Abstract
Triple-negative breast cancer (TNBC) is a particularly aggressive and therapeutically challenging subtype of breast cancer, defined by the lack of estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 expression. This absence of actionable molecular targets contributes to its resistance to conventional treatments. This review provides an overview of the mechanistic functions, interrelated processes, and therapeutic implications of several programmed cell death (PCD) pathways—including apoptosis, pyroptosis, necroptosis, autophagy, and ferroptosis—in the context of TNBC pathogenesis and treatment. A conceptual framework is proposed for leveraging these interconnected cell death pathways as a basis for novel targeted interventions. Given the complex interplay among various PCD forms characterized by shared features such as inflammation, mitochondrial dysfunction, and overlapping molecular mediators, this integrated network offers promising opportunities for combinatorial therapeutic strategies. Modulation of one cell death pathway may influence others, potentially amplifying therapeutic efficacy. Furthermore, these PCD pathways are highly relevant to immunotherapy outcomes, offering a foundation for synergistic treatment modalities. This review provides an in-depth analysis of the crosstalk between immune-based therapies and PCD, along with a comprehensive discussion of derived therapeutic approaches. However, tumor diversity, resistance mechanisms, and discrepancies between preclinical models and human physiology pose major challenges in applying these findings clinically. The overarching goal is to present innovative insights and strategies to enhance the clinical management of TNBC and ultimately improve patient outcomes.
Graphical Abstract
Keywords: Triple-negative breast cancer, Cell death, Immunotherapy, Programmed cell death, TNBC, PCD
Introduction
Triple-negative breast cancer (TNBC) is a highly aggressive breast cancer subtype [1], characterized by the absence of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) expression [2]. TNBC accounts for approximately 15–20% of all invasive breast cancer cases and is associated with heightened invasion, metastasis, recurrence, and poor survival [3]. It is marked by rapid tumor progression and early dissemination, contributing to significantly reduced overall survival. In the absence of specific therapeutic targets, conventional cytotoxic chemotherapy remains the mainstay of treatment for TNBC [4]. However, clinical outcomes remain suboptimal due to the frequent development of multidrug resistance.
Two primary challenges complicate the management of TNBC. First, the lack of hormone receptors and HER2 amplification renders hormone therapies and HER2-targeted treatments ineffective, leaving systemic chemotherapy as the only nonsurgical option [5]. Second, TNBC exhibits substantial heterogeneity at both intertumoral and intratumoral levels, manifesting in divergent clinical, pathological, histological, and molecular profiles [6]. Over time, the efficacy of chemotherapy diminishes as TNBC cells adapt and develop resistance mechanisms, underscoring the urgent need to identify novel therapeutic strategies and unique molecular targets to improve prognosis.
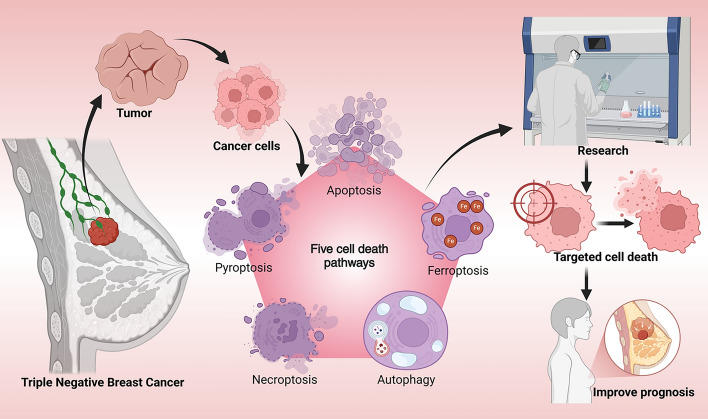
Cell death can be broadly categorized into accidental cell death (ACD) and programmed cell death (PCD) on the basis of morphological features, environmental triggers, and regulatory mechanisms [7]. ACD is an unregulated response to exogenous insults—such as mechanical injury, toxins, or oxidative stress—that overwhelm cellular defense mechanisms and result in rapid demise [7–9]. Conversely, PCD is a genetically regulated and biologically purposeful process, essential for tissue homeostasis, embryonic development, and immune surveillance [10]. Several PCD modalities have been identified, including apoptosis, pyroptosis, necroptosis, ferroptosis, autophagy-dependent cell death, lysosome-dependent cell death (LCD), parthanatos, and panoptosis [9, 11]. Among these, apoptosis, pyroptosis, necroptosis, autophagy, and ferroptosis are particularly relevant in cancer biology, each influencing tumor cell fate through distinct yet sometimes overlapping pathways. Understanding their regulatory networks and interactions is crucial for developing novel therapeutic paradigms for TNBC. Inducing PCD in cancer cells can suppress tumor growth and inhibit metastasis [12]. These cell death pathways also mediate antitumor effects by reshaping the tumor microenvironment, activating immune responses through the release of pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs) [13, 14], and reversing drug resistance by inducing the release of bioactive compounds [11]. Importantly, recent studies have underscored the potential of integrating PCD mechanisms with immunotherapy. The emergence of immune checkpoint inhibitors, T cell therapies, and natural killer (NK) cell therapies has opened new avenues for combining immunotherapeutic strategies with cell death induction to enhance the overall effectiveness of TNBC treatment. This dual-pronged approach may not only facilitate tumor eradication but also help overcome immune evasion by modulating key components of the tumor microenvironment. Recent research has underscored the potential therapeutic benefits of modulating these pathways to enhance treatment outcomes. Despite the promising nature of preclinical findings, the translation of these discoveries into clinical practice poses significant challenges. This difficulty is largely attributed to the intricate interplay between various cell death pathways and the resistance mechanisms inherent in TNBC. Although research tools, including animal models and organoid models, facilitate the screening of potential therapies, their limited applicability to human physiology continues to be a substantial impediment to clinical translation.
Ongoing research aimed at elucidating the molecular underpinnings of cell death in TNBC is expected to reveal novel drug targets, potentially overcoming existing therapeutic limitations and driving the development of next-generation treatments. Overall, leveraging the interconnectivity of cell death pathways holds great promise for advancing both basic understanding and clinical management of TNBC. These topics are discussed at greater length within the body of this review.
The roles of different cell death pathways in TNBC
Multiple programmed cell death mechanisms play diverse and complex roles in TNBC progression and treatment. Apoptosis, the most well-characterized form of PCD, contributes significantly to TNBC cell clearance. However, TNBC frequently acquires resistance to apoptotic signals, diminishing the efficacy of therapies reliant on this mechanism [15]. Pyroptosis, a lytic and inflammatory form of cell death, exhibits dual roles. While it can eliminate tumor cells [16], it may also promote tumor growth under certain conditions [17]. Necroptosis has emerged as a pivotal compensatory mechanism in TNBC, particularly in cases where apoptotic pathways are impaired. Activation of necroptosis has been linked to improved responsiveness in resistant tumor phenotypes [18]. Autophagy, meanwhile, plays a paradoxical role in TNBC [19]. On one hand, it can promote cell survival by recycling intracellular components; on the other, excessive autophagic activity may trigger cell death by degrading essential cellular structures [20]. Ferroptosis, a recently characterized iron-dependent form of cell death, is increasingly recognized as a critical process in TNBC pathogenesis [14]. It is driven by lipid peroxidation and oxidative stress, which can directly kill tumor cells and modulate the tumor microenvironment to suppress progression [21, 22].
These five forms of cell death—apoptosis, pyroptosis, necroptosis, autophagy, and ferroptosis—have been the focus of intensive research, as each uniquely contributes to TNBC cell survival or death. The intricate interplay among these pathways provides a rich foundation for novel therapeutic interventions. Exploring their regulatory crosstalk and functional convergence is key to identifying new drug targets and formulating therapies. A deeper understanding of these cell death pathways and their integration into the tumor landscape can ultimately lead to more effective, personalized treatments for TNBC.
Apoptosis
Apoptosis is a fundamental form of PCD that plays a vital role in maintaining tissue homeostasis. It is a gene-regulated and orderly process involving nuclear fragmentation, cytoplasmic shrinkage, and cell disassembly into apoptotic bodies [23, 24]. A key distinction between apoptosis and necrosis is that apoptosis does not induce inflammation [25]. In cancer, where uncontrolled cell proliferation is a hallmark, inducing apoptosis has emerged as a central therapeutic strategy [26]. In TNBC, apoptosis is particularly crucial, as it promotes the elimination of malignant cells through tightly regulated signaling pathways. Dysregulation of apoptotic processes contributes to tumor progression and therapeutic resistance in TNBC [27].
Apoptosis is primarily triggered through two major signaling pathways: the extrinsic (death receptor-mediated) and intrinsic (mitochondrial) pathways [28]. The extrinsic pathway is initiated by the binding of extracellular death ligands—including Fas ligand (FasL), tumor necrosis factor (TNF)-α, and TNF-related apoptosis-inducing ligand (TRAIL)—to their corresponding death receptors such as Fas receptor (FasR), tumor necrosis factor receptor 1 (TNFR1), death receptor 4 (DR4), and death receptor 5 (DR5) [9, 29]. Ligand binding leads to the assembly of a death-inducing signaling complex (DISC) [30], which recruits and activates caspase-8. Activated caspase-8 then cleaves and activates caspase-3, a key executioner caspase responsible for the morphological and biochemical features of apoptosis [8, 31]. The intrinsic pathway, in contrast, is activated in response to intracellular stress signals such as DNA damage, oxidative stress, and endoplasmic reticulum dysfunction [32, 33]. Oxidative stress, characterized by the excessive accumulation of reactive oxygen species (ROS) [34], can compromise mitochondrial membrane integrity [14], leading to increased permeability and the release of pro-apoptotic factors such as cytochrome c (CytC) into the cytoplasm [35]. DNA damage triggers the activation of ATM and Rad3-related (ATR) proteins, which stabilize and activate the tumor suppressor p53 [36]. Activated p53 promotes the transcription of pro-apoptotic Bcl-2 family proteins, such as Bad and Bid, which antagonize anti-apoptotic proteins such as Bcl-2 [37, 38]. This interaction facilitates the oligomerization of Bax and Bak on the outer mitochondrial membrane, further enhancing membrane permeability [39]. The subsequent release of CytC into the cytoplasm leads to the formation of the apoptosome complex, composed of apoptotic protease activating factor 1 (Apaf-1), CytC, and procaspase-9 [40]. This complex activates caspase-9, which in turn activates caspase-3, thereby initiating the apoptotic cascade [41].
Apoptosis is essential for eliminating genetically unstable or damaged cells, thereby preventing malignant transformation and tumor development [33]. In TNBC, impairment of apoptotic signaling enables tumor cells to evade immune-mediated clearance, promoting sustained proliferation, metastasis, and resistance to therapy [42]. Tumor cells primarily circumvent destruction by upregulating anti-apoptotic proteins while downregulating pro-apoptotic proteins [43]. Differential expression of Bcl-2 family proteins plays a significant role in TNBC progression and drug resistance [44]. For instance, anti-apoptotic Bcl-2 and Bcl-xL overproduction inhibits the function of pro-apoptotic proteins, preventing the cytosolic release of CytC and thereby abrogating apoptotic signaling [45].
TNBC is additionally characterized by the overexpression of the mucin MUC1 [46], and its C-terminal subunit acts as an oncoprotein by interacting with various kinases and effectors [47]. Notably, MUC1 interacts with the nuclear transcription factor NF-κB p65 (RelA), a key regulator of cell survival and inflammation [9, 48]. This interaction enhances NF-κB activation and nuclear translocation, allowing it to bind the promoter of the gene encoding Bcl-2, leading to the upregulation of this anti-apoptotic protein and the consequent inhibition of CytC release and apoptosis induction [49, 50]. Additionally, MUC1 facilitates the activation of the epidermal growth factor receptor (EGFR)/PI3K/Akt signaling pathway [14], which further promotes the transcription of genes that inhibit apoptosis and enhance cell survival [51]. Given the central role of apoptotic dysregulation in TNBC pathogenesis, restoring apoptotic signaling is a promising therapeutic avenue. Current strategies include chemotherapeutic agents, targeted molecular therapies, and immunotherapeutics that aim to modulate key apoptotic regulators, including Bcl-2 family proteins, caspases, and noncoding RNAs involved in cell death regulation.
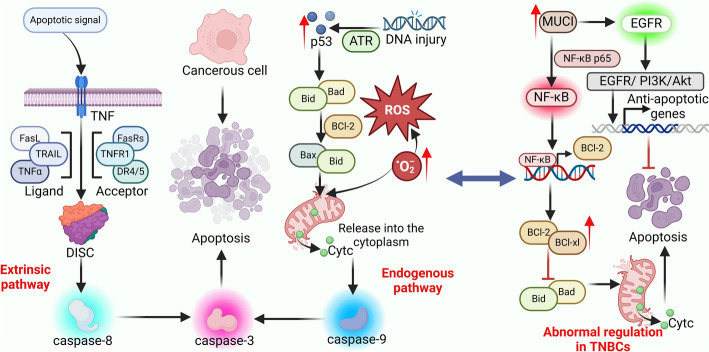
In summary, apoptosis is a critical cellular process that prevents the survival of aberrant cells and maintains tissue integrity. In TNBC, disruption of apoptotic signaling contributes significantly to tumor development, metastasis, and treatment resistance. Present understanding primarily centers on the extrinsic and intrinsic pathways and their molecular regulators. Continued investigation into the mechanisms governing apoptosis in TNBC is essential for the development of innovative, mechanism-based therapies aimed at improving clinical outcomes and patient survival (Fig. 1).
Fig. 1.
Apoptotic Activity and Regulation in TNBC. Apoptosis is predominantly activated via two distinct pathways: the extrinsic and intrinsic pathways. The extrinsic pathway is initiated by receptors of the TNF family, DISC, which subsequently activates caspase-8, leading to the activation of caspase-3. Conversely, the intrinsic pathway is activated by cellular stressors, including oxidative stress and DNA damage, which engage the mitochondrial pathway. Stress-induced alterations in mitochondrial membrane permeability result in the release of pro-apoptotic factors, such as CytC, which facilitates the activation of caspase-9, subsequently leading to caspase-3 activation. The convergence of both pathways at the activation of caspase-3 culminates in apoptosis. In TNBC cells, the overexpression of MUC1 directly interacts with the NF-κB p65 subunit. By activating NF-κB signaling and upregulating the expression of Bcl-2 family proteins, the release of CytC is inhibited. Additionally, MUC1 activates the EGFR/PI3K/Akt signaling pathway, collectively inhibiting apoptosis and promoting tumor cell survival and drug resistance
Pyroptosis
Pyroptosis is a highly inflammatory form of PCD, primarily initiated by the activation of inflammasomes [52]. This process initiates the activation of caspase family proteins, which subsequently cleave Gasdermin proteins [53], leading to pore formation in the cell membrane, ultimately causing cell swelling and lysis [14]. This process results in the release of intracellular contents, including DAMPs and pro-inflammatory cytokines, thereby triggering an acute inflammatory response in the surrounding tissue that resembles necrotic cell death [54, 55]. While both apoptosis and pyroptosis involve the activation of caspase family members, a key distinction is that pyroptosis culminates in lytic cell membrane rupture and the release of numerous inflammatory mediators, including interleukin-1β (IL-1β) and IL-18 [56]. Pyroptosis is characterized by membrane pore formation [14], as well as mitochondrial dysfunction, and robust cytokine release, which collectively contribute to inflammation and immune activation [57, 58]. In TNBC, pyroptosis plays a complex, dualistic role in tumor progression. On one hand, pro-inflammatory cytokines such as IL-1β can enhance tumor invasiveness and metastatic potential [59]. For instance, IL-1β has been shown to promote epithelial-mesenchymal transition (EMT) and cancer stem cell (CSC) traits in TNBC. Moreover, pyroptosis can induce the expression of chemokines such as C–C motif chemokine ligand 2 (CCL2) [60], which activates the Akt signaling pathway and elevates β-catenin levels, further driving EMT and CSC phenotypes [61]. On the other hand, pyroptosis can also initiate potent anti-tumor immune responses. IL-18, a cytokine released during pyroptosis, enhances the cytotoxic activity of NK cells and cytotoxic T lymphocytes (CTLs) against tumor cells [14, 62]. Additionally, the inflammatory milieu generated during pyroptosis facilitates the recruitment of immune effector cells into the tumor microenvironment [63], which can enhance immune-mediated tumor clearance [64]. Pyroptosis can be triggered by multiple upstream pathways, providing opportunities to selectively induce cell death in tumor cells [65].
In tumor cells, pyroptosis is regulated by multiple pathways and factors, which can be broadly classified into three major mechanisms: the classical inflammasome pathway (caspase-1-dependent), the nonclassical inflammasome pathway (dependent on caspase-4/-5 in humans and caspase-11 in mice), and a caspase-3-dependent pathway. The classical, or canonical, inflammasome pathway is primarily activated by the formation of multiprotein complexes known as inflammasomes [66]. These inflammasomes typically consist of three key components: sensor proteins—such as NOD-like receptor thermal protein domain-associated protein 3 (NLRP3) or absent in melanoma 2 (AIM2); the adaptor protein apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC); and the effector protein caspase-1 [67]. Sensor proteins detect PAMPs or DAMPs, leading to conformational changes that promote the assembly of the inflammasome complex. Specifically, NLRP3 utilizes its pyrin domain (PYD) to bind the PYD domain of ASC, initiating inflammasome assembly [68]. AIM2, on the other hand, detects intracellular double-stranded DNA through its hematopoietic interferon-inducible nuclear protein with a 200-amino acid repeat (HIN200) domain and similarly interacts with ASC [69]. ASC functions as a molecular bridge, linking sensor and effector proteins through its N-terminal PYD and C-terminal caspase recruitment domain (CARD) [70]. The PYD of ASC binds to the PYD of sensor proteins [71], while its CARD domain interacts with the CARD domain of caspase-1, thereby recruiting and activating the effector protease [72]. Once activated, caspase-1 processes pro-inflammatory cytokines, including pro-IL-1β and pro-IL-18, into their mature forms [73]. Caspase-1 also cleaves GSDMD, a critical executor of pyroptosis [74, 75]. Gasdermin family proteins generally contain an N-terminal domain, which has pore-forming capabilities, and a C-terminal domain, which acts as an autoinhibitory region that keeps the protein in an inactive state [76]. Upon cleavage, the inhibitory C-terminal domain is removed, liberating the active N-terminal domain. This fragment embeds into the plasma membrane to form transmembrane pores [77], allowing ions and water to enter the cell, ultimately causing cell swelling, lysis, and the release of inflammatory mediators, such as IL-1β and IL-18 [78, 79], triggering a strong inflammatory response. The nonclassical inflammasome pathway is initiated through direct activation of caspase-4/-5 in humans (or caspase-11 in mice) by intracellular lipopolysaccharide (LPS) [80]. Upon recognition of LPS, these caspases cleave GSDMD, leading to membrane pore formation and pyroptosis, similar to the classical pathway. Furthermore, caspase-4/5/11 activation can lead to secondary activation of caspase-1, thereby linking the nonclassical and classical pathways [81]. Leading to effects similar to the classical pathway. The caspase-3-dependent pathway is typically activated by external stimuli, such as chemotherapeutic agents (e.g., cisplatin and doxorubicin), oxidative stress, and DNA damage, all of which can activate caspase-3 [82, 83]. Once activated, caspase-3 cleaves Gasdermin E (GSDME), releasing its pore-forming N-terminal fragment. This process also leads to the activation of caspase-1 and triggers pyroptotic cell death [84].
There is a strong association between pyroptosis and the initiation, progression, and treatment of TNBC. Pyroptosis is characterized by the release of abundant pro-inflammatory cytokines such as IL-1β and IL-18. Within the tumor microenvironment, IL-1β has been implicated in promoting tumor growth, invasion, and metastasis and is correlated with unfavorable clinical outcomes [17]. In contrast, IL-18 has demonstrated the capacity to activate NK cells and CTLs [62, 85], thereby enhancing immune-mediated tumor cell destruction. GSDMD plays a central role in pyroptosis-mediated tumor suppression. Following cleavage by caspase-1 or caspase-4/5/11, the GSDMD N-terminal fragment forms pores in the tumor cell membrane, leading to osmotic imbalance, cell lysis, and the release of tumor-associated antigens [86]. These antigens are subsequently captured by DCs and presented to T cells, triggering a robust adaptive immune response that strengthens antitumor immunity [87]. Additionally, pyroptosis contributes to macrophage polarization toward the M1 phenotype, which is known for its pro-inflammatory and tumor-inhibitory effects. M1 macrophages secrete cytokines that suppress tumor proliferation and metastasis while promoting immune activation within the tumor microenvironment [88]. Pyroptosis can activate tumor suppressor genes such as p53, which not only promotes the apoptosis of tumor cells but also inhibits the growth of TNBC [89]. It has also been shown to induce mitochondrial dysfunction, thereby reducing ATP production and impairing energy metabolism, which limits tumor cell survival [90].
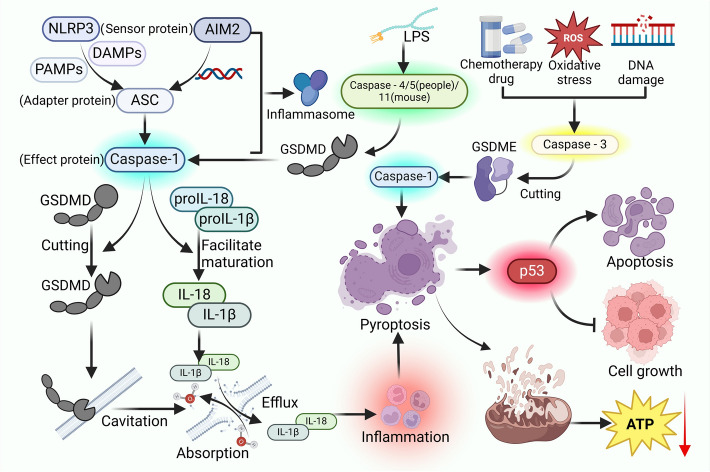
It serves as an important antitumor mechanism in TNBC by both directly eliminating cancer cells and enhancing the immune system’s ability to target tumors. A growing body of research is focusing on the molecular regulation of pyroptosis—particularly gene expression profiles and associated signaling pathways—in the context of TNBC. These insights are essential for guiding the development of innovative therapeutic strategies aimed at leveraging pyroptosis for more effective cancer treatment (Fig. 2).
Fig. 2.
The Mechanistic Activation of Pyroptosis. Pyroptosis is primarily regulated through three distinct pathways: the canonical pathway, which is dependent on caspase-1; the noncanonical pathway, reliant on caspase-4/5 in humans and caspase-11 in mice; and a pathway dependent on caspase-3. The canonical pathway is initiated when sensor proteins such as NLRP3 or AIM2 recognize PAMPs or DAMPs, resulting in the assembly of an inflammasome complex comprising ASC and caspase-1. This complex promotes the maturation of pro-inflammatory cytokines pro-IL-18 and pro-IL-1β and cleaves GSDMD, culminating in the disruption of the plasma membrane and the subsequent release of IL-18 and IL-1β, ultimately leading to cell death. The noncanonical pathway is triggered by LPS, which directly activates caspase-4/-5 or caspase-11, leading to the cleavage of GSDMD and the induction of pyroptosis. Additionally, chemotherapeutic agents can activate caspase-3, which subsequently cleaves GSDME and activates caspase-1, thereby inducing pyroptosis via a caspase-3-dependent mechanism
Necroptosis
Necroptosis is a regulated form of cell death that occurs independently of caspase activity. As a recently characterized form of programmed cell death, necroptosis is mechanistically distinct from both apoptosis and classical necrosis. It is predominantly governed by signaling pathways involving receptor-interacting protein kinases (RIPK1 and RIPK3) and the mixed lineage kinase domain-like protein (MLKL) [91]. Unlike apoptosis, which is a noninflammatory and orderly process that removes cells without disrupting tissue homeostasis, necroptosis is inherently pro-inflammatory [92]. This inflammatory nature arises from the release of intracellular contents following membrane rupture, a morphological hallmark that necroptosis shares with necrosis [75]. However, while necrosis is an uncontrolled, passive response to extreme cellular injury [31], necroptosis is an active, regulated process orchestrated by specific molecular mechanisms [93]. The release of cellular contents during necroptosis intensifies the surrounding inflammatory response [94]. The regulation of necroptosis is primarily mediated by the phosphorylation of MLKL, facilitated by apoptosis-regulating proteins such as RIPK1 and RIPK3 [95], and it interacts with ROS to modulate cell death and inflammatory signaling [96]. The incidence of necroptosis in TNBC provides a novel opportunity for patient treatment.
