Abstract
Background
This study aimed to develop interpretable machine learning models using radiomic and dosiomic features from radiotherapy target volumes to predict treatment response in glioma patients.
Methods
A retrospective analysis was conducted on 176 glioma patients. Treatment response was categorized into disease control rate (DCR) and non-DCR groups (training cohort: 71 vs. 44; validation cohort: 34 vs. 27). Five regions of interest (ROIs) were identified: gross tumor volume (GTV), gross tumor volume with tumor bed (GTVtb), clinical target volume (CTV), GTV-GTV and CTV-GTVtb. For each ROI, radiomic features and dosiomic features were separately extracted from CT images and dose maps. Feature selection was performed. Six dosimetric parameters and six clinical variables were also included in model development. Five predictive models were constructed using four machine learning algorithms: Radiomic, Dosiomic, Dose-Volume Histogram (DVH), Combined (integrating clinical, radiomic, dosiomic, and DVH features), and Clinical models. Model performance was evaluated using accuracy, precision, recall, F1-score, and area under the curve (AUC). SHAP analysis was applied to explain model predictions.
Results
The CTV_combined support vector machine (SVM) model achieved the best performance, with an AUC of 0.728 in the validation cohort. SHAP summary plots showed that dosiomic features contributed significantly to prediction. Force plots further illustrated how individual features affected classification outcomes.
Conclusion
The SHAP-interpretable CTV_combined SVM model demonstrated strong predictive ability for treatment response in glioma patients. This approach may support radiation oncologists in identifying the underlying pathological mechanisms of poor treatment response and adjusting dose distribution accordingly, thereby aiding the development of personalized radiotherapy strategies.
Clinical trial number
Not applicable.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12880-025-01955-z.
Keywords: Radiomics, Dosiomics, Computed tomography, Dose map, Glioma
Introduction
Glioma, a tumor originating from glial cells, accounts for approximately 80% of all central nervous system malignant tumors in adults [1]. Radiotherapy remains a standard treatment for glioma. However, nearly 70% of patients experience recurrence, leading to poor clinical outcomes [2–4]. Clinically, increasing the radiation dose to the target area can improve local control and long-term survival rates [5]. However, increasing the total dose to the target area can damage the normal tissue surrounding the tumor, affecting patient prognosis [6]. As a result, glioma radiotherapy poses several clinical challenges. There is an urgent need for effective models to predict treatment response. Such models could help radiation oncologists design individualized treatment plans, ultimately improving therapeutic efficacy and reducing recurrence.
At present, clinical features, such as or gene signature or conventional imaging markers have been used to predictive glioma radiotherapy response [7–10]. Compared with approaches based on biopsy or general clinical data, radiomics offers a noninvasive strategy for assessing treatment efficacy. It has shown promising predictive performance in glioma patients [11, 12].
However, predicting treatment response solely from radiomics cannot fully elucidate the predictive model. The distribution of the radiotherapy dose is crucial for treatment response, so it is necessary to incorporate information from dose maps [13]. Dosimetric features calculated from the dose-volume histogram (DVH) curve have been used to predict the prognosis of glioma, but these factors cannot quantitatively analyze the spatial information of dose distribution [14]. The dosiomics method aims to reveal spatial features from dose maps for radiotherapy response prediction [15], which may help radiation oncologists understand how dose distribution affects the treatment response of radiotherapy.
Dosiomics is widely used in predicting the side effects of radiotherapy [15–17]. Recently, studies have also employed dosiomics to predict the treatment response to radiotherapy, with predictive models showing good performance [18–21]. However, there are some limitations: (1) only a limited number of studies have used dosiomics to predict the treatment response to radiotherapy for gliomas [22]; and (2) most predictive dosiomic models lack interpretability, which hinders their general application.
SHapley Additive exPlanations (SHAP) method originates from game theory, which defines how features interact and contribute to the model’s predictions [23]. The SHAP method has been successfully used to help radiation oncologists understand predictive radiomic models [24].
Therefore, we aimed to construct a dosiomic model to predict the treatment response to radiotherapy for gliomas. We also compared this model with radiomic models, clinical models, DVH models, and models integrating all features. Additionally, we used the SHAP method to interpret the model. We hypothesized that a dosiomic-based model, when combined with the SHAP method, would be both effective and interpretable for assessing the treatment response to radiotherapy.
Materials and methods
Study population
This study was retrospectively conducted at Hefei Cancer Hospital, under the Chinese Academy of Sciences. A total of 176 patients with histopathologically confirmed glioma were included. Detailed patients’ enrollment criteria are available in Supplementary Information 1. Patients diagnosed between January 2018 and December 2022 formed the training cohort (Cohort 1) used for model development, while those diagnosed from January 2023 to May 2024 comprised the validation cohort (Cohort 2) to assess model performance. This study was conducted in accordance with the ethical principles outlined in the Declaration of Helsinki. Ethical approval was obtained from the Ethics Committee of Hefei Cancer Hospital, Chinese Academy of Sciences (Approval No. PJ-KY2024-004). Informed consent was obtained from all individual participants included in the study.
Radiotherapy and radiotherapy target volume definition
Volumetric modulated arc therapy was delivered using 6 MV X-rays produced by Elekta Axesse (4D) medical accelerators. The gross tumor volume (GTV) was defined as the visible glioma tumor. The gross tumor volume tumor bed (GTVtb) was defined as the visible tumor bed. The clinical target volume (CTV) was defined as a 1-cm to 2.5-cm uniform expansion of the GTV or GTVtb. The GTVs, GTVtbs and CTVs received doses ranging from 40 Gy to 70 Gy, administered in 30–33 fractions. Delineation of the radiotherapy target volume was manually performed by a junior radiation oncologist and subsequently reviewed by a senior experienced radiation oncologist following Radiation Therapy Oncology Group guidelines.
Assessment of radiotherapy treatment response
Each patient’s response to radiotherapy was evaluated through a follow-up MRI conducted typically between 12 and 24 weeks post-treatment. The Response Assessment in Neuro-Oncology (RANO) criteria were employed to categorize outcomes as complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD) [25], with detailed criteria provided in Supplementary Information 2.
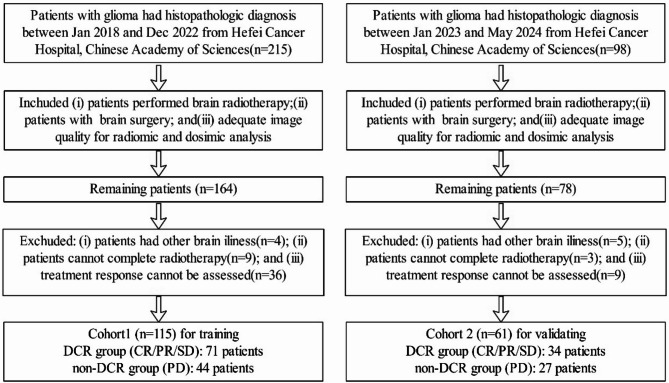
The disease control rate (DCR), which represents the proportion of patients achieving CR, PR, or SD, was used to evaluate treatment response [26]. Consequently, patients classified as CR/PR/SD were grouped under the DCR group, while those with PD were placed in the non-DCR group. Figure 1 illustrates the patient enrollment and grouping process in detail.
Fig. 1.
Patient enrollment and grouping process
Clinical features
Demographic and clinical data, such as gender, age, KPS, tumor type, tumor grade, and chemotherapy history, were gathered from electronic medical records. Initially, a univariate logistic regression analysis was conducted to identify significant clinical factors (p < 0.1). Subsequently, a multivariable logistic regression analysis using forward selection (likelihood ratio) was performed to select clinical factors with p < 0.05. Given the clinical significance of these factors, they were all incorporated into the clinical model, irrespective of their statistical relevance in the training and validation cohorts [27–29].
CT and dose map data acquisition, image processing
The study collected the planning CT scans and associated dose maps for analysis. All images were retrieved from MONACA. The detailed data acquisition and image processing were described in Supplementary Information 3.
Extraction of dosimetric features
Six dosimetric features were derived from the dose-volume histograms (DVH) of GTVs, GTVtbs, CTVs, CTV-GTVs and CTV-GTVtbs: mean dose (Gy), maximum dose (Gy), minimum dose (Gy), D2% (Gy), D50% (Gy), and D98% (Gy), where Dx% denotes the dose received by at least x% of the target volume. Initially, a univariate logistic regression analysis was conducted to identify significant clinical factors (p < 0.1). Subsequently, a multivariable logistic regression analysis using forward selection (likelihood ratio) was performed to select clinical factors with p < 0.05. Owing to their clinical significance, these dosimetric features were incorporated into the DVH model construction, irrespective of their statistical significance within the training and validation cohorts.
The definition of region of interests
Considering GTV, GTVtb, and CTV as radiotherapy target volumes, imaging features were separately extracted from CT images and dose maps using these three radiotherapy target volumes as regions of interest (ROIs). In addition, feature extraction and modeling based on the CTV–GTV and CTV–GTVtb regions can better reflect the pathological characteristics of the peritumoral microenvironment; therefore, these two regions were also defined as ROIs for subsequent analysis.
Extraction of radiomic and dosiomic features
Radiomic and dosiomic features (shown in Supplementary Table 1) categorized into three classes according to their mathematical expression, including : (i) 18 first-order features (describe the distribution of the intensity within the tumor); (ii) 14 shape-based features (describing the shape, including voxel volume, surface area); and (iii) 75 textural features (describe the statistical relationships between voxels and their surroundings as a function of both distance and intensity), including gray-level co-occurrence matrix (GLCM, 24 features), gray-level run length matrix (GLRLM, 16 features), gray-level size zone matrix (GLSZM, 16 features), gray-level dependence matrix (GLDM, 14), and neighbouring gray tone difference matrix (NGTDM,5 features).The detailed features were described in Supplementary Information 4. For each ROI, 107 radiomic features and 107 dosiomic features were extracted separately.
Features selection
For radiomic and dosiomic model construction, the selection of quantitative radiomic or dosiomic features in the training cohort followed a four-step process to minimize bias and reduce the risk of overfitting.
First, all features underwent z-score normalization to eliminate the effects of differing scales. Second, due to the imbalanced distribution in radiotherapy target volumes, the Synthetic Minority Oversampling Technique (SMOTE) was employed to balance the number of radiotherapy target volumes in the training cohort. SMOTE is a widely used data augmentation method designed to address class imbalance, particularly in classification tasks where the minority class is underrepresented. By generating synthetic samples based on feature space similarities between existing minority instances, SMOTE enhances the representation of the minority class, thereby improving model performance and reducing bias during training. Third, a univariate analysis was performed using either the independent sample t-test or the Mann–Whitney U test to compare radiomic features between non-DCR and DCR group. Features that failed to meet the criteria of either of the aforementioned tests were excluded. Fourth, Relief and Recursive Feature Elimination (RFE) methods were utilized for feature selection. RFE are commonly used feature selection algorithms aimed at identifying the most relevant variables for predictive modeling. According to the commonly accepted “rule of thumb” in prognostic research, which recommends a minimum of ten events per predictor variable, and given the sample size in this study, we retained only the five most significant radiomic or dosiomic features after RFE [30–32]. Finally, spearman correlation coefficients between each pair of features were calculated to avoid the underlying severe linear dependence. All pairs of features with spearman correlation coefficient >0.9 were selected, if two features with similar biophysiological characteristics and one feature of each pair was randomly excluded, if two features with different biophysiological characteristics, two features would be included in the further analysis. The remaining features were then used for constructing machine learning-based models.
For the construction of the combined model, the feature selection process proceeded as follows:
(1) Given that shape features derived from radiomics and dosiomics are identical in value, including both would introduce substantial multicollinearity, potentially compromising the validity of feature importance analysis and model interpretability. Therefore, all radiomic shape features (14 in total) were excluded from the combined model. As a result, a total of 93 shape-excluded radiomic features remained for subsequent selection in the combined model.
