Abstract
Background
Childhood cancers contribute significantly to child morbidity and mortality worldwide, with an even greater burden in resource-limited settings. However, there is limited research documenting the incidence and patterns of childhood cancers in Ghana.
Aim
We aimed to examine the trends and patterns of childhood cancers in Northern Ghana over an eight-year period.
Methods
We conducted a retrospective descriptive analysis of medical records from the pediatric oncology unit at Tamale Teaching Hospital in Northern Ghana. The study included children diagnosed with cancer and admitted to the oncology unit between January 2016 and December 2023. We classified cancers based on the International Classification of Childhood Cancer and quantified the number of cases of each type, both overall and stratified by time and child characteristics. SAS JMP Professional Software (version 17.1) was used to analyze the data.
Results
A total of 216 child medical records were analyzed. Most (62.5%) children were male, with 48.1% aged 0 to 3 years. The number of children admitted with cancer increased progressively over time, from 15 cases in 2016/2017 to 82 in 2022/2023. Males and those 0 to 7 years were more likely to be admitted with cancer. Ten cancer types were identified, with retinoblastoma being the most commonly diagnosed cancer (30.1%), followed by lymphomas (23.1%) and renal tumors (15.7%). Of the 184 children with admission outcome data, 56.5% (n = 104) died. Cancer-related deaths were highest among those diagnosed with lymphomas and reticuloendothelial neoplasms (31 out of 104 cases; 29.8%) and retinoblastoma (30 out of 104 cases; 28.8%), as well as those aged 0–3 years (47 out of 104 cases; 45.2%) and among males (70 out of 104 cases; 67.3%). Overall, cancer deaths declined steadily from 71.4% in 2016/2017 to 44.4% in 2022/2023.
Conclusion
Our findings indicate a high burden of childhood cancers in Northern Ghana, with a greater proportion of cases occurring among male children. The observed increase in the number of childhood cancer admissions to the oncology unit may reflect improved case detection, greater awareness among caregivers, and increased referrals to specialized care, rather than a definitive rise in cancer incidence. These findings highlight the urgent need for population-based cancer registries and further research to accurately track and understand childhood cancer trends in the region.
Keywords: Childhood, Cancer, Incidence, Treatment outcome, Ghana
Introduction
Childhood cancers are a leading cause of morbidity and mortality among children worldwide [1–3]. Despite being less common than adult cancers, childhood cancers still pose a substantial global health burden, with nearly 400,000 new cases diagnosed annually [4, 5]. They are the ninth leading cause of disease burden in children [6] and contribute substantially to disability-adjusted life years (DALYs) [7], with incidence rates varying across countries [8]. In the United States, approximately 15,000 children are diagnosed with cancer each year [9], whereas in Kenya, 3200 new cases are diagnosed annually [10]. Notably, 80% of all childhood cancer cases occur in low- and middle-income countries (LMICs) [11, 12] where diagnostic services and treatment options are often limited [13, 14], leading to higher mortality than in high-income countries [15].
In Ghana, leukemia, lymphoma, retinoblastoma, Wilms’ tumor, soft tissue sarcoma, and neuroblastoma are among the most frequently diagnosed childhood cancers, with an estimated 1,200 children under age 15 diagnosed annually [16]. However, the true burden is likely underestimated due to underdiagnosis and inadequate reporting systems. Recent studies indicate a concerning increase in cases. For instance, Owusu et al. [12] found in two tertiary hospitals an increase in age-specific cancer incidence from 1.6 per 100,000 person-years in 2015 to 2.41 per 100,000 person-years in 2017, followed by a decline in 2019, highlighting fluctuations that require further investigation. Their findings also revealed a higher incidence among male children and those under five. Paintsil et al. [17] also observed a rise in childhood cancer cases from 27.2% in 2012 to 43.0% in 2014 at a tertiary hospital, with the highest prevalence among children aged 5–9 years and Burkitt’s lymphoma as the most common cancer.
The continued rise in childhood cancer cases highlights the need for further investigations and a review of Ghana’s national strategy for cancer control to prioritize childhood cancers and the effective allocation of resources [18]. Understanding national trends and high-risk populations is important for developing effective control strategies, ensuring equitable resource distribution, facilitating early diagnosis and treatment, and ultimately reducing childhood cancer mortality. However, existing research is disproportionately concentrated in southern Ghana, leading to a skewed understanding of childhood cancer epidemiology and limiting the effectiveness of national control strategies. The sole study available from northern Ghana was a histopathological review that did not assess patient outcomes [19], thereby limiting its impact on the design of targeted interventions. This lack of nationally representative data limits national estimates and risks excluding northern Ghana from policy and resource allocation decisions. Therefore, in this study, we examined childhood cancer patterns, trends, and admission outcomes in Northern Ghana over a seven-year period using clinical data from the pediatric oncology unit at Tamale Teaching Hospital, the primary referral center for Northern Ghana.
Methods
Study setting and population
This study is a retrospective analysis of medical records from the pediatric oncology unit of Tamale Teaching Hospital (TTH), the only tertiary referral and teaching hospital in Northern Ghana. This hospital serves a wide catchment area covering five of Ghana’s sixteen administrative regions, representing a geographically and socioeconomically diverse population that is underserved by specialized pediatric oncology services. The unit provides specialized services, including histopathological diagnostics, surgical interventions, chemotherapy, endocrine therapy, and follow-up care. Given its central role in pediatric oncology service delivery across the northern regions, the patient population at TTH can be considered broadly representative of the pediatric cancer burden in Northern Ghana.
For this analysis, we included medical records of all children under 16 years who were diagnosed with any form of cancer and admitted to the pediatric oncology unit at TTH between January 2016 and December 2023. Children who did not have confirmed cancer diagnoses were excluded.
Data abstraction and study variables
The primary data source for this analysis was the clinical records of children admitted to the pediatric oncology unit. Data abstraction from the records was carried out using a structured, pre-designed form with clear guidelines developed to ensure consistency and completeness. The form was refined in consultation with clinicians at the oncology unit to ensure clinical relevance and contextual appropriateness. Three members of our study team, who have clinical backgrounds and are familiar with the oncology unit, carried out the data extraction. Training sessions were held prior to the commencement of data collection.
The extracted data included clinical and sociodemographic variables of pediatric patients, notably the exact age at admission, which was categorized into three groups: 0–3 years (infancy and toddlerhood), 4–7 early childhood (preschool to early school age), and 8–15 years (middle childhood to early adolescence), sex, date of admission, cancer type, and admission outcome. Cancer diagnoses were classified according to the International Classification of Childhood Cancer (ICCC) [20]. Treatment outcomes at discharge were recorded as completed treatment, referred, or died during admission.
