Abstract
Background
Autism and ADHD are heritable, co-occurrent, and associated with difficulties with executive functioning (cognitive and self-regulation skills which enable us to set and work toward goals). Executive function difficulties, and their negative impacts across cognitive, health and social domains, extend to individuals with first-degree relatives who are autistic or have ADHD, even if they do not meet thresholds for a clinical diagnosis themselves. Supporting executive function development in children with elevated autism traits, or a first-degree relative with autism or ADHD, addresses community priorities for early support to help achieve the best mental health, education and life outcomes.
Methods
This study will evaluate the feasibility and acceptability of a randomized controlled trial (RCT) of a parent-toddler programme entitled “Supporting Toddlers with a connection to autism or ADHD to develop strong Attention, Regulation and Thinking skills” (START). START is a neurodiversity-affirming programme, co-refined through extensive Patient and Public Involvement. Sixty parent-child dyads, in Oxford or Southampton (UK), will be randomized using Sealed Envelope by a researcher not involved in recruitment, delivery or outcome data collection to receive START or usual practice, on a 1:1 ratio. Children (20 months old) will be assessed using questionnaires completed by the parent (not blind to allocation) post-intervention (within 2 weeks of the end of the active intervention wave, when children are aged 27–31 months), and using parent questionnaires and a battery of executive function measures administered by researchers blind to allocation at baseline and follow-up (36 months old). START will be delivered in small groups to 30 parent-child dyads, in community settings.
Discussion
We will assess the feasibility of recruiting eligible participants to the study, the reliability of measures of implementation fidelity and degree of implementation fidelity achieved, the appropriateness of proposed outcome and mechanism measures, the acceptability of an RCT of the programme, parental adherence to the programme, logistics of programme delivery, and the acceptability of START, using mixed-method measures of engagement and satisfaction. Results will inform the design and implementation of a definitive RCT of START, and yield broader insights into the delivery and evaluation of complex early-years interventions in community settings.
Trial registration
ISRCTN registry ISRCTN99820028 https://doi.org/10.1186/ISRCTN99820028.
Keywords: Autism, ADHD, Executive functions, Emotion regulation, Cognitive development, Toddler, Parent, Feasibility, Randomized controlled trial, Neurodiversity
Background
Executive functions are the higher-order cognitive skills that allow us to set and work toward goals. Executive functions include inhibitory control, cognitive flexibility, and working memory. Executive functions develop rapidly in the first few years of life, supported by improvements in attentional control [1], and early variation in these skills set the stage for later cognitive and socio-emotional development [2]. Executive function difficulties are linked to poor mental and physical health and lower quality of life, across a range of populations [2–4].
Autism and ADHD are both associated with difficulties with executive functions, and these difficulties appear to negatively impact on wellbeing, above and beyond the impacts of the core diagnostic traits associated with autism and ADHD [5, 6]. Autism and ADHD often co-occur [7] and tend to cluster in families; a child with an autistic first-degree relative is more likely than average to have ADHD, and vice versa [8, 9]. Those with a family history of autism or ADHD (i.e., a first degree relative who is autistic and/or has ADHD) are also more likely to experience executive function difficulties, even if they do not meet clinical cut-offs for a diagnosis of autism or ADHD themselves [10–13]. Recent work suggests that upwards of 6% of children under 3 years in the UK have a first degree relative who is autistic and/or has ADHD [14]. Thus, a significant proportion of children are at risk for executive function difficulties and associated challenges.
A protocol for a parent-mediated group-based programme to support the development of early executive functions in toddlers with a family history of autism or ADHD was developed. This protocol was informed by community priorities [15, 16], the existing literature on executive function interventions [17], and broader research into executive function development in infants and toddlers [1, 18], and was then refined through piloting and extensive patient and public involvement (PPI) work to result in a neurodiversity-affirming programme as is described in depth in [BLINDED FOR REVIEW]. During this process, the target population was extended to include toddlers who are suspected by their parents to be autistic, whether or not they have a first-degree relative with suspected or diagnosed autism or ADHD, as these children may be particularly in need of early support.
As no previous studies have investigated a parent-mediated early intervention to support the development of executive functions in this population, this initial study will be conducted as a feasibility trial. This will provide valuable insights into the feasibility and acceptability of a randomized controlled trial of this programme specifically, and early parent-mediated group interventions more generally, as well as monitor potential deficiencies in the structure of the programme. The study aims to contribute to the evidence base on improving outcomes for children with a family history of autism or ADHD and to inform a potential, definitive RCT of the effectiveness and cost-effectiveness of START.
Study aims and objectives
The study’s primary objectives are to assess the following:
- The feasibility of an RCT of the START programme, in terms of:
- The feasibility of recruiting eligible participants to the study.
- The reliability of measures of implementation fidelity, and the degree of implementation fidelity achieved.
- The feasibility of proposed outcome measures for a definitive RCT, completion rates, and loss of data due to participant refusal or invalid administration.
- The feasibility of proposed measures of mediating factors, completion rates, and loss of data due to participant refusal or invalid administration.
- The acceptability of an RCT of the programme, in terms of:
- Retention through randomization, intervention or usual practice, and follow-up.
- Satisfaction with study processes.
- Feasibility of the programme, in terms of:
- Parental adherence.
- The logistics of programme delivery.
- Acceptability of the programme, in terms of:
- Engagement with the programme, and barriers and facilitators to engagement.
- Satisfaction with ethos of the programme, and with the programme materials and delivery approach.
Secondary aims of the study are:
To gain insight into usual parenting practice, and access to services and informal support for parents of toddlers with a family history of autism/ADHD.
To inform changes to delivery and implementation processes, and to the intervention logic model prior to a definitive trial.
To gain preliminary insights into potential mechanisms of change
To gain insights into enabling factors/conditions for success for the START programme specifically, and parent-mediated complex early-years interventions more generally.
Design and method
Study design
The study is a 2-arm feasibility randomized controlled trial of the START programme (plus usual practice) versus usual practice. All participants will continue to have access to usual support and advice services (i.e., outside of participation in START).
