted to maintain ETCO2 within the normal range while administering 0.8 MAC sevoflurane. Subsequently, the patient was positioned in the left lateral position for an ultrasound-guided caudal block, using 1% lidocaine at a dosage of 1 ml/kg (maximum dose of 30 ml).
No additional muscle relaxants were administered during surgery. If analgesia from the sacral block was unsuccessful, as indicated by a heart rate increase exceeding 20% from baseline, 0.1 µg/kg sufentanil was given, and the patient was withdrawn from the study. The heart rate immediately after surgery was considered the baseline heart rate.
In our hospital, the standard intravenous dose of neostigmine for reversing muscle relaxants is 40 µg/kg, with concomitant doses of atropine and glycopyrrolate at 20 µg/kg and 8 µg/kg, respectively. Based on the drug’s prescribing information, in Group G, intravenous administration included glycopyrrolate (8 µg/kg) and neostigmine (40 µg/kg), while the Group A received atropine (20 µg/kg) and neostigmine (40 µg/kg), each administered over a period of 1 min. Heart rates were recorded every minute for 15 min post-administration. After 15 min, sevoflurane was discontinued, and the respiratory rate was reduced to 10 breaths per minute until the patient regained spontaneous breathing, at which point the LMA was removed. The patient was then transferred to the post-anesthesia recovery room (PACU) and returned to the ward when the Aldrete score ≥ 9. The Cornell Assessment of Pediatric Delirium (CAPD) was used to evaluate the occurrence of delirium postoperatively, recording agitation during the awakening period (RASS score ≥ 2), as well as dry mouth, nausea, vomiting, facial flushing, and delirium events within 24 h after surgery.
Demographic data and procedure-related information, such as body weight and operative time, time in PACU were collected.
Primary efficacy endpoints
The area under the time curve (AUC) for the difference in heart rate between baseline and the values measured within 15 min after administration of the trial drug.
Secondary efficacy endpoints
Actual heart rate values measured every minute within 15 min post-administration, changes in heart rate compared to baseline, duration of recovery, and any adverse events occurring within 24 h post-surgery, such as flushing, dry mouth, and delirium.
Statistical analysis
Due to the limited availability of pediatric-specific data for this comparison, sample size estimation was based on the effect size and variability observed in our pilot study and relevant literature. A two-sample independent t-test was used for the primary outcome of mean heart rate during the procedure. To achieve 80% statistical power for detecting a clinically relevant mean difference of 12 bpm between the two sedation groups, with an estimated common standard deviation of 16 bpm (as suggested by pilot data and pediatric sedation studies), at a two-tailed significance level (α) of 0.05, a minimum of 92 patients per group was calculated using PASS. To account for an anticipated dropout/attrition rate of approximately 8–9% (e.g., due to protocol deviations, incomplete data, withdrawal of consent), a final sample size of 100 patients per group was enrolled.
Data analysis was performed using SPSS 26.0. Normally distributed continuous data were described as mean ± standard deviation, while categorical data were described using frequency counts. Analysis of the primary efficacy endpoint was based on an analysis of covariance (ANCOVA) model, with the AUC for heart rate difference from baseline within 15 min as the dependent variable and baseline values as covariates. The least squares mean of the relative baseline changes were calculated for each group, providing the least squares means of the AUC for heart rate differences from baseline within 15 min.
Non-inferiority testing was conducted between the primary efficacy endpoints of the two groups. Based on previous clinical trial data, the standard deviation for the AUC of heart rate difference from baseline within 15 min was assumed to be 80, with α = 0.025 and β = 0.20. Based on clinical judgment, the non-inferiority margin was set at 30, with p < 0.05 considered statistically significant. For secondary efficacy endpoints, comparisons between groups will be performed using t-tests, chi-square tests, or Fisher’s exact test, all employing two-sided tests with α = 0.05. Bonferroni adjustment was applied for multiple AUC comparisons (α = 0.0167). Effect sizes (Cohen’s d) with 95% CIs were calculated.
