Abstract
Background
The aim of this study was to compare the effects of systemic amifostine (Ami) and melatonin (Mel) alone and the combined use of the drugs on apoptotic, oxidative, biochemical and morphometric outcomes caused by radiotherapy (RT)-induced salivary gland injury.
Methods
40 female Sprague-Dawley rats were divided into 5 groups: Control, RT, RT + Ami, RT + Mel, RT + Ami + Mel. 30 min before single dose 5 Gy RT, Ami (200 mg/kg) was administered for 3 days and Mel (10 mg/kg) was administered for 15 days. Caspase-3 and Ki-67 apoptotic activity markers and NF-Κb/p65, a transcription factor, were analyzed immunohistochemically, and serum inflammatory cytokine (IL-1β, IL-10, IL-6, TNF-α) and oxidative stress (8-OHdG and OSI) levels were analyzed biochemically.
Results
The destructive effect of RT on the salivary gland has been clearly demonstrated at the level of histopathological damage, apoptotic activity, oxidative stress and biochemical parameters. When the two drug groups were compared, no statistical difference was found (p > 0.05); Mel prophylaxis provided significant decreases, especially in IL-1β and OSI levels (p < 0.05). With the combined use of both drugs, a slightly synergistic effect at the inflammatory, oxidative and apoptotic levels has been proven.
Conclusion
Mel has a salivary radioprotective activity comparable to Ami. The combined use of drugs has not provided a significant therapeutic advantage in preventing salivary inflammatory stress and apoptotic changes. Thus, Mel may be an alternative to Ami to prevent RT-induced salivary gland damage.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12903-025-06859-6.
Keywords: Radiotherapy, Amifostine, Melatonin, Salivary gland, Rat
Introduction
Head and neck cancer is a worldwide disease that is the seventh most common among all cancer types, with more than 660.000 new cases annually and approximately half of these resulting in death [1, 2]. Radiotherapy (RT) is a treatment for head and neck cancer, used alone or in combination with chemotherapy and/or surgery, and is effective in destroying the tumor and preventing cancer recurrence [3, 4]. However, unfortunately, it can damage adjacent healthy tissues as well as cancer cells.
In the head and neck region, the salivary glands are indirectly damaged by RT due to their proximity to the target area. They lose their secretory function due to high levels of radiation sensitivity [5–7]. Studies have shown that severe radiation damage to the salivary glands leads to an increase in fibrosis and inflammation, and eventually to salivary gland dysfunction [7–9]. This results in dry mouth (xerostomia). Patients with radiation-induced salivary hypotension may experience rapidly progressing dental caries, oral mucosal ulceration, oral infections such as candidiasis, burning sensation in the mouth and changes in taste, trauma, and difficulty eating and swallowing, which directly affect the patient’s quality of life [10].
The prophylactic use of radioprotectants to prevent the side effects of RT is a current area of research [11–15]. One of the best known radioprotective drugs is amifostine (Ami). It is a cytoprotective free radical scavenger that preferentially accumulates in the salivary glands when administered immediately before RT [16]. It is a prodrug that is dephosphorylated by alkaline phosphatase to an active metabolite and selectively accumulates in normal tissues. Studies have shown that Ami reduces severe mucositis and dry mouth and significantly protects secretory cells against radiation-induced hyposalivation [17, 18]. In this context, Ami is the only known FDA-approved agent with radioprotective properties for the prevention of dry mouth [19, 20].
Melatonin (Mel) is a neuroendocrine hormone that is synthesized from tryptophan, an amino acid [21]. Under a wide range of physiological and pathological conditions, Mel and its metabolites modulate multiple mechanisms, including the immune response, inflammation, oxidative stress, apoptosis and sleep [22, 23]. Mel, a well-known powerful antioxidant [24, 25], has potential antioxidative [26] and radioprotective properties [11, 27, 28]. A paucity of studies has been observed in the examination of the effect of Mel on the destruction of the salivary gland after RT [11, 12, 27]. Cakmak Karaer et al. [12] demonstrated that Mel has the capacity to reduce RT-associated salivary gland damage in an animal model. In view of the aforementioned data, this experimental study was planned based on the hypothesis that systemic Ami and Mel administration would limit RT-associated salivary gland damage.
The present study has two main aims: (1) To investigate the destructive effects of RT on the salivary gland, (2) to comparatively investigate the salivary radioprotective efficacy of systemic Ami and Mel administered alone or in combination. In this context, we evaluate and compare the limiting effects of Ami and Mel on the destructive effect of RT on the salivary glands by analyzing apoptotic mechanisms (Caspase-3, Ki-67), oxidative (Oxidative Stress Index (OSI), 8-hydroxy deoxyguanosine (8-OHdG)) and inflammatory markers (Interleukin (IL)−1β, IL-10, Tumor necrosis factor alpha (TNF-α), IL-6) in the tissue.
Materials and methods
Ethics approval and consent to participate
This study was approved by the Recep Tayyip Erdoğan University (RTEU) Animal Experiments Ethics Committee with the ethics committee decision numbered 2023/45. The experimental study was carried out considering the quality of life and welfare of experimental animals. All animals received humane care as specified in the Guide for the Care and Use of Laboratory Animals prepared by the National Academy of Sciences and published by the National Institutes of Health. The ARRIVE (Animal Research: Reporting In Vivo Experiments) guidelines were meticulously adhered to throughout all phases of the study [29]. RTEU Scientific Research Projects Unit TSA-2024-1622 project contributed to the study.
Supply and living environment of rats
All experimental stages of this 22-day animal study, completed in June 2023, were carried out at the RTEU Experimental Animal Center, where 40 female Sprague-Dawley rats were provided. Sample size estimation was based on Total Oxidative Status (TOS) and OSI data from a similar rat model [12], which showed large effect sizes (RT vs RT+Mel: TOS d≈2.36, OSI d≈1.90). With two-sided α=0.05, G*Power 3.1 indicated that 5–6 animals per group (≈6–7 after a nonparametric adjustment for Mann–Whitney/Kruskal–Wallis) would achieve 80% power for the RT vs RT+Mel contrasts. Therefore, the chosen sample size of n=8 per group was considered sufficient to detect large differences in TOS and OSI for RT+Mel. Moreover, the sample size for the study was calculated in accordance with the work of Charan et al. [30] Thus, our study groups were designed with eight rats in each group. The rats were provided with unlimited access to food and water. They were housed in an environment with a 12-h Light-dark cycle with controlled room temperature. The animals were allowed to adapt to environmental conditions for 7 days.