In contrast to apoptosis, necroptosis does not depend on caspases, particularly caspase-8. Instead, it is triggered through the activation of death receptors on the cell surface. When these receptors, such as FasR, TNFR, and DR4/DR5 bind their respective ligands, including FasL, TNF-α, and TRAIL, they typically initiate apoptosis through the formation of a DISC. This complex activates caspase-8, thereby promoting apoptotic cell death [93]. However, in cancer cells where caspase-8 expression is deficient or inhibited, the apoptotic cascade is disrupted. Under these conditions, RIPK1 becomes activated and undergoes deubiquitination, which facilitates its interaction with RIPK3 [97, 98]. This RIPK1–RIPK3 complex undergoes mutual phosphorylation and subsequently recruits MLKL. Phosphorylated MLKL oligomerizes and translocates to the plasma membrane, where it disrupts membrane integrity, culminating in necroptosis [99].
The activation of necroptosis holds particular significance in the context of TNBC, as it offers a potential strategy to overcome the resistance of tumor cells to apoptosis-based treatments [18]. Dysregulation of apoptotic pathways is a common feature in TNBC, often leading to chemoresistance and enabling tumor cells to evade cell death induced by conventional chemotherapeutic agents [92]. This evasion promotes uncontrolled tumor proliferation and contributes to aggressive disease progression [100]. Necroptosis addresses this therapeutic challenge by inducing irreversible cancer cell death via a mechanism distinct from apoptosis [101]. For instance, TNBC cells frequently exhibit elevated levels of the anti-apoptotic protein Bcl-2, which inhibits the activation of the caspase family and thereby blocks the apoptotic cascade [102]. In contrast, necroptosis proceeds independently of caspase activity and is instead mediated by a distinct set of signaling molecules, including receptor-interacting protein kinases RIPK1 and RIPK3, as well as MLKL. As a result, conventional anti-apoptotic resistance mechanisms in TNBC do not interfere with the necroptotic pathway. In its initial stages, necroptosis increases the permeability of tumor cell membranes, leading to the leakage of intracellular contents and ultimately resulting in cell death. This not only suppresses tumor proliferation and growth but also triggers immunological consequences that enhance anti-tumor responses [18]. Specifically, necroptosis promotes the release of high-mobility group box 1 protein (HMGB1) [14], which plays a central role in immune activation in TNBC. HMGB1 recruits immune cells and induces the release of cytokines such as IL-1α and IL-1β, both of which are involved in targeting tumor cells [103, 104]. HMGB1 is recognized by DCs, which are pivotal in orchestrating the adaptive immune response [105]. In TNBC patients, higher levels of DC infiltration are strongly correlated with improved clinical prognosis [106]. DCs engulf antigenic substances released from necrotic tumor cells, they undergo maturation and gain the ability to specifically recognize and target tumor cells presenting those antigens, thereby initiating a robust adaptive immune response [107]. Additionally, DCs secrete various cytokines, including IL-12 [108], which promotes the proliferation and activation of T cells and NK cells, further enhancing anti-tumor immunity [109].
As a caspase-independent programmed cell death pathway, necroptosis provides a promising alternative to trigger tumor cell death when apoptosis is suppressed in TNBC. Further exploration into the regulatory mechanisms of necroptosis and its interplay with immunotherapeutic strategies is expected to offer new theoretical insights for innovative treatments, potentially addressing the therapeutic limitations posed by apoptosis resistance in TNBC (Fig. 3).
Fig. 3.
The Activation and Anti-Tumor Mechanisms of Necroptosis. Necroptosis is initiated when death receptors on the cell surface, such as FasRs and TNFR1, bind to their respective ligands, leading to the formation of the DISC. In the absence or inhibition of caspase-8, RIPK1 is recruited and interacts with RIPK3, resulting in the phosphorylation of MLKL. The necrosome, a multiprotein complex, subsequently translocates to the plasma membrane, thereby initiating necroptosis. This pathway represents a caspase-independent mechanism of cell death with significant potential for anti-tumor applications
Autophagy
Autophagy is an evolutionarily conserved cellular degradation process that plays a fundamental role in maintaining homeostasis and cellular integrity by recycling cytoplasmic components through the formation of autophagosomes [110]. These vesicles encapsulate and degrade damaged proteins and organelles, contributing to intracellular stability and nutrient metabolism [111, 112]. In recent years, autophagy has emerged as a promising therapeutic target in TNBC, where it plays a paradoxical role by both promoting cancer cell survival and facilitating their death under certain conditions [113, 114]. In TNBC, autophagy is recognized as a double-edged sword [19]. While it promotes programmed cancer cell death by increasing autophagic activity, it also sustains tumor survival by providing essential nutrients through the recycling of cellular components, particularly under conditions of metabolic stress [20]. The regulation of autophagy significantly influences tumor proliferation, invasiveness, and therapeutic responsiveness.
The process of autophagy is tightly regulated by a series of autophagy-related genes (ATGs) and signaling pathways [115, 116]. ATG1, also known as UNC-51-like kinase 1 (ULK1), acts as a core regulator, responding to intracellular nutrient levels and upstream signaling cues [117]. Under nutrient-rich conditions, the mammalian target of rapamycin complex 1 (mTORC1) phosphorylates ULK1, thereby inhibiting autophagy [118]. In contrast, nutrient deprivation activates AMP-activated protein kinase (AMPK) [119], which suppresses mTORC1 activity and relieves its inhibition of the ULK1 complex composed of ULK1, ATG13, FIP200, and ATG101 [120, 121]. This ULK1 complex then translocates to the site of autophagosome initiation [122]. At this site, the ULK1 complex recruits the class III phosphatidylinositol 3-kinase (PI3K) complex, facilitating the formation of the phagophore, the precursor to the autophagosome [31]. The phagophore’s expansion requires two additional protein complexes: the ATG5-ATG12-ATG16L1 complex and the LC3 (microtubule-associated protein 1 light chain 3) protein [123]. This ATG5-ATG12-ATG16L1 complex localizes to the autophagosomal membrane, promoting the lipidation of LC3 family proteins [124]. Initially, LC3 is cleaved by ATG4 to form LC3-I, which is then conjugated with phosphatidylethanolamine (PE) to produce LC3-II, the membrane-bound form essential for autophagosome maturation [125]. The autophagosome then engages lysosomal fusion proteins while concurrently disassembling the ATGs on its outer membrane [111]. This process involves specific fusion proteins, such as Syntaxin 17 (STX17), which interacts with SNAP29 and VAMP8 to form the SNARE complex. The SNARE complex mediates membrane fusion, allowing lysosomal enzymes to degrade the autophagosomal contents into smaller molecules, which are then recycled for cellular use [115].
In TNBC, elevated expression of autophagy-related genes such as LC3 and Beclin-1 has been observed, leading to increased autophagic activity [126]. This heightened activity contributes to chemotherapy resistance and metastatic potential [127]. Clinically, downregulating the expression of LC3 and Beclin-1 has become a key strategy in TNBC therapy [128, 129]. While autophagy can suppress tumor initiation by eliminating defective organelles and proteins, it can also support tumor cell survival under stress by generating metabolic substrates through catabolic degradation [130]. In response to nutrient deprivation, TNBC cells activate autophagy to sustain growth and proliferation, converting macromolecules into reusable substrates [131]. Throughout tumor progression, cancer cells encounter various stressors such as chemotherapy, radiotherapy, and oxidative damage. Autophagy alleviates this stress by removing damaged cellular components, thereby enhancing tumor cell resilience [110]. Notably, autophagy in TNBC can suppress apoptosis by downregulating apoptosis-related proteins. For example, overexpression of Beclin 1 has been shown to reduce the levels of pro-apoptotic proteins such as BAX, caspase-3, and caspase-8, thereby impairing apoptosis [132]. Moreover, autophagy influences immune evasion mechanisms in TNBC by regulating immune cell function and differentiation [133]. This includes modulation of T cells and macrophages, allowing tumor cells to circumvent immune surveillance [134, 135]. However, excessive autophagy can be detrimental to both tumor and normal cells [136]. In conditions of extreme nutrient deprivation, for example, autophagy may lead to the degradation of essential cellular components, ultimately resulting in cell death [137, 138].
In summary, autophagy plays a dual and complex role in TNBC pathogenesis. Its intricate regulation underscores the necessity for further research aimed at converting its tumor-promoting effects into therapeutic advantages. A deeper understanding of autophagy’s molecular mechanisms and its dynamic interactions with tumor biology will be essential for designing safe and effective autophagy-targeted therapies (Fig. 4).
Fig. 4.
The Regulation and Function of Autophagy in TNBC. The regulation of autophagy is primarily mediated by ATGs and various signaling pathways. Under conditions of nutrient sufficiency, mTORC1 inhibits ULK1, thereby suppressing autophagy. Conversely, nutrient deprivation activates AMPK, which alleviates the inhibition of ULK1, facilitating the activation of the ULK1 complex, comprising ULK1, ATG13, FIP200, and ATG101. This activation leads to the recruitment of the PI3K complex and the subsequent formation of a phagophore. The elongation of the phagophore necessitates the involvement of the ATG5-ATG12-ATG16L1 complex and microtubule-associated proteins 1A/1B LC3 to develop an autophagosome. The autophagosome subsequently fuses with a lysosome to form an autolysosome, thereby initiating autophagy. In TNBC, the upregulation of Beclin 1 expression inhibits apoptosis, facilitating immune evasion by tumor cells and resulting in excessive autophagy
Ferroptosis
Ferroptosis is a distinct form of regulated cell death characterized by the excessive accumulation of intracellular iron and the subsequent peroxidation of lipids [125, 139]. It plays a critical role in the treatment of TNBC [21]. Unlike apoptosis and autophagy, ferroptosis is specifically driven by iron dependency and lipid peroxidation [140]. In TNBC, ferroptosis is intricately involved in tumor suppression through multiple mechanisms. First, ferroptosis inhibits TNBC cell proliferation by catalyzing lipid peroxidation through iron overload, ultimately disrupting cellular membrane integrity and function [141–143]. Second, ferroptosis can reshape the tumor microenvironment, thereby affecting the migration and invasiveness of TNBC cells [22]. Third, ferroptosis is closely tied to therapeutic responsiveness, with its induction enhancing the cytotoxic effects of anticancer treatments. Thus, modulating ferroptosis pathways offers a promising therapeutic avenue for TNBC management.
Ferroptosis is primarily regulated by two interconnected pathways: the classical iron metabolism pathway and the lipid metabolism pathway [144]. In the iron metabolism pathway, intracellular iron accumulation plays a central role. Transferrin, a major iron transport protein in the bloodstream, binds ferric ions and interacts with transferrin receptor 1 (TFR1) on the cell surface [145, 146]. During periods of high iron demand, such as during rapid tumor cell proliferation, TFR1 expression is upregulated to enhance iron uptake [147]. Upon cellular internalization via endocytosis, ferric ions are released in the acidic endosomal environment, while transferrin is recycled extracellularly [148]. The surplus iron is typically sequestered by ferritin, preventing iron-induced ROS generation [149, 150]. However, under proliferative or hypoxic conditions, TFR1 expression is further increased [151, 152], while pro-inflammatory cytokines, such as TNF-α and IL-6 [153], along with persistent iron demands, downregulate ferroportin (FPN1), the primary iron exporter, leading to iron overload [154]. Excess intracellular iron leads to the production of hydroxyl radicals through the Fenton reaction [155], thereby catalyzing ROS formation [156]. These ROS can target ferritin [157], releasing stored iron and expanding the labile iron pool (LIP). The tumor cell membrane, especially in TNBC, is enriched in polyunsaturated fatty acids (PUFAs), such as arachidonic acid and adrenic acid [158, 159], which are susceptible to ROS-induced lipid peroxidation [160]. This results in lipid peroxidation and the accumulation of lipid peroxides, a hallmark of ferroptosis [161]. The intracellular antioxidant defense system, primarily comprising glutathione peroxidase 4 (GPX4) and glutathione (GSH), plays a vital role in neutralizing lipid peroxides [162]. GPX4 utilizes GSH to reduce lipid peroxides into nontoxic lipid alcohols, thereby preventing ferroptosis [163, 164]. However, when GPX4 activity or GSH availability is diminished, lipid peroxide accumulation increases, facilitating ferroptosis initiation [21, 165].
Ferroptosis also significantly contributes to TNBC development and progression. Its induction requires both elevated free iron levels and excessive lipid peroxidation [166]. TNBC cells are particularly prone to ferroptosis due to their high iron and lipid content [167]. Iron overload in these cells promotes ROS generation and lipid peroxide accumulation, driving ferroptotic cell death. Concurrently, excessive lipid substrates enhance susceptibility to ROS-induced lipid peroxidation. Aberrations in iron regulatory proteins (IRPs) further exacerbate this process; overexpression of IRP1 and IRP2 in breast cancer disrupts iron homeostasis by increasing iron import and reducing iron storage, thereby promoting iron overload and ferroptosis [168]. GPX4 plays a pivotal antioxidant role in inhibiting ferroptosis by detoxifying lipid peroxides [169]. In TNBC, reduced GPX4 expression or activity impairs this defense mechanism, promoting ferroptotic vulnerability. Furthermore, nuclear factor erythroid 2-related factor 2 (Nrf2), a master regulator of oxidative stress responses, can limit ferroptosis by upregulating antioxidant genes. However, depletion of Nrf2 leads to ferritin accumulation within autophagosomes and enhances cellular sensitivity to ferroptosis [170, 171]. In TNBC cells, Nrf2 upregulation contributes to ferroptosis resistance, whereas its inhibition may enhance therapeutic efficacy [172]. Additionally, the tumor suppressor p53 can indirectly regulate ferroptosis by modulating Nrf2 signaling [173]. Notably, p53 is frequently inactivated in TNBC [174], suggesting that restoring p53 function may augment ferroptosis in TNBC cells through Nrf2 inhibition.
In summary, ferroptosis represents a promising anti-tumor mechanism in TNBC by inducing cancer cell death through iron accumulation, lipid peroxidation, and impairment of antioxidant defenses. Compared with conventional cell death pathways, ferroptosis exhibits higher specificity and therapeutic potential in targeting TNBC cells [175, 176]. Future research should focus on identifying novel regulatory factors of ferroptosis and exploring the synergistic effects of combining ferroptosis-inducing therapies with immunotherapy to improve clinical outcomes in TNBC (Fig. 5).
Fig. 5.
The Mechanistic Regulation of Ferroptosis. The initiation of ferroptosis can proceed through the classical iron metabolism pathway and the lipid metabolism pathway. In the iron metabolism pathway, transferrin interacts with the transferrin receptor TFR1 to facilitate the transport of iron ions. The intracellular accumulation of these iron ions, coupled with the reduced expression of the iron export protein FPN1, results in iron overload within the cell. This condition leads to the generation of ROS via the Fenton reaction, subsequently causing lipid peroxidation. In the lipid metabolism pathway, an imbalance in antioxidant system homeostasis, particularly involving GPX4 and GSH, leads to the accumulation of lipid peroxides, thereby inducing ferroptosis
Epigenetic regulation and potential pathways for treating TNBC
Epigenetic regulation governs gene expression without altering the underlying DNA sequence, primarily through mechanisms, such as DNA methylation, histone modifications, and the action of noncoding RNA [177]. DNA methylation typically represses gene expression by interfering with transcriptional activity [178], while histone modifications remodel chromatin architecture to either facilitate or restrict transcription factor access to DNA [179]. Non-coding RNAs, including microRNAs (miRNAs) and long noncoding RNAs (lncRNAs), regulate gene expression post-transcriptionally by either inhibiting mRNA translation or promoting mRNA degradation [180, 181]. These epigenetic processes play critical roles in TNBC, particularly in modulating cell death pathways through the regulation of key gene expression.
In the context of TNBC, aberrant DNA methylation has been shown to suppress the expression of apoptosis-related genes, such as caspase-8, thereby impairing apoptotic cell death [182]. Additionally, the tumor suppressor gene p53, which is central to genomic stability and the induction of apoptosis, can be transcriptionally silenced via hypermethylation of its promoter region. This downregulation of p53 contributes to genomic instability, enhanced tumorigenic potential, and diminished apoptotic responses [183, 184]. The methylation-mediated silencing of caspase-8 further endows tumor cells with anti-apoptotic properties, facilitating their survival and progression [185]. Conversely, in therapeutic settings, apoptosis in TNBC can be promoted through the assembly of the DISC with Fas-associated protein with death domain (FADD), caspase-8, and DR5 [186]. Epigenetic silencing is also implicated in the regulation of pyroptosis. For example, DNA methylation can suppress the transcription of GSDME thereby reducing pyroptotic activity in tumor cells and promoting immune evasion [84]. Similarly, zinc finger DHHC-type containing 1 (ZDHHC1), a putative tumor suppressor gene, is frequently inactivated through promoter methylation. Restoration of ZDHHC1 expression has been shown to enhance both apoptosis and pyroptosis in TNBC cells, contributing to its anti-tumor effects [187]. Moreover, promoter methylation of necroptosis-associated genes also plays a significant role in tumor development [188]. For example, in acute myeloid leukemia (AML) cells, DNA methylation silences RIPK3, reducing necroptosis and leading to drug resistance [189].
Additionally, histone modifications play a pivotal role in regulating cell death pathways, such as ferroptosis and autophagy, thereby influencing tumor cell proliferation and survival [190]. Specific histone modifications can modulate the expression of genes involved in iron metabolism and lipid peroxidation. For example, in pancreatic cancer, the upregulation of the histone acetyltransferase p300 enhances the acetylation of heat shock protein family A (Hsp70) member 5 (HSPA5), thereby promoting lipid peroxidation and sensitizing tumor cells to ferroptosis-induced cell death [191]. Similarly, CBP/p300, a well-characterized histone acetyltransferase, facilitates acetylation of histone H3 at lysine 27 (H3K27ac) [192]. In prostate cancer, CBP/p300-mediated histone acetylation activates autophagy pathways, which in turn suppress tumorigenic potential [193]. Furthermore, targeting histone methylation has emerged as a promising strategy to modulate ferroptosis. For instance, ORY-1001, a selective inhibitor of lysine-specific demethylase 1 (LSD1), inhibits the demethylation of histone marks H3K4me1/2 and H3K9me1/2. This leads to increased methylation at the promoter regions of glutathione synthase genes [194], thereby reducing glutathione synthesis, weakening the antioxidant defense of tumor cells, and enhancing their sensitivity to ferroptosis [195].
Non-coding RNAs also play a critical role in regulating programmed cell death pathways and modulating therapeutic responses in TNBC [196]. MicroRNAs (miRNAs), such as miR-15a and miR-16-1, have been shown to downregulate the anti-apoptotic protein Bcl-2, thereby promoting apoptotic cell death [197, 198]. Low expression levels of miR-30a have been associated with poor prognosis in TNBC, as miR-30a targets Beclin-1, a key regulator of autophagy. Downregulation of miR-30a leads to enhanced autophagic activity and tumor progression [199, 200]. Additionally, the long noncoding RNA (lncRNA) GAS5 promotes apoptosis by modulating the expression of pro-apoptotic proteins, thereby increasing the sensitivity of TNBC cells to chemotherapeutic agents [201, 202].
Ongoing research continues to explore the influence of epigenetic regulation on cell death pathways in TNBC, with the goal of developing more effective therapeutic strategies. Several epigenetically targeted drugs and interventions have been identified that can modulate key cell death mechanisms. These insights underscore the significance of epigenetic regulation in determining tumor progression and therapeutic response, providing new opportunities for improving clinical outcomes in TNBC (Table 1).
Table 1.
Applications of Programmed Cell Death Pathways in Epigenetic Regulation of TNBC
| Cell death pathway involved | Types of epigenetic regulation | Drugs or molecules | Mechanism | References |
|---|---|---|---|---|
| Apoptosis | DNA methylation | Decitabine | TRAF6 triggers the breakdown of DNMT1, DNMT3A, and DNMT3B via the lysosome-dependent protein degradation pathway | [358] |
| DNA methylation | Dazemetostat | The degradation of EZH2 leads to persistent disruption of endoplasmic reticulum function and excessive activation of the unfolded protein response, thereby initiating the apoptosis program of cancer cells | [359] | |
| Histone modification | Hydralazine | Induction of PPARγ up-regulation increases the expression of cyclin- dependent kinase inhibitors p21 and p27, thereby arresting the cell cycle at the G0/G1 phase | [360] | |
| Histone modification | YZ-836P | Induce TNBC cells to reduce the expression of CDK4, CDK6 and Cyclin D1, and upregulate p21 and p27, leading to cell arrest at the G1 phase and inducing apoptosis | [361] | |
| Histone modification | JQ1 | Blocking Brd4 can interfere with cell cycle and apoptosis signaling pathways, leading to tumor cell apoptosis | [362] | |
| Non-coding RNA | miR-34a | Inducing the apoptosis of TNBC cells through the CD44 and NOTCH signaling pathways | [363] | |
| Pyroptosis | DNA methylation | Decitabine | CP@Gel.CP@Gel facilitates the self-catalyzed production of reactive oxygen species, which triggers the activation of caspase-3. DAC prevents the methylation of GSDME, thereby elevating the protein level of GSDME and causing significant cell pyroptosis | [364] |
| Histone modification | HDACi | Following HDACi treatment, the levels of GSDMA, GSDMB, and GSDME rise, which encourages immune cell infiltration and boosts the effectiveness of anti-cancer immunity | [365] | |
| Non-coding RNA | miR-1290 | MiR-1290 can directly interact with the mRNA of NLRP3. It binds to a particular segment of the NLRP3 mRNA, hindering its translation and leading to reduced NLRP3 protein expression | [366] | |
| miR-155-5p | When used with Cetuximab, it enhances the pyroptosis of TNBC cells that overexpress the epidermal growth factor receptor by increasing GSDME-N and cleaved caspase-1 | [367] | ||
| Necroptosis | Histone modification | ZMF-23 | Inhibiting the activities of PAK1 and HDAC6 induces TNFα-regulated necroptosis, thereby further enhancing apoptosis | [368] |
| Autophagy | DNA methylation | DNMT1 inhibitor | Demethylate the CpG island in the promoter region of the Beclin-1 gene | [369] |
| Histone modification | Pan-HDI | It interferes with the hsp90/histone deacetylase 6/HSF1/p97 complex, resulting in increased hsp levels. It also boosts autophagic flux, raises LC3B-II expression, and causes the breakdown of the autophagic substrate p62 | [370] | |
| Histone modification | SAHA | When combined with olaparib, it enhances autophagy in TNBC cells and works together to inhibit the growth of TNBC cells with functional PTEN | [371] | |
| Non-coding RNA | circ-DNMT1 | It can bind to both p53 and AUF1 simultaneously, and the nuclear translocation of p53 and AUF1 promotes autophagy | [372] | |
| Ferroptosis | DNA methylation | SSZ | It enhances histone and DNA methylation at the MUC1 promoter, suppresses MUC1 gene transcription, reduces intracellular GSH levels, and heightens cell sensitivity to erastin-induced ferroptosis | [373] |
| Non-coding RNA | LncFASA | LncFASA attaches directly to the Ahpc-TSA domain of PRDX1, hindering its peroxidase function by inducing liquid–liquid phase separation, which disrupts intracellular ROS balance, controls ferroptosis, and ultimately leads to the destruction of TNBC cells | [374] | |
| Non-coding RNA | POU2F2 | It enhances the transcription of PTPRG-AS1, influences miR-376c-3p to increase SLC7A11 levels, thus preventing ferroptosis and aiding the progression of TNBC | [375] |
Interactions among programmed cell death pathways
The crosstalk among various forms of PCD constitutes a complex and dynamic regulatory network in TNBC therapy [41]. Interactions among apoptosis, pyroptosis, necroptosis, ferroptosis, and autophagy can profoundly influence disease progression and therapeutic efficacy [97]. The boundaries between these pathways are often blurred, and their interplay complicates mechanistic distinctions. Among these, apoptosis serves as a central node with extensive cross-regulatory interactions [56, 203].