Study setting
The intervention will be delivered to families face-to-face in one of two local community settings in the UK. Participants assigned to the intervention arm will be informed of the location and schedule for the appropriate group according to their child’s age. Evaluation data will be collected online and in participants’ homes, or at the Oxford BabyLab.
Participant selection
Recruitment for this feasibility trial will be centered around 2 UK locations (Oxford and Southampton), chosen to have relatively high population density, and socio-economic and ethnic diversity.
Eligibility criteria
Inclusion criteria for the child-participant are:
Has either (1) at least 1 first-degree relative with a community diagnosis (as reported by the parent) OR (2) at least 1 first-degree relative with presumed autism who scores above the clinical threshold on an autism screening measure OR (3) at least 1 first-degree relative with presumed ADHD who scores above the clinical threshold on an ADHD screening measure OR (4) has been identified by their parent, or health or Early Years practitioner as showing high autistic traits (note that due to the young age of the child-participant, ADHD traits in the child-participant are not an inclusion criterion).
Is aged 22-months or less at the time of the eligibility screen, and older than 18 months at the time of the final scheduled intervention start date.
Exclusion criteria for the child-participant are:
Has significant uncorrected visual or hearing problems.
Has a known genetic condition associated with developmental delay (e.g., Fragile X Syndrome, Down’s Syndrome, Neurofibromatosis type 1, Tuberous sclerosis complex).
Is currently placed in a 24-h residential placement, or a foster placement due to end before the 36-month follow-up data collection point.
Any parent in their family has already participated in the START trial.
They, or any parent in their family has participated in a research trial involving a parenting intervention or other EF-related intervention (i.e., via an Early Childhood Education and Care setting) within 3 months of the baseline assessment, or during the course of the START trial.
Inclusion criteria for the parent-participant are:
Is aged 18 years or over
Has permanent or temporary guardianship of the child-participant
Provides solo or joint childcare for the child-participant for at least 1 day per week (totaled across the week)
Has at least conversational-level spoken English
Has capacity to attend 12 weekly 1-h intervention sessions located within 60 min average travel time (based on the parent’s anticipated mode of travel) of the relevant delivery venue.
No exclusions or restrictions will be made for concomitant care or community-based interventions, but parent-participants will be asked to report on this during and post intervention. If a parent-participant has more than one eligible child (e.g., multiple births), all eligible children will be invited to participate in the intervention if randomized to the active arm, but research data will be collected only for the eldest eligible child and siblings not included in the research data collection will not be included in the group size limit.
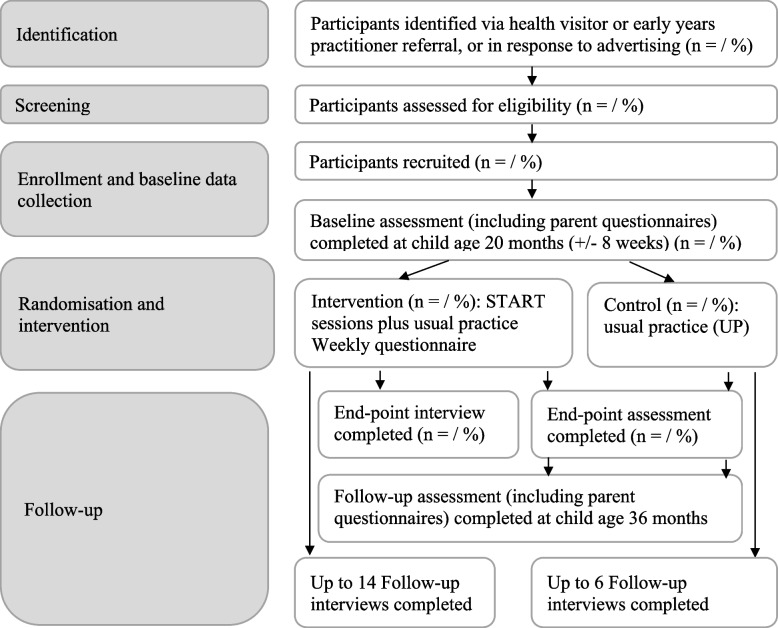
Study flowchart
Figure 1 illustrates the study flowchart.
Fig. 1.
Study flowchart
Intervention
The Supporting Toddlers to develop strong Attention Regulation and Thinking skills (START) module is a manualised programme. It is an extension of the Peep Learning Together Programme (but prior-completion of the Learning Together Programme or Learning Together Programme practitioner training is not a pre-requisite for parents or practitioners), designed to target executive functions, and was developed specifically with toddlers with a family history of autism or ADHD in mind. The programme has been refined through community consultation, as described in detail in Hendry et al. [19].
Intervention design and delivery
START comprises 12 weekly 1-h group sessions, weekly ideas sheets and take-home resources. The sessions are semi-structured parent-child play sessions, comprising facilitator-led group discussion with parents (designed to outline core concepts and to elicit peer-to-peer support), parent-child activities, songs and story sharing relating to a particular attention, regulation, or thinking skill—all of which are designed to support the parent-child relationship and provide enjoyable opportunities for toddlers’ skill development. Each session within the 12-week programme targets a different aspect of executive function development, with initial sessions targeting foundational skills such as attentional control and emotion regulation, and later sessions targeting more-complex skills such as problem-solving. The project ethos is to support all children to thrive, whether they are neurodivergent or neurotypical, and the programme materials emphasize inclusivity (e.g., through making necessary adjustments to the setting and to the activities) and valuing neurodiversity. Facilitator session notes outline the key learning objectives and essential components, but also encourage facilitators to adapt the specific activities to suit their group with some suggestions on why and how to do this.