Study groups
The subjects were randomly distributed into 5 groups of 8 rats each: Control, RT, RT+Ami, RT+Mel, RT+Ami+Mel.
Radiotherapy, amifostine and melatonin administration protocol
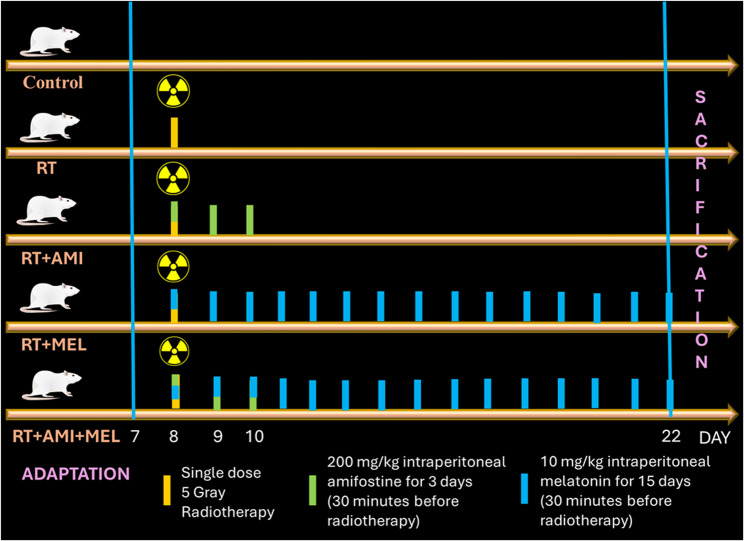
In accordance with our preceding studies [31, 32], a single dose of RT (5 Gray) was dispensed to rats 30 minutes following the intraperitoneal administration of Ami (200 mg/kg) [32, 33] and Mel (10 mg/kg) [31, 32] on the 8th day. The administration of drugs was continued for the subsequent 2 days for Ami [32, 33] and for the following 14 days for Mel [31, 32]. Mel was freshly dissolved in physiological saline solution (0.01%) and administered at night (23:00) [31, 32]. The groups that did not receive the drug (Control and RT) were administered saline intraperitoneally at 11:00 at night for 15 days, and the RT+Ami group was administered saline intraperitoneally for the remaining 12 days after the 3-day Ami protocol [32]. RT planning was performed using 6 MV photon irradiation, 4 Gy/min dose rate linear accelerator (Elekta Synergy; Elekta, Crawley, United Kingdom) and CMS XiO planning system (version 13.2) [34]. According to our previous study, [31, 32] whole cranial irradiation was applied with a single dose of 5 Gray (Fig. 1).
Fig. 1.
Experiment scheme
Sacrification, sample supply and analysis
Intraperitoneal injections of xylazine hydrochloride (10 mg/kg, Rompun, Bayer, Istanbul, Turkey) and ketamine hydrochloride (40 mg/kg, Ketalar, Pfizer, Istanbul, Turkey) were used to induce anesthesia in all groups on the day after Mel administration (day 22). Additionally, intracardiac left ventricular blood samples were obtained using 10-ml syringes. Serum samples were obtained by centrifuging collected blood samples at 1739 g for 15 minutes at +4°C. The acquired blood serum (supernatants) was put into Eppendorf tubes and kept in a Thermo Scientific TLE series deep freezer at -80°C until the day of analysis. As soon as the rats' blood samples were taken, decapitation was administered.
Carefully separated from the surrounding tissues, the right salivary glands were taken out for histological (immunohistochemical) examination. After that, they were put in containers with a 10% neutral formaldehyde solution. Biochemical analysis was performed using the acquired supernatants. Expert investigators (biochemistry: A.Y., K.A.; histology: T.M., L.T.) who were blind to the research group assignment conducted the biochemical and histological analyses. Using immunohistochemical analyses, the densities of Caspase-3, Ki-67, and NF-κB/p65 cells were assessed. Using ELISA and spectrophotometric techniques, the levels of IL-1β, IL-10, IL-6, TNF-α, 8-OHdG, TOS, and Total Antioxidant Status (TAS) were biochemically analyzed. The ratios of TOS/TAS and IL-1β/IL-10 were computed.
Histopathological analysis
Parotid gland tissues were cut into 1.5 cm³ pieces and fixed in 10% formaldehyde solution for 24 hours. Following the fixation process, routine histological follow-up procedures including dehydration, mordanting and paraffin inclusion were performed using a tissue tracking device (Thermo Scientific, Shendon Citadel 2000, England). In the subsequent stage of the procedure, parotid gland tissue samples were embedded in hard paraffin in a tissue embedding device (Leica 1150EG, Leica Biosystems, Germany) and blocked in tissue embedding cassettes (Merck KGAa, Darmstadt, Germany). Using a rotary microtome (Leica RM2255, Germany), 4-5 µm thick sections were taken from the paraffin blocks and stained with hematoxylin & eosin (Merck GmbH, Darmstadt, Germany).
Immunohistochemical procedure
Caspase-3 (rabbit polyclonal, ab16888, Abcam, UK) was used to identify endoplasmic apoptotic cells, Ki-67 primary antibody (rabbit polyclonal, ab21685, Abcam, UK) was used to evaluate proliferation in acinar and ductal epithelial cells, and NF-Kβ/p65 primary antibody kits were used to evaluate pro-inflammatory cytokines (rabbit polyclonal, ab227078, Abcam, UK). Secondary antibody (Goat Anti-Rabbit IgG H&L (HRP), ab205718, Abcam, UK) kits were utilized in conjunction with the primary kits.
Sections measuring 2-3 µm in thickness were obtained from paraffin blocks of parotid gland tissue using a rotary microtome (Leica RM2525, Leica Biosystems, Germany). The obtained sections were incubated with primary and secondary antibodies for 60 minutes after deparaffinization and antigen retrieval procedure using the ISH/ICH device (Bond Max, Leica Biosystems, Australia) in accordance with the manufacturer's instructions. In the next step, sections of the parotid gland tissue were stained with diaminobenzidine tetrahydrochloride (DAB ultraview, Leica Biosystems, Germany) and Harris hematoxylin (Merck KGAa, Darmstadt, Germany).