In TNBC, apoptosis and pyroptosis are interlinked and can be co-activated in response to certain stimuli [41]. Apoptotic processes can trigger pyroptosis via mitochondrial dysfunction and the release of ROS and ATP, which subsequently activate inflammasomes [204, 205]. In turn, pyroptosis induces the release of pro-inflammatory cytokines, such as IL-1β and TNF-α, which can promote apoptosis. IL-1β enhances apoptosis through NF-κB pathway activation [206], while TNF-α can activate caspase-8 [207], promoting apoptosis. Certain treatments can simultaneously induce both apoptosis and pyroptosis, thereby enhancing tumor cell death and improving therapeutic outcomes [92]. For example, caspase-3, a key executor of apoptosis, also cleaves GSDME, which activates caspase-1 and promotes pyroptosis [208], highlighting this as a promising target in TNBC. Autophagy and apoptosis also exhibit bidirectional regulatory interactions. Autophagy can facilitate apoptosis by degrading anti-apoptotic factors, whereas apoptosis may inhibit autophagy through caspase-mediated cleavage of autophagy-related proteins. In TNBC, prolonged chemotherapy often leads to multidrug resistance (MDR) [209]. While autophagy can support cancer cell survival under chemotherapeutic stress [210], it can also induce apoptosis in MDR cells, thereby counteracting resistance [211]. For instance, in TRAIL-resistant lung adenocarcinoma, metformin enhances TRAIL-induced apoptosis by downregulating c-FLIP and increasing autophagic flux [211, 212]. Furthermore, autophagy regulated by ATG1 has been linked to the activation of caspases, DNA fragmentation, and cytoskeletal disruption—hallmarks of apoptosis [213]. Overexpression of Bcl-2, an anti-apoptotic protein, inhibits autophagy by binding to Beclin 1 and reducing PI3K activity [214, 215], underscoring the therapeutic potential of targeting this axis in cancer treatment.
The balance between apoptosis and necroptosis is also essential for effective tumor cell elimination in TNBC. Resistance to traditional chemotherapeutic and targeted therapies often manifests as a decrease in apoptotic susceptibility. In such cases, redirecting cell death toward necroptosis offers an alternative strategy. Caspase-8 functions as a molecular switch between apoptosis and necroptosis [216]. When caspase-8 is inhibited, such as by the pan-caspase inhibitor Q-VD-OPh, RIPK1 is activated, initiating necroptosis via the RIPK1-RIPK3-MLKL axis [217]. AddFerroptosis is also intricately connected to apoptosis in TNBC. Compounds like erastin and its derivatives induce ferroptosis by inhibiting the cystine/glutamate antiporter System Xc− , thereby depleting intracellular cystine and suppressing GSH synthesis [218]. This leads to increased oxidative stress and lipid peroxidation, hallmarks of ferroptosis [219, 220]. The resulting ROS can damage mitochondria [221], triggering the release of CytC and activating downstream caspase cascades culminating in apoptosis [222]. These pathways present novel therapeutic targets and strategies for the treatment of TNBC.
In TNBC therapy, pyroptosis and necroptosis may act synergistically. Pyroptosis involves the release of inflammatory cytokines and intracellular contents, which can stimulate necroptotic pathways [223]. Doxorubicin, a commonly used chemotherapeutic agent, induces ROS accumulation, leading to caspase-3-mediated cleavage of GSDME and the onset of pyroptosis [224]. This is accompanied by the release of pro-inflammatory cytokines, such as IL-18, IL-1β, TNF-α, and IFN-γ [63], with TNF-α binding to TNFR1 and activating the RIPK1-RIPK3-MLKL signaling axis to induce necroptosis [225, 226]. Furthermore, pyroptosis regulators such as ATP can activate the NLRP3 inflammasome, initiating pyroptosis and potentially influencing other cell death pathways [212, 227]. Pyroptosis and autophagy are also interconnected in TNBC. Autophagy can suppress pyroptosis by degrading inflammasome components such as TRIM32 [228], which inhibits pyroptosis [229]. Conversely, pyroptosis-induced cytokines like IL-18 can activate NF-κB signaling, which upregulates autophagy-related genes and promotes autophagic activity [230, 231]. Interestingly, in hepatocellular carcinoma, ferroptosis and pyroptosis may exhibit antagonistic effects, with certain enzymes simultaneously promoting pyroptosis and inhibiting ferroptosis [232]. For example, 4-hydroxynonenal, a lipid peroxidation byproduct, can inhibit NLRP3 inflammasome activation and suppress pyroptosis in macrophages [233].
Autophagy also modulates necroptosis and ferroptosis. By degrading necroptosis-associated proteins such as RIPK1 and RIPK3, autophagy inhibits necroptotic signaling. This effect can be enhanced by agents, such as 3′-epi-12β-hydroxyfroside and HyFS, which suppress the Akt/mTOR pathway [234, 235]. Similarly, RUBCNL/PACER proteins downregulate RIPK1 activity, further inhibiting necroptosis [236]. In the context of ferroptosis, Anomanolide C (AC) induces autophagy-dependent ferroptosis by downregulating GPX4, leading to Fe2+ accumulation and suppression of TNBC cell growth [237]. Specific forms of autophagy, including ferritinophagy, mitophagy, and chaperone-mediated autophagy, can also promote ferroptosis by elevating intracellular free iron or lipid levels [238]. While oxidative stress and lipid peroxidation can trigger autophagy, excessive autophagy may, in turn, enhance ferroptosis [239]. Moreover, oxidative stress associated with ferroptosis can simultaneously activate apoptosis and autophagy, highlighting the interconnected nature of these pathways [204, 240].
A notable interplay exists between necroptosis and ferroptosis in TNBC, with RIPK1 and RIPK3 kinases modulating ferroptotic responses via regulation of iron metabolism-related proteins [241]. Evidence from ischemic stroke models shows that HSP90 can activate both necroptosis and ferroptosis by promoting RIPK1 phosphorylation and inhibiting GPX4 activity, suggesting that activation of necroptosis may also potentiate ferroptosis [242].
Investigating the interactions among different cell death mechanisms offers valuable insights for enhancing cancer treatment, particularly by overcoming tumor resistance and optimizing therapeutic strategies. Combining conventional treatments—such as chemotherapy, radiotherapy, targeted therapy, and immunotherapy—can induce multiple forms of cell death, potentially improving therapeutic efficacy and patient outcomes. This integrative approach is especially pertinent in TNBC, where elucidating the crosstalk among cell death pathways may shed light on tumor pathogenesis and therapeutic resistance, thereby guiding more effective interventions. Currently, TNBC treatment regimens include surgical resection, chemotherapy, radiation therapy, molecularly targeted agents, and immune-based approaches. The ongoing research aims to inform the development of innovative therapeutic strategies, establish comprehensive treatment frameworks, and promote precision medicine, ultimately expanding therapeutic options and improving the quality of life for patients with TNBC.
Immunotherapy and programmed cell death
Immunotherapy, as an emerging modality in TNBC treatment, plays a pivotal role in modulating PCD pathways [243, 244]. Immune checkpoint blockade, T cell-based therapies, and NK cell therapies interact with the regulation of cell death, the tumor microenvironment, and immune escape mechanisms [245].
Immune checkpoint inhibitors (ICIs) enhance antitumor immunity by blocking inhibitory signaling pathways between tumor cells and immune effector cells, thereby allowing cytotoxic T cells to exert apoptotic effects more effectively [246]. This is mediated through the activation of the caspase cascade [247]. Activated T cells can also produce ROS, elevating oxidative stress within tumor cells and inducing ferroptosis [248]. Moreover, ICIs may modulate the expression of iron metabolism-related genes, further promoting ferroptotic cell death [249]. Simultaneously, inflammatory cytokines such as IL-1β and IL-18, secreted by immune cells, can trigger pyroptosis in tumor cells [62, 250]. Blockade of the PD-1/PD-L1 axis has been shown to augment T cell activity and downregulate Beclin 1 expression, thereby suppressing autophagy in tumor cells [251]. In contrast, anti-CTLA-4 therapy enhances the infiltration of macrophages and dendritic cells (DCs) into the tumor microenvironment, whereupon they secrete cytokines that modulate autophagic activity [252]. In TNBC and other malignancies, high expression of the critical immunosuppressive checkpoint molecule T cell immunoglobulin and mucin domain-containing protein 3 (TIM-3) on tumor-associated macrophages (TAMs) is associated with immune evasion [253, 254]. Therapeutic targeting of TIM-3 with monoclonal antibodies can block its signaling, leading to macrophage reactivation [255], culminating in the induction of necroptosis in tumor cells, thereby enhancing their elimination [256].
T cell-based therapies, particularly chimeric antigen receptor T cell (CAR-T) and T cell receptor-engineered T cell (TCR-T) therapies, have demonstrated promise in TNBC management [257, 258]. CAR-T therapy utilizes genetically modified T cells that express receptors targeting specific surface antigens on TNBC cells [259], leading to their activation when they recognize these antigens on the surface of TNBC cells [260]. Upon antigen recognition, CAR-T cells release cytotoxic granules containing perforin and granzymes [261, 262]. Perforin forms pores in tumor cell membranes [263], facilitating the entry of granzyme B, which subsequently activates caspase-3 and caspase-8, initiating apoptosis [264, 265]. TCR-T therapy involves engineering T cells to express tumor-specific TCRs, enabling recognition of intracellular antigens presented by MHC molecules [266]. Ropporin-1, highly expressed in TNBC, has been identified as a target for TCR-T cells, allowing selective cytotoxicity against tumor cells [267]. This approach has already shown clinical utility in other solid tumors, such as melanoma, synovial sarcoma, nonsmall cell lung cancer, and hepatocellular carcinoma [268], underscoring its potential for TNBC therapy.
NK cells are innate immune effectors that exert potent cytotoxic effects against tumor cells [269, 270]. They eliminate cancer cells by releasing perforin and granzyme, promoting apoptosis, and by secreting TRAIL, which binds to death receptors on tumor cells [264, 271, 272]. Additionally, NK cells produce IFN-γ, which activates p53 signaling in tumor cells, upregulating pro-apoptotic proteins and downregulating anti-apoptotic ones, thereby enhancing apoptosis [38, 273, 274]. IFN-γ can also promote autophagy, aiding in macrophage-mediated phagocytosis and tumor cell clearance [275]. Furthermore, NK cells can trigger pyroptosis by secreting pro-inflammatory cytokines and activating DCs to enhance antigen presentation, leading to robust T cell responses [276]. These activated T cells, in turn, release various effector molecules and cytokines that induce additional forms of cell death, including necroptosis and ferroptosis, thereby amplifying tumor cell destruction [277].
The interplay between immunotherapy and cell death pathways is thus critical for the effective treatment of TNBC. Given the complex tumor microenvironment and myriad mechanisms that underlie immune evasion, such as PD-L1 overexpression, targeting immune checkpoints and enhancing immune cell function represent promising therapeutic avenues. Modulating cell death pathways can further alter the tumor milieu and reduce immune resistance. Future research should continue to explore these dynamic interactions, aiming to develop novel drugs, optimize therapeutic regimens, and implement personalized treatment strategies that consider both the tumor microenvironment and mechanisms of immune escape (Table 2).
Table 2.
Applications of programmed cell death pathways in immunotherapy for TNBC
| Cell death pathway involved | Drugs or molecules | Mechanism | References |
|---|---|---|---|
| Apoptosis | PD-1/PD-L1 inhibitors | Inhibiting the interaction between PD-1 and PD-L1 boosts T cell activity, enabling immune cells to more effectively identify and destroy tumor cells, thereby encouraging tumor cell apoptosis | [376] |
| anti-CTLA-4 antibody | Inhibiting the CTLA-4 immune checkpoint pathway decreases Treg cell numbers, boosts T cell activation and proliferation, and encourages tumor cell apoptosis | [377] | |
| IFN-γ | IFN-γ induces endoplasmic reticulum stress thereby hindering the formation of autophagosomes, resulting in the misfolded proteins, damaged organelles and other materials wrapped in autophagosomes not being degraded in a timely manner, interfering with the normal metabolism and function of cells, and triggering apoptotic signaling | [378] | |
| PARP inhibitor | Causes DNA damage, halts the cell cycle, and triggers apoptosis in cancer cells while boosting the immune system’s capacity to target tumor cells | [379, 380] | |
| TNF-α | Binding of TNF-α to TNFR1 induces caspase-8 activation, which ultimately leads to apoptosis | [381] | |
| M1 macrophages | Induces iNOS to produce large amounts of NO and increases toxic effects on tumor cells to promote apoptosis | [382] | |
| Pyroptosis | NLRP3 | The NLRP3 inflammasomes trigger caspase-1 to initiate gasdermin D-dependent pyroptosis and facilitate the release of IL-1β and IL-18 | [227, 383] |
| CDK inhibitors | CDK inhibitor treatment increases the levels of caspase-3 and N-terminal fragments cleaved by GSDME | [384] | |
| TMAO | Inducing endoplasmic reticulum stress kinase PERK triggers pyroptosis in tumor cells, which boosts CD8 T cell-mediated anti-tumor immunity against TNBC in living organisms | [385] | |
| GM@LR | GM@LR delivers plasmid expressing GSDME and MnCO into TNBC cells to activate caspase-3, thereby converting apoptosis to pyroptosis in 4T1 cells. In addition, Mn2+ promotes the maturation of DCs through activation of the STING signaling pathway leading to the infiltration of large numbers of cytotoxic lymphocytes | [386] | |
| Necroptosis | Shikonin | Increased expression of RIPK3, p-RIPK3, and MLKL worked together to induce necroptosis in tumor cells, effectively triggering ICD, boosting CD8 and CD4 T cell infiltration in the tumor, and suppressing Treg cells. | [281] |
| RIPK3 | An alternative pathway, independent of necroapoptosis, activates PGAM5 to control NKT cell activity and enhance NKT cell-driven anti-tumor immunity | [387] | |
| Caspase-8 | Activation of specific NK cells and CD8 T cells to improve recognition of tumor antigens | [388] | |
| GSDMC | GSDMC increases PARPi sensitivity in multiple cancer types by expanding memory CD8T cells in lymphoid tissues and tumors | [389] | |
| Autophagy | PD-1/PD-L1 inhibitors | Combined administration with endostatin has a synergistic effect, leading to a decrease in the levels of IL-17 and TGF-β1, an increase in the secretion of IFN-γ, a reduction in the accumulation of MDSCs, and a reversal of the inhibition of CD8+ T cells. The expressions of vascular endothelial growth factor (VEGF), CD34, and CD31 are significantly downregulated, while apoptosis in tumor cells and autophagy mediated by the PI3K/AKT/mTOR pathway are upregulated | [390] |
| HCQ | Increase the visibility of colon cancer antigens triggered by ICD inducers, and boost ICD-based anti-tumor immunity in both laboratory and live settings | [391] | |
| DOX | Reducing tenascin-C protein levels alongside PD-1 inhibitors elevates CD4 and CD8 tumor-infiltrating lymphocytes (TILs) and significantly boosts granzyme B release | [392] | |
| DMKG | This results in a heightened release of antigens and inflammatory factors, activating DCs. It can also enhance the infiltration of CD8+ T cells into the tumor area, decrease the proportion of Tregs following radiotherapy, and remodel the tumor immune environment | [393] | |
| Ferroptosis | GPX4 inhabitors | Combined use with immune checkpoint inhibitors can promote ferroptosis | [21] |
| CD8 T cell | The discharge of perforin-granzyme and Fas-FasL can trigger cell death and boost the lipid peroxidation reaction specific to ferroptosis in tumor cells | [387] | |
| HIFU | Induce the expression of ferroptosis-related genes such as HOX1, GST, and SQSTM to enhance drug accumulation and penetration in tumors, and stimulate effective ferroptosis-mediated anti-tumor immunity | [394] |
Regulators of cell death
Targeting specific regulators of programmed cell death offers a promising strategy for advancing TNBC therapy. Agents targeting the apoptotic machinery, such as Bcl-2 and Bcl-xL inhibitors, counteract anti-apoptotic signaling and promote tumor cell apoptosis [278]. Regulators of pyroptosis, including small-molecule inflammasome inhibitors like MCC950 and OLT1177, can suppress inflammation and enhance the effectiveness of TNBC treatment [279]. In addition to apoptosis and pyroptosis, necroptosis, autophagy, and ferroptosis pathways are gaining recognition as viable therapeutic targets [280, 281]. For example, drugs targeting necroptosis can trigger cell death by influencing pathways involving key molecules like RIPK1, RIPK3, and MLKL [282, 283]. Although small molecule inhibitors specific to these pathways remain limited, manipulating their core signaling components can still promote tumor cell death. For instance, TNF-α-driven activation of RIPK3 through the PI3K/AKT axis induces necroptosis, while ZBP1 cooperates with MLKL to potentiate this process in cancer therapy [284, 285]. Autophagy is modulated by regulators such as Beclin 1. The mTOR signaling pathway inhibits Beclin 1, but mTOR inhibitors, such as rapamycin, can restore autophagic activity and promote tumor cell degradation [286, 287]. Moreover, agents, such as chloroquine and hydroxychloroquine, which disrupt autophagosome-lysosome fusion, have been tested in TNBC clinical trials, as their inhibition of autophagy can induce tumor cell death [288, 289]. Research on ferroptosis-inducing drugs is rapidly evolving. Compounds such as Erastin and its derivatives initiate ferroptosis by inhibiting System Xc-, thereby reducing cystine uptake and GSH synthesis, which leads to lipid peroxidation and oxidative cell death [219, 220, 290]. Sulfasalazine induces ferroptosis by targeting the mitochondrial voltage-dependent anion channel (VDAC), disrupting mitochondrial function, releasing iron ions, and exacerbating oxidative stress [14, 291]. Additionally, RSL3, a direct inhibitor of GPX4 [292, 293], reduces the enzyme’s activity and promotes lipid peroxidation, thereby enhancing ferroptotic death in TNBC cells.
Despite significant advances, research on cell death regulators continues to face substantial challenges, particularly with the emergence of therapeutic resistance. Future investigations must prioritize understanding the molecular underpinnings of resistance and developing strategies to overcome it. One promising approach involves combination therapies that simultaneously target multiple cell death pathways, thereby enhancing therapeutic efficacy and circumventing resistance. For instance, cisplatin, which is a widely used chemotherapeutic agent in lung and ovarian cancer, induces cell death by interfering with DNA repair mechanisms but is often limited by the onset of resistance [294, 295]. Co-administering cisplatin with ferroptosis inducers such as Erastin or Sulfasalazine has shown promise in overcoming this resistance. These agents enhance tumor suppression by modulating iron metabolism and lipid peroxidation, ultimately triggering ferroptosis [296, 297]. Similarly, TRAIL induces necroptosis in colorectal and breast cancers but also faces therapeutic resistance [298, 299]. This limitation can be addressed by combining TRAIL with chloroquine, which inhibits autophagy in tumor cells, thereby sensitizing them to necroptosis and enhancing anti-tumor activity [300, 301]. In gastric and pancreatic cancers, oxaliplatin has been shown to activate necroptosis-related pathways [302, 303]. When combined with the GPX4 inhibitor RSL3, oxaliplatin’s efficacy is further improved by promoting ferroptosis through the inhibition of antioxidant defenses [304]. These multimodal strategies underscore the therapeutic potential of targeting multiple cell death pathways simultaneously to address resistance. However, in the context of TNBC, tumor heterogeneity significantly impacts drug responsiveness, and pharmacokinetics may vary due to metabolic differences across organs [305]. Furthermore, the dense cellular architecture and complex extracellular matrix of TNBC hinder drug penetration [306, 307]. Therefore, identifying novel molecular targets and developing precisely targeted therapies are crucial to overcoming these barriers and enhancing the therapeutic efficacy of cell death modulators (Table 3).
Table 3.
Application of different cell death regulators in TNBC
| Cell death pathway involved | Drugs | Mechanism | Reference |
|---|---|---|---|
| Apoptosis | ABT-737 | ABT-737 attaches to Bcl-2/Bcl-xL/Bcl-w, stopping them from interacting with Bax and Bak, which in turn lifts the suppression of apoptosis | [395] |
| LCL161 | LCL161 blocks IAPs, lifting their inhibition of apoptosis, and alters the tumor microenvironment by drawing macrophages to the tumor location | [396] | |
| APR-246 | When p53 is activated, it increases the levels of pro-apoptotic proteins, such as Bax and PUMA, and decreases the levels of anti-apoptotic proteins such as Bcl-2 and Bcl-xL | [397, 398] | |
| Pembrolizumab | Blocking the PD-1/PD-L1 immune checkpoint activates CTLs and triggers cancer cell apoptosis via T cell-mediated cytotoxicity | [376, 399] | |
| Epothilone B | Induction of cancer cell apoptosis via the PI3K/AKT/mTOR signaling pathway enhances the apoptotic effects of ABT-737 | [286] | |
| Pyroptosis | Cisplatin | Activation of the MEG3/NLRP3/caspase-1/GSDMD pathway in TNBC induces pyroptosis to exert anti-tumor effects | [383] |
| AZU1 | Regulation of the pNF-κB/NLRP3/caspase-1/GSDMD axis in TNBC promotes pyroptosis | [400] | |
| MCC950 | By binding to the NACHT domain of NLRP3, its oligomerization and activation are prevented, which inhibits its function and makes TNBC cells more sensitive to PTX | [279] | |
| CY-09 | Inhibiting the Walker B motif of NLRP3 reduces its activity, which in turn suppresses IL-1β/EMT/Wnt/β-catenin signaling and makes TNBC cells more sensitive to GDC-0941 | [401] | |
| OLT1177 | Inhibition of NLRP3-induced IL-1β prevents the progression of TNBC | [402] | |
| Autophgy | CQ | Preventing the fusion of autophagosomes with lysosomes results in the buildup of autophagosomes inside the cell, which encourages the death of tumor cells | [288, 289] |
| Toosendanin | Raising lysosomal pH inhibits autolysosome maturation, thereby suppressing late-stage autophagy in TNBC cells | [403] | |
| circEGFR | The translocation of annexin A2 (ANXA2) to the plasma membrane in TNBC cells facilitates the dissociation of the transcription factor TFEB from the ANXA2-TFEB complex, subsequently promoting autophagy. Concurrently, circEGFR acts as a competing endogenous RNA (ceRNA) by directly binding to miR-224-5p, thereby inhibiting its expression and mitigating the suppression of autophagy mediated by the miR-224-5p/ATG13/ULK1 axis | [404] | |
| UBC12 | Enhancing TRIM25 sumoylation boosts its interaction with TFEB, which promotes the transcription and activation of genes related to autophagy | [405] | |
| Necroptosis | ZBP1 | When glucose is deprived, mtDNA is released into the cytoplasm, where it attaches to ZBP1, triggering MLKL activation in a manner dependent on Bcl-2 family proteins and NOXA | [406] |
| Curcuma longa | Enhanced expression of TRAIL under acidic conditions induces necroptotic apoptosis in a RIPK1- and RIPK3-dependent manner | [407] | |
| Ferroptosis | Erastin | Blocking System Xc- on the cell membrane lowers cystine absorption, which results in reduced GSH production inside the cell and weakened antioxidant defenses, thus encouraging lipid peroxidation and ferroptosis | [218, 219] |
| Sulfasalazine | Blocking VDAC in mitochondria impairs their function, leading to the release of iron ions and heightened oxidative stress | [408, 409] | |
| RSL3 | Inhibition of GPX4 activity elevates intracellular lipid peroxidation levels, thereby promoting ferroptosis | [292, 293] | |
| Liproxstatin-1 | In TNBC cells, the activation of cPLA2α leads to the activation of ACSL4, which facilitates the attachment of PUFAs to coenzyme A, thereby raising lipid peroxidation levels and inducing ferroptosis | [410] | |
| Eupaformosanin | Ubiquitination of mutant p53 induces apoptosis and ferroptosis in TNBC cells | [320] |
Clinical translation of cell death pathways in the treatment of TNBC
In the context of TNBC, investigating mechanisms of cell death not only offers a pivotal direction for identifying therapeutic targets but also establishes the groundwork for the development of novel therapies. Nevertheless, much of the research in this area remains at the preclinical stage [41].