START sessions are led by 2 facilitators, who are trained early-years professionals, working collaboratively. In the event of one of the facilitators being unavailable to lead a session (e.g., due to illness), the session may proceed with only one trained facilitator, with a temporary facilitator experienced in working with parents or toddlers assisting if possible. One pair of facilitators will be recruited to lead 3 rounds of the START programme in recruitment area 1 and one pair to lead 2 rounds of the START programme in recruitment area 2. The sessions will be scheduled for dates and times likely (on the basis of findings from pre-pilots) to be most convenient to the majority of families, as well as on the basis of venue and practitioner availability. The sessions may be delivered in a range of community settings such as children’s centres or community centers, but will be consistent within each round. Parent-participants are encouraged to attend every session (with the child-participant) in the 12-week cycle they have been allocated to, but it is explicitly acknowledged that there will be opportunities to revisit core content if sessions are missed—and sessions 6 and 12 are designated “recap weeks” for this purpose. If fewer than 2 parents are able to attend a session (i.e., they phone ahead to let the facilitator know they are ill/unavailable), the session will be postponed if the remaining participant prefers; where possible the programme will be extended by a week to accommodate this, but if that is not possible the missed material will be incorporated into the week 6 or week 12 recap. Facilitators will liaise with parent-participants on a regular basis to identify and problem-solve barriers to engagement. At a minimum, parent-participants in an active group will be contacted 1–2 days before each session (by the facilitator or a researcher not involved in outcome data collection), to be reminded of the next session and asked to complete a reflection from the previous week.
Prior to delivering the sessions, both pairs of facilitators will be trained in the principles and approaches of group delivery generally, and START specifically. Additionally, practitioners—and research team members—will receive neurodiversity-awareness training (with a focus on autism and ADHD, but inclusion of relevant commonly co-occurring conditions).
Preparatory interview
Shortly before the first group session in each wave, facilitators will contact parent-participants allocated to that group for a one-to-one telephone (or virtual meeting) interview. In this interview, the facilitator will reiterate the aims of the programme, identify any accessibility needs relating to the sessions or follow-up materials, and discuss the parent-participant’s aims and expectations for the programme. The aim of this interview is to encourage attendance and engagement with the programme.
Usual practice/comparator
The comparator arm will be usual practice (UP). UP includes any service (mainstream and specialised) provided to families and their children as a part of an education, health and care plan or via any other mechanism, as well as early childcare and education (e.g., nursery), and informal activities. Allocation to a mainstream toddler-and-parent programme was initially considered as a comparator arm. However, the Patient and Public Involvement panel for the study indicated that such programmes are often experienced by parents with a family connection to autism or ADHD (who are at an increased likelihood of neurodivergence themselves, and who are raising a child at increased likelihood of neurodivergence) as aversive and non-inclusive due to lack of accommodations for sensory sensitivity, and a promotion of neurotypical norms (e.g., expected milestones and behaviors for toddlers). Therefore, using a mainstream toddler-and-parent programme as comparator would not be appropriate both for ethical reasons and because attrition to the comparator arm would likely be high. To our knowledge, there are no established toddler-and-parent programmes specially-developed for families with a connection to autism and ADHD, and due to the young age of the child-participants, it is anticipated that most will not currently receive any formal specialist support (thus further justifying the omission of an active control comparator arm). However, this will be determined systematically though our measures of UP in order to inform the definitive RCT design.
Randomization/sequence generation
The study is a 2-arm, randomized controlled feasibility trial. Participant dyads (parent-participant and child-participant) will be randomized within 4 weeks post completion of baseline measures. Participants will be randomized using randomly permuted blocks stratified by recruitment area with an equal allocation 1:1 ratio to START in addition to usual practice (UP), or UP alone. Block sizes will be of 2 and 4. This is because long runs pose a potential threat to interpretation if there were a temporal event which could influence child outcomes (e.g., localized lockdowns linked to the Covid-19 pandemic) and there was an imbalance in exposure to that event between conditions. Each recruitment area will be allocated randomization assignments of up to 60, to allow for over-recruitment in one site if necessary. A researcher who is part of the process evaluation but not the assessment team will conduct randomization, using the Sealed Envelope text messaging service, and contact participants to inform them of their allocation. Intervention facilitators will not be involved in recruitment or randomization. The research team responsible for collecting follow-up data (excluding qualitative interviews), and all remaining study team members (including the trial statistician) will remain blind to participants’ allocation until primary analyses are complete. Parent-participants will not be blind to allocation.
Participants randomized to the intervention arm after a group has already begun may join a group if no more than 1 session has already been held. Participants who have provided baseline data but who cannot be randomized (e.g., because the intervention group appropriate for their child’s age has hit capacity of n = 8) will not be randomized to the trial but will be offered the option of continuation within the study as part of a parallel cohort of participants from which follow-up data is still collected. Data from this parallel cohort will not be used to evaluate trial feasibility but may be used to inform a definitive trial (i.e., through better understanding of usual practice).
Retention strategy
To minimize barriers to attendance of the interventions, all participants in the intervention arm will be eligible for reimbursement of the cost of travel to and from the sessions. These costs can be claimed on a weekly basis following attendance of a session, up to a limit of £5 per session without receipt, or £15 per session if a receipt is presented. To increase home use of the suggested activities, after some sessions parent-participants will be provided with relevant resources, up to a value of £5 per session.
Additionally, to encourage retention to study follow-up and to thank participants for their time, child-participants will be provided with a small BabyLab-branded gift with a value of up to £5 after each assessment visit, and parent-participants will be given a £10 online shopping voucher after completion of the questionnaires at the baseline, intervention end-point and follow-up timepoints.
Contact details will be collected during recruitment, and parent-participants will be reminded by email and text message when a data collection follow-up is due. To maximize equity of accessibility of the parent-report measures, given the likely variation in literacy, communication skills, and digital access amongst parent-participants, 3 methods of data collection for questionnaires will be offered: postal, telephone interview, or online (all online questionnaires will be suitable for smartphones). For face-to-face assessments, parent-participants will be offered the option of an assessment in their home, or to come to the University of Oxford BabyLab (in which case travel expenses will be reimbursed). For participants who decline the standard assessments, a minimum dataset consisting of 2 prioritized outcome measures (BRIEF-P and SDQ) will be offered to reduce participant burden and maximize follow-up rates.