Semi-quantitative analysis
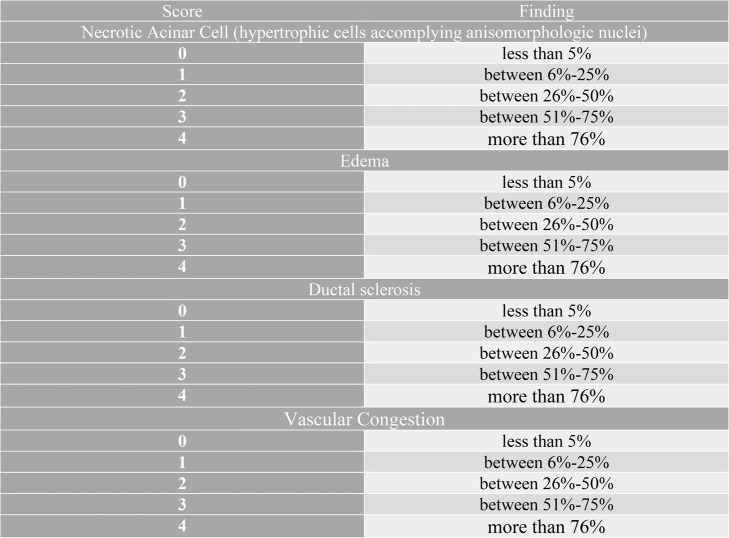
Scoring of parotid tissue sections: Parotid Histopathological Damage Score (PDS) was calculated in accordance with studies on parotid tissue damage due to x-ray irradiation, necrosis in serous acini, intralobular ductus sclerosis, edematous areas and vascular congestion [35, 36] (Table 1.). In the parotid tissue sections of each rat, 20 different areas were randomly scored by a double-blind histopathologist under a x40 objective.
Table 1.
Parotid gland histopathological damage score (PDS)
Biochemical analyses
Analysis of serum IL-1β, IL-10, TNF-α, IL-6 and 8-OHdG levels with ELISA kit
A rat-specific ELISA kit from Elabscience (Houston, Texas, USA) was utilized to assess the serum levels of IL-1β (catalog number: E-EL-R0012), IL-10 (catalog number: E-EL-R0016), TNF-α (catalog number: E-EL-R2856), IL-6 (catalog number: E-EL-R0015) and 8-OHdG (catalog number: E-EL-0028). Results were expressed as pg/mL and ng/mL
Determination of TOS and TAS levels and calculation of OSI
The total amounts of the relevant molecules measured as a whole thanks to an automatic method devised by Erel that uses the principle of light absorption to determine the TOS and TAS levels of the supernatants [37, 38]. OSI= [(TOS (µmol H2O2 equivalent/L)/TAS (µmol Trolox equivalent/L)] was used to compute the TOS/TAS percentage ratio [39]
Statistical analysis
All data obtained as a result of quantitative and semi-quantitative analyses were evaluated by performing the Kruskal-Wallis test using the SPSS 29.0 (IBM Corp., Armonk, NJ, USA) statistical program to determine whether the data conformed to a normal distribution. Significance values were adjusted for multiple testing with Bonferroni correction. The data obtained as a result of quantitative analysis were reported as median, minimum and maximum. The relationship between the parameters was evaluated by Spearman correlation analysis and rho values were reported. Non-parametric data obtained as a result of semi-quantitative analyses were calculated as median, 25% and 75% interquartile ranges, taking into account the maximum and minimum values. Regarding statistical significance, the type-1 error level was set at 5%.
Results
Histomorphometric results
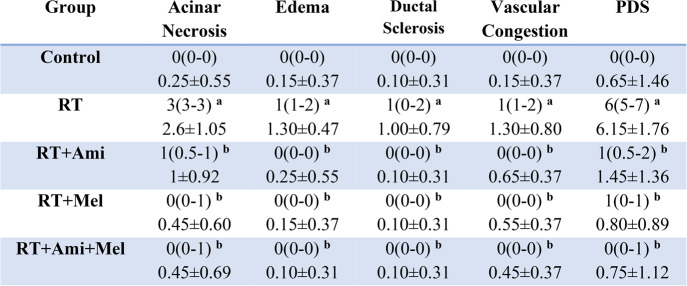
Regarding acinar necrosis, edema, ductal sclerosis, vascular congestion and total PDS score; irradiated group showed significant differences compared to the control group. Ami and Mel applications, both individually and in combination, have shown that they significantly suppress the destructive effects of RT (p < 0.05) (Fig. 2.) (Table 2.)
Fig. 2.
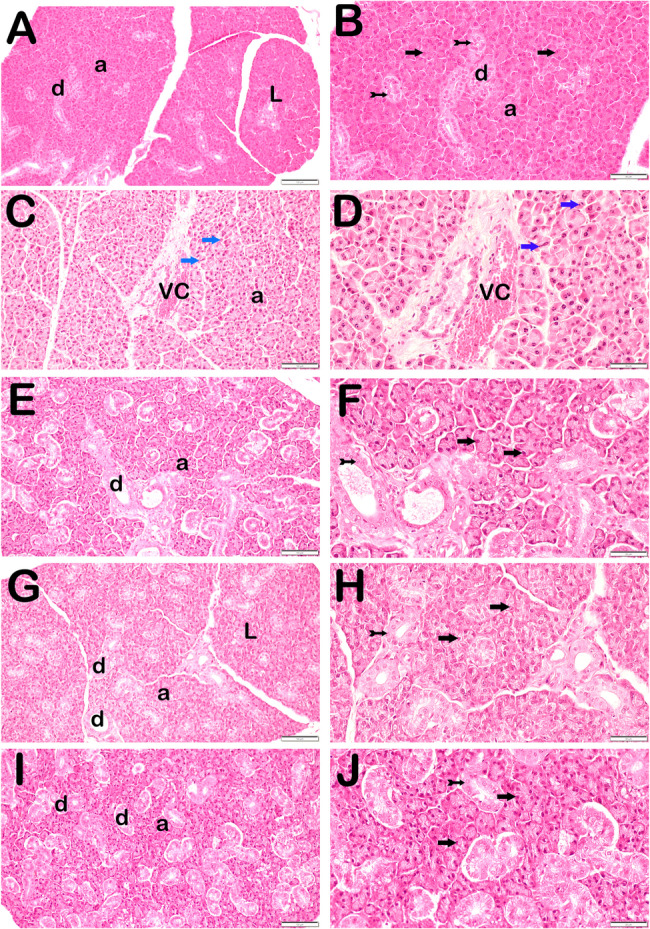
Representative light microscopic image of sections of parotid gland tissue stained with hematoxylin and eosin (RT: Radiotherapy, Ami: Amifostine, Mel: Melatonin; A: Control(x20), B: Control(x40), C: RT(x20), D: RT(x40), E: RT+Ami(x20),F: RT+Ami(x40), G: RT+Mel(x20), H: RT+Mel(x40), I: RT+Ami+Mel(x20), J: RT+Ami+Mel(x40). a: Secretory Acini of Parotid, d: Intralobular Ductus, L: Lobules, VC: Vascular Congestion; black arrow: secretory acinus epithelial cell, tailed arrow: intralobular ductus epithelial cell, blue arrow: necrotic acinus)
Table 2.