(2) To minimize bias and reduce the risk of overfitting, feature selection for these 93 radiomic features in the training cohort followed the same four-step pipeline used for the independent selection of radiomic and dosiomic features. The selected features were then utilized to construct the combined model.
(3) To enhance the comprehensiveness and interpretability of the predictive model, a total of 22 features were included in the final combined model, comprising clinical variables (n = 6), radiomic features (n = 5), dosiomic features (n = 5), and dosimetric metrics (n = 6).
Model Building
For each of the three regions of interest (ROIs)—CTV, GTV, and GTVtb—five predictive models were developed based on distinct feature domains. In addition, to further explore the peritumoral microenvironment, five corresponding models were also constructed for the two subtracted ROIs, CTV–GTV and CTV–GTVtb. The five types of models were as follows:
1) Clinical model (C): Constructed using six clinical variables that reflect patient-specific characteristics and baseline disease status.
2) Radiomic model (R): Developed based on five quantitative features extracted from pre-treatment CT images, characterizing tumor phenotype and intratumoral heterogeneity.
3) Dosiomic model (D): Built using five dosiomic features extracted from the spatial dose distribution map, providing information on local dose heterogeneity within the ROI.
4) DVH-based model (DVH): Based on six conventional dosimetric parameters derived from dose–volume histograms (DVHs), representing global dose characteristics delivered to the target and surrounding organs at risk.
5) Combined model (C + R + D + DVH): Integrating all four feature domains, this model was developed using six clinical variables, five radiomic features (selected from the shape-feature-excluded radiomic feature set), five dosiomic features, and six DVH metrics. This comprehensive feature integration aimed to enhance both the predictive power and the clinical interpretability of the model.
In this study, four machine learning algorithms—Support Vector Machine (SVM), Random Forest (RF), Extreme Gradient Boosting (XGBoost), and Logistic Regression (LR)—were employed to classify patients into DCR and non-DCR groups.
Model evaluation
We performed comprehensive hyperparameter optimization for three classifiers using grid search with 5-fold StratifiedKFold cross-validation to maintain class distribution across folds. Multiple random seeds ensured robustness against initialization variance, with optimal configurations selected based on average validation performance. All implementations used scikit-learn 1.3.2 and XGBoost (scikit-learn compatible API), with detailed hyperparameter spaces explored for each model.
For SVM, we evaluated both linear and radial basis function (RBF) kernels while testing the regularization parameter C across six values (0.01, 0.1, 1.0, 10, and 100) to balance model complexity and generalization.
For the RF classifier, we systematically evaluated tree ensembles with 2–20 estimators (n_estimators), tree depth constraints (max_depth: 1, 2, 3, or unconstrained), and node splitting requirements including min_samples_leaf (3,5,6,7,8,12) and min_samples_split (2–10). Feature selection strategies were assessed through max_features (‘sqrt’ or all features), with all models using Gini impurity criterion and bootstrap aggregation.
For XGBoost, we tuned gradient boosting dynamics through tree quantity (n_estimators: 2–20), depth constraints (max_depth: 1–5), and learning rates (0.01–0.30 in 0.01 increments). Structural parameters included feature discretization bins (max_bin: 5–10) and terminal node limits (max_leaves = 3), with all models configured for binary classification using the ‘binary: logistic’ objective.
For LR, we explored regularization strategies combining penalty types (L1, L2, or no regularization) with compatible solvers - ‘liblinear’ (L1/L2), ‘lbfgs’ and ‘newton-cg’ (L2/no penalty). The regularization strength C was finely tuned from 0.1 to 20, with fixed parameters including maximum 1000 iterations and fit_intercept = True.
Receiver-operating characteristic (ROC) curves, calibration curves and decision curve analysis (DCA) were calculated to assess the SVM model performance [33].Delong test was performed to compare the ROC curves of the interested model with others.
Model interpretability
To address the black-box nature of machine learning models, SHAP (Shapley Additive Explanations), derived from coalitional game theory, was employed to identify and prioritize features that contribute to classification. The SHAP method has been successfully applied in radiomic studies to interpret prediction models.
Consequently, the best performing CTV_combined SVM model (incorporating clinical, radiomic, dosiomic, and DVH features) and other CTV _based SVM models were evaluated using SHAP kernel explainer to analyze feature attributions. The workflow for model construction and evaluation is illustrated in Fig. 2.
Fig. 2.
Workflow of constructing and evaluating predictive models
Furthermore, in addition to SHAP-based attribution analysis, logistic regression was performed for the features included in the CTV_combined model. The contribution of each feature to predictive performance was quantified using the coefficient of determination (R²). Considering that age and tumor grade may serve as potential confounding clinical variables, subgroup analyses were conducted accordingly. Finally, we generated feature maps using PyRadiomics to visualize and better understand the spatial implications of the features contributing to the model’s predictions.
Statistical analysis
Python version 3.8 (https://www.python.org) were used for model building, model evaluation, SHAP analysis and statistical analyses. Continuous variables were reported as mean (SD) and analyzed using an unpaired, two-tailed t-test for normally distributed data or the Mann–Whitney test for data that did not follow a normal distribution. Categorical variables were evaluated using either the χ2 test or Fisher’s exact test. Statistical significance was determined with a P-value threshold of < 0.05.
Results
Patient characteristics and dosimetric features
115 eligible patients were included in cohort 1, comprising 71 patients in the DCR group (CR/PR/SD) and 44 patients in the non-DCR group (PD). Cohort 2 consisted of 61 eligible patients, with 34 in the DCR group and 27 in the non-DCR group.
Clinical characteristics—including age, gender, Karnofsky Performance Status (KPS), tumor type, tumor grade, and chemotherapy use—showed no statistically significant differences between the training and validation cohorts.
Regarding the prescribed dose and dosimetric features of GTV, GTVtb, and CTV, a significant difference was observed in the prescribed dose of GTV within the training cohort and in the prescribed dose of GTVtb within the validation cohort. However, no statistically significant differences were found in the DVH-derived dosimetric features between the training and validation cohorts.
With respect to the dosimetric features of CTV-GTV, CTV-GTVtb, DVH-derived dosimetric features showed no statistically significant differences between the two cohorts.
Following both univariate and multivariable logistic regression analyses of clinical variables and dosimetric features derived from the GTV, GTVtb, CTV, CTV-GTV and CTV-GTVtb (as detailed in Supplementary Tables 2–7), GTVtb_Min dose was identified as an independent risk factor for tumor recurrence (OR: 0.343; 95% CI: 0.205–0.696). Similarly, CTV–GTVtb_D98% was also found to be an independent risk factor for recurrence (OR: 0.581; 95% CI: 0.360–0.919).
Performance of clinical and DVH models in assessing radiotherapy response