Sociodemographic variables of caregivers were captured including their relationship to the child (mother, father, or other), sex, highest educational attainment (none, primary, secondary, tertiary), occupational status (government-employed, self-employed, unemployed), religious affiliation (Muslim or Christian), and ethnic group (Mole-Dagbani, Gurunsi, Guan, Akan, or other). The caregivers’ region of residence was also recorded and categorized into three major geographic belts consistent with Ghana’s administrative classification: Northern Belt (comprising Northern, North East, Upper East, Upper West, and Savannah regions), Middle Belt (Ashanti, Bono, and Bono East), and Southern Belt (Western, Western North, Central, Greater Accra, Eastern, and Volta).
Data management and analysis
The extracted data were cleaned and validated prior to the analysis to ensure completeness and internal consistency. Records were examined for duplication, missingness, and logical inconsistencies. No duplicate or ambiguous entries were identified. Descriptive analyses were performed to summarize the distribution of cancer cases by patient demographics and clinical characteristics. Categorical variables were presented as frequencies and percentages. Childhood cancer types were classified based on the International Classification of Childhood Cancer (ICCC), with case counts reported overall and stratified by sex and age at diagnosis.
We examined trends in cancer distribution over the seven-year study period, stratified by sex and age at diagnosis to identify potential changes in diagnostic patterns or the burden of disease over time. Clinical outcomes at discharge, defined as completion of treatment, referral to another facility, or in-hospital death, were analyzed in relation to child-level variables (such as age at diagnosis, sex, and cancer type), in addition to the year of admission and caregiver characteristics, to investigate potential variations in outcomes. The results are presented in cross-tabulation tables, clustered bar charts, and pie charts. All data management and statistical analysis were conducted using SAS JMP Pro, version 17.
Results
Sociodemographic characteristics
Overall, 216 children diagnosed with cancer and admitted to the pediatric oncology unit between January 2016 and December 2023 were included in this analysis. The sociodemographic characteristics of the children and their caregivers are presented in Table 1. The majority of the children were male (62.5%), and nearly half (48.2%) were 0 to 3 years at the time of diagnosis. Two-thirds of the caregivers were mothers (67.6%). Among the caregivers, 59.7% had no formal education, 74.5% were self-employed, 66.2% identified as Muslim, 52.8% belonged to the Mole-Dagomba ethnic group, and 94.3% lived in the Northern belt of Ghana.
Table 1.
Demographic characteristics of children with cancer and their caregivers
| Variable | Number | Percentage |
|---|---|---|
| Child characteristics: | ||
| Sex | ||
| Male | 135 | 62.5 |
| Female | 81 | 37.5 |
| Age | ||
| 0–3 years | 104 | 48.2 |
| 4–7 years | 70 | 32.4 |
| 8–15 years | 42 | 19.4 |
| Caregiver characteristics: | ||
| Relationship with child | ||
| Father | 66 | 30.6 |
| Mother | 145 | 67.1 |
| Others | 5 | 2.3 |
| Sex | ||
| Male | 70 | 32.4 |
| Female | 146 | 67.6 |
| Educational level | ||
| No formal education | 129 | 59.7 |
| Primary | 49 | 22.7 |
| Secondary | 19 | 8.8 |
| Tertiary | 19 | 8.8 |
| Occupation | ||
| Government employee | 14 | 6.5 |
| Self-employed | 161 | 74.5 |
| Unemployed | 41 | 19.0 |
| Religion | ||
| Christian | 73 | 33.8 |
| Muslim | 143 | 66.2 |
| Ethnic group | ||
| Akan | 4 | 1.9 |
| Guan | 7 | 3.2 |
| Gurunsi | 38 | 17.6 |
| Mole-Dagbani | 114 | 52.8 |
| Others | 53 | 24.5 |
| Regional belt 1 | ||
| Northern belt | 199 | 94.3 |
| Middle belt | 9 | 4.3 |
| Southern belt | 3 | 1.4 |
1Northern belt (Northern, Upper East, Upper West, North East, and Savannah region); Middle belt (Ashanti, Bono, and Bono East); Southern belt (Western, Western North, Central, Greater Accra, Eastern, and Volta region). Missing values = 5
Trends in childhood cancer admissions by sex and age at diagnosis
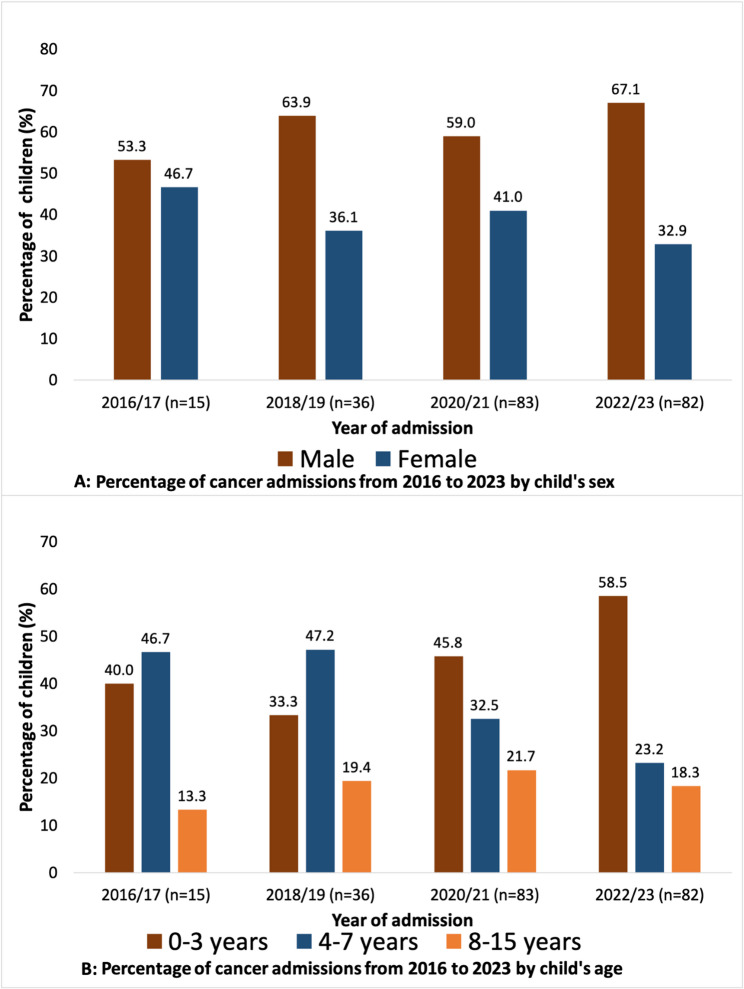
Figure 1 illustrates trends in childhood cancer admissions from January 2016 to December 2023, stratified by sex and age at diagnosis. Male admissions consistently exceeded female admissions across all periods, increasing from 53.3% in 2016/17 to 67.1% in 2022/23. In contrast, female cancer admissions declined from 46.7 to 32.9% over the same period (Fig. 1A). The distribution of cancer admissions by child’s age indicates that these admissions were increasingly dominated by children aged 0–3 at diagnosis, rising from 40.0% in 2016/17 to 58.5% in 2022/23. Conversely, the proportion of children aged 4–7 years declined from 46.7 to 23.2%, while those aged 8–15 years remained relatively stable, showing minor fluctuations between 13.3% and 18.3% (Fig. 1B).