Sample size
A total of 60 families (30 families in the START programme arm, 30 in the usual practice (UP) arm) will be recruited. The purpose of this feasibility study is to provide estimates of key parameters for a future trial rather than to detect statistically significant differences [20]. Therefore, formal a priori power calculations have not been computed. Instead, the recruitment target was selected to ensure that if the lower bounds of the target were met (80% of 60 = 48 with a 95% confidence interval around 80% as 68–89% giving a total n of 41–53), sufficient parent-child dyads would be likely to be randomized to the intervention group to be able to run five groups (> 2 dyads per group). Our target of five groups was set to accommodate and monitor variation in fidelity and acceptability across groups, within the pragmatic limitations (budget and time constraints) of the study.
Study outcomes
The primary objectives of this study are to assess the feasibility and acceptability of the START programme, and of an RCT of the programme. Table 1 outlines the definition and criteria for each aspect of the feasibility and acceptability evaluation. Where a criterion is not met, we will review issues that may have affected meeting the criterion for the sample overall, and for sub-sets based on participant characteristics and contextual factors, and consider steps that can be taken to overcome these issues within a full RCT. The study Steering Committee will review this information, along with reports of adverse events, in order to come to a recommendation regarding progression to a definitive trial.
Table 1.
Trial evaluation components, measurement definition, and feasibility criteria
| Evaluation component | Measurement definition | Feasibility criteria |
|---|---|---|
| Feasibility of recruiting eligible participants to the study | Number of eligible participants who agree to participate in the trial |
• Recruitment of at least 80% of the target sample of 60 parent-child dyads is achieved within the study recruitment period • Sufficient child-participants with a birthdate within 6 months of each other are recruited in the same locality to achieve at least 3 families per intervention group after randomization |
| Reliability of measures of implementation fidelity | Fidelity rating scores for sessions | • At least 60% of researcher and practitioner ratings in complete agreement as to the extent to which (a) broad session aims and (b) specific session aims were met |
| Degree of implementation fidelity | Fidelity rating scores for sessions | • At least 70% of active intervention components are rated as partially- or fully-present in all coded group sessions |
|
Feasibility of proposed outcome measures for a definitive RCT and proposed measures of mediating factors |
For each measure: • Completion rates • Loss of data due to participant refusal or invalid administration. |
• The amount of valid data collected on a measure, as a proportion of the number of participants contributing data at that timepoint, is > 70% |
| Acceptability of an RCT of the programme |
Retention: through randomization, intervention or usual practice, and follow-up Satisfaction with study processes (e.g., randomization and data collection), of parent-participants |
• At least 70% of parent-participants seen at baseline are retained for follow-up at the 31-month time-point, and 60% for follow-up at the 36-month time-point • Acceptability of study processes will be assessed through qualitative feedback via the parent-participant interviews at 37 months, taking into account attrition rates and feedback from the Study Leaver questionnaire where available |
| Feasibility of the programme |
Parental adherence to the intervention through parent- and facilitator questionnaires, qualitative interviews and evaluation of session recordings. Logistics of programme delivery, including venue hire, scheduling, practitioner recruitment and training, and delivery costs |
• No set criteria, but barriers and facilitators of programme feasibility will be considered |
| Acceptability of the programme |
Engagement with the intervention: number of sessions attended (captured via attendance logs by the delivery team) and qualitative interviews. Satisfaction with the intervention: questionnaire and qualitative interviews. |
• At least 60% of parent-participants randomized to the intervention, and who confirm they can attend regularly, attend a minimum of 7 sessions • START programme content factors (i.e., The START programme sessions are not enjoyable for me/The START programme sessions are not enjoyable for my child/The START programme sessions are not relevant for my child) are not listed as a strong influence for leaving the study (in the leaver questionnaire) in more than 10% of • A maximum of 30% of parent-participants rate the acceptability (a composite of enjoyability and usefulness ratings) of the sessions as low (average scores of less than 2 on a scale of 1–4) • Acceptability of the intervention will also be assessed through qualitative feedback via free text comments in the Post-Session Feedback Questionnaire and verbal comments during sessions and the parent-participant interviews |
A secondary aim of the study is to gain insight into usual parenting practice, and access to services and informal support for parents of toddlers with a family history of autism/ADHD.
This will be achieved through collecting data on Early Childhood Education and Care and parent-child activities at each timepoint, as well as access to formal and informal services at intervention end-point.
Additional aims of the study are to inform changes to delivery and implementation processes, and to the intervention logic model prior to a definitive trial, and to gain preliminary insights into potential mechanisms of change.
Intervention outcomes
As detailed in Appendix B, the primary outcomes for the intervention itself is the child’s day-to-day executive function skills, as reported via questionnaire by a primary caregiver (the parent-participant; unblind to allocation) at endpoint and follow-up. Secondary intervention outcomes include the child’s day-to-day executive function skills, as reported via questionnaire by a secondary caregiver (e.g., childminder, nursery key person or grandparent), their performance on a problem-solving task, and a battery of executive function tasks, and their socio-emotional strengths and difficulties (see Appendix B for details). Proposed measures have been chosen based on experience in research with toddlers with a family history of autism/ADHD, and of assessment of emergent executive functions. An overview of the measures and timetabling of data collection is presented in Table 2 (SPIRIT figure).
Table 2.