Histopathological damage score results in salivary gland tissue
Data are presented as median(25%-75% interquartile range) and Mean ± SD. RT: Radiotherapy, Ami: Amifostine, Mel: Melatonin. x(y-z): median(25%-75% interquartile range), SD: Standard Deviation. Footnote symbols (a, b) signify statistically significant differences between the groups; (a) p=0.01 versus to Control Group, (b) p=0.01 versus to RT Group, Kruskal Wallis/Tamhane T2 test
Immunohistochemical results
Tissue Caspase-3, Ki-67 and NF-κB/p65
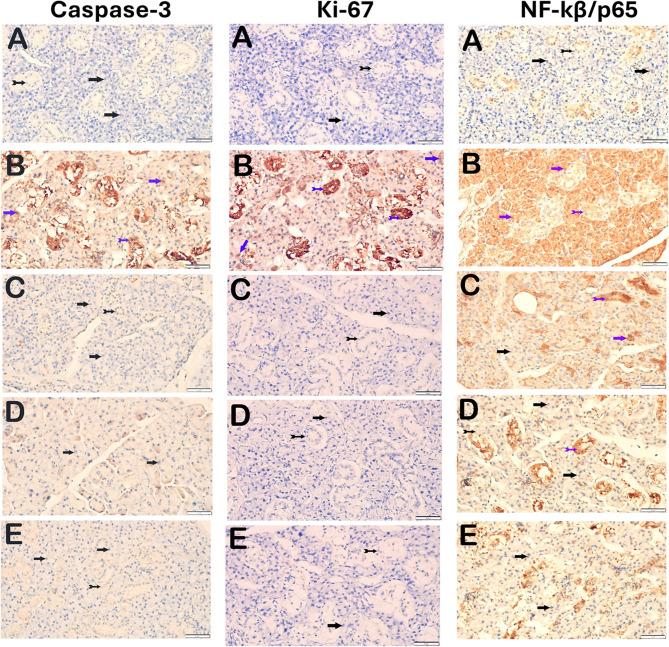
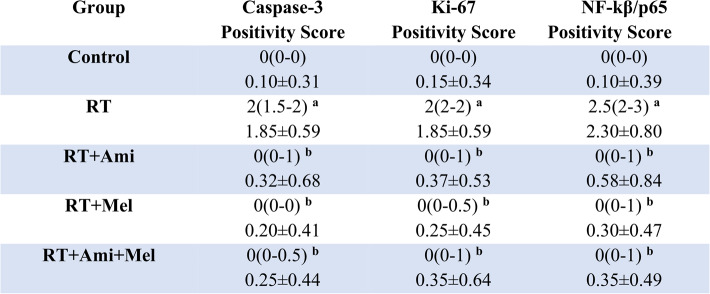
A significant difference was observed between the irradiated group and the control group at the level of all immunohistochemical parameters examined. It was determined that the difference was statistically significant in the groups receiving single and combined drugs compared to the RT group (p<0.05) (Fig. 3) (Table 3).
Fig. 3.
Representative light microscopic image of sections of parotid gland tissue incubated with Caspase-3, Ki-67, NF-kβ/p65 primary antibody (RT: Radiotherapy, Ami: Amifostine, Mel: Melatonin; A: Control, B: RT, C: RT+Ami, D: RT+Mel, E: RT+Ami+Mel; black arrow: serous acinus epithelial cell, tailed arrow: ductal epithelial cell, blue arrow: atypical acinar epithelial cell, blue tailed arrow: atypical ductal epithelial cell)
Table 3.
Immunohistochemical score results in salivary gland tissue
Data are presented as median(25%-75% interquartile range) and Mean ± SD. RT: Radiotherapy, Ami: Amifostine, Mel: Melatonin. x(y-z): median(25%-75% interquartile range), SD: Standard Deviation. Footnote symbols (a, b) signify statistically significant differences between the groups; (a) p=0.01 versus to Control Group, (b) p=0.01 versus to RT Group, Kruskal Wallis/Tamhane T2 test
Biochemical results
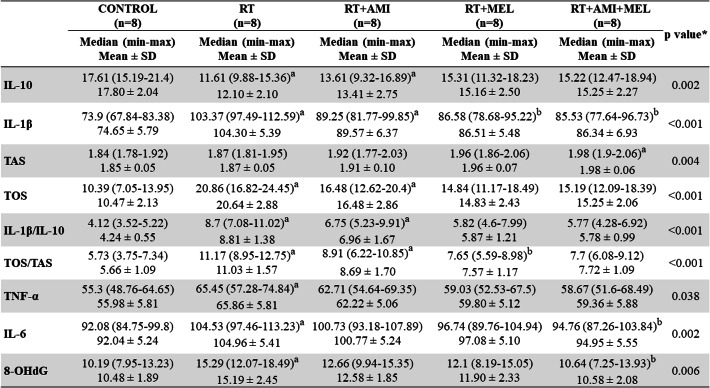
Serum IL-1β, IL-10, IL-1β/IL-10, TNF-α, IL-6 and 8-OHdG
Important differences were detected between the irradiated group and the control group in all inflammatory parameters. It was proven that the combined use of the two agents suppressed the negative effect of RT, with significant decreases in IL-1β, IL-6 and 8-OHdG levels. While the difference in IL-1β, IL-10 and IL-1β/IL-10 levels between the control group and the RT + Ami group was statistically significant (p < 0.05), the difference was not considerable in the Mel group and was found to be at a level approaching the control group (p > 0.05). With Mel prophylaxis, a substantial difference was detected only in the IL-1β level compared to the irradiated group (p < 0.05) (Table 4).
Table 4.