Although there were no clinical features were significantly related to treatment response after univariate and multivariable logistic analysis within the training cohort (Table 1 and Supplementary Table 2). Owing to their clinical significance, all clinical features were included in constructing the clinical model.
Table 1.
Comparison of clinical characteristics and dosimetric features between Non-DCR and DCR groups
| Characteristic | Training cohort | Validation cohort | |||||
|---|---|---|---|---|---|---|---|
| DCR group | non-DCR group | p value | DCR group | non-DCR group | p value | ||
| (N = 71) | (N = 44) | (N = 34) | (N = 27) | ||||
| Age, years | 51.774 ± 14.547 | 52.909 ± 11.585 | 0.063 | 50.294 ± 16.410 | 51.481 ± 12.813 | 0.112 | |
| Gender (%) | 0.958 | 0.553 | |||||
| Male | 42(59.154) | 25(56.818) | 19(55.882) | 18(66.667) | |||
| Female | 29(40.846) | 19(43.182) | 15(44.118) | 9(33.333) | |||
| KPS | 0.142 | 0.424 | |||||
| ≥ 80 | 54(76.056) | 27(61.364) | 28(82.353) | 19(70.370) | |||
| <80 | 17(23.944) | 17(38.636) | 6(17.647) | 8(29.630) | |||
| Tumor type (%) | 0.123 | 0.194 | |||||
| astrocytoma | 23(32.395) | 6(13.636) | 9(26.470) | 7(25.926) | |||
| oligodendroglioma | 7(9.859) | 8(18.182) | 7(20.588) | 1(3.703) | |||
| glioblastoma | 29(40.845) | 22(50.000) | 16(47.059) | 15(55.556) | |||
| glioma | 12(16.901) | 8(18.182) | 2(5.883) | 4(14.815) | |||
| Tumor grade (%) | 0.601 | 0.199 | |||||
| Grade1 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||
| Grade2 | 22(30.986) | 6(13.636) | 11(32.353) | 6(22.222) | |||
| Grade3 | 11(15.493) | 10(22.728) | 4(11.764) | 3(11.111) | |||
| Grade4 | 32(45.070) | 24(54.545) | 16(47.058) | 17(62.963) | |||
| Unknown | 6(8.451) | 4(9.091) | 3(4.225) | 1(3.704) | |||
| Chemotherapy use (%) | 0.492 | 0.725 | |||||
| yes | 66(92.958) | 43(97.727) | 28(82.352) | 24(88.889) | |||
| no | 5(7.042) | 1(2.273) | 6(17.648) | 3(11.111) | |||
| GTV (%) | 0.006 | 0.161 | |||||
| have | 35(49.296) | 33(75.000) | 14(41.176) | 16(59.259) | |||
| don’t have | 36(5.0704) | 11(25.000) | 20(58.824) | 11(40.741) | |||
| GTVtb (%) | 0.19 | 0.002 | |||||
| have | 33(46.479) | 15(34.091) | 20(58.824) | 5(18.519) | |||
| don’t have | 38(53.521) | 29(65.909) | 14(41.176) | 22(81.481) | |||
| CTV (%) | 0.586 | 0.842 | |||||
| have | 66(92.958) | 42(95.454) | 21(61.765) | 16(59.259) | |||
| don’t have | 5(7.042) | 2(4.546) | 13 (38.235) | 11(40.741) | |||
| Prescribed dose of GTV | < 50 Gy | 1 (1.408) | 4 (9.091) | 0.015 | 0 (0.0) | 0 (0.0) | 0.354 |
| 50–59.9 Gy | 6 (8.451) | 3 (6.818) | 2 (5.882) | 3 (11.111) | |||
| ≥ 60 Gy | 28 (39.437) | 26 (59.091) | 12 (35.294) | 13 (48.148) | |||
| 0 Gy | 36 (50.704) | 11 (25.0) | 20 (58.824) | 11 (40.741) | |||
| GTV | Mean dose (Gy) | 60.905 ± 4.792 | 59.423 ± 9.901 | 0.440 | 61.61 ± 3.687 | 60.964 ± 3.934 | 0.646 |
| Min dose (Gy) | 55.618 ± 6.841 | 53.165 ± 13.101 | 0.342 | 58.74 ± 3.722 | 58.009 ± 5.884 | 0.684 | |
| Max dose (Gy) | 63.129 ± 4.771 | 61.826 ± 10.1 | 0.504 | 63.443 ± 3.914 | 62.739 ± 3.969 | 0.629 | |
| D2% (Gy) | 62.27 ± 4.768 | 60.967 ± 9.973 | 0.499 | 62.799 ± 3.778 | 62.152 ± 3.983 | 0.651 | |
| D50% (Gy) | 60.931 ± 4.824 | 59.483 ± 9.922 | 0.452 | 61.662 ± 3.669 | 60.97 ± 3.927 | 0.622 | |
| D98% (Gy) | 59.159 ± 4.866 | 57.304 ± 9.937 | 0.338 | 60.23 ± 3.773 | 59.67 ± 4.169 | 0.702 | |
| Prescribed dose of GTVtb | < 50 Gy | 0 (0.0) | 0 (0.0) | 0.42 | 0 (0.0) | 0 (0.0) | 0.006 |
| 50–59.9 Gy | 6 (8.451) | 3 (6.818) | 6 (17.647) | 2 (7.407) | |||
| ≥ 60 Gy | 27 (38.028) | 12 (27.273) | 14(41.176) | 3 (11.111) | |||
| 0 Gy | 38 (53.521) | 29 (65.909) | 14(41.176) | 22(81.481) | |||
| GTVtb | Mean dose (Gy) | 61.806 ± 2.94 | 60.852 ± 4.486 | 0.460 | 60.701 ± 4.12 | 59.589 ± 6.799 | 0.741 |
| Min dose (Gy) | 58.973 ± 3.166 | 53.597 ± 10.952 | 0.082 | 57.67 ± 4.299 | 54.949 ± 10.772 | 0.607 | |
| Max dose (Gy) | 63.885 ± 3.18 | 63.755 ± 4.571 | 0.921 | 62.98 ± 4.451 | 61.033 ± 6.613 | 0.560 | |
| D2% (Gy) | 63.177 ± 3.129 | 62.791 ± 4.51 | 0.767 | 62.224 ± 4.193 | 60.562 ± 6.755 | 0.623 | |
| D50% (Gy) | 61.836 ± 2.937 | 60.875 ± 4.491 | 0.457 | 60.724 ± 4.171 | 59.627 ± 6.758 | 0.743 | |
| D98% (Gy) | 60.25 ± 3.025 | 58.342 ± 5.561 | 0.228 | 58.996 ± 4.146 | 57.855 ± 7.774 | 0.765 | |
| Prescribed dose of CTV | < 50 Gy | 4 (5.634) | 6 (12.766) | 0.463 | 0 (0.0) | 0 (0.0) | 0.655 |
| 50–59.9 Gy | 21 (29.577) | 13 (27.66) | 11 ((32.353) | 6 (22.222) | |||
| ≥ 60 Gy | 41 (57.746) | 23 (48.936) | 10 (29.412) | 10 (37.037) | |||
| 0 Gy | 5 (7.042) | 5 (10.638) | 13 (38.235) | 11 (40.741) | |||
| CTV | Mean dose (Gy) | 59.445 ± 4.641 | 57.479 ± 8.969 | 0.194 | 59.798 ± 3.861 | 60.096 ± 3.799 | 0.816 |
| Min dose (Gy) | 47.203 ± 12.723 | 42.049 ± 17.683 | 0.106 | 53.191 ± 4.612 | 51.285 ± 6.757 | 0.342 | |
| Max dose (Gy) | 63.481 ± 4.157 | 62.511 ± 8.889 | 0.510 | 63.130 ± 4.265 | 63.380 ± 3.813 | 0.852 | |
| D2% (Gy) | 62.302 ± 4.104 | 61.162 ± 8.766 | 0.433 | 62.018 ± 4.104 | 62.309 ± 3.913 | 0.827 | |
| D50% (Gy) | 59.784 ± 4.201 | 57.915 ± 8.659 | 0.198 | 59.849 ± 3.930 | 60.251 ± 3.764 | 0.754 | |
| D98% (Gy) | 54.623 ± 9.749 | 50.778 ± 14.264 | 0.130 | 57.051 ± 3.955 | 57.190 ± 4.444 | 0.922 | |
| CTV-GTV | Mean dose (Gy) | 58.457 ± 5.772 | 56.238 ± 9.757 | 0.2625 | 60.12 ± 3.422 | 59.810 ± 3.88 | 0.818 |
| Min dose (Gy) | 44.129 ± 15.655 | 40.323 ± 18.876 | 0.3705 | 53.100 ± 5.210 | 51.285 ± 6.757 | 0.414 | |
| Max dose (Gy) | 63.200 ± 4.674 | 61.903 ± 9.684 | 0.4895 | 63.617 ± 3.920 | 63.241 ± 3.763 | 0.792 | |
| D2% (Gy) | 61.817 ± 4.587 | 60.325 ± 9.484 | 0.4178 | 62.425 ± 3.703 | 62.129 ± 3.886 | 0.833 | |
| D50% (Gy) | 58.905 ± 4.977 | 56.719 ± 9.349 | 0.2386 | 60.218 ± 3.427 | 59.857 ± 3.852 | 0.788 | |
| D98% (Gy) | 52.247 ± 13.15 | 48.750 ± 15.708 | 0.3248 | 57.078 ± 3.726 | 56.986 ± 4.605 | 0.952 | |
| CTV-GTVtb | Mean dose (Gy) | 59.689 ± 3.047 | 57.959 ± 5.557 | 0.273 | 59.583 ± 3.86 | 58.826 ± 5.968 | 0.798 |
| Min dose (Gy) | 50.796 ± 7.185 | 43.249 ± 16.392 | 0.106 | 53.237 ± 4.734 | 52.326 ± 9.922 | 0.850 | |
| Max dose (Gy) | 63.965 ± 3.048 | 63.713 ± 4.694 | 0.851 | 63.254 ± 4.236 | 61.525 ± 6.101 | 0.575 | |
| D2% (Gy) | 62.623 ± 2.910 | 62.008 ± 4.621 | 0.640 | 62.088 ± 4.029 | 60.575 ± 6.363 | 0.634 | |
| D50% (Gy) | 59.678 ± 3.175 | 58.173 ± 5.143 | 0.308 | 59.521 ± 3.932 | 58.834 ± 5.895 | 0.815 | |
| D98% (Gy) | 56.498 ± 3.367 | 51.673 ± 12.836 | 0.172 | 56.891 ± 4.130 | 56.525 ± 6.634 | 0.911 | |
Univariate analysis of dosimetric parameters (Table 1) revealed no statistically significant differences in DVH-derived dosimetric features between the two cohorts for the GTV, GTVtb, CTV, CTV–GTV, and CTV–GTVtb regions. Furthermore, both univariate and multivariable logistic regression analyses (Supplementary Tables 3–7) identified two dosimetric features—GTVtb_Min dose and CTV–GTVtb_D98%—as being significantly associated with treatment response. Given their potential clinical relevance, all DVH-derived dosimetric features were included in the DVH model construction, regardless of their statistical significance.
Performances of clinical model and DVH model from each ROI in the training cohort and validation cohort are shown in Tables 2 and 3. The ROC curves with clinical models and DVH models from each radiotherapy target volume in the training and validation cohorts are shown in Figs. 3 and 4.
Table 2.
Predictive performance of models based on CTV, GTV, and GTVtb for radiotherapy treatment response assessment
| Models | Training cohort | Validation cohort | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AUC (95%CI) | Accuracy | Precision | SENS | SPEC | AUC (95%CI) | Accuracy | Precision | SENS | SPEC | |
| GTV_Combined SVM model | 0.797 (0.400, 1.000) | 0.797 | 0.797 | 0.797 | 0.797 | 0.635 (0.451, 0.761) | 0.599 | 0.643 | 0.493 | 0.691 |
| GTV_Radiomic SVM model | 0.546 (0.286, 0.801) | 0.554 | 0.487 | 0.444 | 0.657 | 0.524 (0.410, 0.638) | 0.571 | 0.613 | 0.494 | 0.637 |