Results
A total of 200 children were included in the study, with 100 in Group G and 100 in Group A. No participants withdrew from the study, and there were no adverse events or refusals of informed consent during the research. The study procedure is shown in Fig. 1. There were no statistically significant differences in the age, body weight, ASA class, operative time, time in PACU and baseline heart rates between the two groups (Table 1). All pediatric patients had postoperative CAPD scores below 10, and no cases of delirium were observed.
Fig. 1.
Flow diagram of study
Table 1.
Patient characteristics and intraoperative date
| Variable | Group G (n = 100) | Group A (n = 100) | P value |
|---|---|---|---|
| Age (yr) | 6.91 ± 2.99 | 7.20 ± 3.10 | 0.501 |
| Body weight (kg) | 30.70 ± 6.50 | 30.67 ± 6.56 | 0.963 |
| ASA class (I/II) | 86/14 | 81/19 | 0.632 |
| Operative time (min) | 36.98 ± 4.63 | 37.47 ± 4.64 | 0.456 |
| Time in PACU (min) | 26.57 ± 4.88 | 27.46 ± 4.93 | 0.202 |
| Baseline heart rates (beats/min) | 104.28 ± 11.75 | 105.20 ± 11.22 | 0.572 |
Data are mean ± SD unless otherwise stated
P >0.05, indicating no statistically significant difference between the two groups
Compared to Group A, the incidence of dry mouth in Group G significantly increased within 24 h postoperatively (p < 0.05). There were no statistically significant differences between the two groups in the incidence of agitation, nausea, vomiting, or delirium. (Table 2)
Table 2.
Comparison of postoperative adverse events between two groups
| Variable | Group G (n = 100) | Group A (n = 100) | P value |
|---|---|---|---|
| Agitation | 10 | 14 | 0.387 |
| Dry mouth | 42 | 21 | 0.001* |
| Nausea | 19 | 18 | 0.856 |
| Vomiting | 13 | 12 | 0.832 |
*P<0.05, statistically significant difference between the two groups
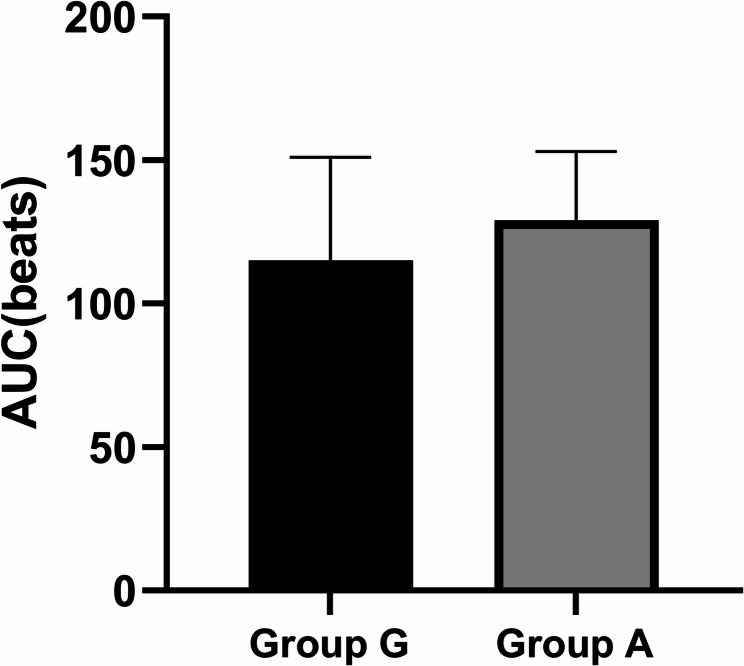
In the Group G, the AUC for the difference in heart rate from baseline within 15 minutes after administration of Glycopyrrolate + Neostigmine was (115 ± 36) bpm, while in the Group A, the AUC was (129± 24) bpm. The heart rate AUC was significantly higher in Group A (129 vs 115 bpm·min; adjusted p = 0.008, Cohen's d = 0.42, 95% CI 0.21–0.63), indicating a moderate effect size, showing a statistically significant difference (p < 0.05) (Figure 2and 3).
Fig. 2.