Comparison of biochemical parameter results between groups
Data are presented as Median(min-max) and Mean ± SD. RT: Radiotherapy, AMI: Amifostine, MEL: Melatonin; min: minimum, max: maximum; SD: Standard Deviation. Footnote symbols (a, b) signify statistically significant differences between the groups. (a) intergroup, compared to Control; (b) intergroup, compared to RT. Kruskal-Wallis test (p <0.05). Significance values have been adjusted by the Bonferroni correction for multiple tests
Serum TOS, TAS and OSI
In terms of TOS and OSI levels, the difference was found to be statistically significant in both the RT and RT + Ami groups in comparison to the control group. The Mel prophylaxis group demonstrated a significant difference compared to the irradiated group only in the OSI level (p = 0.034). With regard to antioxidant capacity, a significant difference was observed in the Ami + Mel group compared to the control group (p < 0.05) (Table 4)
Discussion
One of the organs most affected by head and neck RT is the salivary glands. Radiation-induced salivary gland dysfunction is characterized by hyposalivation, loss of acinar cells, apoptosis, oxidative damage, inflammation, and fibrosis [40, 41]. Xerostomia may be encountered as a clinical reflection of salivary gland damage. This situation is detrimental to the patients’ overall quality of life. Therefore, a considerable volume of research has been conducted on the management of xerostomia [42, 43]. Nevertheless, it would be more efficacious to prevent the occurrence of the problem before treating it. Therefore, we preferred to apply two different agents prophylactically in our study. Ami is a drug that stands out with its cytoprotective activity, but in its current use it is only approved by the FDA for mucositis and xerostomia [19, 20]. Mel is an agent with a dominant antioxidant role and almost no side effects when used correctly [23]. Moreover, the radioprotective properties of Mel, like Ami, have been reported [11, 27]. Therefore, we aimed to compare the efficacy of these two agents and to demonstrate the potential synergistic effect when used together. To the best of our knowledge, our study is the first to comparatively examine the use of systemic Ami and Mel, as well as the combined use of these drugs, in preventing the destructive effects of RT on the salivary gland.
According to the current findings in our study; apoptosis, necrosis, edema, sclerosis and vascular congestion were observed in the irradiated group in histological examinations. In both prophylactic drug groups, apoptosis and salivary gland damage were suppressed thanks to their radioprotective properties. A recently published study by Ren et al. [11] revealed that Mel inhibits whole neck irradiation-induced fibrosis. This study demonstrated that Mel could partially alleviate RT-induced xerostomia. Sagowski et al. [44] showed that late effects such as fibrosis and necrosis developed less in the rat salivary gland with Ami application 6 months after irradiation. In our study, early effects of RT on salivary gland histomorphometric damage could be observed.
In the present study, the destructive effect of RT on the salivary gland was also proven by changes in biochemical parameters. Although there was no statistically significant difference in terms of immunohistochemistry outcomes between the Ami and Mel groups, Mel was found to be superior to Ami at the level of biochemical parameters, probably due to its antioxidant role. In the group where two drugs are used in combination (RT + Ami + Mel), no statistically significant difference was observed in comparison to the drug-alone groups (RT + Ami and RT + Mel). The RT + Ami + Mel group was found to demonstrate a slight biochemical superiority over the RT + Ami group. In the process of inflammation, TNF-α and IL-1 cytokines have been demonstrated to facilitate the proinflammatory mechanism by inducing alterations in cell phenotype through the activation of the NF-κB pathway [45, 46]. In our study findings, it has been proven that the increased NF-κB/p65 positivity as a result of irradiation is significantly suppressed using both agents alone and in combination. However, a review of the literature reveals an absence of animal studies that directly compare our results at this parameter level.
It is widely acknowledged that RT induces oxidative stress [13, 47]. In our study, the levels of OSI and 8-OHdG, an oxidative DNA destruction marker, were tested as indicators of oxidative stress. A significant difference was observed between the irradiated group and the control group with regard to both parameters. Mel prophylaxis significantly reduced oxidative stress levels. Ami, on the other hand, was not superior to Mel in terms of antioxidant effects. Cakmak Karaer et al. [12] showed that Mel reduces radiation-induced histological damage in the parotid and submandibular glands. Additionally, Mel reduced oxidative stress markers such as MDA, TOS and OSI and increased the levels of antioxidant markers. Our results are consistent with evidence of the protective role of melatonin in radiotherapy-associated oxidative damage. One of the numerous studies on Mel conducted by our team investigated the role of Mel in the destructive effects of RT on periodontal tissues [31]. It has been determined that preventive Mel application significantly reduces oxidative parameters and prevents periodontal damage. However, unlike our study findings, no statistically significant difference was found between the RT and control groups in terms of OSI, 8-OHdG and TNF-α levels. It was determined that Mel application significantly reduced 8-OHdG and IL-1β levels. In another recent study [32] investigating the therapeutic efficacy of AMI and Mel in the RT-associated periodontal tissue destruction model, the partial superiority of Mel in preventing periodontal inflammatory stress and apoptosis was determined. In the study by Zhang et al. [13], comparing the effect of irradiation on parotid gland damage with AMI and another antioxidant agent, it was proven that ROS levels increased in the irradiated group. Similar to our findings, AMI was found to be effective on the inhibition of oxidative stress and apoptosis, but did not have the same effect on fibrosis.
There are molecules that have been investigated for years for -associated apoptosis. The most well-known apoptosis markers are the Caspase and Ki-67 families. Caspase-3 is a member of the cysteine protease family, which plays a role in apoptotic mechanisms [48]. Ki-67 is a non-histone nuclear protein that is expressed in actively proliferating cells in all phases of the cell cycle except the G0 resting phase [49]. These two markers have been examined in numerous studies as apoptosis markers due to their roles in the mechanisms of apoptosis [11, 14, 50]. Ren et al. [11] observed modulation of oxidative stress in the salivary glands through effects on 8-OHdG, as well as inhibition of DNA damage and apoptosis, in mice receiving Mel compared with mice receiving RT. They found that the expression of Ki-67, a marker of cell proliferation, was significantly increased in mice receiving RT, as was the number of TUNEL-positive cells. In the RT + Mel group, the number of apoptotic cells was observed to be significantly reduced. Similarly, the antiapoptotic and antioxidant properties of melatonin were emphasized in our study. Additionally, apoptotic activity was suppressed in the amifostine group and the combined group. Conversely, although melatonin and amifostine administered individually resulted in a substantial decline in 8-OHdG levels, a more pronounced decrease was observed in the RT + Ami + Mel group. In another study [50], the preventive effect of Mel on radiation-induced oral mucositis was investigated. It has been shown that radiation-induced oral mucositis is suppressed in mice administered Mel. In the RT group, a significantly higher number of TUNEL positive cells were detected compared to the control group; in the RT + Mel group, the number of TUNEL positive cells was higher than in the control group, but significantly lower than in the RT group. Moreover, the determination of Caspase-3 yielded analogous results. Furthermore, an analysis of the 8-OHdG results, a marker of necrotic cells, revealed a substantial difference in the entire mucosa exclusively between the control group and the RT group; no significant variations were observed among the other groups. In this sense, our Mel findings overlap at the Caspase-3 level. Furthermore, the incorporation of Ami into our study, in conjunction with the comparison and combined utilization of the two agents, yielded valuable results. Moreover, combined administration has been proven to significantly suppress radiation-induced apoptotic mechanisms. Aras et al. [28] found that Mel application could provide a significant reduction in RT-induced inflammation, vacuolization, disruption, swelling, necrosis and apoptotic activity. In a recent study by Safak et al. [14], it was found that Ami significantly reduced salivary Caspase-3 activity in rats receiving RT, similar to our findings. In addition, Ami was able to significantly limit acinar epithelial cell vacuolization, vascular occlusion and fibrosis in the salivary gland tissue. In the study by Zhang et al. [13], a comparison was made between Ami and a different drug group in terms of salivary radioprotective effect, with the observation of similar results in terms of apoptosis inhibition.