Within preclinical studies, animal models and organoid models are indispensable for assessing potential therapeutic agents and targets [308, 309]. The traditional immunodeficient xenograft mouse model, a well-established animal model for TNBC research, effectively simulates the growth, metastasis, and therapeutic response of TNBC in vivo. By inoculating immunodeficient mice with human-derived TNBC cell lines (such as MDA-MB-231 and BT-549), researchers can evaluate the effects of potential drugs or targets on tumor growth, metastasis, and survival outcomes in mice [310, 311]. For example, administering the ferroptosis inducer Erastin to mice inoculated with TNBC cells demonstrated that Erastin facilitates TNBC cell death by inhibiting the expression of GPX4, thereby providing preliminary evidence for the potential application of ferroptosis in TNBC therapy [312]. In recent years, organoid models, as innovative three-dimensional cell culture systems, have increasingly emerged as a pivotal platform for TNBC research due to their enhanced ability to accurately replicate the biological characteristics of patient tumors [313]. These organoids not only preserve the heterogeneity inherent in the patient’s tumor[314]but can also be co-cultured with immune cells to develop models that closely mimic the in vivo tumor immune microenvironment. This capability provides unique insights into the effects of cell death mechanisms on tumor immunity [315]. In the context of screening ferroptosis inducers, research has identified significant variability in the sensitivity of TNBC organoids derived from different patients to these inducers, thereby supporting the advancement of personalized treatment strategies [21]. Compared with animal models, organoid models present distinct advantages in terms of cost-effectiveness, reduced experimental duration, and suitability for high-throughput screening [316]. Nonetheless, while these models can replicate certain biological aspects of TNBC, they still fall short of capturing the intricate physiological and pathological environment of humans, posing a challenge for the direct translation of preclinical findings into clinical practice. Furthermore, the pronounced heterogeneity of TNBC complicates the identification of universal treatment strategies. Consequently, accurately classifying and treating patients on the basis of their specific cell death characteristics remains a formidable challenge in clinical translation.
In summary, preclinical investigations into cell death mechanisms in TNBC serve as a crucial conduit for translating foundational research into clinical practice. Animal models provide initial evidence of the therapeutic potential of cell death mechanisms through in vivo simulations, while organoid models, with their capacity for personalized and efficient screening, offer substantial support for the development of precision therapeutic strategies. Nonetheless, discrepancies between models and human biology, alongside the inherent heterogeneity of TNBC, continue to impede clinical translation. Future endeavors should prioritize the optimization of research models and the enhancement of our understanding of cell death mechanisms to facilitate the progression of related therapies from preclinical research to clinical application. This advancement holds the promise of offering new survival prospects for TNBC patients and achieving the clinical translation of basic research(Table 4).
Table 4.
Different research models for five types of PCD in TNBC
| Types of model research | Cell death pathway involved | Targeted drugs/strategies | Mechanism | References |
|---|---|---|---|---|
| Animal model | Apoptosis | USP19 | USP19 interacts with deubiquitinating enzymes to stabilize the chaperone regulator BAG6. BAG6 promotes the ubiquitination and degradation of BCL2, a process that elevates ER calcium levels, thereby inducing ER stress and increasing cellular apoptosis | [411] |
| Overexpression of PTPN14 | Overexpression of PTPN14 acts as a key regulatory factor in dephosphorylation in estrogen receptor-negative breast cancer (ER- BC) resistance, inducing anoikis and inhibiting proliferation in TNBC cells by suppressing the PI3K/AKT and ERK signaling pathways. This process has no significant impact on normal breast cells | [412] | ||
| lasiokaurin | LA effectively inhibits the activation of the PI3K/Akt/mTOR and STAT3 signaling pathways, inducing cell cycle arrest, apoptosis, and DNA damage in TNBC cells, while also suppressing cellular migration | [413] | ||
| A1155463 + R547 | Targeting BCL-XL enhances the sensitivity of TNBC cells to CDK1/2/4 inhibition by synergistically depleting survival signals and the RTK/AKT pathway, while restoring the tumor-suppressive function of FOXO3a. This results in the suppression of cellular viability, accompanied by the accumulation of DNA damage and the induction of apoptosis | [414] | ||
| PBCH | PBCH is a hyaluronic acid-modified nanocomposite targeting CD44, serving as an oxidative stress amplifier. In the acidic tumor microenvironment, it first releases Ca2⁺, leading to mitochondrial dysfunction and apoptosis. The PdAg nanoenzyme within the composite can also be activated by near-infrared (NIR) light, mediating a burst of ROS and promoting apoptosis through immunogenic cell death | [415] | ||
| Pyroptosis | CP@Gel combined with DAC | Following the injection of CP@Gel, CP continuously releases to catalyze the self-generation of ROS, thereby activating caspase-3. Pre-treatment with DAC enhances the protein levels of GSDME by inhibiting its methylation, inducing robust pyroptosis and anti-tumor immunity, and eliminating residual cancer cells that survive owing to apoptosis resistance | [364] | |
| Autophagy | CQ | CQ inhibits autophagy, and when combined with the PI3K inhibitor ipatasertib and the AKT inhibitor taselisib, it alleviates the therapeutic limitations induced by autophagy activation in response to PI3K/AKT inhibition, thereby enhancing the anti-tumor effects of conventional chemotherapy | [416] | |
| STA1 | In TNBC, the loss of autophagy leads to the high expression of STA1. SAT1 activates a negative regulatory mechanism of autophagy by stabilizing mTOR mRNA, promoting cell proliferation and metastasis in TNBC. Therefore, targeting STA1 may represent a key strategy for targeted therapy in TNBC | [417] | ||
| ADT-OH | ADT-OH improves autophagic flux by inhibiting autophagosome formation, while simultaneously downregulating dynactin subunit 1 and upregulating mitochondrial fusion proteins to induce mitochondrial elongation. Additionally, it reduces mitochondrial autophagy flux and inhibits mitochondrial function, ultimately suppressing migration and invasion in TNBC cells | [418] | ||
| PC3-15 | By inhibiting UbcH5b and its mediated p62 ubiquitination, autophagy induced by lapatinib is suppressed, thereby increasing the sensitivity of TNBC to lapatinib | [419] | ||
| Necroptosis | NP-ALT | Inhibition of tyrosine phosphorylation of p27Kip1 suppresses CDK4/6 and CDK2, inducing ROS-dependent necroptosis. This mechanism subsequently blocks the proliferation of HR + breast cancer cells and CDK4i-resistant cell types, including TNBC | [420] | |
| Ferroptosis | JQ1 + BTZ | Low-dose combination therapy targeting Brd4 and the proteasome (JQ1 + BTZ) triggers lysosomal metabolic reprogramming, promoting the accumulation of LIP. Simultaneously, glutamine catabolism provides substrates for the TCA cycle, exacerbating lipid peroxidation | [421] | |
| Curcumenol | Cur reduces mitochondrial membrane potential and promotes the accumulation of lipid ROS. It also reverses the inhibitory effect of ferrostatin-1 on ferroptosis. By inhibiting the SLC7A11/NF-κB/TGF-β pathway, Cur effectively blocks the progression of TNBC | [422] | ||
| HFPN | Targeted modulation of the core kinase Aurora A in TNBC disrupts the tumor redox balance, promoting ferroptosis in tumor cells, thereby enhancing the efficacy of radiotherapy in TNBC | [423] | ||
| ZC3H13 | ZC3H13-mediated m6A modification promotes ferroptosis through the KCNQ1OT1/TRABD axis, thereby reducing DOX resistance in TNBC | [424] | ||
| OST-01 | Downregulation of LRP8 reduces selenium uptake, lowering the expression of the selenium-dependent protein GPX4. This diminishes the cellular antioxidant capacity, increases lipid peroxidation, and elevates malondialdehyde levels, ultimately inducing ferroptosis in breast cancer cells and inhibiting tumor growth | [425] | ||
| Organoid model | Apoptosis | Restoration of CircRNA-CREIT expression | CircRNA-CREIT promotes the ubiquitination and degradation of PKR by binding to it and recruiting HACE1, thereby inhibiting the PKR/eIF2α signaling pathway and stress granule assembly. The released RACK1 activates the MTK1/JNK pathway, upregulating pro-apoptotic proteins such as Bim, while simultaneously enhancing doxorubicin-induced DNA damage and inhibiting autophagy. This synergistically activates the caspase cascade, leading to apoptosis | [314] |
| Nicotinamide | NAM reduces mitochondrial membrane potential and ATP production, while enhancing the activity of reverse electron transport, fatty acid β-oxidation, and glycerophospholipid/sphingolipid metabolic pathways in TNBC. This collectively increases ROS levels, subsequently triggering apoptosis | [426] | ||
| YZ-836P | Targeted degradation of PRMT5 via PROTACs relieves its inhibition of the p53 pathway, inducing G1-phase cell cycle arrest and significantly promoting apoptosis in TNBC cells | [361] | ||
| LXG6403 | Targeting lysyl oxidases first disrupts the extracellular matrix (ECM), leading to the inactivation of FAK/Src signaling. This subsequently promotes chemotherapy-induced ROS production and DNA damage, ultimately resulting in G1/S-phase cell cycle arrest and apoptosis | [427] | ||
| CDDD11-8 | Selective inhibition of CDK9 downregulates the mRNA and protein levels of oncogenes, such as Myc and MCL1, leading to cell cycle arrest and induction of apoptosis in TNBC cells | [428] | ||
| Autophagy | lncRNA DDIT4-AS1 | PTX chemotherapy increases the expression of DDIT4-AS1. Silencing its expression inhibits the activation of autophagy, thereby sensitizing TNBC cells to PTX treatment | [429] | |
| JNK-IN-8 | Inhibition of mTOR blocks the phosphorylation of TFEB, inducing the nuclear translocation of both TFEB and TFE3. This upregulates their target genes, promoting lysosomal biogenesis and autophagy, ultimately triggering cell death in TNBC cells | [430] | ||
| CDDO-Me | By binding to EGFR and promoting its interaction with KEAP1, K63 ubiquitination of EGFR is induced, leading to its degradation via the autophagy-lysosome pathway. This process blocks the inhibitory effect of the PI3K/AKT/mTOR pathway on autophagy, activates autophagic flux, and synergistically suppresses the progression of TNBC | [431] | ||
| cGAS-STING | In the context of serum deprivation, the inhibition of the cGAS-STING axis leads to cell death through an autophagy-dependent mechanism | [432] | ||
| Ferroptosis | α-Hederin | Activation of IRF1 and promotion of its nuclear translocation directly bind to the GPX4 promoter, inhibiting its transcription and reducing GPX4 protein levels. This weakens the glutathione system’s capacity to detoxify lipid peroxides, triggering iron-dependent lipid peroxidation and inducing ferroptosis in TNBC | [433] | |
| IN10018 combined with crizotinib | Upregulation of p53 inhibits G2/M phase cell cycle arrest, enhances apoptosis by increasing the Bax/Bcl-2 ratio, and induces ferroptosis through the regulation of the SLC7A11/GSH/GPX4 pathway | [434] | ||
| PSMD14 | Silencing PSMD14 reduces its deubiquitinase-mediated stabilization of SF3B4, disrupting the SF3B4/HNRNPC complex that mediates m6A modification and exon inclusion of FADS1 mRNA, leading to downregulation of FADS1. Combined with AA supplementation, this results in decreased FADS1-mediated unsaturated fatty acid production, weakening the glutathione system’s capacity to counter lipid peroxidation, and inducing ferroptosis in TNBC | [435] | ||
| Clinical research model | Apoptosis | TAK-228 combined with Alisertib | TAK-228 enhances the anti-tumor effects of alisertib in vivo. Alisertib induces cell cycle arrest at the G2/M phase, while TAK-228 inhibits the mTORC1/2 pathway, disrupting cellular metabolism and severing survival signals in senescent cells, thereby promoting apoptosis | [436] |
| Omeprazole | By inhibiting the expression of FASN and reducing fatty acid synthesis, the mTORC1 pathway is suppressed, leading to the activation of the mitochondrial apoptotic pathway and promoting cell cycle arrest. This ultimately inhibits the growth and survival of TNBC cells | [437] | ||
| Tigatuzumab | Nab-paclitaxel induces cell death through microtubule stabilization and the mitochondrial apoptotic pathway, while tigatuzumab triggers the extrinsic apoptotic pathway by activating DR5. The combination of these two agents achieves synergistic enhancement through the cross-activation of the caspase cascade, cell cycle synchronization, and modulation of the immune microenvironment | [438] | ||
| Autophagy | TRF2 | Overexpression of TRF2 following PTX exposure results in a reduction of LC3 levels, which is associated with the accumulation of p62SQSTM1, thereby inhibiting the autophagic response induced by paclitaxel treatment | [439] | |
| Pyroptosis | IRE1α RNase | Inhibition of IRE1α RNase activity synergizes with taxane-induced NLRP3 inflammasome-mediated pyroptosis, promoting the conversion of PD-L1-negative “cold tumors” in TNBC to a PD-L1-high expressing immunogenic phenotype. This significantly enhances the sensitivity of TNBC to PD-1 inhibitor therapy | [16] |
Integrated therapeutic strategies for TNBC
TNBC is a highly heterogeneous and aggressive subtype of breast cancer, posing substantial challenges for treatment. Recent research efforts have focused on integrating cell death modulators with conventional therapies such as chemotherapy, radiotherapy, and immunotherapy to achieve personalized treatment approaches that enhance tumor cell death and therapeutic efficacy [281, 317].
Chemotherapeutic agents, such as doxorubicin, induce apoptosis in TNBC cells through the activation of the ERK1/2 signaling pathway, which enhances treatment sensitivity and reduces resistance [318]. Olaparib, a poly (ADP-ribose) polymerase (PARP) inhibitor, has demonstrated efficacy in BRCA-mutated TNBC by impairing DNA repair and inducing apoptosis [319]. Other approaches involve the induction of pro-apoptotic genes or the inhibition of anti-apoptotic proteins. For example, Eupaformosanin restores p53 function, promoting both apoptosis and ferroptosis [174, 320]. In addition, targeting Bcl-2 using siRNAs or inhibitors, such as Venetoclax, increases TNBC cell sensitivity to standard treatments like paclitaxel and cisplatin, highlighting a promising avenue for cancer therapy [321].
Inducing pyroptosis also holds potential for TNBC treatment. Oxaliplatin, a platinum-based chemotherapeutic, elevates ROS production by inhibiting mitochondrial respiration, forcing cancer cells to depend on glycolysis. This metabolic stress activates the MAPK pathway and enhances ROS-related gene expression, triggering pyroptosis [322–324]. Doxorubicin similarly elevates ROS levels and causes DNA damage, releasing mitochondrial DNA and DAMPs, which in turn activate the NLRP3 inflammasome and promote necroptosis [325, 326]. These processes can potentiate the effects of other treatments by increasing tumor cell death and reducing resistance, particularly when apoptosis modulators are used in combination with chemotherapy.
Targeting necroptosis through modulation of proteins, such as RIPK1, RIPK3, and MLKL, offers another promising strategy. Although direct targeting of RIPK1 and RIPK3 is limited due to their roles in normal cellular processes, the mitochondrial protein Smac can inhibit inhibitor of apoptosis proteins (IAPs), thereby enabling RIPK1 and RIPK3 activation and promoting necroptosis [327–330]. Smac mimetics such as LCL161 induce apoptosis via caspase activation while also indirectly activating necroptotic pathways [331]. Ongoing research aims to exploit necroptosis to improve TNBC sensitivity to chemotherapy [332].
Autophagy modulation has also emerged as a key strategy to enhance chemotherapy sensitivity and reduce resistance. Inhibiting ULK1 using MRT68921 disrupts autophagy initiation, leading to the accumulation of cytotoxic substances and increased tumor cell death [333, 334]. Additionally, targeting the PI3KC1-Akt-mTORC1 signaling pathway can inhibit autophagy [335]. Metformin activates AMPK, which downregulates mTORC1 activity and promotes autophagy by suppressing the PI3K/Akt pathway [336, 337]. NVP-BEZ235, a dual PI3K/mTOR inhibitor, also facilitates autophagy and holds promise for TNBC therapy [338]. Although autophagy can enhance the effects of anti-cancer drugs, there are currently no direct small molecule modulators for Beclin 1, a key autophagy regulator in TNBC [339]. However, agents such as 3-methyladenine, used in conjunction with chemotherapy, inhibit PI3KC3 and indirectly affect the Beclin-1-PI3KC3 complex, providing a potential avenue for regulation [340]. Researchers are exploring drugs targeting the autophagy pathway to enhance traditional therapies and improve TNBC patient outcomes.
Targeting ferroptosis by managing iron balance and altering tumor cell metabolism can enhance chemosensitivity [341]. Targeting TFR1 within the iron transport pathway has been shown to trigger ferroptosis [342]. Roxadustat enhances iron uptake and ferroptosis by stabilizing HIF and upregulating TFR1 expression [343, 344]. Moreover, GPX4 inhibitors such as RSL3 and FIN56 promote ferroptosis by inhibiting glutathione peroxidase activity and increasing ROS production [345, 346]. Buthionine sulfoximine (BSO), a synthetic amino acid, suppresses γ-glutamylcysteine synthetase (γ-GCS), thereby reducing glutathione synthesis, promoting lipid peroxidation, and inducing ferroptosis in TNBC cells [347, 348].
The need for personalized treatment approaches is particularly urgent in TNBC, given its high degree of inter- and intra-tumor heterogeneity [349]. Tailoring therapy on the basis of individual genotypic, phenotypic, and pathological characteristics can significantly enhance treatment precision and efficacy. Advances in genomics, transcriptomics, and proteomics have enabled a more comprehensive understanding of TNBC biology, facilitating the development of targeted and personalized treatment strategies. These include novel molecular targets such as ICIs, which provide greater specificity and reduced toxicity compared with traditional chemotherapeutics [350, 351]. A deeper understanding of cell death mechanisms and their associated drug actions will pave the way for more effective and safer treatments.
In summary, the future of TNBC therapy lies in the integration of cell death modulators with personalized treatment regimens. By combining multiple therapeutic modalities and tailoring strategies to individual patients, it is possible to improve treatment outcomes and survival rates. Future research should prioritize the identification of novel targets, development of innovative drugs, optimization of clinical trial design, and refinement of therapeutic protocols.
Challenges and future directions
TNBC treatment continues to face significant challenges, particularly in the areas of drug resistance and heterogeneous patient responses. Resistance to therapy not only compromises treatment efficacy but also contributes to disease progression, while individual variability results in widely differing clinical outcomes [352]. Overcoming these challenges requires innovative research strategies centered on the discovery of novel molecular targets, the refinement of therapeutic approaches, and the development of personalized treatment regimens. Advanced technologies now offer the ability to tailor therapies more precisely, with the potential to improve therapeutic efficacy while minimizing adverse effects.
Owing to the absence of hormone receptors and human epidermal growth factor receptor 2 (HER2), TNBC does not respond to endocrine therapy or HER2-targeted agents, making chemotherapy and certain targeted therapies the primary treatment options [353]. However, prolonged administration of agents that regulate cell death pathways may lead to the development of resistance. A thorough understanding of resistance mechanisms is therefore essential. Combining cell death modulators with other modalities—such as chemotherapy or immunotherapy—may allow for synergistic effects that attack tumor cells through multiple pathways, thereby helping to overcome resistance [41]. To more effectively eliminate resistant tumor cell populations, it is crucial to identify novel molecular targets and design therapies that specifically disrupt these resistance mechanisms. Emerging technologies in genomics and proteomics provide powerful tools to characterize the molecular profiles of resistant tumors, facilitating the development of individualized treatment strategies.
Furthermore, TNBC is not a single disease entity but consists of at least six distinct molecular subtypes, each exhibiting unique biological features and differential responses to therapy [354]. The heterogeneity of these subtypes necessitates the use of personalized treatment approaches informed by comprehensive genetic profiling. Incorporating genotypic and phenotypic data, along with patient-specific clinical characteristics, is essential to improve therapeutic outcomes [355]. Future research should focus on elucidating TNBC heterogeneity through the application of cutting-edge technologies such as single-cell RNA sequencing, metabolomics, and transcriptomics. These approaches can aid in the identification of predictive biomarkers and therapeutic targets, ultimately enhancing the precision and effectiveness of treatment. By leveraging these insights, it may be possible to significantly improve the safety, efficacy, and personalization of TNBC therapies, thereby optimizing patient outcomes.
Current research efforts are increasingly focused on elucidating the pathogenesis and signaling pathways involved in TNBC, with the goal of developing novel therapeutics or repurposing existing drugs. Although preclinical models have yielded promising results—for example, the histone deacetylase inhibitor WW437 and the RNA helicase EIF4A inhibitor Zotatifin—these findings have not yet translated effectively into clinical application, largely due to species-specific differences between human and animal models, as well as a lack of robust clinical data [356, 357]. Future studies should prioritize the identification and validation of novel molecular targets, given the critical role of cell death regulation in the initiation, progression, and therapeutic response of TNBC. Investigating these targets could pave the way for innovative and more effective treatment modalities. The integration of emerging technologies such as artificial intelligence (AI) and high-throughput screening has the potential to revolutionize the drug discovery process. AI enables the analysis of vast biological datasets to identify potential targets, while high-throughput screening facilitates the rapid and systematic assessment of these targets’ biological relevance and therapeutic potential. Addressing the therapeutic challenges posed by TNBC requires a comprehensive understanding of resistance mechanisms, patient-specific variability, and the development of novel, multifaceted therapeutic strategies.
Conclusions
This review underscores the central importance of cell death pathways in TNBC and advocates for the development of integrated therapeutic strategies to address the multifaceted challenges associated with this aggressive cancer subtype. We call for a reassessment of the role of cell death in disease progression and therapeutic response, with the aim of generating new insights into both basic research and clinical practice.
Traditionally considered as discrete processes, apoptosis, pyroptosis, necroptosis, ferroptosis, and autophagy are now recognized to be interrelated through intricate signaling networks. These interactions complicate therapeutic targeting, as the induction of one type of cell death—such as apoptosis—may inadvertently trigger others, including pyroptosis or ferroptosis. This interconnectedness may reflect compensatory feedback mechanisms or the activation of shared signaling pathways. Consequently, focusing exclusively on a single form of cell death may limit therapeutic efficacy. We propose an integrative approach that treats cell death as a coordinated and dynamic process, highlighting the importance of cross-talk between different forms.
By targeting one pathway, it may be possible to modulate others, thereby amplifying the therapeutic impact. We emphasize the importance of integrated therapeutic strategies, as cell death modulators that target a single pathway often encounter resistance. Combining such modulators with other treatment modalities—particularly immunotherapy—can enhance their overall efficacy in TNBC. Immune checkpoint inhibitors, for instance, activate the host immune response, promote tumor cell apoptosis, influence iron metabolism to induce ferroptosis, and may also trigger pyroptosis through the upregulation of inflammatory mediators, thereby indirectly affecting autophagy. In cellular immunotherapy, CAR-T and TCR-T cells recognize tumor-associated antigens and release cytotoxic molecules that initiate apoptosis. NK cells can induce both apoptosis and pyroptosis, activate dendritic cells, and further promote necroptosis and ferroptosis. Given the high degree of heterogeneity in TNBC, personalized therapeutic strategies should be developed on the basis of individual patient profiles. This precision medicine approach holds the potential to improve treatment efficacy while minimizing adverse effects. Moreover, a deeper understanding of cell death mechanisms can aid in disease progression monitoring and early intervention strategies.
The clinical translation of cell death mechanisms in TNBC continues to pose a substantial challenge, as initial investigations predominantly utilize animal and organoid models. Animal models, such as immunodeficient xenograft mice, are instrumental in replicating in vivo tumor growth and treatment responses, while organoid models maintain the heterogeneity of patient tumors and facilitate high-throughput screening. These models offer preliminary evidence of the therapeutic potential inherent in cell death pathways. Nonetheless, significant challenges persist, such as the discrepancies between model systems and human physiology, alongside the pronounced heterogeneity of TNBC, which impede the clinical translation of preclinical findings. To address these issues, future research should focus on optimizing these models and enhancing the comprehension of underlying molecular mechanisms.By addressing these challenges, we aim to facilitate the progression of cell death-related therapies from preclinical research to clinical application, thereby offering novel therapeutic prospects for patients with TNBC.
In summary, we highlight the crucial role of cell death pathways in the treatment of TNBC and advocate for an integrated, systems-level approach that challenges traditional single-pathway paradigms. By targeting interconnected signaling networks, we propose a comprehensive therapeutic strategy that enhances our understanding of disease mechanisms and facilitates the development of innovative treatments. Such an approach aims to expand our knowledge of cell death regulation, uncover novel drug targets, and support the personalization of cancer therapies. Ultimately, modulating multiple forms of programmed cell death may improve tumor cell cytotoxicity and overcome therapeutic resistance, potentially driving breakthroughs in the treatment of complex malignancies such as TNBC.
Acknowledgements
We thank Biorender (https://www.biorender.com/) for helping us with our drawing. We thank LetPub (www.letpub.com.cn) for linguistic assistance and pre-submission expert review.