Fig. 1.
Trends in childhood cancer admissions by (A) child’s sex and (B) age from 2016 to 2023. The numbers on the bars are percentages
Childhood cancer classifications according to the international classification of childhood cancer
Of the 216 pediatric cancer cases (Table 2), the most prevalent childhood cancer was retinoblastoma (30.1%). This was followed by lymphomas and reticuloendothelial neoplasms (23.1%), renal tumors (15.7%), and leukemias, myeloproliferative diseases, and myelodysplastic diseases (14.4%). Other cancers included neuroblastoma and other peripheral nervous cell tumors (6.9%), soft tissue and other extraosseous sarcomas (5.1%), and hepatic tumors (1.9%). Less common types were malignant bone tumors (1.4%), germ cell tumors, trophoblastic tumors, and neoplasms of the gonads (0.9%). Other and unspecified malignant neoplasms represented just 0.5% of cases.
Table 2.
Distribution of childhood cancer types by year of admission, child’s sex, and age cancer types were classified based on the international childhood cancer classification (Steliarova-Foucher et al., 2005)
| Cancer types | Total (n = 216) | Year of admission | Child’s sex | Child age | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2016/17 | 2018/19 | 2020/21 | 2022/23 | Male | Female | 0–3 years | 4–7 years | 8–15 years | ||
| (n = 15) | (n = 36) | (n = 83) | (n = 82) | (n = 135) | (n = 81) | (n = 104) | (n = 70) | (n = 42) | ||
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | ||
| Retinoblastoma | 65 (30.1) | 4 (26.7) | 7 (19.4) | 27 (32.5) | 27 (32.9) | 37 (27.4) | 28 (34.6) | 47 (45.2) | 16 (22.9) | 2 (4.8) |
| Germ cell tumors, trophoblastic tumors, and neoplasms of gonads | 2 (0.9) | 0 (0) | 0 (0) | 1 (1.2) | 1 (1.2) | 2 (1.5) | 0 (0) | 2 (1.9) | 0 (0) | 0 (0) |
| Lymphomas and reticuloendothelial neoplasms | 50 (23.1) | 4 (26.7) | 15 (41.7) | 16 (19.3) | 15 (18.3) | 37 (27.4) | 13 (16.0) | 16 (15.4) | 16 (22.9) | 18 (42.9) |
| Hepatic tumors | 4 (1.9) | 2 (13.3) | 0 (0) | 1 (1.2) | 1 (1.2) | 2 (1.5) | 2 (2.5) | 2 (1.9) | 2 (2.9) | 0 (0) |
| Leukemias, myeloproliferative diseases, and myelodysplastic diseases | 31 (14.4) | 0 (0) | 4 (11.1) | 9 (10.8) | 18 (22.0) | 23 (17.0) | 8 (9.9) | 6 (5.8) | 14 (20.0) | 11 (26.2) |
| Malignant bone tumors | 3 (1.4) | 0 (0) | 0 (0) | 3 (3.6) | 0 (0) | 2 (1.5) | 1 (1.2) | 1 (1.0) | 1 (1.4) | 1 (2.4) |
| Neuroblastoma and other peripheral nervous cell tumors | 15 (6.9) | 2 (13.3) | 1 (2.8) | 8 (9.6) | 4 (4.9) | 9 (6.7) | 6 (7.4) | 2 (1.9) | 8 (11.4) | 5 (11.9) |
| Renal tumors | 34 (15.7) | 1 (6.7) | 8 (22.2) | 12 (14.5) | 13 (15.9) | 19 (14.1) | 15 (18.5) | 22 (21.2) | 8 (11.4) | 4 (9.5) |
| Soft tissue and other extraosseous sarcomas | 11 (5.1) | 2 (13.3) | 1 (2.8) | 6 (7.2) | 2 (2.4) | 3 (2.2) | 8 (9.9) | 5 (4.8) | 5 (7.1) | 1 (2.4) |
| Other and unspecified malignant neoplasms | 1 (0.5) | 0 (0) | 0 (0) | 0 (0) | 1 (1.2) | 1 (0.7) | 0 (0) | 1 (1.0) | 0 (0) | 0 (0) |
Retinoblastoma showed a steady increase over time, with cases rising from 26.7% in 2016/17 to 32.9% in 2022/23. The highest incidence was observed in children aged 0–3 years (45.2%), and among females (34.6%). Lymphomas and reticuloendothelial neoplasms followed a consistent pattern, peaking at 41.7% in 2018/19 and declining to 18.3% in 2022/23. This type was more prevalent in males (27.4%) compared to females (16.0%) and was distributed relatively evenly across age groups, with a higher incidence in children aged 8–15 years (42.9%). Leukemias, myeloproliferative diseases, and myelodysplastic diseases surged in later years, from 0% in 2016/17 to 22.0% in 2022/23. These conditions were more prevalent in older children, particularly those aged 4–7 years (20.0%) and 8–15 years (26.2%). Males (17.0%) had a higher incidence of these diseases compared to females (9.9%).