Ami, a radioprotective and cytoprotective agent, was examined, and it was clearly shown that it had a significant cytoprotective effect on the late toxicity of irradiated salivary glands [51–54]. However, there are some studies that do not support the radioprotective and xerostomia-preventing effects of Ami. In two different studies conducted by Sagowski et al. [44, 55], the cytoprotective effect of Ami on acute toxicity in the rat salivary gland could not be detected. This result may be due to the difference in radiation dose and indeed suggests that Ami is more effective on late effects.
Ami is the only FDA-approved pharmaceutical agent employed to prevent xerostomia resulting from salivary gland involvement, particularly in patients undergoing head and neck RT [19, 20]. Two different studies [56, 57] found low-quality evidence that Ami prevents dry mouth. In a study conducted by Varghese et al. [18] on mice, Ami or its active metabolite was observed to protect the salivary gland acinar cell count and saliva secretion levels upon radiation. Another study [52] demonstrated that Ami provided long-term protection against loss of salivary secretion. However, in the study by Zhang et al. [13], it was observed that Ami had no significant effect on salivary secretion function at the very early stage in radiation-induced parotid gland damage. Considering the different study results, Ami protection may be considered more effective in the long term.
The present study is not without its limitations. In the present study, RT was administered as a single dose. Observing the effects of different doses and/or fractionation will add value to the study. Secondly, unfortunately, there is no consensus on Mel and Ami administration protocols in experimental animal models. Finally, the present study incorporated female rats. It may not be accurate to generalize the results for both genders. However, for the purpose of standardization, it is necessary and important that the groups are of the same gender.
In this context, a comprehensive review of the extant literature revealed a paucity of studies addressing this subject. In a review discussing agents to prevent dry mouth in head and neck cancer patients, no routine systemic chemical treatment protocol was specified that could prevent the development of xerostomia in clinical practice [15]. Considering the available studies, although Ami is an approved drug for xerostomia, some studies [44, 55] have reported that it did not show sufficient effectiveness. Moreover, Ami has side effects such as hypotension, nausea, and vomiting [18]. In contrast, Mel has emerged as a promising agent with a favorable profile. When both drugs are evaluated in terms of side effects and clinical use, it can be concluded that Mel is superior to Ami. However, further research is required on this subject.
Conclusion
This experimental study concluded that Mel exhibited comparable radioprotective activity to Ami. Interestingly, it was seen that it was able to suppress RT-related salivary inflammatory stress more significantly compared to Ami, especially in biochemical analysis. Considering its very limited side effect profile, it may form the basis for more extensive in vivo studies and further clinical trials for its prophylactic application in preventing the destructive effects of head and neck RT on the salivary glands
Supplementary Information
Acknowledgements
This study has been supported by the Recep Tayyip Erdogan University Development Foundation (Grant number: 02025004024445). We would like to thank Lect. PhD Kerimali AKYILDIZ for performing the biochemical analysis of the study, Prof. Dr. Levent TUMKAYA for histological examinations, and Assoc. Prof. Dr. Medeni ARPA for performing the statistical data analysis.
Abbreviations
- RT
Radiotherapy
- Ami
Amifostine
- Mel
Melatonin
- RTEU
Recep Tayyip Erdogan University
- IL
Interleukin
- TNF-α
Tumor necrosis factor alpha
- TOS
Total Oxidative Status
- TAS
Total Antioxidant Status
- FDA
Food and Drug Administration
Authors’ contributions
N.Y.: Conceptualization, methodology, writing, editing, prepared all figures; O.K.: Conceptualization, methodology, writing, editing; S.Y.R.: Radiotherapy protocol; M.T.: Methodology, editing; M.Z.K.: Methodology, editing; T.M.: Histological analysis. A.Y.: Biochemical analysis. All authors reviewed the manuscript.