Abbreviation
- TNBC
Triple-negative breast cancer
- BC
Breast cancer
- ER
Estrogen receptor
- PR
Progesterone receptor
- HER2
Human epidermal growth factor receptor 2
- ACD
Accidental cell death
- PCD
Programmed cell death
- LCD
Lysosome-dependent cell death
- PAMPs
Pathogen-associated molecular patterns
- DAMPs
Damage-associated molecular patterns
- TNF
Tumor necrosis factor
- FasL
Fas ligand
- TRAIL
TNF-related apoptosis inducing ligand
- FasRs
Fas receptors
- TNFR1
Tumor necrosis factor receptor 1
- DR4
Death receptor 4
- DR5
Death receptor 5
- DISC
Death-inducing signal complex
- ROS
Reactive oxygen species
- Cytc
Cytochrome C
- ATR
ATM and rad3-related
- CCL2
C–C motif chemokine ligand 2
- EMT
Epithelial mesenchymal transition
- CSC
Cancer stem cell
- CTL
Cytotoxic T-lymphocyte
- NLRP3
NOD-like receptor thermal protein domain associated protein 3
- AIM2
Absent in melanoma 2
- ASC
Apoptosis-associated speck-like protein containing a CARD
- PYD
Pyrin domain
- HIN200
Hematopoietic interferon-inducible nuclear 200 proteins
- CARD
Caspase recruitment domain
- LPS
Lipopolysaccharides
- DC
Dendritic cell
- RIPK
Receptor interacting protein kinase
- MLKL
Mixed lineage kinase domain-like
- HMGB1
High mobility group box-1 protein
- ATGs
Autophagy-related genes
- mTORC1
Mechanistic target of rapamycin complex 1
- PI3K
Phosphatidylinositol 3 kinase
- LC3
Microtubule associated protein light chain 3
- PE
Phosphatidylethanolamine
- STX17
Syntaxin-17
- SNAP29
Synaptosome associated protein 29
- VAMP8
Vesical-associated membrane protein 8
- TFR1
Transferrin receptor
- FPN1
Ferroportin 1
- LIP
Labile iron pool
- GPX4
Glutathione peroxidase 4
- GSH
Glutathione
- IRP
Iron regulatory protein
- Nrf2
Nuclear factor erythroid 2-related factor 2
- FADD
Fas-associated protein with death domain
- ZDHHC1
Zinc finger DHHC-type containing 1
- AML
Acute myeloid leukemia
- HSPA5
Heat shock protein family A member 5
- LSD1
Lysine-specific demethylase 1
- TRAF6
TNF receptor-associated factor 6
- MDR
Multiple drug resistance
- AC
Anomanolide C
- TIM-3
T cell immunoglobulin and mucin domain-containing protein 3
- TAMs
Tumor-associated macrophages
- CAR-T
Chimeric antigen receptor T cell immunotherapy
- TCR-T
Receptor-engineered T cell
- MDSCs
Myeloid-derived suppressor cells
- VDAC
Voltage-dependent anion channel
- MAPK
Mitogen-activated protein kinase
- ERK
Extracellular regulated protein kinases
- JNK
C-Jun n-terminal kinase
- IAPs
Inhibitor of apoptosis proteins
- γ-GCS
γ-Glutamylcysteine synthetase
- RSL3
RAS-selective lethal 3
Author contributions
Y.L. carried out manuscript writing and chart drawing; J.H. and J.C. carried out manuscript review and editing; T.C. and W.L. carried out figure drawing; Z.Y. and F.Z. carried out supervision and funding acquisition.
Funding
This research was supported by the Sichuan Science and Technology Program (2022YFS0623) and the Natural Science Foundation of Southwest Medical University (2022QN079).
Data availability
Data sharing does not apply to this article as no new datasets were created or analyzed in this study. All information is derived from publicly published articles.
Declarations
Ethics approval and consent to participate
Since this article does not pertain to research involving humans or animals, the review and/or approval of an ethics committee is not required.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yaqi Liu and Jinwei He have contributed equally to this research.
Yaqi Liu and Jinwei He are co-first author.
Contributor Information
Zhihui Yang, Email: yzhih73@126.com.
Fancai Zeng, Email: zfcai@163.com.
References
- 1.Leon-Ferre RA, Goetz MP. Advances in systemic therapies for triple negative breast cancer. BMJ. 2023;381: e071674. [DOI] [PubMed] [Google Scholar]
- 2.Carvalho FM. Targeting low-risk triple-negative breast cancer: a review on de-escalation strategies for a new era. Transl Breast Cancer Res. 2025;6:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cortes J, Rugo HS, Cescon DW, et al. Pembrolizumab plus chemotherapy in advanced triple-negative breast cancer. N Engl J Med. 2022;387(3):217–26. [DOI] [PubMed] [Google Scholar]
- 4.He S, Sharpless NE. Senescence in health and disease. Cell. 2017;169(6):1000–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Y, Zhang H, Merkher Y, et al. Recent advances in therapeutic strategies for triple-negative breast cancer. J Hematol Oncol. 2022;15(1):121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garrido-Castro AC, Lin NU, Polyak K. Insights into molecular classifications of triple-negative breast cancer: improving patient selection for treatment. Cancer Discov. 2019;9(2):176–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang X, Li X, Xia R, et al. Ferroptosis resistance in cancer: recent advances and future perspectives. Biochem Pharmacol. 2024;219: 115933. [DOI] [PubMed] [Google Scholar]
- 8.Zhang W, Zhu C, Liao Y, et al. Caspase-8 in inflammatory diseases: a potential therapeutic target. Cell Mol Biol Lett. 2024;29(1):130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peng F, Liao M, Qin R, et al. Regulated cell death (RCD) in cancer: key pathways and targeted therapies. Signal Transduct Target Ther. 2022;7(1):286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christgen S, Tweedell RE, Kanneganti TD. Programming inflammatory cell death for therapy. Pharmacol Ther. 2022;232: 108010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu J, Hong M, Li Y, et al. Programmed cell death tunes tumor immunity. Front Immunol. 2022;13: 847345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zou Y, Xie J, Zheng S, et al. Leveraging diverse cell-death patterns to predict the prognosis and drug sensitivity of triple-negative breast cancer patients after surgery. Int J Surg. 2022;107: 106936. [DOI] [PubMed] [Google Scholar]
- 13.Sun H, Hu W, Yan Y, et al. Using PAMPs and DAMPs as adjuvants in cancer vaccines. Hum Vaccin Immunother. 2021;17(12):5546–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yin Q, Song SY, Bian Y, et al. Unlocking the potential of pyroptosis in tumor immunotherapy: a new horizon in cancer treatment. Front Immunol. 2024;15:1381778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu H, Zhu J, Zhong Y, et al. PIK3CA mutation confers resistance to chemotherapy in triple-negative breast cancer by inhibiting apoptosis and activating the PI3K/AKT/mTOR signaling pathway. Ann Transl Med. 2021;9(5):410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu L, Peng F, Luo Q, et al. IRE1α silences dsRNA to prevent taxane-induced pyroptosis in triple-negative breast cancer. Cell. 2024;187(25):7248-66.e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sonnessa M, Cioffi A, Brunetti O, et al. NLRP3 inflammasome from bench to bedside: new perspectives for triple negative breast cancer. Front Oncol. 2020;10:1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gong Y, Fan Z, Luo G, et al. The role of necroptosis in cancer biology and therapy. Mol Cancer. 2019;18(1): 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kenific CM, Debnath J. Cellular and metabolic functions for autophagy in cancer cells. Trends Cell Biol. 2015;25(1):37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Debnath J, Gammoh N, Ryan KM. Autophagy and autophagy-related pathways in cancer. Nat Rev Mol Cell Biol. 2023;24(8):560–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang F, Xiao Y, Ding JH, et al. Ferroptosis heterogeneity in triple-negative breast cancer reveals an innovative immunotherapy combination strategy. Cell Metab. 2023. 10.1016/j.cmet.2022.09.021. [DOI] [PubMed] [Google Scholar]
- 22.Xu H, Ye D, Ren M, et al. Ferroptosis in the tumor microenvironment: perspectives for immunotherapy. Trends Mol Med. 2021;27(9):856–67. [DOI] [PubMed] [Google Scholar]
- 23.Li X, Pu W, Zheng Q, et al. Proteolysis-targeting chimeras (PROTACs) in cancer therapy. Mol Cancer. 2022;21(1):99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He Q, Liu H, Deng S, et al. The golgi apparatus may be a potential therapeutic target for apoptosis-related neurological diseases. Front Cell Dev Biol. 2020;8: 830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao R, Kaakati R, Lee AK, et al. Novel roles of apoptotic caspases in tumor repopulation, epigenetic reprogramming, carcinogenesis, and beyond. Cancer Metastasis Rev. 2018;37(2–3):227–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ismail Z, Dam J, Penny C, et al. Copper-imidazo[1,2-a]pyridines differentially modulate pro- and anti-apoptotic protein and gene expression in HL-60 and K562 leukaemic cells to cause apoptotic cell death. Biochim Biophys Acta Mol Cell Res. 2022;1869(1): 119160. [DOI] [PubMed] [Google Scholar]
- 27.Lin H, Wang Y, Wang P, et al. Mutual regulation between N6-methyladenosine (m6A) modification and circular RNAs in cancer: impacts on therapeutic resistance. Mol Cancer. 2022;21(1):148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu Z, Deng J, Zhou H, et al. Programmed cell death in sepsis associated acute kidney injury. Front Med (Lausanne). 2022;9: 883028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fulda S. Targeting extrinsic apoptosis in cancer: challenges and opportunities. Semin Cell Dev Biol. 2015;39:20–5. [DOI] [PubMed] [Google Scholar]
- 30.Kischkel FC, Hellbardt S, Behrmann I, et al. Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. EMBO J. 1995;14(22):5579–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.D’arcy MS. Cell death: a review of the major forms of apoptosis, necrosis and autophagy. Cell Biol Int. 2019;43(6):582–92. [DOI] [PubMed] [Google Scholar]
- 32.Brenner D, Mak TW. Mitochondrial cell death effectors. Curr Opin Cell Biol. 2009;21(6):871–7. [DOI] [PubMed] [Google Scholar]
- 33.Cheeran MC, Lokensgard JR, Schleiss MR. Neuropathogenesis of congenital cytomegalovirus infection: disease mechanisms and prospects for intervention. Clin Microbiol Rev. 2009;22(1):99–126 (Table of Contents). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsutsui H, Kinugawa S, Matsushima S. Oxidative stress and heart failure. Am J Physiol Heart Circ Physiol. 2011;301(6):H2181–90. [DOI] [PubMed] [Google Scholar]
- 35.Shi TF, Zhou Z, Jiang WJ, et al. Hyperglycemia-induced oxidative stress exacerbates mitochondrial apoptosis damage to cochlear stria vascularis pericytes via the ROS-mediated Bcl-2/CytC/AIF pathway. Redox Rep. 2024;29(1):2382943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carlsson MJ, Vollmer AS, Demuth P, et al. p53 triggers mitochondrial apoptosis following DNA damage-dependent replication stress by the hepatotoxin methyleugenol. Cell Death Dis. 2022;13(11):1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Panda SK, Sahoo G, Swain SS, et al. Anticancer activities of mushrooms: a neglected source for drug discovery. Pharmaceuticals (Basel). 2022. 10.3390/ph15020176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hao Q, Chen J, Lu H, et al. The ARTS of p53-dependent mitochondrial apoptosis. J Mol Cell Biol. 2023. 10.1093/jmcb/mjac074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fakhri S, Abbaszadeh F, Moradi SZ, et al. Effects of polyphenols on oxidative stress, inflammation, and interconnected pathways during spinal cord injury. Oxid Med Cell Longev. 2022;2022:8100195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perini GF, Ribeiro GN, Pinto Neto JV, et al. BCL-2 as therapeutic target for hematological malignancies. J Hematol Oncol. 2018;11(1):65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liao M, Qin R, Huang W, et al. Targeting regulated cell death (RCD) with small-molecule compounds in triple-negative breast cancer: a revisited perspective from molecular mechanisms to targeted therapies. J Hematol Oncol. 2022;15(1):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wong RS. Apoptosis in cancer: from pathogenesis to treatment. J Exp Clin Cancer Res. 2011;30(1):87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koff JL, Ramachandiran S, Bernal-Mizrachi L. A time to kill: targeting apoptosis in cancer. Int J Mol Sci. 2015;16(2):2942–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nocquet L, Roul J, Lefebvre CC, et al. Low BCL-xL expression in triple-negative breast cancer cells favors chemotherapy efficacy, and this effect is limited by cancer-associated fibroblasts. Sci Rep. 2024;14(1):14177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee A, Jin HO, Masudul Haque M, et al. Synergism of a novel MCL-1 downregulator, acriflavine, with navitoclax (ABT-263) in triple-negative breast cancer, lung adenocarcinoma and glioblastoma multiforme. Int J Oncol. 2022. 10.3892/ijo.2021.5292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hou R, Jiang L, Liu D, et al. Lewis(y) antigen promotes the progression of epithelial ovarian cancer by stimulating MUC1 expression. Int J Mol Med. 2017;40(2):293–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Siroy A, Abdul-Karim FW, Miedler J, et al. MUC1 is expressed at high frequency in early-stage basal-like triple-negative breast cancer. Hum Pathol. 2013;44(10):2159–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morgan MJ, Liu ZG. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011;21(1):103–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li W, Han Y, Sun C, et al. Novel insights into the roles and therapeutic implications of MUC1 oncoprotein via regulating proteins and non-coding RNAs in cancer. Theranostics. 2022;12(3):999–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hiraki M, Maeda T, Mehrotra N, et al. Targeting MUC1-C suppresses BCL2A1 in triple-negative breast cancer. Signal Transduct Target Ther. 2018;3:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ashrafizadeh M, Zarrabi A, Hushmandi K, et al. Association of the epithelial-mesenchymal transition (EMT) with cisplatin resistance. Int J Mol Sci. 2020. 10.3390/ijms21114002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu Z, Wang C, Yang J, et al. Caspase-1 engages full-length Gasdermin D through two distinct interfaces that mediate caspase recruitment and substrate cleavage. Immunity. 2020;53(1):106-14.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lawrence SM, Corriden R, Nizet V. How neutrophils meet their end. Trends Immunol. 2020;41(6):531–44. [DOI] [PubMed] [Google Scholar]
- 54.Shi J, Gao W, Shao F. Pyroptosis: gasdermin-mediated programmed necrotic cell death. Trends Biochem Sci. 2017;42(4):245–54. [DOI] [PubMed] [Google Scholar]
- 55.Voet S, Mc Guire C, Hagemeyer N, et al. A20 critically controls microglia activation and inhibits inflammasome-dependent neuroinflammation. Nat Commun. 2018;9(1): 2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Place DE, Kanneganti TD. Cell death-mediated cytokine release and its therapeutic implications. J Exp Med. 2019;216(7):1474–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen X, He WT, Hu L, et al. Pyroptosis is driven by non-selective gasdermin-D pore and its morphology is different from MLKL channel-mediated necroptosis. Cell Res. 2016;26(9):1007–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Miao R, Jiang C, Chang WY, et al. Gasdermin D permeabilization of mitochondrial inner and outer membranes accelerates and enhances pyroptosis. Immunity. 2023;56(11):2523-41.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jeon M, Han J, Nam SJ, et al. Elevated IL-1β expression induces invasiveness of triple negative breast cancer cells and is suppressed by zerumbone. Chem Biol Interact. 2016;258:126–33. [DOI] [PubMed] [Google Scholar]
- 60.Guo Q, Zhou C, Xiang Y, et al. Pyroptosis orchestrates immune responses in endometriosis. Int Immunopharmacol. 2023;118: 110141. [DOI] [PubMed] [Google Scholar]
- 61.Chen X, Yang M, Yin J, et al. Tumor-associated macrophages promote epithelial-mesenchymal transition and the cancer stem cell properties in triple-negative breast cancer through CCL2/AKT/β-catenin signaling. Cell Commun Signal. 2022;20(1):92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sun Y, Hu H, Liu Z, et al. Macrophage STING signaling promotes NK cell to suppress colorectal cancer liver metastasis via 4–1BBL/4-1BB co-stimulation. J Immunother Cancer. 2023. 10.1136/jitc-2022-006481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li X, Zou Y, Fu YY, et al. Ibudilast attenuates folic acid-induced acute kidney injury by blocking pyroptosis through TLR4-mediated NF-κB and MAPK signaling pathways. Front Pharmacol. 2021;12: 650283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zheng Y, Lakshmanan S. Dose-dependent efficacy of umbelliferone and gelatin-coated ZnO/ZnS core-shell nanoparticles: a novel arthritis agent for severe knee arthritis. Oxid Med Cell Longev. 2022;2022:7795602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen W, Yang KB, Zhang YZ, et al. Synthetic lethality of combined ULK1 defection and p53 restoration induce pyroptosis by directly upregulating GSDME transcription and cleavage activation through ROS/NLRP3 signaling. J Exp Clin Cancer Res. 2024;43(1):248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rao Z, Zhu Y, Yang P, et al. Pyroptosis in inflammatory diseases and cancer. Theranostics. 2022;12(9):4310–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fang Y, Tian S, Pan Y, et al. Pyroptosis: A new frontier in cancer. Biomed Pharmacother. 2020;121: 109595. [DOI] [PubMed] [Google Scholar]
- 68.Toldo S, Abbate A. The role of the NLRP3 inflammasome and pyroptosis in cardiovascular diseases. Nat Rev Cardiol. 2024;21(4):219–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Costa Franco MMS, Marim FM, Alves-Silva J, et al. AIM2 senses Brucella abortus DNA in dendritic cells to induce IL-1β secretion, pyroptosis and resistance to bacterial infection in mice. Microbes Infect. 2019;21(2):85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fu J, Wu H. Structural mechanisms of NLRP3 inflammasome assembly and activation. Annu Rev Immunol. 2023;41:301–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lu A, Magupalli VG, Ruan J, et al. Unified polymerization mechanism for the assembly of ASC-dependent inflammasomes. Cell. 2014;156(6):1193–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lu A, Li Y, Schmidt FI, et al. Molecular basis of caspase-1 polymerization and its inhibition by a new capping mechanism. Nat Struct Mol Biol. 2016;23(5):416–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lin C, Zhang J. Inflammasomes in inflammation-induced cancer. Front Immunol. 2017;8: 271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kovacs SB, Miao EA. Gasdermins: effectors of pyroptosis. Trends Cell Biol. 2017;27(9):673–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang W, Wu H, Liao Y, et al. Caspase family in autoimmune diseases. Autoimmun Rev. 2025;24(2): 103714. [DOI] [PubMed] [Google Scholar]
- 76.Zuo Y, Chen L, Gu H, et al. GSDMD-mediated pyroptosis: a critical mechanism of diabetic nephropathy. Expert Rev Mol Med. 2021;23: e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zheng Z, Deng W, Bai Y, et al. The lysosomal rag-ragulator complex licenses RIPK1 and caspase-8-mediated pyroptosis by Yersinia. Science. 2021. 10.1126/science.abg0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shi J, Zhao Y, Wang K, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526(7575):660–5. [DOI] [PubMed] [Google Scholar]
- 79.Li Z, Ji S, Jiang ML, et al. The regulation and modification of GSDMD signaling in diseases. Front Immunol. 2022;13: 893912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rathinam VAK, Zhao Y, Shao F. Innate immunity to intracellular LPS. Nat Immunol. 2019;20(5):527–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang K, Sun Q, Zhong X, et al. Structural mechanism for GSDMD Targeting by autoprocessed caspases in pyroptosis. Cell. 2020;180(5):941-55.e20. [DOI] [PubMed] [Google Scholar]
- 82.Zhang CC, Li CG, Wang YF, et al. Chemotherapeutic paclitaxel and cisplatin differentially induce pyroptosis in A549 lung cancer cells via caspase-3/GSDME activation. Apoptosis. 2019;24(3–4):312–25. [DOI] [PubMed] [Google Scholar]
- 83.Xu D, Liu L, Zhao Y, et al. Melatonin protects mouse testes from palmitic acid-induced lipotoxicity by attenuating oxidative stress and DNA damage in a SIRT1-dependent manner. J Pineal Res. 2020;69(4): e12690. [DOI] [PubMed] [Google Scholar]
- 84.Pan J, Li Y, Gao W, et al. Transcription factor Sp1 transcriptionally enhances GSDME expression for pyroptosis. Cell Death Dis. 2024;15(1): 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Arnold D, Wasem C, Juillard P, et al. IL-18-independent cytotoxic T lymphocyte activation and IFN-gamma production during experimental acute graft-versus-host disease. Int Immunol. 2002;14(5):503–11. [DOI] [PubMed] [Google Scholar]
- 86.Fang Y, Tang Y, Huang B. Pyroptosis: a road to next-generation cancer immunotherapy. Semin Immunol. 2023;68: 101782. [DOI] [PubMed] [Google Scholar]
- 87.DEL Prete A, Salvi V, Soriani A, et al. Dendritic cell subsets in cancer immunity and tumor antigen sensing. Cell Mol Immunol. 2023;20(5):432–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li N, Chen J, Geng C, et al. Myoglobin promotes macrophage polarization to M1 type and pyroptosis via the RIG-I/caspase1/GSDMD signaling pathway in CS-AKI. Cell Death Discov. 2022;8(1):90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yi J, Li H, Chu B, et al. Inhibition of USP7 induces p53-independent tumor growth suppression in triple-negative breast cancers by destabilizing FOXM1. Cell Death Differ. 2023;30(7):1799–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Feng WQ, Zhang YC, Xu ZQ, et al. IL-17A-mediated mitochondrial dysfunction induces pyroptosis in colorectal cancer cells and promotes CD8 + T-cell tumour infiltration. J Transl Med. 2023;21(1): 335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li Q, Tong Y, Chen J, et al. Targeting programmed cell death via active ingredients from natural plants: a promising approach to cancer therapy. Front Pharmacol. 2024;15: 1491802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gielecińska A, Kciuk M, Yahya EB, et al. Apoptosis, necroptosis, and pyroptosis as alternative cell death pathways induced by chemotherapeutic agents? Biochim Biophys Acta (BBA). 2023;1878(6): 189024. [DOI] [PubMed] [Google Scholar]
- 93.Newton K, Dixit VM, Kayagaki N. Dying cells fan the flames of inflammation. Science. 2021;374(6571):1076–80. [DOI] [PubMed] [Google Scholar]
- 94.Yang J, Hu S, Bian Y, et al. Targeting cell death: pyroptosis, ferroptosis, apoptosis and necroptosis in osteoarthritis. Front Cell Dev Biol. 2021;9: 789948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Khoury MK, Gupta K, Franco SR, et al. Necroptosis in the pathophysiology of disease. Am J Pathol. 2020;190(2):272–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hsu SK, Chang WT, Lin IL, et al. The role of necroptosis in ROS-mediated cancer therapies and its promising applications. Cancers (Basel). 2020. 10.3390/cancers12082185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yuan J, Ofengeim D. A guide to cell death pathways. Nat Rev Mol Cell Biol. 2024;25(5):379–95. [DOI] [PubMed] [Google Scholar]
- 98.Ai Y, Meng Y, Yan B, et al. The biochemical pathways of apoptotic, necroptotic, pyroptotic, and ferroptotic cell death. Mol Cell. 2024;84(1):170–9. [DOI] [PubMed] [Google Scholar]
- 99.Ye K, Chen Z, Xu Y. The double-edged functions of necroptosis. Cell Death Dis. 2023;14(2): 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Campbell KJ, Dhayade S, Ferrari N, et al. MCL-1 is a prognostic indicator and drug target in breast cancer. Cell Death Dis. 2018;9(2): 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Annibaldi A, Meier P. Checkpoints in TNF-induced cell death: implications in inflammation and cancer. Trends Mol Med. 2018;24(1):49–65. [DOI] [PubMed] [Google Scholar]