Comparison of the AUC for heart rate change from baseline between two groups within 15 min post-administration
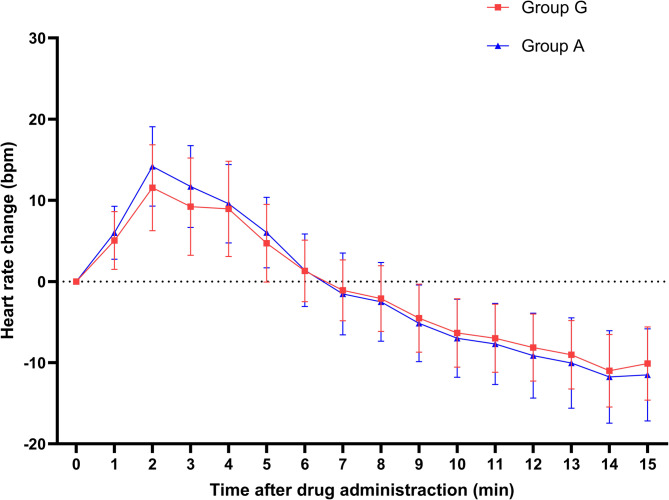
Fig. 3.
Per-minute heart rate changes within 15 min post-administration
Comparison of the AUC of the difference in heart rate from baseline between the two groups within 15 min after administration of the trial drug. In Group G, the AUC was (115 ± 36) bpm, while in Group A, the AUC was (129 ± 24) bpm, showing a statistically significant difference (p < 0.05).
Both groups reached peak heart rates at 2 min post-administration, after which heart rates gradually decreased below baseline. Significant differences in heart rates between the two groups were observed at 2, 3, 4 and 5 min post-administration (p < 0.05). Comparisons of heart rate changes at 2, 3, 5, 9, 10, 11, 12, 13, 14, and 15 min also showed statistically significant differences (p < 0.05).
Measured heart rate values per minute for both groups within 15 min after administration. The heart rates of children in both groups peaked at 2 min after drug administration, with the atropine group exhibiting a higher heart rate. Subsequently, the heart rates gradually decreased, with a more pronounced reduction observed in the atropine group.
Discussion
Neostigmine is a commonly used cholinesterase inhibitor, but it can lead to muscarinic side effects such as bradycardia and increased airway secretions, which necessitates co-administration with anticholinergic drugs. Glycopyrrolate and atropine are both widely used anticholinergics; however, glycopyrrolate is a potent, long-acting option. Compared to atropine, glycopyrrolate’s pharmacodynamics and pharmacokinetics make it more suitable for use with neostigmine in reversing neuromuscular blockade during anesthesia. Glycopyrrolate has a stronger and longer-lasting peripheral anticholinergic effect, being 5–6 times more potent than atropine and having a duration that is 3–4 times longer [1]. When combined with neostigmine to reverse non-depolarizing muscle relaxants, glycopyrrolate results in fewer episodes of tachycardia and is more effective at reducing salivary secretions, effectively countering the muscarinic symptoms caused by neostigmine.
In pharmacokinetics, glycopyrrolate takes effect within 1 min after administration and lasts for 2–4 h, with its inhibition of salivary secretion persisting for up to 7 h. It has a limited ability to cross the blood-brain barrier, and in infants and children, it exhibits a higher volume of distribution and clearance compared to adults [2, 3].
The primary advantage of glycopyrrolate is its minimal impact on heart rate, resulting in more stable hemodynamics [4]. Evidence suggests atropine administration during pediatric anesthesia may precipitate cardiac arrhythmias [5]. Conversely, clinical data indicate glycopyrrolate demonstrates superior cardiac rhythm stability [6]. Due to shortages of glycopyrrolate and its established clinical use, there has been limited research in recent years [7]. Currently, there is a lack of studies on the optimal dosing of glycopyrrolate when used in conjunction with neostigmine in pediatric patients.