Funding
This study was supported by the Scientific Research Fund of Recep Tayyip Erdogan University (Bap-TSA-2024-1622). The funder had no role in the design, analysis, and reporting of the study.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by the RTEU Local Ethics Committee with the decision letter 2023/45 on 14.11.2023.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. [DOI] [PubMed] [Google Scholar]
- 2.Johnson DE, Burtness B, Leemans CR, Lui VWY, Bauman JE, Grandis JR. Head and neck squamous cell carcinoma. Nat Rev Dis Primers. 2020;6(1):92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poole ME, Sailer SL, Rosenman JG, Tepper JE, Weissler MC, Shockley WW, et al. Chemoradiation for locally advanced squamous cell carcinoma of the head and neck for organ preservation and palliation. Arch Otolaryngol Head Neck Surg. 2001;127(12):1446–50. [DOI] [PubMed] [Google Scholar]
- 4.Xiang M, Colevas AD, Holsinger FC, Le QX, Beadle BM. Survival after definitive chemoradiotherapy with concurrent cisplatin or carboplatin for head and neck cancer. J Natl Compr Canc Netw. 2019;17(9):1065–73. [DOI] [PubMed] [Google Scholar]
- 5.Davy C, Heathcote S. A systematic review of interventions to mitigate radiotherapy-induced oral mucositis in head and neck cancer patients. Support Care Cancer. 2021;29(4):2187–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jensen SB, Vissink A, Limesand KH, Reyland ME. Salivary gland hypofunction and Xerostomia in head and neck radiation patients. J Natl Cancer Inst Monogr. 2019;2019(53):95–106. [DOI] [PubMed]
- 7.Jasmer KJ, Gilman KE, Munoz Forti K, Weisman GA, Limesand KH. Radiation-induced salivary gland dysfunction: mechanisms, therapeutics and future directions. J Clin Med. 2020. 10.3390/jcm9124095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abedi SM, Yarmand F, Motallebnejad M, Seyedmajidi M, Moslemi D, Ashrafpour M, et al. Vitamin E protects salivary glands dysfunction induced by ionizing radiation in rats. Arch Oral Biol. 2015;60(9):1403–9. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka J, Mishima K. Application of regenerative medicine to salivary gland hypofunction. Jpn Dent Sci Rev. 2021;57:54–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heller D, Al-Hashimi I, Pacheco VB. Saliva and its importance in sjögren’s syndrome. In: Cha S, editor. Sjögren’s Syndrome and Oral Health. Springer; 2021. p. 21–30. [Google Scholar]
- 11.Ren J, Zhu T, Wang Z, Lu L, Fan S. Amelioration of gamma irradiation-induced salivary gland damage in mice using melatonin. J Pineal Res. 2023;75(2):e12897. [DOI] [PubMed] [Google Scholar]
- 12.Cakmak Karaer I, Simsek G, Yildiz A, Vardi N, Polat A, Tanbek K, et al. Melatonin’s protective effect on the salivary gland against ionized radiation damage in rats. J Oral Pathol Med. 2016;45(6):444–9. [DOI] [PubMed] [Google Scholar]
- 13.Zhang T, Liu C, Ma S, Gao Y, Wang R. Protective effect and mechanism of action of Rosmarinic acid on radiation-induced parotid gland injury in rats. Dose Response. 2020;18(1):1559325820907782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Safak G, Celiker M, Tumkaya L, Mercantepe T, Rakici S, Cinar S, et al. Comparison of effects of dexmedetomidine and amifostine against X-ray radiation-induced parotid damage. Radiat Environ Biophys. 2022;61(2):241–53. [DOI] [PubMed] [Google Scholar]
- 15.Sardellitti L, Bortone A, Filigheddu E, Serralutzu F, Milia EP. Xerostomia: from pharmacological treatments to traditional medicine-an overview on the possible clinical management and prevention using systemic approaches. Curr Oncol. 2023;30(5):4412–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scrimger R. Salivary gland sparing in the treatment of head and neck cancer. Expert Rev Anticancer Ther. 2011;11(9):1437–48. [DOI] [PubMed] [Google Scholar]
- 17.Gu J, Zhu S, Li X, Wu H, Li Y, Hua F. Effect of amifostine in head and neck cancer patients treated with radiotherapy: a systematic review and meta-analysis based on randomized controlled trials. PLoS One. 2014;9(5):e95968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Varghese JJ, Schmale IL, Mickelsen D, Hansen ME, Newlands SD, Benoit DSW, et al. Localized delivery of amifostine enhances salivary gland radioprotection. J Dent Res. 2018;97(11):1252–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brizel DM, Wasserman TH, Henke M, Strnad V, Rudat V, Monnier A, et al. Phase III randomized trial of amifostine as a radioprotector in head and neck cancer. J Clin Oncol. 2000;18(19):3339–45. [DOI] [PubMed] [Google Scholar]
- 20.Andreassen CN, Grau C, Lindegaard JC. Chemical radioprotection: a critical review of amifostine as a cytoprotector in radiotherapy. Semin Radiat Oncol. 2003;13(1):62–72. [DOI] [PubMed] [Google Scholar]
- 21.Fiore A, Murray PJ. Tryptophan and indole metabolism in immune regulation. Curr Opin Immunol. 2021;70:7–14. [DOI] [PubMed] [Google Scholar]
- 22.Reiter RJ, Tan DX, Galano A. Melatonin: exceeding expectations. Physiol (Bethesda). 2014;29(5):325–33. [DOI] [PubMed] [Google Scholar]
- 23.Cipolla-Neto J, Amaral FGD. Melatonin as a hormone: new physiological and clinical insights. Endocr Rev. 2018;39(6):990–1028. [DOI] [PubMed] [Google Scholar]
- 24.Tordjman S, Chokron S, Delorme R, Charrier A, Bellissant E, Jaafari N, et al. Melatonin: pharmacology, functions and therapeutic benefits. Curr Neuropharmacol. 2017;15(3):434–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang HM, Zhang Y. Melatonin: a well-documented antioxidant with conditional pro-oxidant actions. J Pineal Res. 2014;57(2):131–46. [DOI] [PubMed] [Google Scholar]
- 26.Tan DX, Manchester LC, Esteban-Zubero E, Zhou Z, Reiter RJ. Melatonin as a potent and inducible endogenous antioxidant: synthesis and metabolism. Molecules. 2015;20(10):18886–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koc M, Deniz CD, Eryilmaz MA, Tezcan Y, Gurbilek M. Radioprotective effects of melatonin and thymoquinone on liver, parotid gland, brain, and testis of rats exposed to total body irradiation. Turk J Med Sci. 2023;53(4):902–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aras S, Tanzer IHO, Karacavus S, Sayir N, Erdem E, Hacimustafaoglu F, et al. Effect of melatonin on low and high dose radiotherapy induced thyroid injury. Biotech Histochem. 2023;98(5):346–52. [DOI] [PubMed] [Google Scholar]