| GTV_Clinical SVM model | 0.776 (0.410, 0.964) | 0.733 | 0.630 | 0.686 | 0.744 | 0.558 (0.502, 0.621) | 0.529 | 0.407 | 0.474 | 0.576 |
| GTV_DVH SVM model | 0.799 (0.782, 0.819) | 0.698 | 0.752 | 0.698 | 0.690 | 0.629 (0.609, 0.664) | 0.543 | 0.532 | 0.430 | 0.640 |
| GTV_Dosiomic SVM model | 0.748 (0.440, 0.924) | 0.610 | 0.508 | 0.543 | 0.673 | 0.582 (0.526, 0.665) | 0.507 | 0.381 | 0.370 | 0.627 |
| GTVtb_Combined SVM model | 0.798 (0.402, 1.000) | 0.806 | 0.684 | 0.797 | 0.779 | 0.619 (0.326, 0.882) | 0.608 | 0.324 | 0.565 | 0.619 |
| GTVtb_Radiomic SVM model | 0.598 (0.311, 0.885) | 0.605 | 0.393 | 0.457 | 0.709 | 0.524 (0.420, 0.629) | 0.580 | 0.108 | 0.333 | 0.644 |
| GTVtb_Clinical SVM model | 0.757 (0.430, 0.933) | 0.716 | 0.747 | 0.767 | 0.646 | 0.625 (0.481, 0.707) | 0.546 | 0.289 | 0.700 | 0.506 |
| GTVtb_DVH SVM model | 0.569 (0.313, 0.825) | 0.588 | 0.609 | 0.711 | 0.432 | 0.522 (0.361, 0.665) | 0.537 | 0.126 | 0.435 | 0.563 |
| GTVtb_Dosiomic SVM model | 0.599 (0.320, 0.876) | 0.564 | 0.548 | 0.489 | 0.630 | 0.563 (0.499, 0.629) | 0.580 | 0.260 | 0.433 | 0.619 |
| CTV_Combined SVM model | 0.815 (0.790, 0.839) | 0.717 | 0.698 | 0.861 | 0.547 | 0.728 (0.717, 0.739) | 0.615 | 0.486 | 0.826 | 0.493 |
| CTV_Radiomic SVM model | 0.726 (0.692, 0.760) | 0.606 | 0.607 | 0.916 | 0.247 | 0.607 (0.562, 0.632) | 0.459 | 0.411 | 0.853 | 0.232 |
| CTV_Clinical SVM mode | 0.806 (0.780, 0.832) | 0.649 | 0.740 | 0.548 | 0.712 | 0.565 (0.497, 0.632) | 0.521 | 0.285 | 0.389 | 0.597 |
| CTV_DVH SVM model | 0.722 (0.441, 0.882) | 0.691 | 0.696 | 0.887 | 0.458 | 0.631 (0.524, 0.717) | 0.542 | 0.476 | 0.640 | 0.485 |
| CTV_Dosiomic SVM model | 0.554 (0.348, 0.743) | 0.534 | 0.533 | 0.820 | 0.192 | 0.551 (0.466, 0.610) | 0.371 | 0.340 | 0.786 | 0.131 |
| GTV_Combined RF model | 0.617 (0.545, 0.691) | 0.562 | 0.560 | 0.749 | 0.376 | 0.555 (0.505, 0.617) | 0.514 | 0.481 | 0.677 | 0.373 |
| GTV_Radiomic RF model | 0.514 (0.428, 0.599) | 0.504 | 0.510 | 0.484 | 0.522 | 0.504 (0.453, 0.576) | 0.493 | 0.445 | 0.399 | 0.573 |
| GTV_Clinical RF model | 0.627 (0.570, 0.687) | 0.556 | 0.581 | 0.734 | 0.364 | 0.587 (0.505, 0.695) | 0.564 | 0.520 | 0.598 | 0.534 |
| GTV_DVH RF model | 0.577 (0.518, 0.623) | 0.563 | 0.558 | 0.433 | 0.684 | 0.566 (0.487, 0.654) | 0.558 | 0.524 | 0.370 | 0.720 |
| GTV_Dosiomic RF model | 0.621 (0.566, 0.677) | 0.555 | 0.550 | 0.674 | 0.431 | 0.577 (0.491, 0.677) | 0.571 | 0.565 | 0.554 | 0.586 |
| GTVtb_Combined RF model | 0.791 (0.734, 0.852) | 0.678 | 0.661 | 0.798 | 0.573 | 0.552 (0.481, 0.633) | 0.586 | 0.247 | 0.433 | 0.626 |
| GTVtb_Radiomic RF model | 0.802 (0.712, 0.888) | 0.722 | 0.680 | 0.894 | 0.557 | 0.517 (0.414, 0.620) | 0.476 | 0.200 | 0.533 | 0.461 |
| GTVtb_Clinical RF model | 0.665 (0.576, 0.756) | 0.582 | 0.519 | 0.896 | 0.310 | 0.567 (0.514, 0.622) | 0.463 | 0.224 | 0.632 | 0.419 |
| GTVtb_DVH RF model | 0.708 (0.654, 0.758) | 0.678 | 0.665 | 0.734 | 0.614 | 0.520 (0.470, 0.577) | 0.455 | 0.205 | 0.533 | 0.435 |
| GTVtb_Dosiomic RF model | 0.875 (0.809, 0.944) | 0.782 | 0.732 | 0.913 | 0.646 | 0.615 (0.557, 0.689) | 0.504 | 0.244 | 0.634 | 0.471 |
| CTV_Combined RF model | 0.617 (0.582, 0.645) | 0.559 | 0.511 | 0.758 | 0.411 | 0.550 (0.454, 0.626) | 0.537 | 0.419 | 0.679 | 0.454 |
| CTV_Radiomic RF model | 0.588 (0.545, 0.632) | 0.567 | 0.519 | 0.710 | 0.461 | 0.555 (0.525, 0.585) | 0.498 | 0.393 | 0.679 | 0.393 |
| CTV_Clinical RF mode | 0.572 (0.541, 0.595) | 0.521 | 0.546 | 0.662 | 0.379 | 0.556 (0.476, 0.636) | 0.503 | 0.382 | 0.614 | 0.439 |
| CTV_DVH RF model | 0.565 (0.538, 0.593) | 0.500 | 0.460 | 0.765 | 0.288 | 0.554 (0.457, 0.657) | 0.462 | 0.391 | 0.773 | 0.283 |
| CTV_Dosiomic RF model | 0.601 (0.558, 0.660) | 0.533 | 0.484 | 0.699 | 0.405 | 0.553 (0.509, 0.608) | 0.556 | 0.423 | 0.574 | 0.546 |
| GTV_Combined XGBoost model | 0.641 (0.588, 0.697) | 0.607 | 0.623 | 0.583 | 0.624 | 0.675 (0.581, 0.747) | 0.614 | 0.593 | 0.539 | 0.680 |
| GTV_Radiomic XGBoost model | 0.530 (0.427, 0.608) | 0.541 | 0.504 | 0.522 | 0.569 | 0.548 (0.495, 0.609) | 0.579 | 0.546 | 0.491 | 0.654 |
| GTV_Clinical XGBoost model | 0.615 (0.571, 0.661) | 0.444 | 0.192 | 0.398 | 0.602 | 0.588 (0.549, 0.623) | 0.507 | 0.185 | 0.398 | 0.602 |
| GTV_DVH XGBoost model | 0.541 (0.441, 0.652) | 0.534 | 0.541 | 0.485 | 0.579 | 0.588 (0.541, 0.636) | 0.586 | 0.577 | 0.477 | 0.680 |
| GTV_Dosiomic XGBoost model | 0.604 (0.535, 0.669) | 0.578 | 0.703 | 0.461 | 0.709 | 0.683 (0.537, 0.768) | 0.571 | 0.609 | 0.464 | 0.665 |
| GTVtb_Combined XGBoost model | 0.768 (0.677, 0.851) | 0.652 | 0.661 | 0.722 | 0.610 | 0.516 (0.428, 0.604) | 0.538 | 0.242 | 0.567 | 0.530 |
| GTVtb_Radiomic XGBoost model | 0.813 (0.693, 0.929) | 0.791 | 0.821 | 0.780 | 0.811 | 0.581 (0.546, 0.620) | 0.434 | 0.221 | 0.700 | 0.365 |
| GTVtb_Clinical XGBoost model | 0.592 (0.504, 0.663) | 0.426 | 0.340 | 0.802 | 0.198 | 0.551 (0.497, 0.609) | 0.323 | 0.166 | 0.802 | 0.198 |
| GTVtb_DVH XGBoost model | 0.740 (0.656, 0.838) | 0.678 | 0.664 | 0.761 | 0.608 | 0.621 (0.564, 0.673) | 0.593 | 0.290 | 0.600 | 0.591 |
| GTVtb_Dosiomic XGBoost model | 0.880 (0.841, 0.916) | 0.748 | 0.787 | 0.719 | 0.796 | 0.593 (0.530, 0.682) | 0.635 | 0.304 | 0.467 | 0.679 |
| CTV_Combined XGBoost model | 0.657 (0.628, 0.690) | 0.563 | 0.514 | 0.731 | 0.437 | 0.666 (0.627, 0.706) | 0.556 | 0.420 | 0.586 | 0.539 |
| CTV_Radiomic XGBoost model | 0.629 (0.594, 0.665) | 0.559 | 0.509 | 0.667 | 0.485 | 0.585 (0.538, 0.635) | 0.498 | 0.388 | 0.652 | 0.409 |
| CTV_Clinical XGBoost mode | 0.656 (0.594, 0.719) | 0.454 | 0.092 | 0.202 | 0.798 | 0.569 (0.536, 0.620) | 0.580 | 0.074 | 0.202 | 0.798 |
| CTV_DVH XGBoost model | 0.654 (0.612, 0.698) | 0.608 | 0.555 | 0.560 | 0.653 | 0.585 (0.528, 0.630) | 0.576 | 0.423 | 0.399 | 0.678 |
| CTV_Dosiomic XGBoost model | 0.641 (0.604, 0.679) | 0.563 | 0.534 | 0.784 | 0.413 | 0.551 (0.485, 0.622) | 0.484 | 0.386 | 0.665 | 0.379 |
| GTV_Combined LR model | 0.832 (0.789, 0.863) | 0.774 | 0.739 | 0.865 | 0.680 | 0.608 (0.575, 0.638) | 0.557 | 0.528 | 0.555 | 0.559 |
| GTV_Radiomic LR model | 0.588 (0.557, 0.627) | 0.610 | 0.604 | 0.660 | 0.559 | 0.535 (0.495, 0.571) | 0.493 | 0.460 | 0.632 | 0.373 |
| GTV_Clinical LR model | 0.763 (0.729, 0.793) | 0.610 | 0.364 | 0.443 | 0.711 | 0.556 (0.540, 0.573) | 0.550 | 0.308 | 0.428 | 0.656 |
| GTV_DVH LR model | 0.615 (0.558, 0.672) | 0.569 | 0.674 | 0.591 | 0.529 | 0.511 (0.471, 0.560) | 0.492 | 0.526 | 0.463 | 0.517 |
| GTV_Dosiomic LR model | 0.637 (0.609, 0.677) | 0.590 | 0.772 | 0.529 | 0.630 | 0.542 (0.513, 0.582) | 0.500 | 0.509 | 0.417 | 0.571 |
| GTVtb_Combined LR model | 0.988 (0.984, 0.994) | 0.945 | 0.920 | 0.973 | 0.915 | 0.698 (0.546, 0.793) | 0.710 | 0.418 | 0.566 | 0.748 |
| GTVtb_Radiomic LR model | 0.866 (0.829, 0.907) | 0.806 | 0.806 | 0.786 | 0.820 | 0.523 (0.472, 0.570) | 0.462 | 0.220 | 0.633 | 0.417 |
| GTVtb_Clinical LR model | 0.832 (0.795, 0.860) | 0.624 | 0.644 | 0.822 | 0.343 | 0.639 (0.607, 0.684) | 0.364 | 0.214 | 0.701 | 0.276 |
| GTVtb_DVH LR model | 0.855 (0.806, 0.904) | 0.782 | 0.827 | 0.703 | 0.853 | 0.666 (0.568, 0.732) | 0.469 | 0.265 | 0.833 | 0.374 |
| GTVtb_Dosiomic LR model | 0.814 (0.780, 0.851) | 0.679 | 0.848 | 0.571 | 0.740 | 0.558 (0.491, 0.632) | 0.461 | 0.180 | 0.568 | 0.434 |
| CTV_Combined LR model | 0.815 (0.789, 0.839) | 0.700 | 0.678 | 0.876 | 0.487 | 0.713 (0.683, 0.743) | 0.571 | 0.450 | 0.772 | 0.455 |
| CTV_Radiomic LR model | 0.713 (0.671, 0.755) | 0.600 | 0.579 | 0.967 | 0.163 | 0.616 (0.604, 0.627) | 0.371 | 0.353 | 0.853 | 0.093 |
| CTV_Clinical LR mode | 0.709 (0.688, 0.737) | 0.623 | 0.688 | 0.494 | 0.717 | 0.565 (0.523, 0.617) | 0.541 | 0.297 | 0.388 | 0.630 |
| CTV_DVH LR model | 0.635 (0.611, 0.658) | 0.560 | 0.572 | 0.687 | 0.399 | 0.558 (0.512, 0.610) | 0.528 | 0.406 | 0.518 | 0.533 |
| CTV_Dosiomic LR model | 0.653 (0.628, 0.674) | 0.580 | 0.566 | 0.961 | 0.127 | 0.631 (0.592, 0.655) | 0.400 | 0.367 | 0.879 | 0.124 |
Table 3.