Renal tumors also showed an increasing trend, rising from 6.7% in 2016/17 to 15.9% in 2022/23. These tumors were most common in children aged 0–3 years (21.2%), with a higher prevalence in males (18.5%) than in females (14.1%). Other cancer types, such as neuroblastoma and peripheral nervous cell tumors, increased from 2.8% in 2018/19 to 9.6% in 2020/21, with a higher incidence in males (6.7%) and children aged 4–7 years (11.4%). Soft tissue sarcomas and hepatic tumors remained relatively rare but were more prevalent among children aged 0–3 years (4.8%) and 4–7 years (7.1%).
Case counts of pediatric cancer subtypes
The cancer subtypes within each major category are presented in Table 3. Retinoblastoma (n = 65) was the single most common individual diagnosis. Among lymphomas and reticuloendothelial neoplasms, Burkitt lymphoma was the predominant subtype (n = 22). In the leukemias category, acute lymphoblastic leukemia accounted for the majority of cases (n = 22). Other notable subtype distributions included nephroblastoma (n = 34) as the leading renal tumor, rhabdomyosarcoma (n = 11) among soft tissue sarcomas, and neuroblastoma (n = 15) among tumors of the peripheral nervous system. Hepatic tumors were exclusively hepatoblastoma (n = 4), while Ewing’s sarcoma (n = 1) and osteosarcoma (n = 2) together represented all malignant bone tumors. Germ cell tumors (n = 2) were the only tumors observed in the Germ cell tumors, trophoblastic tumors, and neoplasms of gonads category.
Table 3.
Case count of childhood cancer subgroups admitted from 2016 to 2023
| Cancer subtypes | Number |
|---|---|
| Retinoblastoma | |
| Retinoblastoma | 65 |
| Germ cell tumors, trophoblastic tumors, and neoplasms of gonads | |
| Germ cell tumor | 2 |
| Lymphomas and reticuloendothelial neoplasms | |
| Burkitt lymphoma | 22 |
| Hodgkin lymphoma | 3 |
| Lymphoma | 13 |
| Non-Hodgkin lymphoma | 12 |
| Hepatic tumors | |
| Hepatoblastoma | 4 |
| Leukemias, myeloproliferative diseases, and myelodysplastic diseases | |
| Acute lymphoblastic leukemia | 22 |
| Acute myeloid leukemia | 9 |
| Malignant bone tumors | |
| Ewing’s sarcoma | 1 |
| Osteosarcoma | 2 |
| Neuroblastoma and other peripheral nervous cell tumors | |
| Neuroblastoma | 15 |
| Renal tumors | |
| Nephroblastoma | 34 |
| Soft tissue and other extraosseous sarcomas | |
| Rhabdomyosarcoma | 11 |
| Other and unspecified malignant neoplasms | |
| Paranasal sinus tumor | 1 |
Clinical outcome on discharge
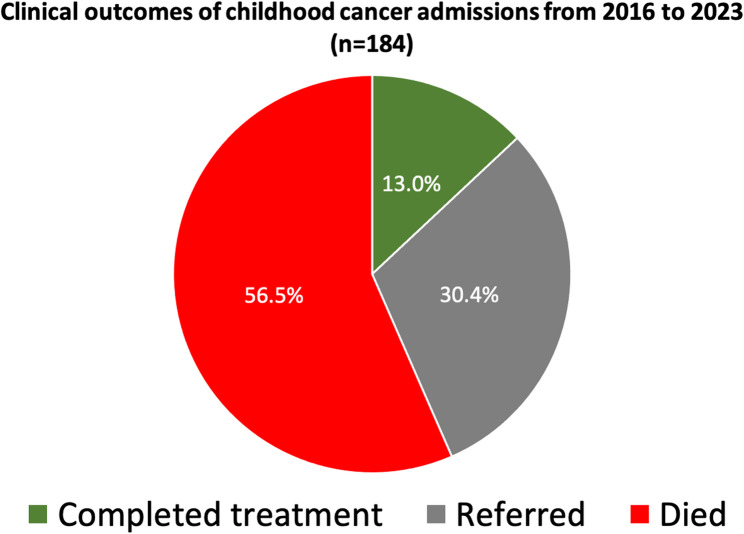
Of the 216 patients, discharge outcome data were available for 184 (85.2%), while data for the remaining 32 patients (14.8%) were unavailable. As shown in Fig. 2, over half of these children 104 (56.5%) died during their admission. Referrals to other facilities were recorded for 56 (30.4%), while only 24 (13.0%) completed treatment and were discharged.
Fig. 2.
Clinical outcomes of childhood cancer admissions from 2016 to 2023
Clinical outcomes on discharge by child and caregiver characteristics
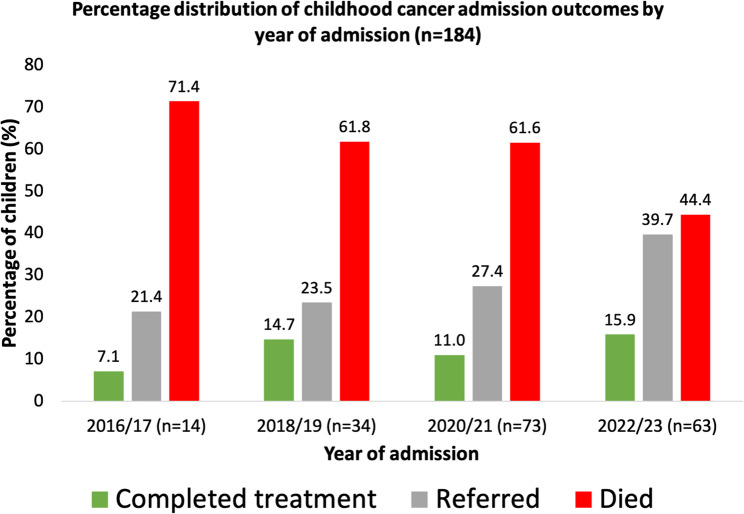
Table 4 presents discharge outcomes by sociodemographic characteristics. Among those who died, the majority were males (67.3%), children aged 0–3 years (45.2%), those whose caregivers were uneducated (66.3%), were employed (80.8%), those from Northern belt (98.0%), and those with lymphomas and reticuloendothelial neoplasms (29.8%). Also, among those referred, the majority were males (57.1%), children aged 0–3 years (48.2%), those whose caregivers were uneducated (55.4%), were employed (80.4%), those from Northern belt (91.1%), and those with leukemias, myeloproliferative diseases, and myelodysplastic diseases (23.2%). In addition, of those who completed treatment were children aged 0–3 years (58.3%), those whose caregivers were educated (62.5%) and employed (87.5%), from Northern belt (87.5%), and had Retinoblastoma (58.3%). Across all outcomes, most children resided in the Northern belt. Figure 3 shows that cancer-related mortality declined from 71.4% in 2016/17 to 44.4% in 2022/23, while referrals increased from 21.4 to 39.7%, and treatment completion rose from 7.1 to 15.9% over the same period, despite some mid-period fluctuations.