- 29.Percie du Sert N, Hurst V, Ahluwalia A, Alam S, Avey MT, Baker M, et al. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. PLoS Biol. 2020;18(7):e3000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Charan J, Biswas T. How to calculate sample size for different study designs in medical research? Indian J Psychol Med. 2013;35(2):121–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kose O, Arabaci T, Kizildag A, Erdemci B, Ozkal Eminoglu D, Gedikli S, et al. Melatonin prevents radiation-induced oxidative stress and periodontal tissue breakdown in irradiated rats with experimental periodontitis. J Periodontal Res. 2017;52(3):438–46. [DOI] [PubMed] [Google Scholar]
- 32.Yorgancilar N, Kose O, Rakici SY, Mercantepe T, Akyildiz K, Tumkaya L, et al. Preventive effects of melatonin and amifostine on irradiated rats with experimental periodontitis. BMC Oral Health. 2024;24(1):1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gezer A, Karadag-Sari E. The role of amifostine in preventing radiotherapy induced testicular tissue damage in rats. Biotech Histochem. 2022;97(3):215–21. [DOI] [PubMed] [Google Scholar]
- 34.Rakici SY, Guzel AI, Tumkaya L, Sevim Nalkiran H, Mercantepe T. Pelvic radiation-induced testicular damage: an experimental study at 1 Gray. Syst Biol Reprod Med. 2020;66(2):89–98. [DOI] [PubMed] [Google Scholar]
- 35.Coppes RP, Vissink A, Konings AW. Comparison of radiosensitivity of rat parotid and submandibular glands after different radiation schedules. Radiother Oncol. 2002;63(3):321–8. [DOI] [PubMed] [Google Scholar]
- 36.Matei LI, Neag MA, Mocan LP, Sufletel RT, Cutas A, Onofrei MM, et al. The effects of radiofrequency electromagnetic radiation emitted by mobile phones on rat Parotid gland histology - an experimental study. Eur Rev Med Pharmacol Sci. 2024;28(20):4405–19. [DOI] [PubMed] [Google Scholar]
- 37.Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem. 2005;38(12):1103–11. [DOI] [PubMed] [Google Scholar]
- 38.Erel O. A novel automated method to measure total antioxidant response against potent free radical reactions. Clin Biochem. 2004;37(2):112–9. [DOI] [PubMed] [Google Scholar]
- 39.Esen C, Alkan BA, Kirnap M, Akgul O, Isikoglu S, Erel O. The effects of chronic periodontitis and rheumatoid arthritis on serum and gingival crevicular fluid total antioxidant/oxidant status and oxidative stress index. J Periodontol. 2012;83(6):773–9. [DOI] [PubMed] [Google Scholar]
- 40.Kim JH, Kim KM, Jung MH, Jung JH, Kang KM, Jeong BK, et al. Protective effects of alpha lipoic acid on radiation-induced salivary gland injury in rats. Oncotarget. 2016;7(20):29143–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu X, Gong B, de Souza LB, Ong HL, Subedi KP, Cheng KT, et al. Radiation inhibits salivary gland function by promoting STIM1 cleavage by caspase-3 and loss of SOCE through a TRPM2-dependent pathway. Sci Signal. 2017. 10.1126/scisignal.aal4064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li Y, Li X, Pang R, Yang G, Tian M, Zhao T, et al. Diagnosis, prevention, and treatment of Radiotherapy-Induced xerostomia: A review. J Oncol. 2022;2022:7802334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hosseini MS, Sanaie S, Mahmoodpoor A, Jabbari Beyrami S, Jabbari Beyrami H, Fattahi S, et al. Cancer treatment-related xerostomia: basics, therapeutics, and future perspectives. Eur J Med Res. 2024;29(1):571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sagowski C, Wenzel S, Metternich FU, Kehrl W. Studies on the radioprotective potency of amifostine on salivary glands of rats during fractionated irradiation: acute and late effects. Eur Arch Otorhinolaryngol. 2003;260(1):42–7. [DOI] [PubMed] [Google Scholar]
- 45.Pober JS, Sessa WC. Evolving functions of endothelial cells in inflammation. Nat Rev Immunol. 2007;7(10):803–15. [DOI] [PubMed] [Google Scholar]
- 46.Zhao H, Wu L, Yan G, Chen Y, Zhou M, Wu Y, et al. Inflammation and tumor progression: signaling pathways and targeted intervention. Signal Transduct Target Ther. 2021;6(1):263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu R, Bian Y, Liu L, Liu L, Liu X, Ma S. Molecular pathways associated with oxidative stress and their potential applications in radiotherapy (review). Int J Mol Med. 2022. 10.3892/ijmm.2022.5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eskandari E, Eaves CJ. Paradoxical roles of caspase-3 in regulating cell survival, proliferation, and tumorigenesis. J Cell Biol. 2022. 10.1083/jcb.202201159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fu DR, Kato D, Endo Y, Kadosawa T. Apoptosis and Ki-67 as predictive factors for response to radiation therapy in feline nasal lymphomas. J Vet Med Sci. 2016;78(7):1161–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tokuyama-Toda R, Umeki H, Okubo M, Terada-Ito C, Yudo T, Ide S, et al. The preventive effect of melatonin on radiation-induced oral mucositis. Cells. 2023. 10.3390/cells12172178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Menard TW, Izutsu KT, Ensign WY, Keller PJ, Morton TH, Truelove EL. Radioprotection by WR-2721 of gamma-irradiated rat parotid gland: effect on gland weight and secretion at 8–10 days post irradiation. Int J Radiat Oncol Biol Phys. 1984;10(9):1555–9. [DOI] [PubMed] [Google Scholar]
- 52.Konings AW, Faber H, Vissink A, Coppes RP. Radioprotective effect of amifostine on Parotid gland functioning is region dependent. Int J Radiat Oncol Biol Phys. 2005;63(5):1584–91. [DOI] [PubMed] [Google Scholar]
- 53.Sagowski C, Wenzel S, Jenicke L, Bohuslavizki KH, Kehrl W, Zywietz F, et al. [Reducing late toxicity with amifostine in fractionated irradiation of the rat salivary glands]. HNO. 2002;50(9):822–8. [DOI] [PubMed] [Google Scholar]
- 54.Joseph LJ, Bhartiya US, Raut YS, Hawaldar RW, Nayak Y, Pawar YP, et al. Radioprotective effect of Ocimum sanctum and amifostine on the salivary gland of rats after therapeutic radioiodine exposure. Cancer Biother Radiopharm. 2011;26(6):737–43. [DOI] [PubMed] [Google Scholar]
- 55.Sagowski C, Wenzel S, Neumann B, Kehrl W, Zywietz F, Roeser K, et al. [Radioprotective effectiveness of amifostine on the salivary glands of the rat during fractionated irradiation]. HNO. 2002;50(2):139–45. [DOI] [PubMed] [Google Scholar]
- 56.Riley P, Glenny AM, Hua F, Worthington HV. Pharmacological interventions for preventing dry mouth and salivary gland dysfunction following radiotherapy. Cochrane Database Syst Rev. 2017;7(7):CD012744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ferraiolo DM, Veitz-Keenan A. Insufficient evidence for interventions to prevent dry mouth and salivary gland dysfunction post head and neck radiotherapy. Evidence-Based Dentistry. 2018;19(1):30–1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.