Predictive performance of models based on CTV-GTV and CTV-GTVtb for radiotherapy treatment response assessment
| Models | Training cohort | Validation cohort | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AUC (95%CI) | Accuracy | Precision | SENS | SPEC | AUC (95%CI) | Accuracy | Precision | SENS | SPEC | |
| CTV-GTV_Combined SVM model | 0.745 (0.403, 0.928) | 0.722 | 0.647 | 0.776 | 0.670 | 0.614 (0.491, 0.724) | 0.564 | 0.519 | 0.614 | 0.520 |
| CTV-GTV_Radiomic SVM model | 0.811 (0.802, 0.819) | 0.703 | 0.665 | 0.803 | 0.601 | 0.580 (0.545, 0.605) | 0.557 | 0.522 | 0.570 | 0.546 |
| CTV-GTV_Clinical SVM model | 0.762 (0.385, 0.957) | 0.738 | 0.832 | 0.698 | 0.744 | 0.575 (0.519, 0.630) | 0.521 | 0.407 | 0.474 | 0.562 |
| CTV-GTV_DVH SVM model | 0.730 (0.460, 0.885) | 0.666 | 0.682 | 0.666 | 0.663 | 0.606 (0.436, 0.753) | 0.507 | 0.503 | 0.431 | 0.572 |
| CTV-GTV_Dosiomic SVM model | 0.646 (0.466, 0.754) | 0.579 | 0.477 | 0.566 | 0.583 | 0.635 (0.526, 0.703) | 0.550 | 0.419 | 0.556 | 0.545 |
| CTV-GTVtb_Combined SVM model | 0.763 (0.429, 0.947) | 0.801 | 0.870 | 0.819 | 0.752 | 0.546 (0.501, 0.591) | 0.552 | 0.086 | 0.265 | 0.627 |
| CTV-GTVtb_Radiomic SVM model | 0.580 (0.262, 0.890) | 0.605 | 0.496 | 0.720 | 0.444 | 0.520 (0.409, 0.632) | 0.551 | 0.240 | 0.434 | 0.582 |
| CTV-GTVtb_Clinical SVM model | 0.814 (0.779, 0.849) | 0.661 | 0.680 | 0.828 | 0.428 | 0.652 (0.604, 0.704) | 0.483 | 0.271 | 0.734 | 0.417 |
| CTV-GTVtb_DVH SVM model | 0.756 (0.440, 0.941) | 0.781 | 0.679 | 0.666 | 0.875 | 0.554 (0.513, 0.600) | 0.697 | 0.284 | 0.266 | 0.810 |
| CTV-GTVtb_Dosiomic SVM model | 0.806 (0.754, 0.844) | 0.764 | 0.887 | 0.614 | 0.899 | 0.551 (0.494, 0.625) | 0.773 | 0.167 | 0.133 | 0.939 |
| CTV-GTV_Combined RF model | 0.575 (0.526, 0.624) | 0.578 | 0.559 | 0.748 | 0.401 | 0.568 (0.481, 0.644) | 0.536 | 0.506 | 0.785 | 0.320 |
| CTV-GTV_Radiomic RF model | 0.581 (0.533, 0.629) | 0.578 | 0.553 | 0.867 | 0.284 | 0.569 (0.522, 0.618) | 0.550 | 0.509 | 0.769 | 0.360 |
| CTV-GTV_Clinical RF model | 0.604 (0.504, 0.703) | 0.563 | 0.575 | 0.774 | 0.333 | 0.552 (0.456, 0.647) | 0.536 | 0.499 | 0.815 | 0.294 |
| CTV-GTV_DVH RF model | 0.556 (0.500, 0.614) | 0.540 | 0.557 | 0.483 | 0.598 | 0.554 (0.521, 0.595) | 0.579 | 0.561 | 0.447 | 0.693 |
| CTV-GTV_Dosiomic RF model | 0.575 (0.478, 0.671) | 0.549 | 0.538 | 0.461 | 0.642 | 0.555 (0.448, 0.646) | 0.550 | 0.525 | 0.385 | 0.693 |
| CTV-GTVtb_Combined RF model | 0.849 (0.735, 0.936) | 0.809 | 0.764 | 0.936 | 0.691 | 0.552 (0.465, 0.658) | 0.545 | 0.215 | 0.467 | 0.565 |
| CTV-GTVtb_Radiomic RF model | 0.863 (0.791, 0.934) | 0.808 | 0.775 | 0.889 | 0.737 | 0.550 (0.523, 0.582) | 0.545 | 0.210 | 0.433 | 0.574 |
| CTV-GTVtb_Clinical RF model | 0.627 (0.536, 0.742) | 0.529 | 0.474 | 0.784 | 0.296 | 0.555 (0.474, 0.634) | 0.427 | 0.218 | 0.699 | 0.357 |
| CTV-GTVtb_DVH RF model | 0.731 (0.641, 0.796) | 0.627 | 0.593 | 0.802 | 0.439 | 0.563 (0.541, 0.578) | 0.551 | 0.238 | 0.534 | 0.556 |
| CTV-GTVtb_Dosiomic RF model | 0.857 (0.812, 0.895) | 0.756 | 0.763 | 0.839 | 0.698 | 0.549 (0.500, 0.606) | 0.455 | 0.253 | 0.668 | 0.399 |
| CTV-GTV_Combined XGBoost model | 0.581 (0.501, 0.657) | 0.511 | 0.504 | 0.487 | 0.537 | 0.635 (0.537, 0.731) | 0.593 | 0.577 | 0.553 | 0.627 |
| CTV-GTV_Radiomic XGBoost model | 0.518 (0.481, 0.554) | 0.482 | 0.470 | 0.451 | 0.528 | 0.502 (0.458, 0.550) | 0.493 | 0.458 | 0.616 | 0.387 |
| CTV-GTV_Clinical XGBoost model | 0.650 (0.586, 0.714) | 0.444 | 0.192 | 0.398 | 0.602 | 0.603 (0.537, 0.668) | 0.507 | 0.185 | 0.398 | 0.602 |
| CTV-GTV_DVH XGBoost model | 0.562 (0.503, 0.626) | 0.503 | 0.484 | 0.432 | 0.587 | 0.544 (0.473, 0.603) | 0.500 | 0.480 | 0.400 | 0.586 |
| CTV-GTV_Dosiomic XGBoost model | 0.568 (0.514, 0.620) | 0.549 | 0.554 | 0.503 | 0.597 | 0.536 (0.455, 0.636) | 0.500 | 0.464 | 0.446 | 0.546 |
| CTV-GTVtb_Combined XGBoost model | 0.814 (0.750, 0.875) | 0.766 | 0.724 | 0.878 | 0.655 | 0.597 (0.526, 0.667) | 0.489 | 0.250 | 0.733 | 0.426 |
| CTV-GTVtb_Radiomic XGBoost model | 0.862 (0.791, 0.939) | 0.739 | 0.817 | 0.672 | 0.819 | 0.503 (0.420, 0.588) | 0.420 | 0.210 | 0.633 | 0.365 |
| CTV-GTVtb_Clinical XGBoost model | 0.574 (0.515, 0.626) | 0.426 | 0.340 | 0.802 | 0.198 | 0.554 (0.526, 0.580) | 0.323 | 0.166 | 0.802 | 0.198 |
| CTV-GTVtb_DVH XGBoost model | 0.790 (0.706, 0.855) | 0.722 | 0.723 | 0.747 | 0.707 | 0.610 (0.551, 0.668) | 0.593 | 0.203 | 0.300 | 0.669 |
| CTV-GTVtb_Dosiomic XGBoost model | 0.856 (0.804, 0.909) | 0.487 | 0.300 | 0.581 | 0.484 | 0.527 (0.430, 0.633) | 0.545 | 0.158 | 0.501 | 0.556 |
| CTV-GTV_Combined LR model | 0.871 (0.848, 0.892) | 0.805 | 0.772 | 0.886 | 0.720 | 0.706 (0.640, 0.774) | 0.593 | 0.567 | 0.740 | 0.466 |
| CTV-GTV_Radiomic LR model | 0.681 (0.651, 0.710) | 0.615 | 0.642 | 0.615 | 0.604 | 0.578 (0.570, 0.588) | 0.500 | 0.452 | 0.571 | 0.439 |
| CTV-GTV_Clinical LR model | 0.769 (0.739, 0.800) | 0.620 | 0.372 | 0.453 | 0.722 | 0.557 (0.543, 0.571) | 0.557 | 0.316 | 0.428 | 0.669 |
| CTV-GTV_DVH LR model | 0.615 (0.538, 0.698) | 0.564 | 0.700 | 0.549 | 0.558 | 0.605 (0.594, 0.622) | 0.535 | 0.784 | 0.464 | 0.597 |
| CTV-GTV_Dosiomic LR model | 0.736 (0.697, 0.771) | 0.656 | 0.641 | 0.711 | 0.599 | 0.551 (0.464, 0.639) | 0.535 | 0.501 | 0.661 | 0.426 |
| CTV-GTVtb_Combined LR model | 0.995 (0.993, 0.998) | 0.964 | 0.977 | 0.952 | 0.974 | 0.512 (0.461, 0.562) | 0.428 | 0.249 | 0.834 | 0.322 |
| CTV-GTVtb_Radiomic LR model | 0.773 (0.742, 0.807) | 0.648 | 0.501 | 0.602 | 0.646 | 0.573 (0.559, 0.583) | 0.482 | 0.301 | 0.734 | 0.416 |
| CTV-GTVtb_Clinical LR model | 0.819 (0.792, 0.843) | 0.612 | 0.635 | 0.834 | 0.303 | 0.646 (0.600, 0.712) | 0.371 | 0.222 | 0.735 | 0.276 |
| CTV-GTVtb_DVH LR model | 0.772 (0.749, 0.797) | 0.685 | 0.695 | 0.678 | 0.679 | 0.591 (0.562, 0.610) | 0.241 | 0.168 | 0.667 | 0.130 |
| CTV-GTVtb_Dosiomic LR model | 0.819 (0.785, 0.854) | 0.751 | 0.733 | 0.793 | 0.707 | 0.574 (0.564, 0.584) | 0.386 | 0.205 | 0.667 | 0.313 |
Fig. 3.
Receiver Operating Characteristic curves for four machine learning models based on GTV, GTVtb, CTV, CTV-GTV and CTV-GTVtb in predicting radiotherapy treatment response within the training cohort
Fig. 4.
Receiver Operating Characteristic curves for four machine learning models based on GTV, GTVtb, CTV, CTV-GTV and CTV-GTVtb in predicting radiotherapy treatment response within the validation cohort
SVM models constructed using clinical and DVH features for each ROI—GTV, GTVtb, CTV, TV-GTV and CTV-GTVtb—demonstrated limited discriminative power. The clinical SVM models yielded integrated AUCs of 0.733,0.716,0.649,0.738 and 0.661, while the corresponding DVH models achieved 0.799,0.569,0.722,0.730 and 0.756 in the training cohort. Consistently, in the validation cohort, AUCs for the clinical models were 0.558,0.625,0.565,0.575 and 0.652, with the DVH models performing similarly at 0.629,0.522,0.631,0.606 and 0.554 suggesting suboptimal predictive capability.
A comparable trend was observed with the RF classifiers. In the training cohort, AUCs for the clinical RF models were 0.627,0.665,0.572,0.604 and 0.627 cross GTV, GTVtb, CTV, TV-GTV and CTV-GTVtb, respectively, whereas the DVH-based models scored 0.577,0.708,0.565,0.556 and 0.731. In the validation cohort, clinical RF models yielded 0.587,0.567,0.556,0.552 and 0.555, while their DVH counterparts reached 0.566, 0.520,0.554, 0.554, and 0.563, again reflecting suboptimal performance.
Similarly, models based on the XGBoost algorithm failed to achieve satisfactory predictive accuracy. For clinical features, the training AUCs were 0.615,0.592,0.656, 0.650 and 0.574, whereas DVH-based XGBoost models produced AUCs of 0.541, 0.740, 0.654, 0.562 and 0.790. In the validation cohort, clinical model AUCs were 0.588,0.551,0.569,0.603 and 0.554, with the DVH models recording 0.588, 0.621, 0.585, 0.500 and 0.593.
LR models followed a similar pattern. Clinical models produced training AUCs of 0.763,0.832,0.709,0.769 and 0.819, and DVH-based models reached 0.615, 0.855, 0.635, 0.615 and 0.772. In the validation cohort, clinical LR models showed AUCs of 0.556,0.639,0.565,0.557 and 0.646, while DVH models attained 0.511, 0.666, 0.558, 0.605, and 0.591. Collectively, these results suggest that both clinical and DVH features, when used independently across multiple classifiers, offer limited prognostic value.
The clinical and DVH models exhibited relatively modest predictive performance, likely reflecting the limited discriminatory capacity of clinical variables and DVH parameters when used in isolation, without the integration of radiomic and dosiomic features.
Feature selection
All features were assessed for missing data before feature selection. Since no variable had more than 10% missingness, none were excluded. Mean imputation was applied to handle the remaining missing values.
Radiomic features, dosiomic features, and radiomic features from a shape-feature-excluded set were initially selected based on univariate analysis. Specifically, 33, 15, 32, 17, and 22 radiomic features; 36, 41, 25, 39, and 29 dosiomic features; and 17, 52, 33, 28, and 13 shape-excluded radiomic features were identified for the GTV, GTVtb, CTV, CTV–GTV, and CTV–GTVtb regions, respectively.
Given the sample size and the commonly recommended rule of at least 10 patients per feature [29], recursive feature elimination (RFE) was performed to reduce dimensionality. As a result, five radiomic features, five dosiomic features, and five shape-excluded radiomic features were retained for model development (Table 4).
Table 4.