Participant timeline (SPIRIT figure): schedule of enrolment, interventions and assessments
| Measure | Type | Target | Expression of interest | Screening | Enrollment and baseline | Randomization | Post-allocation | ||
|---|---|---|---|---|---|---|---|---|---|
| Intervention | Intervention end-point | Follow-up | |||||||
| Time-point (age in weeks) | 1–20 | 1–20 | 20 (± 8) | 20 (± −8) |
18–25 until 21–28 |
21–29 | 36 (± 4) | ||
| Contact data | Q | P | x | x | x | ||||
| Consent for eligibility screen | Q | P | x | ||||||
| Consent for study | Q | x | |||||||
| Screening questionnaire | Q | C, P | x | ||||||
| ASD and/or ADHD screener | Q | FM | x | ||||||
| Randomization allocation | NA | x | |||||||
| Preparatory interview | I | x | |||||||
| Child Details Questionnaire | Q | x | |||||||
| Household Questionnaire | Q | x | |||||||
| Early Childhood Behavior Questionnaire (Short Form scales for Activity, Inhibitory Control, Attention Shifting and Attentional Focus only) | Q | C | x | x | |||||
| Sensory Profile 2 | Q | C | x | x | |||||
| Early Executive Functions Questionnaire | Q | C | x | x | x | ||||
| Behavior Rating Inventory of Executive Function –Preschool version (BRIEF-P); parent participant | Q | C | x | x | |||||
| BRIEF-P; secondary carer | Q | C | x | ||||||
| Quantitative Checklist for Autism in Toddlers | Q | C | x | ||||||
| Social Responsiveness Scale, Second Edition (SRS-2) | x | ||||||||
| PROMIS Early Childhood Parent Report of Persistence, Flexibility, and Frustration tolerance | x | ||||||||
| Parental mental health (PHQ9, GAD-7) | Q | P | x | x | |||||
| Parental Self-Efficacy and Barriers Questionnaire | Q | P | x | x | |||||
| Early Childhood Education and Care questionnaire | Q | C | x | x | x | x | |||
| EF behavioral assessment- Set 1 | BA | C | x | x | |||||
| EF behavioral assessment- Set 2 | BA | C | x | ||||||
| Problem-solving tasks | C | x | x | ||||||
| Mullen Scales of Early Learning | BA | C | x | x | |||||
| ASQ Gross Motor | Q | C | x | x | |||||
| Autism Diagnostic Observation Schedule | BA | C | x | x | |||||
| Observer ratings of child behavior and regulation | BA | C | x | x | x | ||||
| Strengths and Difficulties Questionnaire | Q | C | x | ||||||
| Parental Support Questionnaire | Q | P | x | x | |||||
| Activities Questionnaire | Q | C | x | x | x | ||||
| Sources of Help Questionnaire | Q | C | x | x | x | ||||
| Post Session Feedback Questionnaire | Q | P | x | x | |||||
| Recent Life Events questionnaire | Q | P | x | x | |||||
| Post Intervention Feedback Questionnaire | Q | P | x | ||||||
| PE – attendance sheets, expense claims | P | x | |||||||
| PE – facilitator session reflections | N/A | x | |||||||
| PE– fidelity reviews | N/A | ||||||||
| PE– facilitator interviews | N/A | x | |||||||
| PE– parent interviews | I | P | x | ||||||
| Study leaver questionnaire (if appropriate) | Q | P | x | x | x | x | |||
BA Behavioral assessment: total anticipated duration 2–2.5 h; see Appendix B for details. C Child, P Parent, FM Family member, F Facilitator, I Interview, PE Process evaluation, Q Questionnaire (completed by the parent-participant, except for the ASD and/or ADHD screener (completed by the relevant adult Family Member) and the Secondary carer BRIEF-P (completed by a carer other than the parent-participant)
In addition, we will collect data relating to 3 hypothesized mechanisms of change: parental self-efficacy (self-reported via questionnaire); levels of parental responsiveness (observed during a semi-standardized parent-child interaction task), and levels of parent-child enriching activities (parent-reported via questionnaire). Further measures relate to contextual factors likely to influence intervention efficacy. These include child factors such as developmental level, sensory profile, early indicators of autism, and ADHD; process factors such as adherence and fidelity; and broader contextual factors such as demographics and family context, language exposure, parenting support, childcare, and concomitant care and services.
Data collection methods
Recruitment
One means of recruitment to the study will be by practitioner recommendation: e.g., a Health Visitor, Special Educational Needs coordinator or nursery practitioner in a recruitment hub area gives the parent an information leaflet and advises them to make contact. A second means of recruitment will be self-referral after seeing a recruitment advertisement (e.g., on social media—including via relevant organizations or peer support groups—or poster in a community setting). Multiple recruitment routes will be used in each area to maximize the generalizability of the sample, and minimize the likelihood of contamination via parents in the intervention group sharing information about the programme with parents in the control group. To further reduce the risk of contamination, parents in the intervention group will be asked not to share details about the programme with anyone other than their child’s co-parents. Parents interested in taking part in the study will contact the study team by phone or email (these contact details will be displayed on recruitment advertisements and information leaflets). Upon expression of interest in the study, respondents will be sent a participant information sheet by email or post (as preferred) and a screening questionnaire will be sent out, or phone interview scheduled (depending on participant preference).
Participant screening
The screening questionnaire will be conducted as soon as possible to establish eligibility after expression of interest by a research team member (i.e., principle investigator (PI) or research assistant (RA)), either via online questionnaire or via telephone as preferred by the respondent. An overview of the study will be provided (to recap the information provided in the participant information sheet) and the screening process will be explained. If the screening questionnaire is being conducted via telephone verbal assent to proceed will be obtained, or if the screening questionnaire is being completed online, respondents will be asked to consent to proceed.
Parent-participants are required to consent on behalf of themselves, and their child. A print version of the consent form will also be completed at the baseline assessment and follow-up assessment visits. If applicable (i.e., family history of autism or ADHD is strongly suspected but no first-degree family member has a confirmed diagnosis), respondents will be asked to complete a screening measure for the family member suspected to be autistic or have ADHD. The respondent will be informed of their eligibility and if applicable, the baseline assessment will be arranged.
Baseline assessment
A baseline assessment will be carried out when the child-participant is 20 months old (± 8 weeks). Prior to the assessment visit, parent-participants will be sent a pre-visit baseline questionnaire to complete, either online or in paper copy (paper versions will be collected at the baseline visit). Parent-participants will be invited to email the research team if they need any clarification about how to interpret or answer any of the questionnaires, and to set up a phone interview to go through the questionnaires verbally if preferred.