Table 4.
Distribution of child and caregiver characteristics by childhood cancer admission outcomes (N = 184)
| Variable | Completed treatment n (%) |
Referred n (%) |
Died n (%) |
|---|---|---|---|
| Child characteristics: | |||
| Sex | |||
| Male | 12 (50.0) | 32 (57.1) | 70 (67.3) |
| Female | 12 (50.0) | 24 (42.9) | 34 (32.7) |
| Age | |||
| 0–3 years | 14 (58.3) | 27 (48.2) | 47 (45.2) |
| 4–7 years | 7 (29.2) | 18 (32.1) | 36 (34.6) |
| 8–15 years | 3 (12.5) | 11 (19.6) | 21 (20.2) |
| Caregiver characteristics: | |||
| Educational level | |||
| Uneducated | 9 (37.5) | 31 (55.4) | 69 (66.3) |
| Educated | 15 (62.5) | 25 (44.6) | 35 (33.7) |
| Occupation | |||
| Unemployed | 3 (12.5) | 11 (19.6) | 20 (19.2) |
| Employed | 21 (87.5) | 45 (80.4) | 84 (80.8) |
| Regional belt | |||
| Northern belt | 21 (87.5) | 51 (91.1) | 98 (98.0) |
| Middle belt | 2 (8.3) | 3 (5.4) | 2 (2.0) |
| Southern belt | 1 (4.2) | 2 (3.6) | 0 (0) |
| Type of childhood cancer | |||
| Retinoblastoma | 14 (58.3) | 11 (19.6) | 30 (28.8) |
|
Germ cell tumors, trophoblastic tumors, and neoplasms of gonads |
0 (0) | 1 (1.8) | 1 (1.0) |
|
Lymphomas and reticuloendothelial neoplasms |
5 (20.8) | 11 (19.6) | 31 (29.8) |
| Hepatic tumors | 1 (4.2) | 1 (1.8) | 2 (1.9) |
|
Leukemias, myeloproliferative diseases, and myelodysplastic diseases |
0 (0) | 13 (23.2) | 12 (11.5) |
| Malignant bone tumors | 0 (0) | 2 (3.6) | 1 (1.0) |
|
Neuroblastoma and other peripheral nervous cell tumors |
1 (4.2) | 2 (3.6) | 8 (7.7) |
| Renal tumors | 3 (12.5) | 11 (19.6) | 14 (13.5) |
|
Soft tissue and other extraosseous sarcomas |
0 (0) | 3 (5.4) | 5 (4.8) |
|
Other and unspecified malignant Neoplasms |
0 (0) | 1 (1.8) | 0 (0) |
Categories recorded: Caregiver’s educational level [Uneducated (No formal education), Educated (Primary Secondary, and Tertiary)] and Caregiver’s occupation [Unemployed, Employed (Government employee, Self-employed)]
Fig. 3.
Distribution of childhood cancer admission outcomes by year of admission
Discussion
This study examined patterns and temporal trends in childhood cancer incidence at the Tamale Teaching Hospital in Northern Ghana from 2016 to 2023. The results of this study indicate a higher incidence of childhood cancer among male children compared to their female counterparts. This trend aligns with previous research by Williams et al. [21], Endalamaw et al. [22], and Williams et al. [23], which consistently reported a greater prevalence of childhood cancer among males. While the reasons for this disparity remain an area of ongoing research, several biological factors have been proposed as potential contributors. Studies suggest that a combination of immune-related, genetic, and hormone-related mechanisms may play a role in the higher incidence observed in males [24]. These findings highlight the need for further investigation into sex-based differences in childhood cancer to support the development of targeted public health interventions.
The study also revealed that the highest number of childhood cancer cases occurred in children aged 0–3 years. This finding is comparable with studies by Owusu et al. [12] and Siegel [25], which reported that pediatric cancer rates are highest within this age group. This could be due to congenital genetic mutations, prenatal environmental exposures, or early-life susceptibility to carcinogens. Certain cancers, such as neuroblastoma, retinoblastoma, and Wilms’ tumor, predominantly occur in infancy and early childhood [5], further supporting this observation. The immaturity of the immune system during this critical developmental phase may also contribute to the increased vulnerability of younger children to malignancies [26]. Hence, public health initiatives should focus on raising awareness about the early signs and symptoms of childhood cancers, promoting genetic screening for high-risk families, and reducing prenatal and early-life exposure to known carcinogens.
The current study showed that the most prevalent childhood cancer was retinoblastoma. Retinoblastoma is a malignant tumor of the retina that primarily affects young children, with both hereditary and non-hereditary forms linked to mutations in the RB1 gene [27, 28]. The high prevalence of retinoblastoma in this study suggests that genetic factors may be contributing to the disease burden in the studied population. In contrast, a study by Owusu et al. [12] found lymphomas as the most prevalent childhood cancer. Moreover, other studies by Ward et al. [15], Lu et al. [29], and Wu et al. [30] identified leukemia as the most common cancer in children. The variation in findings could be due to differences in geographic distribution and genetic predisposition.
The mortality rate for childhood cancer in this study was high, with more than half of the affected children dying during the course of treatment. This finding is concerning and highlights significant challenges in childhood cancer management within the studied population. This was similarly observed in the study by Slone et al. [31]. However, the observed mortality rate is higher than reported in studies by Loeffen et al. [32] and Horn et al. [33], indicating a discrepancy in findings. These studies revealed that childhood cancer mortality was relatively low. The disparity in findings may be attributed to differences in healthcare infrastructure, access to specialized pediatric oncology care, and the availability of advanced treatment options and supportive care services. This highlights the urgent need for strengthened healthcare policies to improve childhood cancer management. Efforts should prioritize early detection programs, timely referral systems, expanded access to chemotherapy and radiotherapy, and enhanced supportive care, including infection control and nutritional support.