Features selected for different rois in Radiomic, Dosiomic, and combined models
| ROIs | Radiomic Features(R) for radiomic model | Dosiomic Features(D) for dosimic model | Radiomic Features(R) for combined model (selected from the shape-feature-excluded radiomic feature set) |
|---|---|---|---|
| GTV | shape_Maximum2DDiameterSlice | firstorder_Kurtosis | firstorder_10Percentile |
| shape_MajorAxisLength | firstorder_Maximum | firstorder_Mean | |
| shape_MeshVolume | shape_MajorAxisLength | firstorder_Energy | |
| shape_VoxelVolume | firstorder_Variance | glszm_GrayLevelNonUniformity | |
| firstorder_TotalEnergy | shape_Maximum2DDiameterSlice | firstorder_TotalEnergy | |
| GTVtb | glszm_GrayLevelNonUniformity | shape_Elongation | ngtdm_Busyness |
| shape_MajorAxisLength | firstorder_Uniformity | gldm_LargeDependenceHighGrayLevelEmphasis | |
| ngtdm_Busyness | firstorder_Minimum | glszm_GrayLevelNonUniformity | |
| shape_Maximum3DDiameter | shape_MajorAxisLength | firstorder_Energy | |
| shape_Elongation | firstorder_Entropy | firstorder_TotalEnergy | |
| CTV | gldm_SmallDependenceLowGrayLevelEmphasis | firstorder_MeanAbsoluteDeviation | gldm_SmallDependenceLowGrayLevelEmphasis |
| firstorder_Kurtosis | shape_MajorAxisLength | glrlm_GrayLevelNonUniformity | |
| shape_Maximum2DDiameterSlice | firstorder_Minimum | firstorder_Kurtosis | |
| firstorder_RootMeanSquared | shape_Maximum2DDiameterSlice | firstorder_Skewness | |
| glrlm_GrayLevelNonUniformity | firstorder_Variance | glszm_ZoneVariance | |
| CTV-GTV | shape_Elongation | shape_Elongation | glrlm_ShortRunLowGrayLevelEmphasis |
| shape_Maximum2DDiameterColumn | glrlm_ShortRunLowGrayLevelEmphasis | glszm_LowGrayLevelZoneEmphasis | |
| firstorder_Kurtosis | glcm_Correlation | glcm_Correlation | |
| firstorder_Maximum | glrlm_RunPercentage | gldm_DependenceEntropy | |
| firstorder_Skewness | gldm_SmallDependenceLowGrayLevelEmphasis | firstorder_Minimum | |
| CTV-GTVtb | shape_Maximum2DDiameterColumn | shape_MeshVolume | glrlm_GrayLevelNonUniformity |
| glszm_ZoneVariance | firstorder_Uniformity | glszm_ZoneVariance | |
| gldm_GrayLevelNonUniformity | firstorder_Entropy | gldm_GrayLevelNonUniformity | |
| glszm_GrayLevelNonUniformity | shape_Maximum2DDiameterSlice | firstorder_Energy | |
| shape_Maximum2DDiameterSlice | shape_MinorAxisLength | glszm_LargeAreaLowGrayLevelEmphasis |
To evaluate potential feature redundancy, Spearman correlation coefficients were calculated between all selected feature pairs in five ROIs (Supplementary Figs. 1–5).
Among the radiomic feature pairs, dosiomic feature pairs, and shape-excluded radiomic feature pairs, no pair exhibited a Spearman correlation coefficient greater than 0.9. Therefore, all selected features were retained for subsequent model construction.
Performance of radiomic models, dosiomic models and combined models to assess radiotherapy treatment response
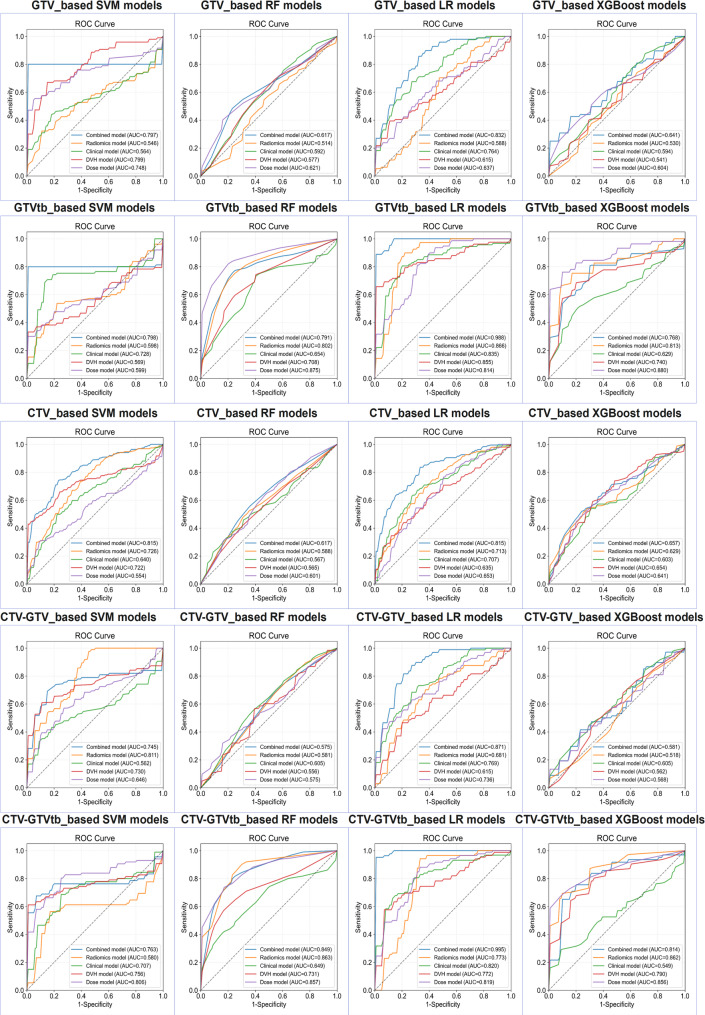
The performance of radiomic, dosiomic, and combined models for evaluating radiotherapy treatment response was assessed using optimal SVM models with a linear kernel. Tables 2 and 3 presents the performance metrics for these models in both the training and validation cohorts. Additionally, Figs. 3 and 4 illustrates the ROC curves for the radiomic, dosiomic, and combined models in both cohorts.
Among all tested models, the CTV-based combined model using an SVM with a linear kernel (C = 1.0) achieved the best overall performance. This model outperformed all other CTV-based models, achieving a notable AUC of 0.815 (95% CI: 0.790–0.839) in the training cohort and 0.728 (95% CI: 0.717–0.739) in the validation cohort, surpassing the clinical, radiomic, dosiomic, and DVH-based models.
For GTV-based models, the GTV_combined model using the XGBoost algorithm demonstrated superior performance, yielding an AUC of 0.675 (95% CI: 0.581–0.747) in the validation cohort.
Among the GTVtb-based models, the GTVtb_combined model with logistic regression achieved the highest AUCs: 0.988 (95% CI: 0.984–0.994) in the training cohort and 0.698 (95% CI: 0.546–0.793) in the validation cohort, outperforming all other models based on the GTVtb region.
To further investigate the predictive value of the peritumoral region, we evaluated models based on the CTV–GTV region. The CTV–GTV_combined LR model achieved strong performance, with an AUC of 0.871 (95% CI: 0.848–0.892) in the training cohort and 0.706 (95% CI: 0.640–0.774) in the validation cohort, outperforming all other models in this region.
In contrast, the predictive performance of models based on the CTV–GTVtb region was generally inferior. Although the CTV–GTVtb_combined LR model achieved a high AUC of 0.995 (95% CI: 0.993–0.998) in the training cohort, most models based on radiomic, dosiomic, or DVH features yielded AUCs below 0.6 in the validation cohort. These results suggest that the CTV–GTVtb region may have limited value in predicting radiotherapy response.
Evaluation and interpretability of the CTV_combined SVM model
To evaluate and interpret the CTV_combined model, calibration plots were generated, visually confirming the alignment between the actual and predicted probabilities of the combined models in both the training and validation cohorts (Supplementary Fig. 6). Decision curves of CTV_combined SVM model (Supplementary Fig. 7) demonstrated stable clinical utility in the low-to-intermediate threshold range, supporting its use in identifying patients at moderate or low risk. These plots highlighted the effectiveness of the combined model, which integrated comprehensive data from dose maps, CT images, and clinical characteristics. The results of the DeLong test (as shown in Supplementary Table 8) demonstrated that the AUC of the CTV_combined SVM model was significantly higher than that of the individual CTV-based SVM models (p < 0.05), indicating its superior discriminatory performance.
The SHAP method was utilized to provide a quantitative explanation for the SVM models. Notably, the CTV_combined SVM model demonstrated the highest integrated AUCs in the validation cohort, making it a prime candidate for SHAP interpretation.
SHAP summary plots (Fig. 5) showed the importance and distribution of each feature’s impact on the model’s output. In Fig. 5, we focused on the top ten features of the CTV_combined SVM model, identifying that four dosiomic features, two dosimetric features, one clinical feature and three radiomic features were particularly effective in distinguishing between DCR and non-DCR classifications. Detailed descriptions of these features can be found in Supplementary Information 5.
Fig. 5.
SHAP summary plots for the CTV_combined SVM model, with dosiomic features (D) and radiomic features (R)
The SHAP summary plots and the SHAP feature importance plots for the CTV_combined SVM model (including all features), as well as for the other CTV-based SVM models, were presented in Supplementary Fig. 8–12, which facilitate further interpretation of the overall model.
The force plot (Fig. 6) provided an individualized interpretation by visualizing how each feature’s SHAP value influenced a specific patient’s assessment, either positively or negatively. The length of each arrow represented the magnitude of a feature’s contribution, with colors indicating positive (red) or negative (blue) contributions. Further details are provided in Supplementary Information 6.
Fig. 6.
SHAP force plots illustrating how the CTV_combined SVM model distinguishes treatment response in two patients, with D: dosiomic features, R: radiomic features, MAL: MajorAxisLength, SDLGLE: SmallDependenceLowGrayLevelEmphasis, MDS: Maximum2DDiameterSlice, GLNU: GrayLevelNonUniformity
SHAP interaction plots (Fig. 7) reveal pairwise feature interactions in the CTV_combined SVM model. The plots highlight how combinations of top-ranked 5 features jointly contribute to model predictions, with color gradients indicating feature value ranges and horizontal dispersion reflecting interaction strength.
Fig. 7.
SHAP interaction plots visualize feature dependencies by illustrating the interaction values between top 5 features, with D: dosiomic features, MDS: Maximum2DDiameterSlice, MAL: MajorAxisLength
Additionally, the results of the logistic regression analysis (Supplementary Table 9) demonstrated that D.firstorder_Variance and D.firstorder_MeanAbsoluteDeviation exhibited notable explanatory power, with a pseudo R² value of 0.118 and 0.175. Although pseudo R² values are typically small and less intuitive than metrics such as AUC, accuracy, sensitivity, and specificity, the pseudo R² of this dosiomic feature ranked among the highest, indicating that dosiomic features contributed substantially to the model’s predictive performance.
To further investigate the potential confounding effects of age and tumor grade, subgroup analyses were conducted. The results demonstrated that the CTV_combined SVM model maintained good predictive performance in the validation cohort, with AUCs of 0.764 and 0.767 in the age subgroup (> 40 years) and across tumor grades (WHO grade 3–4), respectively (Supplementary Fig. 13 and Supplementary Table 10; Supplementary Fig. 14 and Supplementary Table 10), supporting the model’s robustness. In contrast, the model showed limited performance in patients aged ≤ 40 years (AUC = 0.585) and comparable performance in those with WHO grade 2 tumors (AUC = 0.581) (Supplementary Table 10). These findings may be partly explained by the relatively small sample sizes in these subgroups, which could have influenced model performance.
Finally, feature maps were employed to visualize the spatial distribution of dosiomic features (as presented in Supplementary Fig. 8(c)), providing insights into the anatomical locations and patterns through which these features influence model predictions.
Discussion
In this study, we demonstrated that dosiomic-based models could effectively predict the treatment response to radiotherapy in patients with glioma. The CTV_combined SVM model, which integrated clinical, radiomic, dosiomic, and DVH information, showed good performance.
Unlike findings reported in other studies, clinical and dosimetric factors in our analysis did not demonstrate strong predictive performance for glioma prognosis [14]. Nevertheless, both the clinical and DVH-based models showed limited effectiveness, likely due to the insufficient biological and dosimetric information captured by these features alone. Although we recognize that clinical variables and dosimetric parameters—such as tumor grade or maximum dose—play a critical role in guiding dose prescription in routine practice [5, 13], they fall short of fully capturing the biological complexity of gliomas and the heterogeneity in treatment response. Therefore, it is necessary to incorporate a broader range of data modalities, including radiomic and dosiomic, features, to enhance model performance.
When radiomic and dosiomic information were integrated, the performance of the CTV_combined model improved compared to using clinical features or dosimetric features alone. This finding underscores the significant contribution of radiomic and dosiomic information to model discrimination. Radiomics can transform complex imaging information of tumors into quantifiable feature data, thereby providing valuable information for clinical decision-making [34, 35]. Similar to our findings, M. Patel et al. used quantitative radiomic features from MRI to predict glioblastoma treatment response, achieving an AUC of 0.80 [36]. Additionally, Liu et al. identified radiomic features derived from brain MRI that showed potential in predicting recurrence in glioma patients after radiotherapy [37].Dosiomics provided the distribution of radiotherapy dose information for radiation oncologists by transforming the complex dose distribution data into quantifiable features [15]. Studies have found that dosiomics can predict the treatment response to radiotherapy for patients with tumors, which is consistent with our findings [19–21, 38].