- 102.Wang S, Wang H, Feng C, et al. The regulatory role and therapeutic application of pyroptosis in musculoskeletal diseases. Cell Death Discov. 2022;8(1): 492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tseng LM, Lau KY, Chen JL, et al. Regorafenib induces damage-associated molecular patterns, cancer cell death and immune modulatory effects in a murine triple negative breast cancer model. Exp Cell Res. 2023;429(1): 113652. [DOI] [PubMed] [Google Scholar]
- 104.Jalali AM, Mitchell KJ, Pompoco C, et al. Therapeutic significance of NLRP3 inflammasome in cancer: friend or foe? Int J Mol Sci. 2024. 10.3390/ijms252413689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Meng X, Na R, Peng X, et al. Musashi-2 potentiates colorectal cancer immune infiltration by regulating the post-translational modifications of HMGB1 to promote DCs maturation and migration. Cell Commun Signal. 2024;22(1):117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hamdan F, Fusciello M, Cerullo V. Personalizing oncolytic virotherapy. Hum Gene Ther. 2023;34(17–18):870–7. [DOI] [PubMed] [Google Scholar]
- 107.Pittet MJ, DI Pilato M, Garris C, et al. Dendritic cells as shepherds of T cell immunity in cancer. Immunity. 2023;56(10):2218–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Garris CS, Arlauckas SP, Kohler RH, et al. Successful anti-PD-1 cancer immunotherapy requires T cell-dendritic cell crosstalk involving the cytokines IFN-γ and IL-12. Immunity. 2018;49(6):1148-61.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Li W, Zhao X, Ren C, et al. The therapeutic role of γδT cells in TNBC. Front Immunol. 2024;15: 1420107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Glick D, Barth S, Macleod KF. Autophagy: cellular and molecular mechanisms. J Pathol. 2010;221(1):3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell. 2011;147(4):728–41. [DOI] [PubMed] [Google Scholar]
- 112.Wu H, Huang S, Zhang D. Autophagic responses to hypoxia and anticancer therapy in head and neck cancer. Pathol Res Pract. 2015;211(2):101–8. [DOI] [PubMed] [Google Scholar]
- 113.Yao J, Jia L, Khan N, et al. Deletion of autophagy inducer RB1CC1 results in degeneration of the retinal pigment epithelium. Autophagy. 2015;11(6):939–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ren W, Sun Y, Zhao L, et al. NLRP3 inflammasome and its role in autoimmune diseases: a promising therapeutic target. Biomed Pharmacother. 2024;175: 116679. [DOI] [PubMed] [Google Scholar]
- 115.Liu S, Yao S, Yang H, et al. Autophagy: regulator of cell death. Cell Death Dis. 2023;14(10):648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yu S, Li Y, Lu X, et al. The regulatory role of miRNA and lncRNA on autophagy in diabetic nephropathy. Cell Signal. 2024;118: 111144. [DOI] [PubMed] [Google Scholar]
- 117.Wang B, Pareek G, Kundu M. ULK/Atg1: phasing in and out of autophagy. Trends Biochem Sci. 2024;49(6):494–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Devenport SN, Shah YM. Functions and implications of autophagy in colon cancer. Cells. 2019. 10.3390/cells8111349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ke F, Zhang R, Chen R, et al. The role of Rhizoma Paridis saponins on anti-cancer: the potential mechanism and molecular targets. Heliyon. 2024;10(17): e37323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kim J, Kundu M, Viollet B, et al. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13(2):132–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Roach PJ. AMPK -> ULK1 -> autophagy. Mol Cell Biol. 2011;31(15):3082–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zachari M, Ganley IG. The mammalian ULK1 complex and autophagy initiation. Essays Biochem. 2017;61(6):585–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Samare-Najaf M, Samareh A, Savardashtaki A, et al. Non-apoptotic cell death programs in cervical cancer with an emphasis on ferroptosis. Crit Rev Oncol Hematol. 2024;194: 104249. [DOI] [PubMed] [Google Scholar]
- 124.Hwang S, Maloney NS, Bruinsma MW, et al. Nondegradative role of Atg5-Atg12/ Atg16L1 autophagy protein complex in antiviral activity of interferon gamma. Cell Host Microbe. 2012;11(4):397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Huang R, Chen H, Liang J, et al. Dual role of reactive oxygen species and their application in cancer therapy. J Cancer. 2021;12(18):5543–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sun S, Yu W, Zhang G, et al. Potential mechanism of traditional Chinese medicine intervention in gastric cancer: targeted regulation of autophagy. Front Pharmacol. 2025;16:1548672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fan M, Gao J, Zhou L, et al. Highly expressed SERCA2 triggers tumor cell autophagy and is a druggable vulnerability in triple-negative breast cancer. Acta Pharm Sin B. 2022;12(12):4407–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chen X, Li S, Li D, et al. Ethanol extract of Brucea javanica seed inhibit triple-negative breast cancer by restraining autophagy via PI3K/Akt/mTOR pathway. Front Pharmacol. 2020;11:606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Xiang H, Zhang J, Lin C, et al. Targeting autophagy-related protein kinases for potential therapeutic purpose. Acta Pharm Sin B. 2020;10(4):569–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Feng Y, He D, Yao Z, et al. The machinery of macroautophagy. Cell Res. 2014;24(1):24–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Abd El-Aziz YS, Gillson J, Jansson PJ, et al. Autophagy: a promising target for triple negative breast cancers. Pharmacol Res. 2022;175: 106006. [DOI] [PubMed] [Google Scholar]
- 132.Domitrović R, Potočnjak I. A comprehensive overview of hepatoprotective natural compounds: mechanism of action and clinical perspectives. Arch Toxicol. 2016;90(1):39–79. [DOI] [PubMed] [Google Scholar]
- 133.Levine B, Mizushima N, Virgin HW. Autophagy in immunity and inflammation. Nature. 2011;469(7330):323–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Dowling SD, Macian F. Autophagy and T cell metabolism. Cancer Lett. 2018;419:20–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Liu C, Xiao K, Xie L. Progress in preclinical studies of macrophage autophagy in the regulation of ALI/ARDS. Front Immunol. 2022;13: 922702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zou R, Wu S, Wang Y, et al. Role of integrin-linked kinase in static compressive stress-induced autophagy via phosphatidylinositol 3 kinase in human periodontal ligament cells. Int J Mol Med. 2021. 10.3892/ijmm.2021.5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhang M, Sui W, Xing Y, et al. Angiotensin IV attenuates diabetic cardiomyopathy via suppressing FoxO1-induced excessive autophagy, apoptosis and fibrosis. Theranostics. 2021;11(18):8624–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Theofani E, Xanthou G. Autophagy: a friend or foe in allergic asthma? Int J Mol Sci. 2021. 10.3390/ijms22126314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Li J, Jia YC, Ding YX, et al. The crosstalk between ferroptosis and mitochondrial dynamic regulatory networks. Int J Biol Sci. 2023;19(9):2756–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Li MH, Yang Y, Dong QQ, et al. Novel epitranscriptomic and epigenetic therapeutic strategies and targets for ferroptosis in liver fibrosis. Eur J Pharmacol. 2025;996: 177344. [DOI] [PubMed] [Google Scholar]
- 141.Zeng L, Ding S, Cao Y, et al. A MOF-based potent ferroptosis inducer for enhanced radiotherapy of triple negative breast cancer. ACS Nano. 2023;17(14):13195–210. [DOI] [PubMed] [Google Scholar]
- 142.Gong D, Chen M, Wang Y, et al. Role of ferroptosis on tumor progression and immunotherapy. Cell Death Discov. 2022;8(1):427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kong L, Xie Y, Hu L, et al. Excessive nitrogen application dampens antioxidant capacity and grain filling in wheat as revealed by metabolic and physiological analyses. Sci Rep. 2017;7:43363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhou P, Zhang S, Wang M, et al. The induction mechanism of ferroptosis, necroptosis, and pyroptosis in inflammatory bowel disease, colorectal cancer, and intestinal injury. Biomolecules. 2023. 10.3390/biom13050820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sherman HG, Jovanovic C, Stolnik S, et al. New perspectives on iron uptake in eukaryotes. Front Mol Biosci. 2018;5:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Garcia-Santos D, Schranzhofer M, Bergeron R, et al. Extracellular glycine is necessary for optimal hemoglobinization of erythroid cells. Haematologica. 2017;102(8):1314–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Candelaria PV, Leoh LS, Penichet ML, et al. Antibodies targeting the transferrin receptor 1 (TfR1) as direct anti-cancer agents. Front Immunol. 2021;12: 607692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hassan W, Ibrahim M, Nogueira CW, et al. Influence of pH on the reactivity of diphenyl ditelluride with thiols and anti-oxidant potential in rat brain. Chem Biol Interact. 2009;180(1):47–53. [DOI] [PubMed] [Google Scholar]
- 149.Dhingra R, Ravandi A, Kirshenbaum LA. Ferroptosis: beating on death’s door. Am J Physiol Heart Circ Physiol. 2018;314(4):H772–5. [DOI] [PubMed] [Google Scholar]
- 150.Leonidova A, Anstaett P, Pierroz V, et al. Induction of cytotoxicity through photorelease of aminoferrocene. Inorg Chem. 2015;54(20):9740–8. [DOI] [PubMed] [Google Scholar]
- 151.Cao J, Hu C, Xu J, et al. Aberrant expression TFR1/CD71 in gastric cancer identifies a novel potential prognostic marker and therapeutic target. Evid Based Complement Alternat Med. 2022;2022: 2022: 4257342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Xiong J, Nie M, Fu C, et al. Hypoxia enhances HIF1α transcription activity by upregulating KDM4A and mediating H3K9me3, thus inducing ferroptosis resistance in cervical cancer cells. Stem Cells Int. 2022;2022:1608806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Urrutia P, Aguirre P, Esparza A, et al. Inflammation alters the expression of DMT1, FPN1 and hepcidin, and it causes iron accumulation in central nervous system cells. J Neurochem. 2013;126(4):541–9. [DOI] [PubMed] [Google Scholar]
- 154.Liu B, Song Z, Fan Y, et al. Downregulation of FPN1 acts as a prognostic biomarker associated with immune infiltration in lung cancer. Aging (Albany NY). 2021;13(6):8737–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zeng Z, Luo Y, Xu X, et al. A mitochondria-targeting ROS-activated nanoprodrug for self-augmented antitumor oxidation therapy. J Control Release. 2023;359:415–27. [DOI] [PubMed] [Google Scholar]
- 156.Jambunathan N. Determination and detection of reactive oxygen species (ROS), lipid peroxidation, and electrolyte leakage in plants. Methods Mol Biol. 2010;639:292–8. [DOI] [PubMed] [Google Scholar]
- 157.Yang X, Chen Y, Guo J, et al. Polydopamine nanoparticles targeting ferroptosis mitigate intervertebral disc degeneration via reactive oxygen species depletion, iron ions chelation, and GPX4 ubiquitination suppression. Adv Sci (Weinh). 2023;10(13): e2207216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.DEL Vento F, Vermeulen M, Ucakar B, et al. Significant benefits of nanoparticles containing a necrosis inhibitor on mice testicular tissue autografts outcomes. Int J Mol Sci. 2019. 10.3390/ijms20235833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Liu X, Tuerxun H, Li Y, et al. Ferroptosis: reviewing CRC with the third eye. J Inflamm Res. 2022;15:6801–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wen J, Aili A, Yan YX, et al. OIT3 serves as a novel biomarker of hepatocellular carcinoma by mediating ferroptosis via regulating the arachidonic acid metabolism. Front Oncol. 2022;12: 977348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ursini F, Maiorino M. Lipid peroxidation and ferroptosis: the role of GSH and GPx4. Free Radic Biol Med. 2020;152:175–85. [DOI] [PubMed] [Google Scholar]
- 162.Angrimani DSR, Brito MM, Rui BR, et al. Reproductive and endocrinological effects of benign prostatic hyperplasia and finasteride therapy in dogs. Sci Rep. 2020;10(1):14834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhang W, Liu Y, Liao Y, et al. GPX4, ferroptosis, and diseases. Biomed Pharmacother. 2024;174: 116512. [DOI] [PubMed] [Google Scholar]
- 164.Ge Y, Jiang L, Yang C, et al. Interactions between tumor-associated macrophages and regulated cell death: therapeutic implications in immuno-oncology. Front Oncol. 2024;14:1449696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Guo N, Chen Y, Zhang Y, et al. Potential role of APEX1 during ferroptosis. Front Oncol. 2022;12: 798304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Battaglia AM, Chirillo R, Aversa I, et al. Ferroptosis and cancer: mitochondria meet the “iron maiden” cell death. Cells. 2020. 10.3390/cells9061505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wang Y, Sun Y, Wang F, et al. Ferroptosis induction via targeting metabolic alterations in triple-negative breast cancer. Biomed Pharmacother. 2023;169: 115866. [DOI] [PubMed] [Google Scholar]
- 168.Wang W, Deng Z, Hatcher H, et al. IRP2 regulates breast tumor growth. Cancer Res. 2014;74(2):497–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Liu X, Tuerxun H, Zhao Y, et al. Crosstalk between ferroptosis and autophagy: broaden horizons of cancer therapy. J Transl Med. 2025;23(1): 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Anandhan A, Dodson M, Shakya A, et al. NRF2 controls iron homeostasis and ferroptosis through HERC2 and VAMP8. Sci Adv. 2023. 10.1126/sciadv.ade9585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Berndt C, Alborzinia H, Amen VS, et al. Ferroptosis in health and disease. Redox Biol. 2024;75: 103211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Xiang D, Zhou L, Yang R, et al. Advances in ferroptosis-inducing agents by targeted delivery system in cancer therapy. Int J Nanomed. 2024;19:2091–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Yang Y, Ren J, Zhang J, et al. FTO ameliorates doxorubicin-induced cardiotoxicity by inhibiting ferroptosis via P53–P21/Nrf2 activation in a HuR-dependent m6A manner. Redox Biol. 2024;70: 103067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Adams CM, Mitra R, Xiao Y, et al. Targeted MDM2 degradation reveals a new vulnerability for p53-inactivated triple-negative breast cancer. Cancer Discov. 2023;13(5):1210–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zhou TJ, Zhang MM, Liu DM, et al. Glutathione depletion and dihydroorotate dehydrogenase inhibition actuated ferroptosis-augment to surmount triple-negative breast cancer. Biomaterials. 2024;305: 122447. [DOI] [PubMed] [Google Scholar]
- 176.Mokhtarpour K, Razi S, Rezaei N. Ferroptosis as a promising targeted therapy for triple negative breast cancer. Breast Cancer Res Treat. 2024;207(3):497–513. [DOI] [PubMed] [Google Scholar]
- 177.Sun L, Zhang H, Gao P. Metabolic reprogramming and epigenetic modifications on the path to cancer. Protein Cell. 2022;13(12):877–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Moore LD, Le T, Fan G. DNA methylation and its basic function. Neuropsychopharmacology. 2013;38(1):23–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Etchegaray JP, Mostoslavsky R. Interplay between metabolism and epigenetics: a nuclear adaptation to environmental changes. Mol Cell. 2016;62(5):695–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Fabian MR, Sonenberg N, Filipowicz W. Regulation of mRNA translation and stability by microRNAs. Annu Rev Biochem. 2010;79:351–79. [DOI] [PubMed] [Google Scholar]
- 181.Fang L, Huang H, Lv J, et al. M5C-methylated lncRNA NR_033928 promotes gastric cancer proliferation by stabilizing GLS mRNA to promote glutamine metabolism reprogramming. Cell Death Dis. 2023;14(8):520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Gopisetty G, Ramachandran K, Singal R. DNA methylation and apoptosis. Mol Immunol. 2006;43(11):1729–40. [DOI] [PubMed] [Google Scholar]
- 183.Arakawa H. P53, apoptosis and axon-guidance molecules. Cell Death Differ. 2005;12(8):1057–65. [DOI] [PubMed] [Google Scholar]
- 184.Mandal R, BARRóN JC, Kostova I, et al. Caspase-8: the double-edged sword. Biochimica et Biophysica Acta (BBA). 2020;1873(2): 188357. [DOI] [PubMed] [Google Scholar]
- 185.Teng Y, Dong YC, Liu Z, et al. DNA methylation-mediated caspase-8 downregulation is associated with anti-apoptotic activity and human malignant glioma grade. Int J Mol Med. 2017;39(3):725–33. [DOI] [PubMed] [Google Scholar]
- 186.Fancy RM, Kim H, Napier T, et al. Calmodulin antagonist enhances DR5-mediated apoptotic signaling in TRA-8 resistant triple negative breast cancer cells. J Cell Biochem. 2018;119(7):6216–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Le X, Mu J, Peng W, et al. DNA methylation downregulated ZDHHC1 suppresses tumor growth by altering cellular metabolism and inducing oxidative/ER stress-mediated apoptosis and pyroptosis. Theranostics. 2020;10(21):9495–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Yang Z, Jiang B, Wang Y, et al. 2-HG inhibits necroptosis by stimulating DNMT1-dependent hypermethylation of the RIP3 promoter. Cell Rep. 2017;19(9):1846–57. [DOI] [PubMed] [Google Scholar]
- 189.Zhu S, Luo Y, Li K, et al. RIPK3 deficiency blocks R-2-hydroxyglutarate-induced necroptosis in IDH-mutated AML cells. Sci Adv. 2024;10(16): eadi1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zhou S, Liu J, Wan A, et al. Epigenetic regulation of diverse cell death modalities in cancer: a focus on pyroptosis, ferroptosis, cuproptosis, and disulfidptosis. J Hematol Oncol. 2024;17(1): 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Wang Y, Liu Y, Wang C, et al. EP300 promotes ferroptosis via HSPA5 acetylation in pancreatic cancer. Sci Rep. 2023;13(1):15004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Chen Q, Yang B, Liu X, et al. Histone acetyltransferases CBP/p300 in tumorigenesis and CBP/p300 inhibitors as promising novel anticancer agents. Theranostics. 2022;12(11):4935–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zhou R, Yang Y, Park SY, et al. P300/CBP-associated factor promotes autophagic degradation of δ-catenin through acetylation and decreases prostate cancer tumorigenicity. Sci Rep. 2019;9(1):3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Fang Y, Liao G, Yu B. LSD1/KDM1A inhibitors in clinical trials: advances and prospects. J Hematol Oncol. 2019;12(1):129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Yang GJ, Lei PM, Wong SY, et al. Pharmacological inhibition of LSD1 for cancer treatment. Molecules. 2018. 10.3390/molecules23123194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Sabit H, Cevik E, Tombuloglu H, et al. Triple negative breast cancer in the era of miRNA. Crit Rev Oncol Hematol. 2021;157: 103196. [DOI] [PubMed] [Google Scholar]
- 197.Ferrari P, Scatena C, Ghilli M, et al. Molecular mechanisms, biomarkers and emerging therapies for chemotherapy resistant TNBC. Int J Mol Sci. 2022. 10.3390/ijms23031665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Youness RA, Hafez HM, Khallaf E, et al. The long noncoding RNA sONE represses triple-negative breast cancer aggressiveness through inducing the expression of miR-34a, miR-15a, miR-16, and let-7a. J Cell Physiol. 2019;234(11):20286–97. [DOI] [PubMed] [Google Scholar]
- 199.Pourhanifeh MH, Mahjoubin-Tehran M, Karimzadeh MR, et al. Autophagy in cancers including brain tumors: role of microRNAs. Cell Commun Signal. 2020;18(1): 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Huang J, Li Y, Zhu S, et al. Mir-30 family: a novel avenue for treating bone and joint diseases? Int J Med Sci. 2023;20(4):493–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Thangavelu L, Moglad E, Gupta G, et al. GAS5 lncRNA: a biomarker and therapeutic target in breast cancer. Pathol Res Pract. 2024;260: 155424. [DOI] [PubMed] [Google Scholar]
- 202.Ni R, Jiang J, Wang F, et al. Treating incurable non-communicable diseases by targeting iron metabolism and ferroptosis. Sci China Life Sci. 2025. 10.1007/s11427-024-2787-y. [DOI] [PubMed] [Google Scholar]
- 203.Booth LA, Tavallai S, Hamed HA, et al. The role of cell signalling in the crosstalk between autophagy and apoptosis. Cell Signal. 2014;26(3):549–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Wang B, Wang Y, Zhang J, et al. ROS-induced lipid peroxidation modulates cell death outcome: mechanisms behind apoptosis, autophagy, and ferroptosis. Arch Toxicol. 2023;97(6):1439–51. [DOI] [PubMed] [Google Scholar]
- 205.Jiang L, Chen HY, He CH, et al. Dual-modal apoptosis assay enabling dynamic visualization of ATP and reactive oxygen species in living cells. Anal Chem. 2023;95(6):3507–15. [DOI] [PubMed] [Google Scholar]
- 206.Shimada K, Crother TR, Karlin J, et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity. 2012;36(3):401–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Roberge S, Roussel J, Andersson DC, et al. TNF-α-mediated caspase-8 activation induces ROS production and TRPM2 activation in adult ventricular myocytes. Cardiovasc Res. 2014;103(1):90–9. [DOI] [PubMed] [Google Scholar]
- 208.Jiang M, Qi L, Li L, et al. The caspase-3/GSDME signal pathway as a switch between apoptosis and pyroptosis in cancer. Cell Death Discov. 2020;6:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Nedeljković M, Damjanović A. Mechanisms of chemotherapy resistance in triple-negative breast cancer-how we can rise to the challenge. Cells. 2019. 10.3390/cells8090957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Mathew R, Karantza-Wadsworth V, White E. Role of autophagy in cancer. Nat Rev Cancer. 2007;7(12):961–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Li YJ, Lei YH, Yao N, et al. Autophagy and multidrug resistance in cancer. Chin J Cancer. 2017;36(1): 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Nazim UM, Moon JH, Lee JH, et al. Activation of autophagy flux by metformin downregulates cellular FLICE-like inhibitory protein and enhances TRAIL- induced apoptosis. Oncotarget. 2016;7(17):23468–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Bai Y, Liu X, Qi X, et al. PDIA6 modulates apoptosis and autophagy of non-small cell lung cancer cells via the MAP4K1/JNK signaling pathway. EBioMedicine. 2019;42:311–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Oberstein A, Jeffrey PD, Shi Y. Crystal structure of the Bcl-XL-Beclin 1 peptide complex: Beclin 1 is a novel BH3-only protein. J Biol Chem. 2007;282(17):13123–32. [DOI] [PubMed] [Google Scholar]
- 215.Xiao X, Wang W, Li Y, et al. HSP90AA1-mediated autophagy promotes drug resistance in osteosarcoma. J Exp Clin Cancer Res. 2018;37(1):201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Zheng M, Kanneganti TD. The regulation of the ZBP1-NLRP3 inflammasome and its implications in pyroptosis, apoptosis, and necroptosis (PANoptosis). Immunol Rev. 2020;297(1):26–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Sittithumcharee G, Kariya R, Kasemsuk T, et al. Antitumor effect of acanthoic acid against primary effusion lymphoma via inhibition of c-FLIP. Phytother Res. 2021;35(12):7018–26. [DOI] [PubMed] [Google Scholar]
- 218.Bordini J, Morisi F, Cerruti F, et al. Iron causes lipid oxidation and inhibits proteasome function in multiple myeloma cells: a proof of concept for novel combination therapies. Cancers (Basel). 2020. 10.3390/cancers12040970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.DE Carvalho MAJ, Chaves-Filho A, DE Souza AG, et al. Proconvulsant effects of sildenafil citrate on pilocarpine-induced seizures: involvement of cholinergic, nitrergic and pro-oxidant mechanisms. Brain Res Bull. 2019;149:60–74. [DOI] [PubMed] [Google Scholar]
- 220.Wang GX, Tu HC, Dong Y, et al. ΔNp63 inhibits oxidative stress-induced cell death, including ferroptosis, and cooperates with the BCL-2 family to promote clonogenic survival. Cell Rep. 2017;21(10):2926–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Li J, Cao F, Yin HL, et al. Ferroptosis: past, present and future. Cell Death Dis. 2020;11(2): 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Redza-Dutordoir M, Averill-Bates DA. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim Biophys Acta. 2016;1863(12):2977–92. [DOI] [PubMed] [Google Scholar]