In addition, this study found that mortality rates among childhood cancer patients were higher in males compared to females. A possible explanation for this trend is that males have poorer survival outcomes for childhood cancer [24]. The increased risk of childhood cancer among males may have a genetic basis, as hormonal differences between males and females during childhood are minimal [21]. This means that factors beyond hormonal influence, such as genetic susceptibility, immune response differences, and variations in tumor biology, could contribute to the observed disparity in survival rates. This result is consistent with previous studies by Curado et al. [34], Gupta et al. [35], Williams and Spector [36], and Siegel et al. [37], all of which reported higher mortality rates among male childhood cancer patients compared to females. This highlights the need for targeted interventions aimed at improving survival outcomes among male children with cancer.
According to the study results, higher mortality and referral rates were predominant among children whose caregivers had no formal education. This finding emphasizes the significant role of caregiver education in determining childhood cancer outcomes. Caregivers with lower educational levels may have limited awareness of early cancer symptoms, delayed healthcare-seeking behavior, and reduced understanding of treatment protocols which can contribute to poor prognosis [38, 39]. This result aligns with previous research by Moore et al. [40] and García-Quintero et al. [41] indicating that caregiver literacy and health knowledge play a crucial role in disease management and survival outcomes in pediatric oncology. Therefore, public health interventions should focus on caregiver education programs to enhance awareness of childhood cancer signs, the importance of early diagnosis, and adherence to treatment.
Furthermore, the study found that cancer treatment completion was higher among children of employed caregivers compared to those of unemployed caregivers. This discovery suggests that the economic stability associated with caregiver employment plays a crucial role in ensuring adherence to cancer treatment. Employed caregivers are more likely to have financial resources which contribute to a higher likelihood of treatment completion [42]. This result is in accordance with studies by Jones [43], Bekui et al. [44], and Tran et al. [45] that have highlighted the influence of socioeconomic status on pediatric cancer treatment outcomes. This underscores the need for government policies and healthcare programs that provide financial assistance, subsidized treatments, and transportation support for unemployed caregivers. This will ultimately improve treatment adherence and outcomes for children with cancer.
Lastly, among childhood cancers, retinoblastoma had the highest treatment completion rate. This may be attributed to its distinct and visible symptoms, such as leukocoria (white pupil) and strabismus (misaligned eyes) [46, 47], which may prompt caregivers to seek medical attention earlier than for other childhood cancers with more subtle presentations [48]. A key factor influencing treatment completion is caregiver awareness and knowledge about childhood cancers [39], as caregivers play a crucial role in ensuring that children receive timely medical attention and adhere to prescribed treatment plans [49]. To improve early recognition, reduce delays in seeking care, and enhance treatment adherence, community outreach initiatives should be implemented to equip caregivers with essential knowledge about childhood cancer symptoms and management.
Limitations
Despite the valuable insights provided, this study has some limitations. The reliance on hospital electronic records may introduce selection bias, as only children who sought care at the facility were included, potentially underrepresenting cases managed elsewhere or undiagnosed cases in the region. Additionally, data completeness and accuracy depended on the quality of medical record entry, which may have led to missing or misclassified information. The data source lacked information on cancer staging and reasons for referral or treatment abandonment, which constrained more in-depth analyses. The retrospective nature of the study also limited the ability to assess causality between demographic factors and clinical outcomes. Moreover, the absence of a formal cancer registry and population-level data limits the ability to determine true incidence rates or trends. Furthermore, some cancer types, like retinoblastoma, may appear more frequently due to easier diagnosis, introducing possible detection bias. Furthermore, socioeconomic variables such as household income and healthcare accessibility, which could influence treatment adherence and outcomes, were not captured. Lastly, while the classification of cancer types followed the International Childhood Cancer Classification (ICCC), potential inconsistencies in diagnostic coding could have impacted the categorization of certain cases.
Clinical implications
Childhood cancer is increasingly prevalent in Northern Ghana, particularly among males and younger children, highlighting an urgent need for improved early detection and treatment strategies. High mortality rates, especially for retinoblastoma and lymphomas and reticuloendothelial neoplasms, suggest gaps in access to timely diagnosis, specialized pediatric oncology care, and supportive therapies. Though a decline in cancer-related deaths indicates some progress, further investments in healthcare infrastructure, capacity building for early diagnosis, and enhanced treatment protocols are crucial to improving survival outcomes for affected children. Clinicians and healthcare facilities are encouraged, where feasible, to update data collection forms and document details on cancer stage at diagnosis, reasons for treatment refusal or abandonment, and reasons for referral.
Conclusion
This study reveals clinical insights into childhood cancers in Northern Ghana between 2016 and 2023. The predominance of retinoblastoma, followed by lymphomas and renal tumors, suggests potential environmental, genetic, or diagnostic trends that warrant further investigation. The disproportionate burden among male children under three years old raises questions about sex-specific vulnerabilities or care-seeking behaviors. Alarmingly, most children either died during treatment or were referred, underscoring systemic limitations in early diagnosis, treatment availability, and continuity of care particularly in the Northern belt, where mortality and referral rates were highest. These adverse outcomes were more pronounced among children of uneducated and employed caregivers, indicating the need for targeted caregiver education and support systems. Notably, retinoblastoma showed better treatment completion, which may reflect earlier detection or more accessible treatment options for this cancer type. These findings highlight the urgent need to strengthen regional healthcare infrastructure, expand public and caregiver education, and ensure timely, accessible pediatric oncology services across all zones. For policymakers and health program designers, this calls for equitable distribution of oncology resources, workforce training, and referral system improvements. Future longitudinal and intervention-based research is essential to better understand the causal pathways of these disparities and to evaluate the effectiveness of targeted health interventions aimed at improving childhood cancer outcomes in Ghana.
Acknowledgements
The authors express their gratitude to the Tamale Teaching Hospital and its Pediatric Oncology Unit for granting access to medical records and supporting this research. Special thanks to the hospital staff for their assistance in data collection and validation. We also acknowledge the caregivers and families whose data contributed to this study.
Abbreviations
- DALYs
Disability-adjusted life years
- LMICs
Low- and middle-income countries
- TTH
Tamale Teaching Hospital
- ICCC
International Childhood Cancer Classification
Authors’ contributions
AW, MMI, ISM, and PST conceived the study. The design of the work was carried out by AW, MMI, ISM, PST, IA, BAN, AA, SM, and WJS. The acquisition and analysis of data were handled by AW, MMI, ISM, SM, WJS, and PST. The interpretation of the data was done by AW, MMI, ISM, SM, WJS, PST, and IA. AW, MMI, ISM, PST, IA, BAN, AA, SM, and WJS drafted the manuscript or substantively revised it. All authors reviewed and approved the final manuscript.
Funding statement
This study did not receive any funding.