Heart rate is one of the most important indicators of hemodynamics in children, and this study monitored heart rate changes as the primary observation metric reflecting the cardiovascular effects of glycopyrrolate. Glycopyrrolate has a weaker effect on M2 receptors, which are involved in heart rate regulation in the heart and presynaptic terminals. Its onset time (1–2 min) and duration of action (2–4 h) are synchronized with those of neostigmine, making the combination beneficial for stabilizing heart rate in patients [8]. In contrast, atropine is a non-selective M receptor antagonist with a rapid onset (30–60 s) and a short duration (15–30 min). To reduce oral secretions, atropine was administered during anesthesia induction in both groups, which can increase heart rate. However, considering that the baseline heart rate in this study was measured immediately after administering neuromuscular blockade reversal agents at the end of surgery—a considerable time after anesthesia induction—and given that both groups received the same medication, the influence of atropine on the baseline can be disregarded. In this study, both groups of children reached peak heart rates 2 min after administration, which is earlier than the 3 min observed in adult studies, indicating differences in drug metabolism between children and adults [9]. When used in combination with neostigmine, atropine can lead to heart rate fluctuations and may induce tachyarrhythmias. Compared to glycopyrrolate, atropine’s quick onset and short duration can significantly increase heart rate when used to reverse muscle relaxation, potentially triggering tachyarrhythmias. Subsequently, as the effects of atropine wane, heart rate may decrease, leading to significant fluctuations that can destabilize hemodynamics. In contrast, the combination of glycopyrrolate with neostigmine results in a more stable heart rate and improved hemodynamic stability during recovery. This combination can significantly reduce the risk of postoperative cardiovascular adverse events, such as tachycardia and myocardial ischemia [10]. The subjects of this study were children undergoing penile flap surgery under laryngeal mask general anesthesia combined with caudal block, which provides effective and comprehensive analgesia. Additionally, maintaining a certain depth of anesthesia before remove the laryngeal mask eliminates hemodynamic fluctuations caused by pain or airway irritation, leading to more accurate results.
A statistically significant difference was observed in heart rate AUC (115 ± 36 vs. 129 ± 24 bpm·min) and peak heart rate at 2 min. Neither group experienced severe arrhythmias or acute heart failure. Glycopyrrolate—a selective muscarinic antagonist with 3- to 5-fold greater affinity for M₃/M₁ receptors than cardiac M₂ receptors (which regulate heart rate)—demonstrated superior heart rate stability compared to atropine, thereby reducing sustained tachycardia and consequent myocardial oxygen demand [1]. However, no clinical evidence establishes the practical significance of this difference in children with higher baseline heart rates and greater physiological variability, particularly in low-risk patients. In high-risk populations (e.g., congenital heart disease or hemodynamic instability), even minor heart rate fluctuations may impact myocardial oxygen demand and perfusion. Further research should examine glycopyrrolate’s effects on myocardial oxygen consumption and clinical outcomes, especially in critically ill children.
Another advantage of glycopyrrolate is that its quaternary ammonium structure makes it less likely to cross the blood-brain barrier, resulting in fewer central nervous system-related adverse events, such as postoperative delirium, and minimal cognitive impact. This makes it an ideal anticholinergic agent to be used in conjunction with neostigmine. In this study, there were no statistically significant differences in recovery time or the incidence of delirium between the two groups, and glycopyrrolate did not demonstrate a clear advantage in reducing postoperative delirium in children. Numerous factors can influence postoperative delirium, including advanced age, cognitive impairment, anemia, infection, and pain, with anticholinergic drugs being only one of the contributing factors [11]. Furthermore, assessing postoperative delirium in children is more challenging than in adults.
Our study observed a significantly higher incidence of dry mouth in the glycopyrrolate group compared to the placebo (42% vs. 21%, P < 0.01). Glycopyrrolate, as an anticholinergic agent, causes dry mouth by competitively blocking M3 muscarinic receptors on the salivary glands. This inhibits the action of acetylcholine, significantly reducing salivary secretion. Dry mouth is a direct manifestation of its peripheral anticholinergic effects and a common side effect. While dry mouth is a known side effect of anticholinergics like glycopyrrolate in adults, its implications in pediatric populations warrant specific emphasis. Age has no prominent effect on the pharmacokinetics of glycopyrrolate in children older than 2 months [3]. In pediatric patients, dry mouth can cause discomfort, irritability, or difficulty swallowing, which may affect postoperative recovery, which can exacerbate anxiety in both the children and their parents. Shortening the preoperative and postoperative fasting periods can partially alleviate dry mouth discomfort in pediatric patients [1]. For example, the preoperative water fasting time may be delayed until 2 h before surgery. Postoperatively, based on the patient’s level of consciousness, the fasting duration may be appropriately reduced, with oral fluid intake attempted as early as 2 h after surgery.