- 223.Zhou J, Qiu J, Song Y, et al. Pyroptosis and degenerative diseases of the elderly. Cell Death Dis. 2023;14(2): 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Zhang Z, Zhang H, Li D, et al. Caspase-3-mediated GSDME induced pyroptosis in breast cancer cells through the ROS/JNK signalling pathway. J Cell Mol Med. 2021;25(17):8159–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Siegmund D, Wajant H. TNF and TNF receptors as therapeutic targets for rheumatic diseases and beyond. Nat Rev Rheumatol. 2023;19(9):576–91. [DOI] [PubMed] [Google Scholar]
- 226.Hou S, Zhang J, Jiang X, et al. PARP5A and RNF146 phase separation restrains RIPK1-dependent necroptosis. Mol Cell. 2024;84(5):938-54.e8. [DOI] [PubMed] [Google Scholar]
- 227.Huang Y, Xu W, Zhou R. NLRP3 inflammasome activation and cell death. Cell Mol Immunol. 2021;18(9):2114–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Yu J, Feng D, Bao L, et al. TRIM32 inhibits NEK7 ubiquitylation-dependent microglia pyroptosis after spinal cord Injury. Mol Biotechnol. 2023. 10.1007/s12033-023-00989-4. [DOI] [PubMed] [Google Scholar]
- 229.Yang Q, Liu TT, Lin H, et al. TRIM32-TAX1BP1-dependent selective autophagic degradation of TRIF negatively regulates TLR3/4-mediated innate immune responses. PLoS Pathog. 2017;13(9): e1006600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Victor AR, Nalin AP, Dong W, et al. IL-18 Drives ILC3 Proliferation and Promotes IL-22 Production via NF-κB. J Immunol. 2017;199(7):2333–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Fang WY, Tseng YT, Lee TY, et al. Triptolide prevents LPS-induced skeletal muscle atrophy via inhibiting NF-κB/TNF-α and regulating protein synthesis/degradation pathway. Br J Pharmacol. 2021;178(15):2998–3016. [DOI] [PubMed] [Google Scholar]
- 232.Wang H, Shu L, Lv C, et al. BRCC36 deubiquitinates HMGCR to regulate the interplay between ferroptosis and pyroptosis. Adv Sci (Weinh). 2024;11(11): e2304263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Hsu CG, CHáVEZ CL, Zhang C, et al. The lipid peroxidation product 4-hydroxynonenal inhibits NLRP3 inflammasome activation and macrophage pyroptosis. Cell Death Differ. 2022;29(9):1790–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Sun Y, Huang YH, Huang FY, et al. 3’-epi-12β-hydroxyfroside, a new cardenolide, induces cytoprotective autophagy via blocking the Hsp90/Akt/mTOR axis in lung cancer cells. Theranostics. 2018;8(7):2044–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Huang FY, Dai SZ, Xu WT, et al. 3’-epi-12β-hydroxyfroside-mediated autophagy degradation of RIPK1/RIPK3 necrosomes leads to anergy of immunogenic cell death in triple-negative breast cancer cells. Pharmacol Res. 2023;187: 106613. [DOI] [PubMed] [Google Scholar]
- 236.Rojas-Rivera D, BELTRáN S, MUñOZ-CARVAJAL F, et al. The autophagy protein RUBCNL/PACER represses RIPK1 kinase-dependent apoptosis and necroptosis. Autophagy. 2024;20(11):2444–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Chen YM, Xu W, Liu Y, et al. Anomanolide C suppresses tumor progression and metastasis by ubiquitinating GPX4-driven autophagy-dependent ferroptosis in triple negative breast cancer. Int J Biol Sci. 2023;19(8):2531–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Tang D, Chen X, Kang R, et al. Ferroptosis: molecular mechanisms and health implications. Cell Res. 2021;31(2):107–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Zhou B, Liu J, Kang R, et al. Ferroptosis is a type of autophagy-dependent cell death. Semin Cancer Biol. 2020;66:89–100. [DOI] [PubMed] [Google Scholar]
- 240.Jiang R, He S, Gong H, et al. Identification of ATG7 as a Regulator of Proferroptosis and Oxidative Stress in Osteosarcoma. Oxid Med Cell Longev. 2022;2022:8441676. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 241.Wu J, Ye J, Xie Q, et al. Targeting regulated cell death with pharmacological small molecules: an update on autophagy-dependent cell death, ferroptosis, and necroptosis in cancer. J Med Chem. 2022;65(4):2989–3001. [DOI] [PubMed] [Google Scholar]
- 242.Zhou Y, Liao J, Mei Z, et al. Insight into crosstalk between ferroptosis and necroptosis: novel therapeutics in ischemic stroke. Oxid Med Cell Longev. 2021;2021:9991001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Sun Y, Lian T, Huang Q, et al. Nanomedicine-mediated regulated cell death in cancer immunotherapy. J Control Release. 2023;364:174–94. [DOI] [PubMed] [Google Scholar]
- 244.Emens LA. Breast cancer immunotherapy: facts and hopes. Clin Cancer Res. 2018;24(3):511–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Dermani FK, Samadi P, Rahmani G, et al. PD-1/PD-L1 immune checkpoint: Potential target for cancer therapy. J Cell Physiol. 2019;234(2):1313–25. [DOI] [PubMed] [Google Scholar]
- 246.Bagchi S, Yuan R, Engleman EG. Immune checkpoint inhibitors for the treatment of cancer: clinical impact and mechanisms of response and resistance. Annu Rev Pathol. 2021;16:223–49. [DOI] [PubMed] [Google Scholar]
- 247.Chow A, Perica K, Klebanoff CA, et al. Clinical implications of T cell exhaustion for cancer immunotherapy. Nat Rev Clin Oncol. 2022;19(12):775–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Han T, Sun Y, Jiang X, et al. Air bag-embedded MIL-101(Fe) metal-organic frameworks for an amplified tumor microenvironment activation loop through strategic delivery of iron ions and lentinan. Theranostics. 2024;14(15):5883–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Cheu JW, Lee D, Li Q, et al. Ferroptosis suppressor protein 1 inhibition promotes tumor ferroptosis and anti-tumor immune responses in liver cancer. Cell Mol Gastroenterol Hepatol. 2023;16(1):133–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Sanchez-Lopez E, Zhong Z, Stubelius A, et al. Choline uptake and metabolism modulate macrophage IL-1β and IL-18 production. Cell Metab. 2019;29(6):1350-62.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Li C, Yao H, Wang H, et al. Repurposing screen identifies Amlodipine as an inducer of PD-L1 degradation and antitumor immunity. Oncogene. 2021;40(6):1128–46. [DOI] [PubMed] [Google Scholar]
- 252.Tsimberidou AM, Alayli FA, Okrah K, et al. Immunologic signatures of response and resistance to nivolumab with ipilimumab in advanced metastatic cancer. J Exp Med. 2024. 10.1084/jem.20240152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Moamin MR, Allen R, Woods SL, et al. Changes in the immune landscape of TNBC after neoadjuvant chemotherapy: correlation with relapse. Front Immunol. 2023;14: 1291643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Zhao L, Cheng S, Fan L, et al. TIM-3: an update on immunotherapy. Int Immunopharmacol. 2021;99: 107933. [DOI] [PubMed] [Google Scholar]
- 255.Gallazzi M, Ucciero MAM, Faraci DG, et al. New Frontiers in Monoclonal Antibodies for the Targeted Therapy of Acute Myeloid Leukemia and Myelodysplastic Syndromes. Int J Mol Sci. 2022. 10.3390/ijms23147542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Wang X, Chen Z, Tang J, et al. Identification and validation of a necroptosis-related prognostic model in tumor recurrence and tumor immune microenvironment in breast cancer management. J Inflamm Res. 2024;17:5057–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Cai H, Lv M, Wang T. PANoptosis in cancer, the triangle of cell death. Cancer Med. 2023;12(24):22206–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Dadgar N, Arunachalam AK, Hong H, et al. Advancing cholangiocarcinoma care: insights and innovations in T cell therapy. Cancers (Basel). 2024. 10.3390/cancers16183232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Xie Y, Hu Y, Zhou N, et al. CAR T-cell therapy for triple-negative breast cancer: where we are. Cancer Lett. 2020;491:121–31. [DOI] [PubMed] [Google Scholar]
- 260.Corti C, Venetis K, Sajjadi E, et al. CAR-T cell therapy for triple-negative breast cancer and other solid tumors: preclinical and clinical progress. Expert Opin Investig Drugs. 2022;31(6):593–605. [DOI] [PubMed] [Google Scholar]
- 261.Zhou R, Yazdanifar M, Roy LD, et al. CAR t cells targeting the tumor MUC1 glycoprotein reduce triple-negative breast cancer growth. Front Immunol. 2019;10: 1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Xia L, Zheng ZZ, Liu JY, et al. EGFR-targeted CAR-T cells are potent and specific in suppressing triple-negative breast cancer both in vitro and in vivo. Clin Transl Immunol. 2020;9(5):e01135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Fabian KP, Padget MR, Donahue RN, et al. PD-L1 targeting high-affinity NK (t-haNK) cells induce direct antitumor effects and target suppressive MDSC populations. J Immunother Cancer. 2020. 10.1136/jitc-2019-000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Prager I, Liesche C, VAN Ooijen H, et al. NK cells switch from granzyme B to death receptor-mediated cytotoxicity during serial killing. J Exp Med. 2019;216(9):2113–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Liu Y, Fang Y, Chen X, et al. Gasdermin E-mediated target cell pyroptosis by CAR T cells triggers cytokine release syndrome. Sci Immunol. 2020. 10.1126/sciimmunol.aax7969. [DOI] [PubMed] [Google Scholar]
- 266.Shafer P, Kelly LM, Hoyos V. Cancer therapy with TCR-engineered T cells: current strategies, challenges, and prospects. Front Immunol. 2022;13: 835762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Kortleve D, Hammerl D, van Brakel M, et al. TCR-engineered T cells directed against Ropporin-1 constitute a safe and effective treatment for triple-negative breast cancer. Cancer Discov. 2024;14(12):2450–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Baulu E, Gardet C, Chuvin N, et al. TCR-engineered T cell therapy in solid tumors: state of the art and perspectives. Sci Adv. 2023;9(7): eadf3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Liu S, Galat V, Galat Y, et al. NK cell-based cancer immunotherapy: from basic biology to clinical development. J Hematol Oncol. 2021;14(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Shimasaki N, Jain A, Campana D. NK cells for cancer immunotherapy. Nat Rev Drug Discov. 2020;19(3):200–18. [DOI] [PubMed] [Google Scholar]
- 271.Sanmarco LM, Wheeler MA, Gutiérrez-Vázquez C, et al. Gut-licensed IFNγ(+) NK cells drive LAMP1(+)TRAIL(+) anti-inflammatory astrocytes. Nature. 2021;590(7846):473–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Vlachava VM, Seirafian S, Fielding CA, et al. HCMV-secreted glycoprotein gpUL4 inhibits TRAIL-mediated apoptosis and NK cell activation. Proc Natl Acad Sci U S A. 2023;120(49): e2309077120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Marin ND, Becker-Hapak M, Song WM, et al. Memory-like differentiation enhances NK cell responses against colorectal cancer. Oncoimmunology. 2024;13(1):2348254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Sajish M, Zhou Q, Kishi S, et al. Trp-tRNA synthetase bridges DNA-PKcs to PARP-1 to link IFN-γ and p53 signaling. Nat Chem Biol. 2012;8(6):547–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Yang SL, Tan HX, Niu TT, et al. The IFN-γ-IDO1-kynureine pathway-induced autophagy in cervical cancer cell promotes phagocytosis of macrophage. Int J Biol Sci. 2021;17(1):339–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Lion E, Smits EL, Berneman ZN, et al. NK cells: key to success of DC-based cancer vaccines? Oncologist. 2012;17(10):1256–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Tang R, Xu J, Zhang B, et al. Ferroptosis, necroptosis, and pyroptosis in anticancer immunity. J Hematol Oncol. 2020;13(1):110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Domínguez-Álvarez E, Rácz B, Marć MA, et al. Selenium and tellurium in the development of novel small molecules and nanoparticles as cancer multidrug resistance reversal agents. Drug Resist Updat. 2022;63:100844. [DOI] [PubMed] [Google Scholar]
- 279.Balahura Stămat LR, Dinescu S. Inhibition of NLRP3 inflammasome contributes to paclitaxel efficacy in triple negative breast cancer treatment. Sci Rep. 2024;14(1):24753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Wang FY, Yang LM, Wang SS, et al. Cycloplatinated (II) complex based on isoquinoline alkaloid elicits ferritinophagy-dependent ferroptosis in triple-negative breast cancer cells. J Med Chem. 2024;67(8):6738–48. [DOI] [PubMed] [Google Scholar]
- 281.Liang J, Tian X, Zhou M, et al. Shikonin and chitosan-silver nanoparticles synergize against triple-negative breast cancer through RIPK3-triggered necroptotic immunogenic cell death. Biomaterials. 2024;309: 122608. [DOI] [PubMed] [Google Scholar]
- 282.Park HH, Kim HR, Park SY, et al. RIPK3 activation induces TRIM28 derepression in cancer cells and enhances the anti-tumor microenvironment. Mol Cancer. 2021;20(1):107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Lee SY, Kim H, Li CM, et al. Casein kinase-1γ1 and 3 stimulate tumor necrosis factor-induced necroptosis through RIPK3. Cell Death Dis. 2019;10(12):923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Xin C, Guangliang S, Qing Z, et al. Astilbin protects chicken peripheral blood lymphocytes from cadmium-induced necroptosis via oxidative stress and the PI3K/Akt pathway. Ecotoxicol Environ Saf. 2020;190: 110064. [DOI] [PubMed] [Google Scholar]
- 285.Yang Y, Wu M, Cao D, et al. ZBP1-MLKL necroptotic signaling potentiates radiation-induced antitumor immunity via intratumoral STING pathway activation. Sci Adv. 2021;7(41): eabf6290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Chen H, Liu Gao MY, Zhang L, et al. MicroRNA-155 affects oxidative damage through regulating autophagy in endothelial cells. Oncol Lett. 2019;17(2):2237–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Li J, Kim SG, Blenis J. Rapamycin: one drug, many effects. Cell Metab. 2014;19(3):373–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Mauthe M, Orhon I, Rocchi C, et al. Chloroquine inhibits autophagic flux by decreasing autophagosome-lysosome fusion. Autophagy. 2018;14(8):1435–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Fraga M, Moradpour D, Artru F, et al. Hepatocellular type II fibrinogen inclusions in a patient with severe COVID-19 and hepatitis. J Hepatol. 2020;73(4):967–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Wang L, Liu Y, Du T, et al. ATF3 promotes erastin-induced ferroptosis by suppressing system Xc(). Cell Death Differ. 2020;27(2):662–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Yang R, Gao W, Wang Z, et al. Polyphyllin I induced ferroptosis to suppress the progression of hepatocellular carcinoma through activation of the mitochondrial dysfunction via Nrf2/HO-1/GPX4 axis. Phytomedicine. 2024;122: 155135. [DOI] [PubMed] [Google Scholar]
- 292.Chen H, Qi Q, Wu N, et al. Aspirin promotes RSL3-induced ferroptosis by suppressing mTOR/SREBP-1/SCD1-mediated lipogenesis in PIK3CA-mutant colorectal cancer. Redox Biol. 2022;55: 102426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Sui X, Zhang R, Liu S, et al. RSL3 drives ferroptosis through GPX4 inactivation and ROS production in colorectal cancer. Front Pharmacol. 2018;9:1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Chow YP, Tan LP, Chai SJ, et al. Exome sequencing identifies potentially druggable mutations in nasopharyngeal carcinoma. Sci Rep. 2017;7:42980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Dasari S, Tchounwou PB. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol. 2014;740:364–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Guo J, Xu B, Han Q, et al. Ferroptosis: a novel anti-tumor action for cisplatin. Cancer Res Treat. 2018;50(2):445–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Su Y, Zhao B, Zhou L, et al. Ferroptosis, a novel pharmacological mechanism of anti-cancer drugs. Cancer Lett. 2020;483:127–36. [DOI] [PubMed] [Google Scholar]
- 298.Kundu M, Greer YE, Dine JL, et al. Targeting TRAIL death receptors in triple-negative breast cancers: challenges and strategies for cancer therapy. Cells. 2022. 10.3390/cells11233717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Laudisi F, Pacifico T, Maresca C, et al. Rafoxanide sensitizes colorectal cancer cells to TRAIL-mediated apoptosis. Biomed Pharmacother. 2022;155: 113794. [DOI] [PubMed] [Google Scholar]
- 300.Helmy SA, El-Mesery M, El-Karef A, et al. Chloroquine upregulates TRAIL/TRAILR2 expression and potentiates doxorubicin anti-tumor activity in thioacetamide-induced hepatocellular carcinoma model. Chem Biol Interact. 2018;279:84–94. [DOI] [PubMed] [Google Scholar]
- 301.Pasquier B. Autophagy inhibitors. Cell Mol Life Sci. 2016;73(5):985–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Kang YK, Yook JH, Park YK, et al. PRODIGY: a phase III study of neoadjuvant docetaxel, oxaliplatin, and S-1 plus surgery and adjuvant S-1 versus surgery and adjuvant S-1 for resectable advanced gastric cancer. J Clin Oncol. 2021;39(26):2903–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Conroy T, Castan F, Lopez A, et al. Five-year outcomes of FOLFIRINOX vs gemcitabine as adjuvant therapy for pancreatic cancer: a randomized clinical trial. JAMA Oncol. 2022;8(11):1571–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Yang C, Zhang Y, Lin S, et al. Suppressing the KIF20A/NUAK1/Nrf2/GPX4 signaling pathway induces ferroptosis and enhances the sensitivity of colorectal cancer to oxaliplatin. Aging (Albany NY). 2021;13(10):13515–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Ge LP, Jin X, Ma D, et al. ZNF689 deficiency promotes intratumor heterogeneity and immunotherapy resistance in triple-negative breast cancer. Cell Res. 2024;34(1):58–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Deepak KGK, Vempati R, Nagaraju GP, et al. Tumor microenvironment: challenges and opportunities in targeting metastasis of triple negative breast cancer. Pharmacol Res. 2020;153: 104683. [DOI] [PubMed] [Google Scholar]
- 307.So JY, Ohm J, Lipkowitz S, et al. Triple negative breast cancer (TNBC): non-genetic tumor heterogeneity and immune microenvironment: Emerging treatment options. Pharmacol Ther. 2022;237: 108253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Abdolahi S, Ghazvinian Z, Muhammadnejad S, et al. Patient-derived xenograft (PDX) models, applications and challenges in cancer research. J Transl Med. 2022;20(1):206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Takahashi T. Organoids for drug discovery and personalized medicine. Annu Rev Pharmacol Toxicol. 2019;59:447–62. [DOI] [PubMed] [Google Scholar]
- 310.Zhang Z, Huang W, Wang L, et al. Ailanthone induces triple-negative breast cancer cells death involving the inhibition of OTUB1-mediated ERRα deubiquitylation. J Adv Res. 2025. 10.1016/j.jare.2025.01.035. [DOI] [PubMed] [Google Scholar]
- 311.Lin Z, Liu Z, Yang X, et al. Simeprevir induces ferroptosis through β-TrCP/Nrf2/GPX4 axis in triple-negative breast cancer cells. Biomed Pharmacother. 2024;180: 117558. [DOI] [PubMed] [Google Scholar]
- 312.Devericks EN, Brosnan BH, Ho AN, et al. Glutathione peroxidase 4 (GPX4) and obesity interact to impact tumor progression and treatment response in triple negative breast cancer. Cancer Metab. 2025;13(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Rossi G, Manfrin A, Lutolf MP. Progress and potential in organoid research. Nat Rev Genet. 2018;19(11):671–87. [DOI] [PubMed] [Google Scholar]
- 314.Wang X, Chen T, Li C, et al. CircRNA-CREIT inhibits stress granule assembly and overcomes doxorubicin resistance in TNBC by destabilizing PKR. J Hematol Oncol. 2022;15(1):122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Liu J, Zhu S, Zeng L, et al. DCN released from ferroptotic cells ignites AGER-dependent immune responses. Autophagy. 2022;18(9):2036–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Hockney S, Parker J, Turner JE, et al. Next generation organoid engineering to replace animals in cancer drug testing. Biochem Pharmacol. 2023;213: 115586. [DOI] [PubMed] [Google Scholar]
- 317.Wang Y, Zhao M, He S, et al. Necroptosis regulates tumor repopulation after radiotherapy via RIP1/RIP3/MLKL/JNK/IL8 pathway. J Exp Clin Cancer Res. 2019;38(1):461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Mehraj U, Mir IA, Hussain MU, et al. Adapalene and doxorubicin synergistically promote apoptosis of TNBC cells by hyperactivation of the ERK1/2 pathway through ROS induction. Front Oncol. 2022;12: 938052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Fang W, Wang J, Ma X, et al. A progressively disassembled DNA repair inhibitors nanosystem for the treatment of BRCA wild-type triple-negative breast cancer. Int J Nanomed. 2023;18:6001–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Wei Y, Zhu Z, Hu H, et al. Eupaformosanin induces apoptosis and ferroptosis through ubiquitination of mutant p53 in triple-negative breast cancer. Eur J Pharmacol. 2022;924: 174970. [DOI] [PubMed] [Google Scholar]
- 321.Hu M, Li W, Zhang Y, et al. Venetoclax in adult acute myeloid leukemia. Biomed Pharmacother. 2023;168: 115820. [DOI] [PubMed] [Google Scholar]
- 322.Kwak AW, Kim WK, Lee SO, et al. Licochalcone B induces ROS-dependent apoptosis in oxaliplatin-resistant colorectal cancer cells via p38/JNK MAPK signaling. Antioxidants (Basel). 2023. 10.3390/antiox12030656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Kwak AW, Park JW, Lee SO, et al. Isolinderalactone sensitizes oxaliplatin-resistance colorectal cancer cells through JNK/p38 MAPK signaling pathways. Phytomedicine. 2022;105: 154383. [DOI] [PubMed] [Google Scholar]
- 324.Jiang X, Li G, Zhu B, et al. p20BAP31 induces cell apoptosis via both AIF caspase-independent and the ROS/JNK mitochondrial pathway in colorectal cancer. Cell Mol Biol Lett. 2023;28(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Jafari M, Sriram V, Premnauth G, et al. Modified peroxamide-based reactive oxygen species (ROS)-responsive doxorubicin prodrugs. Bioorg Chem. 2022;127: 105990. [DOI] [PubMed] [Google Scholar]
- 326.Kuno A, Hosoda R, Tsukamoto M, et al. SIRT1 in the cardiomyocyte counteracts doxorubicin-induced cardiotoxicity via regulating histone H2AX. Cardiovasc Res. 2023;118(17):3360–73. [DOI] [PubMed] [Google Scholar]
- 327.Zhuang Y, Che J, Wu M, et al. Altered pathways and targeted therapy in double hit lymphoma. J Hematol Oncol. 2022;15(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Yang C, Ran Q, Zhou Y, et al. Doxorubicin sensitizes cancer cells to Smac mimetic via synergistic activation of the CYLD/RIPK1/FADD/caspase-8-dependent apoptosis. Apoptosis. 2020;25(5–6):441–55. [DOI] [PubMed] [Google Scholar]
- 329.Kieckhöfer E, Slaats GG, Ebert LK, et al. Primary cilia suppress Ripk3-mediated necroptosis. Cell Death Discov. 2022;8(1):477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Weigert M, Binks A, Dowson S, et al. RIPK3 promotes adenovirus type 5 activity. Cell Death Dis. 2017;8(12):3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Yang L, Kumar B, Shen C, et al. LCL161, a SMAC-mimetic, preferentially radiosensitizes human papillomavirus-negative head and neck squamous cell carcinoma. Mol Cancer Ther. 2019;18(6):1025–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Feoktistova M, Makarov R, Yazdi AS, et al. RIPK1 and TRADD regulate TNF-induced signaling and ripoptosome formation. Int J Mol Sci. 2021. 10.3390/ijms222212459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Ouyang L, Zhang L, Fu L, et al. A small-molecule activator induces ULK1-modulating autophagy-associated cell death in triple negative breast cancer. Autophagy. 2017;13(4):777–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Abd El-Aziz YS, Toit-Thompson TD, McKay MJ, et al. Novel combinatorial autophagy inhibition therapy for triple negative breast cancers. Eur J Pharmacol. 2024;973:176568. [DOI] [PubMed] [Google Scholar]
- 335.Wang SY, Zhao JM, Zhou CL, et al. Herbal cake-partitioned moxibustion inhibits colonic autophagy in Crohn’s disease via signaling involving distinct classes of phosphatidylinositol 3-kinases. World J Gastroenterol. 2020;26(39):5997–6014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115(5):577–90. [DOI] [PubMed] [Google Scholar]