Baseline assessments will be conducted by the research team either in the participants’ home, or at the University of Oxford BabyLab as preferred. Both the parent-participant and child-participant are required to attend the baseline assessment. If the assessment is being carried out in the home, participants will be asked to ensure that other family members, including other children, do not come into the room that the assessment is being carried out in for the duration of the assessment. During the assessment, parent-participants will remain in the room with the child-participant but will be primarily occupied in completing the during-visit baseline questionnaire. The assessments will be led by a trained member of the research team (including training to research reliability on standardized measures) and will include a battery of age-appropriate child-friendly tasks and play-based assessments. During the assessment visit, researchers will monitor children for assent by sensitive attention to any signs, verbal or non-verbal, that they are not wholly willing to continue with the data collection. If withdrawal of assent if observed, the data collection will be paused and the child given an opportunity to rest, snack, or play, as appropriate. If, after a break, the child-participant continues to show signs of withdrawal of assent to a particular task or measure, this will be skipped and the child-participant given the opportunity to try the next task. If the child-participant shows withdrawal of assent across multiple measures, the testing session will be terminated. The total duration of the tasks and behavioral assessments is anticipated to be 2 h, but visits will be scheduled for 2.5 h to allow plenty of time for breaks.
End-point
Within 2 weeks of the final intervention session for the current wave (regardless of whether the participant has been randomized to the active or control arm), the parent-participant will be asked to complete the intervention end-point assessment (via parent questionnaire).
Participants enrolled to the active arm will also be invited to take part in a one-to-one interview (online or face to face, as preferred) at the intervention end-point (see process evaluation).
Follow-up
A further follow-up assessment will be carried out when the child-participant is 36 months old (± 4 weeks). Prior to the assessment visit, parent-participants will be sent a pre-visit follow-up questionnaire to complete, either online or in paper copy (paper versions will be collected at the face-to-face assessment). During the assessment, parent-participants will complete the during-visit questionnaire. Additionally, probes for changes in important aspects that might not be captured in the standardized questionnaires will be included in qualitative interviews conducted as part of the process evaluation, as described below.
Follow-up assessments will be conducted either in the participants’ home or at the University of Oxford BabyLab as preferred and will follow the processes outlined for the baseline assessment. To reduce the risk of bias, the researcher will ask participants not to reveal their allocation. If allocation is revealed, this will be reported and taken into account as an indication of potential bias.
Delivery and implementation process evaluation
Quantitative methods include attendance logs (collected by the facilitators and supplied to the research team directly) and fidelity self-report checklists completed by facilitators. Intervention audio recordings will be used to assess observer ratings of intervention fidelity in a subset of at least 33% of all sessions. Each facilitator pair will have at least 3 sessions reviewed and we will aim to review each session with unique content (i.e., not sessions 6 and 12) on two different occasions (i.e., with different participants). Using a pre-defined checklist, fidelity will be assessed by determining the proportion of discussion topics and activities which are completed in a way appropriate to achieve the stated session and programme aims. Fidelity reviews will be completed by trained (using pre-pilot material) graduate students not involved in the study assessments, and reviewed against self-reported fidelity scores completed by facilitators (together) after each session. Agreement between self-reported and externally-rated fidelity will be reported.
At regular intervals (aiming for fortnightly but with a minimum of monthly) during active programme delivery, a researcher (a trained graduate student with experience in intervention delivery) will hold an online debrief meeting with practitioners. In these calls, the researcher will elicit practitioners’ reflections on how the session went, whether it was delivered as intended, and their observations about the parents and children (e.g., receptiveness to materials, engagement, barriers, and facilitators to engagement). These debrief calls will not be video or audio recorded but the researcher will make notes in a reflective log. Notes will be dated, but will not include parent or child names. These notes will be used to inform and help interpret other elements of the process evaluation.
Intervention adherence
Parent-participants will be asked to complete a weekly online questionnaire during the active intervention to report whether they attended the last session and, if not, reasons for non-attendance. If they did attend the last session parent-participants will be asked to report:
What they have learned from the last session
Perceptions of the usefulness and enjoyability of the last session (including their perceptions of their child’s enjoyment)
- How much time they have spent that week trying each of the suggested activities from the intervention (including adaptations):
- Reasons for low use (e.g., no time, not appropriate for child, not important)
- The extent to which each activity was used as suggested, or adapted, and reasons for adaptation
This weekly questionnaire will be sent out only in online format but will be sufficiently brief that participants can fill it in via smartphone with minimal data costs. If a parent-participant is not able (e.g., for time or access reasons) to complete the online form in advance of the next session, they will be asked to complete a hard-copy version at the start of the session and to post it into a sealed box which is opened only at the Process evaluation stage. Descriptive data summaries and questionnaire completion rates for the weekly online questionnaires will be reported.
Contextual factors and intervention mechanisms
Quantitative data on contextual factors which may influence intervention adherence, acceptability and efficacy (e.g., socio-economic status, recent life events, parental mental health) will be collected via enrollment questionnaire, post-session questionnaire and end-point interview (participants randomized to the intervention arm only), end-point assessment, and follow-up assessment (see Table 2). These contextual factors will be used to assess patterns of attrition and adherence.
Approximately 1 month after the 36-month follow-up assessment, a subset of participants (n = 14 in the intervention arm, 6 in the comparator arm, selected to ensure diversity of perspectives in terms of neurodivergence/typicality of the parent-participant, neurodivergence/typicality of the child-participant, and adherence to the intervention) will be invited to take part in a one-to-one interview, via telephone or video conferencing or face to face (as preferred by the participant). The interview schedule will include: parents’ motivations for taking part in the study; the perceived burden and appropriateness of the intervention, and its impact on their parenting and their child (for those in the intervention arm specifically); acceptability and experience of being randomized to the comparator arm (for those in the comparator arm specifically); parents’ experience of the study as a whole and its impact on their parenting and their child; and perceived appropriateness of the outcome measures, including probes for changes in important aspects that might not be captured in the standardized questionnaires. The interviews will also be used as an opportunity to interrogate patterns in the quantitative data, which will be analyzed prior to the qualitative interviews.