There were also some limitations in the present study. First, this study only included boys scheduled to undergo penile flap surgery, sex may exert an influence on drug metabolism [12], these findings may not generalize to girls, other age groups, or patients undergoing higher-stress procedures (e.g., thoracic/abdominal surgeries). Future research should include more diverse pediatric populations. Second, during the pre-experiment, excessive salivation occurred when attempting to induce anesthesia without atropine, so atropine was used for both groups during anesthesia induction in this study. Although this may have impacted baseline heart rate [9], the medication was consistent across both groups and is unlikely to affect the study’s outcomes. Third, routine neuromuscular blockade monitoring should be performed, but due to the institution’s lack of adequate monitoring equipment, the failure to conduct such monitoring represents a limitation of this study. Without objective assessment of blockade reversal (e.g., train-of-four monitoring), the efficacy of both drug combinations in achieving adequate reversal cannot be confirmed, which weakens the clinical relevance of the safety outcomes reported. Future research should employ more objective criteria for evaluating neuromuscular blockade [13]. Fourth, the exact ratio of atropine and glycopyrrolate to neostigmine had not been conclusively determined. In numerous studies, nuanced variations exist in the dosing ratios of atropine or glycopyrrolate to neostigmine. Ramamurthy got similar conclusions, suggesting that a dosage of 0.2 mg of glycopyrrolate mixed with 1 mg of neostigmine appeared to be optimal for achieving the most stable heart rate and the lowest incidence of arrhythmias. Our adopted dosing regimens in this study were guided by clinical evidence from both adult and pediatric populations, as well as approved drug labeling in our jurisdiction [1, 14]. Future studies should further investigate drug dosing in pediatric patients. Fifth, this study only demonstrated a statistically significant difference, which should be viewed as a physiological signal worthy of investigation rather than a definitive clinical indicator. While maintaining stable heart rates may be advantageous for pediatric patients, there is insufficient data to determine to what extent it influences clinical outcomes. Further research is needed to establish this relationship.
Conclusion
Glycopyrrolate has minimal central anticholinergic effects, a lesser impact on cardiac conduction, and a stronger inhibitory effect on salivary secretion, making it an ideal anticholinergic agent. Glycopyrrolate may be preferred in scenarios where hemodynamic stability is prioritized, considering its higher risk of dry mouth.
Acknowledgements
This study was supported by department of Anesthesiology, Shanghai Children’s Hospital. The authors would like to thank the patients who participated in this trial.
Authors’ contributions
ZTX and WR designed the study, conducted the study, acquired the data and revised the manuscript. YXT acquired the data, analyzed the data, prepared figures and tables, wrote the manuscript and modified the language. RLL and WSS acquired the data and analyzed the data. WGQ analyzed the data and wrote the manuscript. YSH revised the manuscript and modified the language. All authors read and approved the final version of the manuscript.
Funding
The study was funded by the Clinical Research Training Program of Shanghai Children’s Hospital (2023YLY08).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
The studies involving human participants were reviewed and approved by Institutional Review Board of Shanghai Children’s Hospital, Shanghai Written informed consent was taken from all the patients. This study was registered in Chinese Clinical Trial Registry (No. ChiCTR2300076270, 28-09-2023) where the trial protocol could be accessed. All adverse events were immediately reported to the Ethics Committee (within 24 h for serious events). Events were categorized by severity with appropriate actions taken. Affected participants were monitored until complete resolution. All cases were reviewed by the research team.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Tianxiao Zou and Xueting Yang contributed equally to this work.