GTV represented the total volume of the visible tumor identified [39], GTVtb referred to the volume of tissue where the primary tumor was located prior to surgical removal. CTV encompassed the volume of tissue containing the GTV or GTVtb, as well as regions at risk of containing microscopic disease [40–42]. Owing to the different physiopathologic information in each radiotherapy target volume, dosiomic and radiomic features within different radiotherapy target volumes were used for predicting the treatment response. We found that the CTV_combined SVM model achieved the best performance. This may be because the CTV_combined SVM model not only integrated clinical information, tumor information, and dose distribution information, but also because CTV contained complete physiopathologic information of the tumor and the tumor microenvironment. To investigate the role of the peritumoral microenvironment in treatment response prediction, we further constructed models based on two ROIs: CTV–GTV and CTV–GTVtb. The results showed that the CTV–GTV_Combined LR model achieved an AUC of 0.706 in the validation cohort, which was slightly lower than that of the best-performing model. This suggests that the CTV–GTV region, representing the peritumoral microenvironment, possesses predictive capability; however, due to the lack of information from the tumor core, its performance was inferior to models based on the entire CTV.
Despite using identical radiomic feature sets for GTV and GTVtb cohorts, we observed noticeable variations in model performance across different machine learning algorithms. This inconsistency can be attributed to three key factors: (1) differences in model assumptions and learning capacity—while logistic regression assumes linearity, tree-based models like random forest and XGBoost capture complex nonlinear interactions more effectively; (2) limited sample size, which increases the risk of overfitting for high-capacity models despite the use of cross-validation and hyperparameter tuning; and (3) sensitivity to data preprocessing—models such as SVM and logistic regression are more influenced by feature scaling, whereas ensemble methods are more robust. These factors collectively contribute to the observed variability in ROC curves, even under consistent feature inputs.
To assess the robustness of the best-performing model across different age groups and tumor grades, and to support individualized clinical decision-making, a subgroup analysis was performed. The results demonstrated that the CTV_combined SVM model maintained favorable predictive performance in both the older age subgroup (> 40 years) and in patients with higher-grade tumors (WHO grade 3–4), indicating strong robustness in these populations. However, a slight decline in performance was observed among patients aged ≤ 40 years and those with lower-grade tumors (WHO grade 2).Several potential explanations may account for this observation. First, the relatively small sample sizes in the ≤ 40-year and WHO grade 2 subgroups may have led to reduced model stability and increased prediction variance, thereby limiting generalizability. Second, younger patients and those with low-grade tumors may exhibit distinct clinical or dosimetric characteristics compared to their counterparts. For example, they tend to have lower tumor grades, smaller target volumes, and may receive lower radiation doses, all of which can affect tumor radiosensitivity and alter treatment response patterns. These differences may challenge the model’s ability to generalize from the overall training cohort to these specific subpopulations. Moreover, unmeasured confounding factors—such as tumor molecular subtypes (e.g., IDH mutation status) or underlying genetic profiles—may also contribute to the observed performance discrepancy. Further validation using larger and more balanced datasets across age and tumor grade subgroups is warranted to enhance model robustness and ensure its applicability to broader patient populations, particularly younger individuals and those with low-grade gliomas.
In this study, SHAP analysis revealed the high importance of dosiomic features in the predictive model. Notably, all three of the top-ranked features contributing to model performance were dosiomic. Furthermore, logistic regression analysis was conducted on features derived from the optimal model (CTV_combined SVM model), and the pseudo-R² values indicated that D.firstorder_Variance and D.firstorder_MeanAbsoluteDeviation exhibited strong explanatory power. These findings collectively suggest that dosiomic features played a substantial role in the model’s predictive capability.
Based on the SHAP analysis, the contribution of individual features to the model’s predictions can be clearly visualized. The SHAP summary plot of the CTV_combined SVM model revealed that patients in the DCR group tended to exhibit higher values of “D.firstorder.Variance” and lower values of “D.shape.Maximum2DDiameterSlice,” suggesting that the radiotherapy dose was concentrated around the peritumoral core region and that a non-uniform dose distribution (e.g., dose gradient) was applied in this area. Additionally, higher “D.firstorder.Minimum” values observed in non-DCR patients further indicated that even the lowest-dose voxels within the CTV received relatively high radiation doses, implying that the treatment plan failed to establish an effective dose gradient. From a radiomics perspective, patients with higher R.glrlm.GrayLevelNonUniformity values (red dot) may exhibit greater gray-level heterogeneity and lower textural complexity within the CTV region on CT images. This feature likely reflects the presence of structurally ambiguous, low-density, hypometabolic, or necrotic areas, which have been associated with poor radiotherapy response and unfavorable prognosis [43, 44], thereby indicating suboptimal treatment outcomes.
Considering that the margin region may reflect the tumor microenvironment, we conducted additional analyses on both the GTV-combined model and the CTV-GTV-combined model using SHAP analysis (Supplementary Fig. 15–16), as well as univariate and multivariable logistic regression (Supplementary Tables 11–12). The pseudo-R² values from the logistic regression indicated that dosiomic features demonstrated relatively strong explanatory power. According to the SHAP summary plots, heterogeneous dose distribution within the GTV region—characterized by high D.Variance and D.Kurtosis—was associated with tumor recurrence (non-DCR group). In the CTV-GTV region, the dose distribution appeared less compact, asymmetric, and elongated in the 2D plane, as evidenced by higher values of D.Maximum2DDiameterColumn, D.Skewness, and D.Elongation. These findings suggest that the corresponding radiotherapy plans may have poor dose conformity, uneven target coverage, and potential hotspot displacement, which may negatively impact treatment efficacy. From a radiomics perspective, patients with poor treatment response also exhibited lower values of R.ShortRunLowGrayLevelEmphasis and R.LowGrayLevelZoneEmphasis in the CTV-GTV (margin) region. This may reflect a more solid tumor texture with higher cellular density, which is commonly associated with more aggressive and radioresistant tumor phenotypes. Clinically, such tumors often exhibit invasive growth patterns and lower rates of local control.
Although the SHAP summary plots for other CTV-based models do not exhibit an identical ranking of feature importance compared to the CTV_combined model, the overall trend in their predictive influence on treatment response remains largely consistent. This discrepancy may stem from the fact that, in single kind of feature modeling, each feature’s contribution is assessed independently without accounting for potential interactions. In contrast, when multiple kinds of features are combined, the model can leverage inter-feature relationships to refine its predictions, potentially amplifying or diminishing the importance of certain features.
A local explanation was provided using the SHAP force plot. In Fig. 6(a), the SHAP value for the patient was higher than the base value, leading the model to classify this patient into the non-DCR group. The patient had a high " D.MDS(Maximum2DDiameterSlice)” value, which made a significant positive contribution (indicated in red) to this classification. The longer arrow for " D.MDS " compared to other positively contributing features demonstrated its substantial influence on the classification. In addition to evaluating individual feature contributions, SHAP interaction analysis was performed to further investigate potential synergistic effects among key predictive features. As illustrated in Fig. 7, most SHAP interaction values were centered around zero, suggesting that the model relied primarily on the independent effects of each feature rather than on feature–feature interactions. However, moderate interactions were observed between shape-related dosiomic features, such as D.MAL (MajorAxisLength) and D.MDS (Maximum2DDiameterSlice), indicating that the geometric characteristics of dose distribution—specifically, elongated versus compact spatial patterns—may jointly influence treatment response prediction. Moreover, weak interactions between D.Variance and patient age suggest that the impact of dose heterogeneity may vary across different age groups, potentially reflecting differences in tissue sensitivity or biological response. Interestingly, the joint presence of high D.Minimum and high D.Variance values appeared to modulate model predictions in a non-linear manner, implying that although high minimum doses might reflect adequate coverage, an overly heterogeneous dose distribution could compromise therapeutic efficacy. These findings underscore the importance of not only achieving sufficient dose levels but also considering spatial dose distribution characteristics in personalized radiotherapy planning.
Therefore, we propose that the CTV_combined SVM model may serve as a non-invasive clinical decision-support tool to help radiation oncologists identify patients who are less likely to benefit from standard dose prescriptions. This might enable early treatment adaptation or intensification strategies. Furthermore, the model holds potential for integration into existing radiotherapy planning systems, offering individualized risk stratification based on pre-treatment CT and dose distribution map.
This research has certain limitations. Primarily, a key focus of this study was leveraging dosiomics to evaluate the spatial distribution of radiation dose and its correlation with treatment response. Consequently, the analysis was confined to CT images and dose maps. Moving forward, future research will integrate other imaging modalities, such as MRI, which offers comprehensive biological insights, to enhance the model’s predictive performance and clinical utility. Secondly, it is a retrospective analysis conducted at a single institution, with training and validation cohorts separated temporally. Additionally, radiomic and dosiomic features were derived from predefined radiotherapy target volumes. To enhance the model’s generalizability, future investigations should incorporate a broader and more heterogeneous patient cohort from multiple institutions. In the future, we plan to collect a larger dataset with consistently annotated radiotherapy target volumes to explore this further. Furthermore, we plan to compute the intraclass correlation coefficient to assess feature reproducibility across different observers and time points, thereby strengthening the reliability of our results. Lastly, while SHAP provided semantic feature-based interpretability for the predictive model, it did not directly link radiomic or dosiomic features to underlying pathological mechanisms. Therefore, future studies should consider integrating local microscopic pathological image analysis to bridge this gap.
Conclusions
The proposed CTV_combined SVM model, which incorporates dosiomic and radiomic features, might be valuable for predicting the treatment response to radiotherapy in patients with glioma. The SHAP method may help interpret the CTV_combined SVM model, providing radiation oncologists with a clearer understanding of the model. Beyond its technical merits, this model has the potential to be incorporated into clinical decision-support systems, aiding radiation oncologists in tailoring radiotherapy strategies based on individualized CT and dose map. Future work should focus on prospective validation across multi-center cohorts and seamless integration into radiotherapy planning workflows, ultimately moving toward more adaptive and biologically informed treatment paradigms.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
None.
Abbreviations
- AUC
Area under the curve
- CI
Confidence interval
- CTV
Clinical target volume
- DCR
Disease control rate
- DVH
Dose-volume histogram
- GTV
Gross tumor volume
- GTVtb
Gross tumor volume tumor bed
- LR
Logistic regression
- MRI
Magnetic resonance imaging
- RANO
Response Assessment in Neuro-Oncology
- RF
Random forest
- ROC
Receiver-operating characteristic
- ROIs
Regions of interest
- RTOG
Radiation Therapy Oncology Group
- SHAP
SHapley Additive exPlanations
- SVM
Support vector machine
Author contributions
Yixin Wang Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Validation, Visualization, Writing – original draft. Yongkang Zhang Methodology, Software, Writing – review & editing. Lin Lin Data curation, Methodology. Zongtao Hu Supervision, Writing – review & editing. Hongzhi Wang Conceptualization, Data curation, Formal analysis, Project administration, Resources, Supervision, Validation, Visualization, Writing – review & editing.
Funding
This work was supported by the CASHIPS Director’s Fund, Grant No.YZJJ2024QN48. Open access funding provided by the CASHIPS Director’s Fund, Grant No.YZJJ2024QN48.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was conducted in accordance with the ethical principles outlined in the Declaration of Helsinki. Ethical approval was obtained from the Ethics Committee of Hefei Cancer Hospital, Chinese Academy of Sciences (Approval No. PJ-KY2024-004). Informed consent was obtained from all individual participants included in the study.