Data availability
The data used to support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
The study received ethical approval from the Kwame Nkrumah University of Science and Technology Committee on Human Research, Publication, and Ethics. Permission to access medical records was granted by Tamale Teaching Hospital, with data anonymized to protect confidentiality. Consent to participate was not applicable, as the study was based solely on the analysis of existing medical records and did not involve any direct interaction with participants or primary data collection. All procedures adhered to the Declaration of Helsinki, ensuring patient rights, data integrity, and confidentiality.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cunningham RM, Walton MA, Carter PM. The major causes of death in children and adolescents in the united States. N Engl J Med. 2018;379(25):246875. 10.1056/NEJMsa1806938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mullen CJ, Barr RD, Franco EL. Timeliness of diagnosis and treatment: the challenge of childhood cancers. Br J Cancer. 2021;125(12):1612–20. 10.1038/s41416-021-01515-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao Y, Sun P, Xiao J, Jin L, Ma N, Li Z, et al. International patterns and trends of childhood and adolescent cancer, 1978–2012. J Natl Cancer Cent. 2022;2(2):78–89. 10.1016/j.jncc.2022.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steliarova-Foucher E, Colombet M, Ries LAG, Moreno F, Dolya A, Bray F, et al. International incidence of childhood cancer, 2001-10: a population-based registry study. Lancet Oncol. 2017;18(6):719–31. 10.1016/S1470-2045(17)30186-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. Childhood cancer. 2025. [Internet]. Available from: https://www.who.int/news-room/fact-sheets/detail/cancer-in-children
- 6.Grabas MR, Kjaer SK, Frederiksen MH, Winther JF, Erdmann F, Dehlendorff C, et al. Incidence and time trends of childhood cancer in Denmark, 1943–2014. Acta Oncol. 2020;59(5):588–95. 10.1080/0284186X.2020.1725239. [DOI] [PubMed] [Google Scholar]
- 7.Sun K, Zheng R, Zhang S, Zeng H, Wang S, Chen R, et al. Patterns and trends of cancer incidence in children and adolescents in China, 2011–2015: a population-based cancer registry study. Cancer Med. 2021;10(13):4575–86. 10.1002/cam4.4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ward ZJ, Yeh JM, Bhakta N, Frazier AL, Atun R. Estimating the total incidence of global childhood cancer: a simulation-based analysis. Lancet Oncol. 2019;20(4):483–93. 10.1016/S1470-2045(18)30909-4. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. Childhood Cancers by Primary Site. 2024. [Internet]. Available from: https://gis.cdc.gov/Cancer/USCS/?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcancer%2Fdataviz%2Findex.htm#/ChildhoodCancerPrimarySite/
- 10.Kenyatta University Teaching, Referral & Research Hospital (KUTRRH). Increasing childhood cancer survival rate from 20% to 90. 2024. Available from: https://www.kutrrh.go.ke/increasing-childhood-cancer-survival-rate-from-20-to-90-article/
- 11.Ortiz R, Vásquez L, Giri B, Kapambwe S, Dille I, Mahmoud L, et al. Developing and sustaining high-quality care for children with cancer: the WHO global initiative for childhood cancer. Rev Panam Salud Publica. 2023;47:e164. 10.26633/RPSP.2023.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Owusu WE, Burger JR, Lubbe MS, Joubert R, Cockeran M. Incidence patterns of childhood cancer in two tertiary hospitals in Ghana from 2015 to 2019: a retrospective observational study. Cancer Epidemiol. 2023;87:102470. 10.1016/j.canep.2023.102470. [DOI] [PubMed] [Google Scholar]
- 13.Olbara G, Martijn HA, Njuguna F, Langat S, Martin S, Skiles J, et al. Influence of health insurance status on childhood cancer treatment outcomes in Kenya. Support Care Cancer. 2020;28(2):917–24. 10.1007/s00520-019-04859-1. [DOI] [PubMed] [Google Scholar]
- 14.Petricca K, Carson L, Kambugu J, Denburg A. Strengthening access to cancer medicines for children in East africa: policy options to enhance medicine procurement, forecasting, and regulations. Glob Health Res Policy. 2024;9(1):24. 10.1186/s41256-024-00365-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ward E, DeSantis C, Robbins A, Kohler B, Jemal A. Childhood and adolescent cancer statistics, 2014. CA Cancer J Clin. 2014;64(2):83–103. 10.3322/caac.21219. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization. Beating childhood cancers through early detection and treatment. 2021. [Internet]. Available from: https://www.afro.who.int/countries/ghana/news/beating-childhood-cancers-through-early-detection-and-treatment
- 17.Paintsil V, Blay Nguah S, Osei-Akoto A, Osei-Tutu L, Hammond C. Pattern of childhood cancers presenting to the paediatric cancer unit of a tertiary hospital in kumasi, Ghana. J Cancer Prev Curr Res. 2015;3(3):00083. 10.15406/jcpcr.2015.03.00083. [Google Scholar]
- 18.Ministry of Health of Ghana. National Strategy for Cancer Control in Ghana. 2011. [Internet]. Available from: https://www.iccp-portal.org/system/files/plans/Cancer%20Plan%20Ghana%20Ministry%20of%20Health.pdf
- 19.Der EM, Abantanga FA. Histopathological review of childhood and adolescent cancers in Northern Ghana. West Afr J Med. 2022;39(12):1229–37. 10.4314/wajm.v39i12.1. [PubMed] [Google Scholar]
- 20.Steliarova-Foucher E, Stiller C, Lacour B, Kaatsch P. International classification of childhood cancer, third edition. Cancer. 2005;103(7):1457–67. 10.1002/cncr.20910. [DOI] [PubMed] [Google Scholar]
- 21.Williams LA, Richardson M, Kehm RD, McLaughlin CC, Mueller BA, Chow EJ, et al. The association between sex and most childhood cancers is not mediated by birthweight. Cancer Epidemiol. 2018;57:7–12. 10.1016/j.canep.2018.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Endalamaw A, Assimamaw NT, Ayele TA, Muche AA, Zeleke EG, Wondim A, et al. Prevalence of childhood cancer among children attending referral hospitals of outpatient department in Ethiopia. BMC Cancer. 2021;21(1):271. 10.1186/s12885-021-08014-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams LA, Richardson M, Marcotte EL, Poynter JN, Spector LG. Sex-ratio among childhood cancers by single-year of age. Pediatr Blood Cancer. 2019;66(6):e27620. 10.1002/pbc.27620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown K. Pediatric cancer is on the rise, with some types becoming more common. Northwell Health. 2023. [Internet]. Available from: https://www.northwell.edu/news/the-latest/pediatric-cancer-is-on-the-rise-with-some-types-becoming-more-common