References
- 1.Tao YB, Tang ZL, Lin ZL, Lei WP, Lu XL, Sun JL. Effects of glycopyrrolate and atropine for oral secretions and perioperative hemodynamics in children undergoing tonsillectomy and adenoidectomy: a prospective, single-center, randomized, double-blind, controlled trial. Front Pharmacol. 2024;15: 1344786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chabicovsky M, Winkler S, Soeberdt M, Kilic A, Masur C, Abels C. Pharmacology, toxicology and clinical safety of glycopyrrolate. Toxicol Appl Pharmacol. 2019;370:154–69. [DOI] [PubMed] [Google Scholar]
- 3.Rautakorpi P, Ali-Melkkilä T, Kaila T, Olkkola KT, Iisalo E, Iisalo E, Kanto J. Pharmacokinetics of glycopyrrolate in children. J Clin Anesth. 1994;6(3):217–20. [DOI] [PubMed] [Google Scholar]
- 4.Ittichaikulthol W, Pisitsak C, Wirachpisit N, Piathong P, Suyawet R, Komonhirun R. A comparison of the combination of Atropine and glycopyrrolate with Atropine alone for the reversal of muscle relaxant. J Med Assoc Thai. 2014;97(7):705–9. [PubMed] [Google Scholar]
- 5.Thurlow AC. Cardiac dysrhythmias in outpatient dental anaesthesia in children. The effect of prophylactic intravenous atropine. Anaesthesia. 1972;27(4):429–35. [DOI] [PubMed] [Google Scholar]
- 6.Mirakhur RK, Clarke RS, Elliott J, Dundee JW. Atropine and glycopyrronium premedication. A comparison of the effects on cardiac rate and rhythm during induction of anaesthesia. Anaesthesia. 1978;33(10):906–12. [PubMed] [Google Scholar]
- 7.Howard J, Wigley J, Rosen G, D’Mello J. Glycopyrrolate: it’s time to review. J Clin Anesth. 2017;36:51–3. [DOI] [PubMed] [Google Scholar]
- 8.Paech MJ, Kaye R, Baber C, Nathan EA. Recovery characteristics of patients receiving either Sugammadex or neostigmine and glycopyrrolate for reversal of neuromuscular block: a randomised controlled trial. Anaesthesia. 2018;73(3):340–7. [DOI] [PubMed] [Google Scholar]
- 9.Yun Y, Cao D, Zhang X, Ouyang W, Min S, Lv J, Li L, Huang F. Glycopyrrolate versus atropine for preventing bradycardia induced by neostigmine injection after general anesthesia surgery: a randomized open, parallel-controlled multicenter clinical trial. Am J Transl Res. 2021;13(11):12996–3002. [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y, Ren L, Li Y, Zhou Y, Yang J. The effect of glycopyrrolate vs. atropine in combination with neostigmine on cardiovascular system for reversal of residual neuromuscular blockade in the elderly: a randomized controlled trial. BMC Anesthesiol. 2024;24(1): 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rössler J, Abramczyk E, Paredes S, Anusic N, Pu X, Maheshwari K, Turan A, Ruetzler K. Association of intravenous neostigmine and anticholinergics or sugammadex with postoperative delirium: a retrospective cohort study. Anesth Analg. 2024. 10.1213/ANE.0000000000006939. [DOI] [PubMed] [Google Scholar]
- 12.Braithwaite HE, Payne T, Duce N, Lim J, McCulloch T, Loadsman J, Leslie K, Webster AC, Gaskell A, Sanders RD. Impact of female sex on anaesthetic awareness, depth, and emergence: a systematic review and meta-analysis. Br J Anaesth. 2023;131(3):510–22. [DOI] [PubMed] [Google Scholar]
- 13.Miller RD, Ward TA. Monitoring and pharmacologic reversal of a nondepolarizing neuromuscular blockade should be routine. Anesth Analg. 2010;111(1):3–5. [DOI] [PubMed] [Google Scholar]
- 14.Ramamurthy S, Shaker MH, Winnie AP. Glycopyrrolate as a substitute for atropine in neostigmine reversal of muscle relaxant drugs. Can Anaesth Soc J. 1972;19(4):399–411. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.