- 337.Zheng X, Li W, Xu H, et al. Sinomenine ester derivative inhibits glioblastoma by inducing mitochondria-dependent apoptosis and autophagy by PI3K/AKT/mTOR and AMPK/mTOR pathway. Acta Pharm Sin B. 2021;11(11):3465–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Li B, Zhang X, Ren Q, et al. NVP-BEZ235 inhibits renal cell carcinoma by targeting TAK1 and PI3K/Akt/mTOR pathways. Front Pharmacol. 2021;12: 781623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Li X, Yang KB, Chen W, et al. CUL3 (cullin 3)-mediated ubiquitination and degradation of BECN1 (beclin 1) inhibit autophagy and promote tumor progression. Autophagy. 2021;17(12):4323–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Wang J, Yang L, You J, et al. Platelet-derived growth factor regulates the biological behavior of oral mucosal fibroblasts by inducing cell autophagy and its mechanism. J Inflamm Res. 2021;14:3405–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Wang X, Zhao L, Wang C, et al. Potent nanoreactor-mediated ferroptosis-based strategy for the reversal of cancer chemoresistance to Sorafenib. Acta Biomater. 2023;159:237–46. [DOI] [PubMed] [Google Scholar]
- 342.Shen Y, Li X, Dong D, et al. Transferrin receptor 1 in cancer: a new sight for cancer therapy. Am J Cancer Res. 2018;8(6):916–31. [PMC free article] [PubMed] [Google Scholar]
- 343.Joharapurkar AA, Pandya VB, Patel VJ, et al. Prolyl hydroxylase inhibitors: a breakthrough in the therapy of Anemia Associated with Chronic Diseases. J Med Chem. 2018;61(16):6964–82. [DOI] [PubMed] [Google Scholar]
- 344.Sanguigno L, Guida N, Anzilotti S, et al. Stroke by inducing HDAC9-dependent deacetylation of HIF-1 and Sp1, promotes TfR1 transcription and GPX4 reduction, thus determining ferroptotic neuronal death. Int J Biol Sci. 2023;19(9):2695–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Sha W, Hu F, Xi Y, et al. Mechanism of ferroptosis and its role in type 2 diabetes mellitus. J Diabetes Res. 2021;2021:9999612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Bekric D, Ocker M, Mayr C, et al. Ferroptosis in hepatocellular carcinoma: mechanisms, drug targets and approaches to clinical translation. Cancers (Basel). 2022. 10.3390/cancers14071826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Dos Reis Oliveira C, Pereira JC, Barros Ibiapina A, et al. Buthionine sulfoximine and chemoresistance in cancer treatments: a systematic review with meta-analysis of preclinical studies. J Toxicol Environ Health B Crit Rev. 2023;26(8):417–41. [DOI] [PubMed] [Google Scholar]
- 348.Haddad JJ. L-Buthionine-(S,R)-sulfoximine, an irreversible inhibitor of gamma-glutamylcysteine synthetase, augments LPS-mediated pro-inflammatory cytokine biosynthesis: evidence for the implication of an IkappaB-alpha/NF-kappaB insensitive pathway. Eur Cytokine Netw. 2001;12(4):614–24. [PubMed] [Google Scholar]
- 349.Xiao Y, Ma D, Yang YS, et al. Comprehensive metabolomics expands precision medicine for triple-negative breast cancer. Cell Res. 2022;32(5):477–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Maqbool M, Bekele F, Fekadu G. Treatment strategies against triple-negative breast cancer: an updated review. Breast Cancer (Dove Med Press). 2022;14:15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Shi HX, Tao HT, He JJ, et al. Targeting DKK1 enhances the antitumor activity of paclitaxel and alleviates chemotherapy-induced peripheral neuropathy in breast cancer. Mol Cancer. 2024;23(1):152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Yi YW. Therapeutic implications of the drug resistance conferred by extracellular vesicles derived from triple-negative breast cancer cells. Int J Mol Sci. 2023. 10.3390/ijms24043704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Chung AH, Leisner TM, Dardis GJ, et al. CIB1 depletion with docetaxel or TRAIL enhances triple-negative breast cancer cell death. Cancer Cell Int. 2019;19:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Zhu S, Wu Y, Song B, et al. Recent advances in targeted strategies for triple-negative breast cancer. J Hematol Oncol. 2023;16(1):100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Martín M, Stecklein SR, Gluz O, et al. TNBC-DX genomic test in early-stage triple-negative breast cancer treated with neoadjuvant taxane-based therapy. Ann Oncol. 2024. 10.1016/j.annonc.2024.10.012. [DOI] [PubMed] [Google Scholar]
- 356.Zhang T, Li J, Ma X, et al. Inhibition of HDACs-EphA2 signaling axis with WW437 demonstrates promising preclinical antitumor activity in breast cancer. EBioMedicine. 2018;31:276–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Cencic R, Im YK, Naineni SK, et al. A second-generation eIF4A RNA helicase inhibitor exploits translational reprogramming as a vulnerability in triple-negative breast cancer. Proc Natl Acad Sci U S A. 2024;121(4): e2318093121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Dahn ML, Cruickshank BM, Jackson AJ, et al. Decitabine response in breast cancer requires efficient drug processing and is not limited by multidrug resistance. Mol Cancer Ther. 2020;19(5):1110–22. [DOI] [PubMed] [Google Scholar]
- 359.Wang C, Chen X, Liu X, et al. Discovery of precision targeting EZH2 degraders for triple-negative breast cancer. Eur J Med Chem. 2022;238: 114462. [DOI] [PubMed] [Google Scholar]
- 360.Jiang Y, Huang Y, Cheng C, et al. Combination of thiazolidinedione and hydralazine suppresses proliferation and induces apoptosis by PPARγ up-expression in MDA-MB-231 cells. Exp Mol Pathol. 2011;91(3):768–74. [DOI] [PubMed] [Google Scholar]
- 361.Guo Y, Li Y, Zhou Z, et al. Targeting PRMT5 through PROTAC for the treatment of triple-negative breast cancer. J Exp Clin Cancer Res. 2024;43(1):314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Bai L, Liu H, You R, et al. Combination nano-delivery systems remodel the immunosuppressive tumor microenvironment for metastatic triple-negative breast cancer therapy. Mol Pharm. 2024;21(5):2148–62. [DOI] [PubMed] [Google Scholar]
- 363.Mekala JR, Naushad SM, Ponnusamy L, et al. Epigenetic regulation of miR-200 as the potential strategy for the therapy against triple-negative breast cancer. Gene. 2018;641:248–58. [DOI] [PubMed] [Google Scholar]
- 364.Rao Z, Zhu Y, Chen Z, et al. Injectable autocatalytic hydrogel triggers pyroptosis to stimulate anticancer immune response for preventing postoperative tumor recurrence. Adv Sci (Weinh). 2024. 10.1002/advs.202408415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Yang X, Cui X, Wang G, et al. HDAC inhibitor regulates the tumor immune microenvironment via pyroptosis in triple negative breast cancer. Mol Carcinog. 2024;63(9):1800–13. [DOI] [PubMed] [Google Scholar]
- 366.Li Y, Li X. miR-1290 modulates the radioresistance of triple-negative breast cancer by targeting NLRP3-mediated pyroptosis. Clin Transl Oncol. 2022;24(9):1764–75. [DOI] [PubMed] [Google Scholar]
- 367.Xu W, Song C, Wang X, et al. Downregulation of miR-155-5p enhances the anti-tumor effect of cetuximab on triple-negative breast cancer cells via inducing cell apoptosis and pyroptosis. Aging (Albany NY). 2021;13(1):228–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Zhang J, Chen X, Chen G, et al. Identification of a novel PAK1/HDAC6 dual inhibitor ZMF-23 that triggers tubulin-stathmin regulated cell death in triple negative breast cancer. Int J Biol Macromol. 2023;251: 126348. [DOI] [PubMed] [Google Scholar]
- 369.Wong KK. DNMT1: a key drug target in triple-negative breast cancer. Semin Cancer Biol. 2021;72:198–213. [DOI] [PubMed] [Google Scholar]
- 370.Rao R, Balusu R, Fiskus W, et al. Combination of pan-histone deacetylase inhibitor and autophagy inhibitor exerts superior efficacy against triple-negative human breast cancer cells. Mol Cancer Ther. 2012;11(4):973–83. [DOI] [PubMed] [Google Scholar]
- 371.Min A, Im SA, Kim DK, et al. Histone deacetylase inhibitor, suberoylanilide hydroxamic acid (SAHA), enhances anti-tumor effects of the poly (ADP-ribose) polymerase (PARP) inhibitor olaparib in triple-negative breast cancer cells. Breast Cancer Res. 2015;17:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Du WW, Yang W, Li X, et al. A circular RNA circ-DNMT1 enhances breast cancer progression by activating autophagy. Oncogene. 2018;37(44):5829–42. [DOI] [PubMed] [Google Scholar]
- 373.Hasegawa M, Takahashi H, Rajabi H, et al. Functional interactions of the cystine/glutamate antiporter, CD44v and MUC1-C oncoprotein in triple-negative breast cancer cells. Oncotarget. 2016;7(11):11756–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Fan X, Liu F, Wang X, et al. LncFASA promotes cancer ferroptosis via modulating PRDX1 phase separation. Sci China Life Sci. 2024;67(3):488–503. [DOI] [PubMed] [Google Scholar]
- 375.Li J, Li PT, Wu W, et al. POU2F2-mediated upregulation of lncRNA PTPRG-AS1 inhibits ferroptosis in breast cancer via miR-376c-3p/SLC7A11 axis. Epigenomics. 2024;16(4):215–31. [DOI] [PubMed] [Google Scholar]
- 376.Stark MC, Joubert AM, Visagie MH. Molecular farming of pembrolizumab and nivolumab. Int J Mol Sci. 2023. 10.3390/ijms241210045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Morihara H, Yamada T, Tona Y, et al. Anti-CTLA-4 treatment suppresses hepatocellular carcinoma growth through Th1-mediated cell cycle arrest and apoptosis. PLoS ONE. 2024;19(8): e0305984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Fang C, Weng T, Hu S, et al. IFN-γ-induced ER stress impairs autophagy and triggers apoptosis in lung cancer cells. Oncoimmunology. 2021;10(1):1962591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Li J, Lan M, Peng J, et al. Cdh1 deficiency sensitizes TNBC cells to PARP inhibitors. Genes (Basel). 2022. 10.3390/genes13050803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Siraj AK, Poyil PK, Padmaja D, et al. PLK1 and PARP positively correlate in Middle Eastern breast cancer and their combined inhibition overcomes PARP inhibitor resistance in triple negative breast cancer. Front Oncol. 2023;13:1286585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Naudé PJ, Den Boer JA, Luiten PG, et al. Tumor necrosis factor receptor cross-talk. Febs J. 2011;278(6):888–98. [DOI] [PubMed] [Google Scholar]
- 382.Vannini F, Kashfi K, Nath N. The dual role of iNOS in cancer. Redox Biol. 2015;6:334–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Yan H, Luo B, Wu X, et al. Cisplatin induces pyroptosis via activation of MEG3/NLRP3/caspase-1/GSDMD pathway in triple-negative breast cancer. Int J Biol Sci. 2021;17(10):2606–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Zhao W, Zhang L, Zhang Y, et al. The CDK inhibitor AT7519 inhibits human glioblastoma cell growth by inducing apoptosis, pyroptosis and cell cycle arrest. Cell Death Dis. 2023;14(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Wang H, Rong X, Zhao G, et al. The microbial metabolite trimethylamine N-oxide promotes antitumor immunity in triple-negative breast cancer. Cell Metab. 2022;34(4):581-94.e8. [DOI] [PubMed] [Google Scholar]
- 386.Zhong H, Chen G, Li T, et al. Nanodrug augmenting antitumor immunity for enhanced TNBC therapy via pyroptosis and cGAS-STING activation. Nano Lett. 2023;23(11):5083–91. [DOI] [PubMed] [Google Scholar]
- 387.Liao P, Wang W, Wang W, et al. CD8(+) T cells and fatty acids orchestrate tumor ferroptosis and immunity via ACSL4. Cancer Cell. 2022;40(4):365-78.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Feng Y, Daley-Bauer LP, Mocarski ES. Caspase-8-dependent control of NK- and T cell responses during cytomegalovirus infection. Med Microbiol Immunol. 2019;208(3–4):555–71. [DOI] [PubMed] [Google Scholar]
- 389.Wang S, Chang CW, Huang J, et al. Gasdermin C sensitizes tumor cells to PARP inhibitor therapy in cancer models. J Clin Invest. 2024. 10.1172/JCI166841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Wu J, Zhao X, Sun Q, et al. Synergic effect of PD-1 blockade and endostar on the PI3K/AKT/mTOR-mediated autophagy and angiogenesis in Lewis lung carcinoma mouse model. Biomed Pharmacother. 2020;125: 109746. [DOI] [PubMed] [Google Scholar]
- 391.Li J, Cai W, Yu J, et al. Autophagy inhibition recovers deficient ICD-based cancer immunotherapy. Biomaterials. 2022;287: 121651. [DOI] [PubMed] [Google Scholar]
- 392.Li ZL, Zhang HL, Huang Y, et al. Autophagy deficiency promotes triple-negative breast cancer resistance to T cell-mediated cytotoxicity by blocking tenascin-C degradation. Nat Commun. 2020;11(1):3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Tan H, Liu J, Huang J, et al. Ketoglutaric acid can reprogram the immunophenotype of triple-negative breast cancer after radiotherapy and improve the therapeutic effect of anti-PD-L1. J Transl Med. 2023;21(1):462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Yu X, Li X, Chen Q, et al. High intensity focused ultrasound-driven nanomotor for effective ferroptosis-immunotherapy of TNBC. Adv Sci (Weinh). 2024;11(15): e2305546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Valcourt DM, Dang MN, Scully MA, et al. Nanoparticle-mediated Co-delivery of notch-1 antibodies and ABT-737 as a potent treatment strategy for triple-negative breast cancer. ACS Nano. 2020;14(3):3378–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Zhang W, Zhai Y, Cai Y, et al. Enhancing immunotherapy efficacy against MHC-I deficient triple-negative breast cancer using LCL161-loaded macrophage membrane-decorated nanoparticles. Acta Pharm Sin B. 2024;14(7):3218–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Michaeli O, Luz I, Vatarescu M, et al. APR-246 as a radiosensitization strategy for mutant p53 cancers treated with alpha-particles-based radiotherapy. Cell Death Dis. 2024;15(6):426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Yu J, Zhang L. PUMA, a potent killer with or without p53. Oncogene. 2008;27(1):S71-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Schadendorf D, Fisher DE, Garbe C, et al. Melanoma. Nat Rev Dis Primers. 2015;1:15003. [DOI] [PubMed] [Google Scholar]
- 400.Lei S, Li S, Xiao W, et al. Azurocidin 1 inhibits the aberrant proliferation of triple-negative breast cancer through the regulation of pyroptosis. Oncol Rep. 2023. 10.3892/or.2023.8625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Zheng Q, Yao D, Cai Y, et al. NLRP3 augmented resistance to gemcitabine in triple-negative breast cancer cells via EMT/IL-1β/Wnt/β-catenin signaling pathway. 2020. Biosci Rep. 10.1042/BSR20200730. [DOI] [PMC free article] [PubMed]
- 402.Chang CM, Liang TR, Lam HYP. The use of schisandrin B to combat triple-negative breast cancers by inhibiting NLRP3-induced interleukin-1β production. Biomolecules. 2024. 10.3390/biom14010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Zhang S, Dong Y, Chen X, et al. Toosendanin, a late-stage autophagy inhibitor, sensitizes triple-negative breast cancer to irinotecan chemotherapy. Chin Med. 2022;17(1):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Song H, Zhao Z, Ma L, et al. Novel exosomal circEGFR facilitates triple negative breast cancer autophagy via promoting TFEB nuclear trafficking and modulating miR-224-5p/ATG13/ULK1 feedback loop. Oncogene. 2024;43(11):821–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Zheng B, Qian F, Wang X, et al. Neddylation activated TRIM25 desensitizes triple-negative breast cancer to paclitaxel via TFEB-mediated autophagy. J Exp Clin Cancer Res. 2024;43(1):177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Baik JY, Liu Z, Jiao D, et al. ZBP1 not RIPK1 mediates tumor necroptosis in breast cancer. Nat Commun. 2021;12(1):2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Jouan-Lanhouet S, Arshad MI, Piquet-Pellorce C, et al. TRAIL induces necroptosis involving RIPK1/RIPK3-dependent PARP-1 activation. Cell Death Differ. 2012;19(12):2003–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Chen X, Kang R, Kroemer G, et al. Broadening horizons: the role of ferroptosis in cancer. Nat Rev Clin Oncol. 2021;18(5):280–96. [DOI] [PubMed] [Google Scholar]
- 409.Costa I, Barbosa DJ, Benfeito S, et al. Molecular mechanisms of ferroptosis and their involvement in brain diseases. Pharmacol Ther. 2023;244: 108373. [DOI] [PubMed] [Google Scholar]
- 410.Xu S, Tuo QZ, Meng J, et al. Thrombin induces ferroptosis in triple-negative breast cancer through the cPLA2α/ACSL4 signaling pathway. Transl Oncol. 2024;39: 101817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Zhang X, Chen X, Qian F, et al. Deubiquitinase USP19 modulates apoptotic calcium release and endoplasmic reticulum stress by deubiquitinating BAG6 in triple negative breast cancer. Clin Transl Med. 2023;13(9): e1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Li W, Huang M, Wu Z, et al. mRNA-lipid nanoparticle-mediated restoration of PTPN14 exhibits antitumor effects by overcoming anoikis resistance in triple-negative breast cancer. Adv Sci (Weinh). 2024;11(32): e2309988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Lin J, Qu Z, Pu H, et al. In vitro and in vivo anti-cancer activity of lasiokaurin in a triple-negative breast cancer model. Molecules. 2023. 10.3390/molecules28237701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Castellanet O, Ahmad F, Vinik Y, et al. BCL-XL blockage in TNBC models confers vulnerability to inhibition of specific cell cycle regulators. Theranostics. 2021;11(19):9180–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Zhao L, Tong Y, Yin J, et al. Photo-activated oxidative stress amplifier: a strategy for targeting glutathione metabolism and enhancing ROS-mediated therapy in triple-negative breast cancer treatment. Small. 2024;20(51): e2403861. [DOI] [PubMed] [Google Scholar]
- 416.Cocco S, Leone A, Roca MS, et al. Inhibition of autophagy by chloroquine prevents resistance to PI3K/AKT inhibitors and potentiates their antitumor effect in combination with paclitaxel in triple negative breast cancer models. J Transl Med. 2022;20(1):290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Tian W, Zhu L, Luo Y, et al. Autophagy deficiency induced by SAT1 potentiates tumor progression in triple-negative breast cancer. Adv Sci (Weinh). 2024;11(36): e2309903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Yu S, Cao Z, Cai F, et al. ADT-OH exhibits anti-metastatic activity on triple-negative breast cancer by combinatorial targeting of autophagy and mitochondrial fission. Cell Death Dis. 2024;15(6):463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Huang M, Zhou Y, Duan D, et al. Targeting ubiquitin conjugating enzyme UbcH5b by a triterpenoid PC3-15 from Schisandra plants sensitizes triple-negative breast cancer cells to lapatinib. Cancer Lett. 2021;504:125–36. [DOI] [PubMed] [Google Scholar]
- 420.Jilishitz I, Quiñones JL, Patel P, et al. NP-ALT, a liposomal: peptide drug, blocks p27Kip1 phosphorylation to induce oxidative stress, necroptosis, and regression in therapy-resistant breast cancer cells. Mol Cancer Res. 2021;19(11):1929–45. [DOI] [PubMed] [Google Scholar]
- 421.Vinik Y, Maimon A, Dubey V, et al. Programming a ferroptosis-to-apoptosis transition landscape revealed ferroptosis biomarkers and repressors for cancer therapy. Adv Sci (Weinh). 2024;11(17): e2307263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Li F, Qi Q, Qiao Y, et al. Curcumenol inhibits malignant progression and promotes ferroptosis via the SLC7A11/NF-κB/TGF-β pathway in triple-negative breast cancer. Int J Mol Med. 2025. 10.3892/ijmm.2025.5552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Guo Y, Wang H, Wang X, et al. Enhancing radiotherapy in triple-negative breast cancer with hesperetin-induced ferroptosis via AURKA targeting nanocomposites. J Nanobiotechnology. 2024;22(1):744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Huang L, Han L, Liang S, et al. Molecular mechanism of ZC3H13 -mediated ferroptosis in doxorubicin resistance of triple negative breast cancer. Cell Biol Toxicol. 2025;41(1):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Kang H, Hoang DH, Valerio M, et al. Pharmacological activity of OST-01, a natural product from baccharis coridifolia, on breast cancer cells. J Hematol Oncol. 2025;18(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Jung M, Lee KM, Im Y, et al. Nicotinamide (niacin) supplement increases lipid metabolism and ROS-induced energy disruption in triple-negative breast cancer: potential for drug repositioning as an anti-tumor agent. Mol Oncol. 2022;16(9):1795–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Cetin M, Saatci O, Rezaeian AH, et al. A highly potent bi-thiazole inhibitor of LOX rewires collagen architecture and enhances chemoresponse in triple-negative breast cancer. Cell Chem Biol. 2024;31(11):1926-41.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Mustafa EH, Laven-Law G, Kikhtyak Z, et al. Selective inhibition of CDK9 in triple negative breast cancer. Oncogene. 2024;43(3):202–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Jiang T, Zhu J, Jiang S, et al. Targeting lncRNA DDIT4-AS1 sensitizes triple negative breast cancer to chemotherapy via suppressing of autophagy. Adv Sci (Weinh). 2023;10(17): e2207257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Soleimani M, Somma A, Kaoud T, et al. Covalent JNK inhibitor, JNK-IN-8, suppresses tumor growth in triple-negative breast cancer by activating TFEB- and TFE3-mediated lysosome biogenesis and autophagy. Mol Cancer Ther. 2022;21(10):1547–60. [DOI] [PubMed] [Google Scholar]
- 431.Wang H, Wang H, Wang R, et al. Discovery of a molecular glue for EGFR degradation. Oncogene. 2025;44(8):545–56. [DOI] [PubMed] [Google Scholar]
- 432.Liu LC, Shen YC, Wang YL, et al. Growth-promoting function of the cGAS-STING pathway in triple-negative breast cancer cells. Front Oncol. 2022;12: 851795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Wu X, Jin L, Ren D, et al. α-Hederin causes ferroptosis in triple-negative breast cancer through modulating IRF1 to suppress GPX4. Phytomedicine. 2025;141: 156611. [DOI] [PubMed] [Google Scholar]
- 434.Tan X, Kong D, Tao Z, et al. Simultaneous inhibition of FAK and ROS1 synergistically repressed triple-negative breast cancer by upregulating p53 signalling. Biomark Res. 2024;12(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Yu Y, Hu J, Wang W, et al. Targeting PSMD14 combined with arachidonic acid induces synthetic lethality via FADS1 m(6)A modification in triple-negative breast cancer. Sci Adv. 2025;11(19):eadr3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Davis SL, Ionkina AA, Bagby SM, et al. Preclinical and dose-finding phase I trial results of combined treatment with a TORC1/2 inhibitor (TAK-228) and aurora A kinase inhibitor (Alisertib) in solid tumors. Clin Cancer Res. 2020;26(17):4633–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Sardesai SD, Thomas A, Gallagher C, et al. Inhibiting fatty acid synthase with omeprazole to improve efficacy of neoadjuvant chemotherapy in patients with operable TNBC. Clin Cancer Res. 2021;27(21):5810–7. [DOI] [PubMed] [Google Scholar]
- 438.Forero-Torres A, Varley KE, Abramson VG, et al. TBCRC 019: a phase II trial of nanoparticle albumin-bound paclitaxel with or without the anti-death receptor 5 monoclonal antibody tigatuzumab in patients with triple-negative breast cancer. Clin Cancer Res. 2015;21(12):2722–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Iachettini S, Terrenato I, Porru M, et al. TRF2 as novel marker of tumor response to taxane-based therapy: from mechanistic insight to clinical implication. J Exp Clin Cancer Res. 2024;43(1):75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Zheng Q, Yao D, Cai Y, et al. NLRP3 augmented resistance to gemcitabine in triple-negative breast cancer cells via EMT/IL-1β/Wnt/β-catenin signaling pathway. 2020. Biosci Rep. 10.1042/BSR20200730. [DOI] [PMC free article] [PubMed]
Data Availability Statement
Data sharing does not apply to this article as no new datasets were created or analyzed in this study. All information is derived from publicly published articles.