All facilitators will also be asked to complete a one-to-one interview, following their final group session. This interview will explore their own and their perceptions of parents’ experience of the intervention, adherence to the protocol, and recommendations for any further adaptations prior to a definitive trial.
Interviews will be completed by trained students not involved in the study assessments to retain masking during outcome data collection.
Data management
Data will be managed according to the project Data Management Plan (see Appendix C).
Statistical methods
All main analysis will be based on the intention-to-treat (ITT) principle. Analysis will take place after full recruitment and follow-up. As a feasibility RCT, the main focus will be on tabulated and graphical summaries of our feasibility outcomes as listed in Table 3. We will report data in accordance with CONSORT (Consolidated Standards of Reporting Trials) 2010 extension statement for Pilot and Feasibility trials.
Table 3.
Feasibility outcomes
| Outcome | Summary statistic |
|---|---|
| Number of eligible participants who agree to participate in the trial | N |
| Fidelity rating scores for sessions |
% of researcher and practitioner ratings in complete agreement as to the extent to which broad session aims % of researcher and practitioner ratings in complete agreement as to the extent to which specific session aims were met |
| Fidelity rating scores for sessions | % of active intervention components rated as partially- or fully-present in all coded group sessions |
|
For each measure: • Completion rates • Loss of data due to participant refusal or invalid administration. |
% of valid data collected on a measure, as a proportion of the number of participants contributing data at that timepoint Count of missing data due to participant refusal Count of missing data due to invalid administration |
|
Retention: through randomization, intervention or usual practice, and follow-up Satisfaction with study processes (e.g., randomization and data collection), of parent-participants |
% of parent-participants seen at baseline who are retained to endpoint % of parent-participants seen at baseline who are retained to follow-up Qualitative analysis of the parent-participant interviews at 37 months, taking into account attrition rates and feedback from the Study Leaver questionnaire where available |
| Parental adherence to the intervention | Qualitative analysis of parent- and facilitator questionnaires, and interviews |
|
Engagement with the intervention: number of sessions attended Satisfaction with the intervention: questionnaire and qualitative interviews |
Number of sessions attended by participant, by group, and by location (Mean and SD) Qualitative analysis of parent- and facilitator questionnaires, and interviews % of parent-participants randomized to the intervention, and who confirm they can attend regularly, who attend a minimum of 7 sessions % of leaver questionnaire responses that endorse START programme content factors as a strong influence for leaving the study % of parent session ratings rated < 2 for enjoyability and usefulness (mean scores by participant) Qualitative analysis of free text comments in the Post-Session Feedback Questionnaire and verbal comments during sessions and the parent-participant interviews |
To inform a definitive trial, we will conduct the following analysis on the intervention outcomes: proposed measures will be analyzed either using mixed effects model analysis or repeated measures using a mixed effects model to take into account discrete timing of the follow-up assessment adjusting for baseline score where appropriate, and including recruitment area and participant as a random effect. The BRIEF-P is not suitable for 20-month-olds [21] but recently, EEFQ scores have been shown to be predictive of BRIEF-P scores [22]; therefore, we will explore including EEFQ baseline scores in the mixed effects model. The presentation of the analysis will focus on point estimates and associated 95% confidence intervals rather than statistical significance (p values).
Qualitative analysis
Interview data (facilitators and parent-participants) will be analyzed using thematic analysis. Qualitative findings will be triangulated with quantitative analysis of process evaluation data (fidelity ratings, acceptability and adherence scores).
Adverse event reporting
Adverse events will be monitored for and reported as per the study Adverse Event Standard Operating Procedure; Appendix D.
Auditing
No independent audits are planned.
Study governance
Ethical approval for this study has been granted from University of Oxford Medical Sciences Interdivisional Research Ethics Committee, reference number R67115. A study steering committee (SSC) will meet annually to provide study oversight. SSC members are listed in the Acknowledgements section. As this study is not classified as a clinical trial an independent data monitoring committee has not been appointed, but during the active data collection and analysis phase the PI will report data quality checks to the SSC, with guidance and oversight from the Centre for Healthcare Randomised Trials, University of Aberdeen a registered UK CRC clinical trials unit (7).
Confidentiality and access to data
De-identified research data will be stored on password-protected servers approved by the University. All hard copy forms will be stored in locked filing cabinets. Audio and video files will be recorded on encrypted devices and securely held in password-protected servers approved by the University. No identifiable data will be published. University of Oxford is the Data Controller with regards to all project data. University of Aberdeen and Peeple are Data Processors for project data.
Dissemination policy
The findings from this feasibility trial will be submitted for publication in a peer-reviewed journal. Journal authorship guidelines will be followed, and a CRediT (Contributor Roles Taxonomy) statement included. Summary findings will be submitted for presentation at stakeholder events, and to national and international academic conferences.
Project updates will be published annually on the project website and via a newsletter, beginning in Summer 22. The newsletter will be distributed to participants in the trial (including the initial feasibility stage), and to community stakeholders (early years practitioners, clinicians, and members of the public) who have either registered interest in receiving project updates or via the Peeple network of early years practitioners.
The code for statistical analyses will be published as an appendix to the final report. Access to the de-identified participant level dataset will be limited to the research team and direct collaborators by default. Participants will be given the option to share their de-identified data with other researchers, and uptake of this option will be used to inform plans for data sharing: i.e., whether there is likely to be sufficient support for making de-identified data more broadly available by default in the event of a definitive trial. De-identified research data for participants who have agreed to data sharing beyond the core research team will be uploaded to the OSF platform (or its equivalent).
Discussion/statement of impact
Executive functions are important for long-term health and wellbeing, as well as academic and economic outcomes. Children with a family history of autism and/or ADHD are at elevated likelihood for executive function difficulties, yet currently are not offered any systematic provision of support. Early parent-mediated intervention offers the possibility for relatively low-cost support that can be adapted to meet children’s individual needs and family context. The START programme is a novel parent-mediated intervention for toddlers with a family history of autism/ADHD. It was designed with the principles of neurodiversity in mind, and with collaborative input from members of the autism and ADHD communities. The aim of the START programme is to support children’s ability to pursue their own goals and to help them as individuals—with unique strengths, challenges and interests—to thrive. The findings from this feasibility study will determine the progression to, and design of, a definitive randomized controlled trial to assess the effectiveness and cost-effectiveness of the START intervention. In addition, the results of this study will yield broader insights into the delivery and evaluation of complex early-years interventions in community settings.