- 25.Siegel DA. Geographic variation in pediatric cancer incidence-United states, 2003–2014. MMWR Morb Mortal Wkly Rep. 2018;67:707–13. 10.15585/mmwr.mm6725a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simon AK, Hollander GA, McMichael A. Evolution of the immune system in humans from infancy to old age. Proc Biol Sci. 2015;282(1821):20143085. 10.1098/rspb.2014.3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yun J, Li Y, Xu CT, Pan BR. Epidemiology and Rb1 gene of retinoblastoma. Int J Ophthalmol. 2011;4(1):103–9. 10.3980/j.issn.2222-3959.2011.01.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.American Cancer Society. What Is Retinoblastoma? 2018. [Internet]. Available from: https://www.cancer.org/cancer/types/retinoblastoma/about/what-is-retinoblastoma.html
- 29.Lu L, Huang C, Huang H. Childhood cancer: an emerging public health issue in China. Ann Transl Med. 2015;3(17):250. 10.3978/j.issn.2305-5839.2015.08.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu Y, Deng Y, Wei B, Xiang D, Hu J, Zhao P, et al. Global, regional, and national childhood cancer burden, 1990–2019: an analysis based on the global burden of disease study 2019. J Adv Res. 2022;40:233–47. 10.1016/j.jare.2022.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Slone JS, Chunda-Liyoka C, Perez M, Mutalima N, Newton R, Chintu C, et al. Pediatric malignancies, treatment outcomes and abandonment of pediatric cancer treatment in Zambia. PLoS ONE. 2014;9(2):e89102. 10.1371/journal.pone.0089102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Loeffen EAH, Knops RRG, Boerhof J, Feijen EAM, Merks JHM, Reedijk AMJ, et al. Treatment-related mortality in children with cancer: prevalence and risk factors. Eur J Cancer. 2019;121:113–22. 10.1016/j.ejca.2019.08.008. [DOI] [PubMed] [Google Scholar]
- 33.Horn SR, Stoltzfus KC, Mackley HB, Lehrer EJ, Zhou S, Dandekar SC, et al. Long-term causes of death among pediatric patients with cancer. Cancer. 2020;126(13):3102–13. 10.1002/cncr.32885. [DOI] [PubMed] [Google Scholar]
- 34.Curado MP, Pontes T, Guerra-Yi ME, Cancela MDC. Leukemia mortality trends among children, adolescents, and young adults in Latin America. Rev Panam Salud Publica. 2011;29(2):96–102. 10.1590/S1020-49892011000200004. [DOI] [PubMed] [Google Scholar]
- 35.Gupta S, Morris SK, Suraweera W, Aleksandrowicz L, Dikshit R, Jha P. Childhood cancer mortality in India: direct estimates from a nationally representative survey of childhood deaths. J Glob Oncol. 2016;2(6):403–11. 10.1200/JGO.2015.000935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williams LA, Spector LG. Survival differences between males and females diagnosed with childhood cancer. JNCI Cancer Spectr. 2019;3(2):pkz032. 10.1093/jncics/pkz032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Siegel DA, Richardson LC, Henley SJ, Wilson RJ, Dowling NF, Weir HK, et al. Pediatric cancer mortality and survival in the United States, 2001–2016. Cancer. 2020;126(19):4379–89. 10.1002/cncr.33080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li S, He Y, Liu J, Chen K, Yang Y, Tao K, et al. An umbrella review of socioeconomic status and cancer. Nat Commun. 2024;15(1):1. 10.1038/s41467-024-54444-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nurhidayah I, Hendriyani D, Adistie F, Nurhaeni N, Mediani HS. Factors influencing treatment-seeking behavior among caregivers of children with cancer: a scoping review. J Multidiscip Healthc. 2025;18:563–78. 10.2147/JMDH.S497004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moore C, Gallagher P, Dunne S. Health literacy, eHealth literacy and their association with burden, distress, and self-efficacy among cancer caregivers. Front Psychol. 2024;15:1283227. 10.3389/fpsyg.2024.1283227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.García-Quintero X, Bastardo Blanco D, Vásquez L, Fuentes-Alabí S, Benites-Majano S, Maza M, et al. Health literacy on quality of life for children with cancer: modules on pediatric palliative care. Rev Panam Salud Publica. 2023;47:e134. 10.26633/RPSP.2023.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fakeye KMB, Samuel LJ, Wolff JL. Financial contributions and experiences of non-spousal, employed family caregivers. J Appl Gerontol. 2022;41(12):2459–68. 10.1177/07334648221115261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jones BL. The challenge of quality care for family caregivers in pediatric cancer care. Semin Oncol Nurs. 2012;28(4):213–. 10.1016/j.soncn.2012.09.003. 20. [DOI] [PubMed] [Google Scholar]
- 44.Bekui BAA, Ohene LA, Badzi C, Ampomah MO, Aziato L. Physical and socioeconomic burden of caregiving on family caregivers of children with cancer at a tertiary hospital in Ghana. Nurs Open. 2023;10(2):915–25. 10.1002/nop2.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tran YH, Coven SL, Park S, Mendonca EA. Social determinants of health and pediatric cancer survival: a systematic review. Pediatr Blood Cancer. 2022;69(5):e29546. 10.1002/pbc.29546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O’Brien JM, Retinoblastoma. Clinical presentation and the role of neuroimaging. AJNR Am J Neuroradiol. 2001;22(3):427–9. PMID: 11237964. [PubMed] [Google Scholar]
- 47.Balmer A, Munier F. Differential diagnosis of leukocoria and strabismus, first presenting signs of retinoblastoma. Clin Ophthalmol. 2007;1(4):431–9. 10.2147/OPTH.S1369. [PMC free article] [PubMed] [Google Scholar]
- 48.Nazemi KJ, Malempati S. Emergency department presentation of childhood cancer. Emerg Med Clin North Am. 2009;27(3):477. 10.1016/j.emc.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 49.Morgan EH, Schoonees A, Sriram U, Faure M, Seguin-Fowler RA. Caregiver involvement in interventions for improving children’s dietary intake and physical activity behaviors. Cochrane Database Syst Rev. 2020;2020(1):CD012547. 10.1002/14651858.CD012547.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon reasonable request.