Consent for publication
The authors consent to publish the manuscript in its current form.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Schaff LR, Mellinghoff IK. Glioblastoma and other primary brain malignancies in adults: A review. JAMA. 2023;329(7):574–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antoni D, Feuvret L, Biau J, Robert C, Mazeron JJ, Noel G. Radiation guidelines for gliomas. Cancer Radiother. 2022;26(1–2):116–28. [DOI] [PubMed] [Google Scholar]
- 3.Tan AC, Ashley DM, Lopez GY, Malinzak M, Friedman HS, Khasraw M. Management of glioblastoma: state of the Art and future directions. CA Cancer J Clin. 2020;70(4):299–312. [DOI] [PubMed] [Google Scholar]
- 4.Zhou X, Liao X, Zhang B, He H, Shui Y, Xu W, Jiang C, Shen L, Wei Q. Recurrence patterns in patients with high-grade glioma following temozolomide-based chemoradiotherapy. Mol Clin Oncol. 2016;5(2):289–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang T, Nam DH, Ram Z, Poon WS, Wang J, Boldbaatar D, Mao Y, Ma W, Mao Q, You Y, et al. Clinical practice guidelines for the management of adult diffuse gliomas. Cancer Lett. 2021;499:60–72. [DOI] [PubMed] [Google Scholar]
- 6.Koutsarnakis C, Neromyliotis E, Komaitis S, Mazarakis N, O’Hara DJ, Stranjalis G, Chumas P. Effects of brain radiotherapy on cognitive performance in adult low-grade glioma patients: A systematic review. Radiother Oncol. 2021;160:202–11. [DOI] [PubMed] [Google Scholar]
- 7.Wu S, Xu J, Li G, Jin X. Integrating radiosensitivity gene signature improves glioma outcome and radiotherapy response prediction. Med (Kaunas). 2022;58(10). [DOI] [PMC free article] [PubMed]
- 8.Lamborn KR, Chang SM, Prados MD. Prognostic factors for survival of patients with glioblastoma: recursive partitioning analysis. Neuro Oncol. 2004;6(3):227–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weller M, van den Bent M, Preusser M, Le Rhun E, Tonn JC, Minniti G, Bendszus M, Balana C, Chinot O, Dirven L, et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat Rev Clin Oncol. 2021;18(3):170–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou Q, Xue C, Ke X, Zhou J. Treatment response and prognosis evaluation in High-Grade glioma: an imaging review based on MRI. J Magn Reson Imaging. 2022;56(2):325–40. [DOI] [PubMed] [Google Scholar]
- 11.Park CJ, Han K, Kim H, Ahn SS, Choi YS, Park YW, Chang JH, Kim SH, Jain R, Lee SK. Radiomics risk score May be a potential imaging biomarker for predicting survival in isocitrate dehydrogenase wild-type lower-grade gliomas. Eur Radiol. 2020;30(12):6464–74. [DOI] [PubMed] [Google Scholar]
- 12.Kocher M, Ruge MI, Galldiks N, Lohmann P. Applications of radiomics and machine learning for radiotherapy of malignant brain tumors. Strahlenther Onkol. 2020;196(10):856–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Unkelbach J, Menze BH, Konukoglu E, Dittmann F, Ayache N, Shih HA. Radiotherapy planning for glioblastoma based on a tumor growth model: implications for Spatial dose redistribution. Phys Med Biol. 2014;59(3):771–89. [DOI] [PubMed] [Google Scholar]
- 14.Chen H, Li C, Zheng L, Lu W, Li Y, Wei Q. A machine learning-based survival prediction model of high grade glioma by integration of clinical and dose-volume histogram parameters. Cancer Med. 2021;10(8):2774–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liang B, Yan H, Tian Y, Chen X, Yan L, Zhang T, Zhou Z, Wang L, Dai J. Dosiomics: extracting 3D Spatial features from dose distribution to predict incidence of radiation pneumonitis. Front Oncol. 2019;9:269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Z, Wang Z, Yan M, Yu J, Dekker A, Zhao L, Wee L. Radiomics and dosiomics signature from whole lung predicts radiation pneumonitis: A model development study with prospective external validation and Decision-curve analysis. Int J Radiat Oncol Biol Phys. 2023;115(3):746–58. [DOI] [PubMed] [Google Scholar]
- 17.Adachi T, Nakamura M, Shintani T, Mitsuyoshi T, Kakino R, Ogata T, Ono T, Tanabe H, Kokubo M, Sakamoto T, et al. Multi-institutional dose-segmented dosiomic analysis for predicting radiation pneumonitis after lung stereotactic body radiation therapy. Med Phys. 2021;48(4):1781–91. [DOI] [PubMed] [Google Scholar]
- 18.Murakami Y, Soyano T, Kozuka T, Ushijima M, Koizumi Y, Miyauchi H, Kaneko M, Nakano M, Kamima T, Hashimoto T, et al. Dose-Based radiomic analysis (Dosiomics) for intensity modulated radiation therapy in patients with prostate cancer: correlation between planned dose distribution and biochemical failure. Int J Radiat Oncol Biol Phys. 2022;112(1):247–59. [DOI] [PubMed] [Google Scholar]
- 19.Wu A, Li Y, Qi M, Lu X, Jia Q, Guo F, Dai Z, Liu Y, Chen C, Zhou L, et al. Dosiomics improves prediction of locoregional recurrence for intensity modulated radiotherapy treated head and neck cancer cases. Oral Oncol. 2020;104:104625. [DOI] [PubMed] [Google Scholar]
- 20.Morelli L, Parrella G, Molinelli S, Magro G, Annunziata S, Mairani A, Chalaszczyk A, Fiore MR, Ciocca M, Paganelli C, et al. A dosiomics analysis based on linear energy transfer and biological dose maps to predict local recurrence in sacral chordomas after carbon-ion radiotherapy. Cancers (Basel). 2022;15(1). [DOI] [PMC free article] [PubMed]
- 21.Liu H, Zhao D, Huang Y, Li C, Dong Z, Tian H, Sun Y, Lu Y, Chen C, Wu H, et al. Comprehensive prognostic modeling of locoregional recurrence after radiotherapy for patients with locoregionally advanced hypopharyngeal squamous cell carcinoma. Front Oncol. 2023;13:1129918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geraghty BJ, Dasgupta A, Sandhu M, Malik N, Maralani PJ, Detsky J, Tseng CL, Soliman H, Myrehaug S, Husain Z, et al. Predicting survival in patients with glioblastoma using MRI radiomic features extracted from radiation planning volumes. J Neurooncol. 2022;156(3):579–88. [DOI] [PubMed] [Google Scholar]
- 23.Lundberg SM, Lee SI. A unified approach to interpreting model predictions. Adv Neur In. 2017;30.
- 24.Wang Y, Lang J, Zuo JZ, Dong Y, Hu Z, Xu X, Zhang Y, Wang Q, Yang L, Wong STC, et al. The radiomic-clinical model using the SHAP method for assessing the treatment response of whole-brain radiotherapy: a multicentric study. Eur Radiol. 2022;32(12):8737–47. [DOI] [PubMed] [Google Scholar]
- 25.Wen PY, van den Bent M, Youssef G, Cloughesy TF, Ellingson BM, Weller M, Galanis E, Barboriak DP, de Groot J, Gilbert MR, et al. RANO 2.0: update to the response assessment in Neuro-Oncology criteria for High- and Low-Grade gliomas in adults. J Clin Oncol. 2023;41(33):5187–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bradbury P, Seymour L. Tumor shrinkage and objective response rates: gold standard for oncology efficacy screening trials, or an outdated end point? Cancer J (Sudbury Mass). 2009;15(5):354–60. [DOI] [PubMed] [Google Scholar]
- 27.Mallett S, Royston P, Dutton S, Waters R, Altman DG. Reporting methods in studies developing prognostic models in cancer: a review. BMC Med. 2010;8:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun GW, Shook TL, Kay GL. Inappropriate use of bivariable analysis to screen risk factors for use in multivariable analysis. J Clin Epidemiol. 1996;49(8):907–16. [DOI] [PubMed] [Google Scholar]
- 29.Halligan S, Menu Y, Mallett S. Why did European radiology reject my radiomic biomarker paper? How to correctly evaluate imaging biomarkers in a clinical setting. Eur Radiol. 2021;31(12):9361–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Smeden M, de Groot JA, Moons KG, Collins GS, Altman DG, Eijkemans MJ, Reitsma JB. No rationale for 1 variable per 10 events criterion for binary logistic regression analysis. BMC Med Res Methodol. 2016;16(1):163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Riley RD, Snell KI, Ensor J, Burke DL, Harrell FE Jr., Moons KG, Collins GS. Minimum sample size for developing a multivariable prediction model: PART II - binary and time-to-event outcomes. Stat Med. 2019;38(7):1276–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vergouwe Y, Steyerberg EW, Eijkemans MJ, Habbema JD. Substantial effective sample sizes were required for external validation studies of predictive logistic regression models. J Clin Epidemiol. 2005;58(5):475–83. [DOI] [PubMed] [Google Scholar]
- 33.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143(1):29–36. [DOI] [PubMed] [Google Scholar]
- 34.Bi WL, Hosny A, Schabath MB, Giger ML, Birkbak NJ, Mehrtash A, Allison T, Arnaout O, Abbosh C, Dunn IF, et al. Artificial intelligence in cancer imaging: clinical challenges and applications. CA Cancer J Clin. 2019;69(2):127–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tomaszewski MR, Gillies RJ. The biological meaning of radiomic features. Radiology. 2021;298(3):505–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patel M, Zhan J, Natarajan K, Flintham R, Davies N, Sanghera P, Grist J, Duddalwar V, Peet A, Sawlani V. Machine learning-based radiomic evaluation of treatment response prediction in glioblastoma. Clin Radiol. 2021;76(8):628. e617-628 e627. [DOI] [PubMed] [Google Scholar]
- 37.Liu C, Li Y, Xia X, Wang J, Hu C. Application of radiomics feature captured from MRI for prediction of recurrence for glioma patients. J Cancer. 2022;13(3):965–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang H, Xue J, Qu T, Bernstein K, Chen T, Barbee D, Silverman JS, Kondziolka D. Predicting local failure of brain metastases after stereotactic radiosurgery with radiomics on planning MR images and dose maps. Med Phys. 2021;48(9):5522–30. [DOI] [PubMed] [Google Scholar]
- 39.Whitfield GA, Kennedy SR, Djoukhadar IK, Jackson A. Imaging and target volume delineation in glioma. Clin Oncol (R Coll Radiol). 2014;26(7):364–76. [DOI] [PubMed] [Google Scholar]
- 40.Niyazi M, Andratschke N, Bendszus M, Chalmers AJ, Erridge SC, Galldiks N, Lagerwaard FJ, Navarria P, Munck Af Rosenschold P, Ricardi U, et al. ESTRO-EANO guideline on target delineation and radiotherapy details for glioblastoma. Radiother Oncol. 2023;184:109663. [DOI] [PubMed] [Google Scholar]
- 41.Nie S, Zhu Y, Yang J, Xin T, Xue S, Sun J, Mu D, Chen Z, Sun P, Yu J, et al. Clinicopathologic analysis of microscopic tumor extension in glioma for external beam radiotherapy planning. BMC Med. 2021;19(1):269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Minniti G, Amelio D, Amichetti M, Salvati M, Muni R, Bozzao A, Lanzetta G, Scarpino S, Arcella A, Enrici RM. Patterns of failure and comparison of different target volume delineations in patients with glioblastoma treated with conformal radiotherapy plus concomitant and adjuvant Temozolomide. Radiother Oncol. 2010;97(3):377–81. [DOI] [PubMed] [Google Scholar]
- 43.Elshafeey N, Kotrotsou A, Hassan A, Elshafei N, Hassan I, Ahmed S, Abrol S, Agarwal A, El Salek K, Bergamaschi S, et al. Multicenter study demonstrates radiomic features derived from magnetic resonance perfusion images identify pseudoprogression in glioblastoma. Nat Commun. 2019;10(1):3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Horsman MR, Overgaard J. The impact of hypoxia and its modification of the outcome of radiotherapy. J Radiat Res. 2016;57(Suppl 1):i90–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.