Acknowledgements
We would like to gratefully acknowledge the contributions of the following people during the development of the START feasibility RCT protocol: Andrew Elders for initial statistical advice, Members of the Steering Committee for the START Co-refinement and RCT Feasibility Trial: Charlotte Featherstone, Frances Gardner, Cat Hughes, Claire Hughes, Sally Smith, Alan Stein.
Appendix A
Spirit checklist
SPIRIT 2013 Checklist: Recommended items to address in a clinical trial protocol and related documents*.
| Section/item | Item No | Description | Addressed on page number |
|---|---|---|---|
| Administrative information | |||
| Title | 1 | Descriptive title identifying the study design, population, interventions, and, if applicable, trial acronym | 1 |
| Trial registration | 2a | Trial identifier and registry name. If not yet registered, name of intended registry | 2 |
| 2b | All items from the World Health Organization Trial Registration Data Set | Appendix E | |
| Protocol version | 3 | Date and version identifier | Title page |
| Funding | 4 | Sources and types of financial, material, and other support | Title page, Appendix E |
| Roles and responsibilities | 5a | Names, affiliations, and roles of protocol contributors | Title page |
| 5b | Name and contact information for the trial sponsor | Appendix E | |
| 5c | Role of study sponsor and funders, if any, in study design; collection, management, analysis, and interpretation of data; writing of the report; and the decision to submit the report for publication, including whether they will have ultimate authority over any of these activities | NA | |
| 5d | Composition, roles, and responsibilities of the coordinating centre, steering committee, endpoint adjudication committee, data management team, and other individuals or groups overseeing the trial, if applicable (see Item 21a for data monitoring committee) | 32-33 | |
| Introduction | |||
| Background and rationale | 6a | Description of research question and justification for undertaking the trial, including summary of relevant studies (published and unpublished) examining benefits and harms for each intervention | 4–5 |
| 6b | Explanation for choice of comparators | 14 | |
| Objectives | 7 | Specific objectives or hypotheses | 5–6 |
| Trial design | 8 | Description of trial design including type of trial (eg, parallel group, crossover, factorial, single group), allocation ratio, and framework (eg, superiority, equivalence, noninferiority, exploratory) | 7 |
| Methods: Participants, interventions, and outcomes | |||
| Study setting | 9 | Description of study settings (eg, community clinic, academic hospital) and list of countries where data will be collected. Reference to where list of study sites can be obtained | 7 |
| Eligibility criteria | 10 | Inclusion and exclusion criteria for participants. If applicable, eligibility criteria for study centres and individuals who will perform the interventions (eg, surgeons, psychotherapists) | 7–9 |
| Interventions | 11a | Interventions for each group with sufficient detail to allow replication, including how and when they will be administered | 11-13 |
| 11b | Criteria for discontinuing or modifying allocated interventions for a given trial participant (eg, drug dose change in response to harms, participant request, or improving/worsening disease) | NA | |
| 11c | Strategies to improve adherence to intervention protocols, and any procedures for monitoring adherence (eg, drug tablet return, laboratory tests) | 15–6, 28–30 | |
| 11d | Relevant concomitant care and interventions that are permitted or prohibited during the trial | 13 | |
| Outcomes | 12 | Primary, secondary, and other outcomes, including the specific measurement variable (eg, systolic blood pressure), analysis metric (eg, change from baseline, final value, time to event), method of aggregation (eg, median, proportion), and time point for each outcome. Explanation of the clinical relevance of chosen efficacy and harm outcomes is strongly recommended | 17–19, Appendix B for intervention outcome measures |
| Participant timeline | 13 | Time schedule of enrolment, interventions (including any run-ins and washouts), assessments, and visits for participants. A schematic diagram is highly recommended (see Figure 3) | 10, 22–24 |
| Sample size | 14 | Estimated number of participants needed to achieve study objectives and how it was determined, including clinical and statistical assumptions supporting any sample size calculations | 16 |
| Recruitment | 15 | Strategies for achieving adequate participant enrolment to reach target sample size | 15, 25 |
| Methods: Assignment of interventions (for controlled trials) | |||
| Allocation: | |||
| Sequence generation | 16a | Method of generating the allocation sequence (e.g., computer-generated random numbers), and list of any factors for stratification. To reduce predictability of a random sequence, details of any planned restriction (e.g., blocking) should be provided in a separate document that is unavailable to those who enrol participants or assign interventions | 14–15 |
| Allocation concealment mechanism | 16b | Mechanism of implementing the allocation sequence (e.g., central telephone; sequentially numbered, opaque, sealed envelopes), describing any steps to conceal the sequence until interventions are assigned | 14–15 |
| Implementation | 16c | Who will generate the allocation sequence, who will enrol participants, and who will assign participants to interventions | 14–15 |
| Blinding (masking) | 17a | Who will be blinded after assignment to interventions (e.g., trial participants, care providers, outcome assessors, data analysts), and how | 14-15 |
| 17b | If blinded, circumstances under which unblinding is permissible, and procedure for revealing a participant’s allocated intervention during the trial | NA | |
| Methods: Data collection, management, and analysis | |||
| Data collection methods | 18a | Plans for assessment and collection of outcome, baseline, and other trial data, including any related processes to promote data quality (e.g., duplicate measurements, training of assessors) and a description of study instruments (eg, questionnaires, laboratory tests) along with their reliability and validity, if known. Reference to where data collection forms can be found, if not in the protocol | 26–31 |
| 18b | Plans to promote participant retention and complete follow-up, including list of any outcome data to be collected for participants who discontinue or deviate from intervention protocols | 15–16 | |
| Data management | 19 | Plans for data entry, coding, security, and storage, including any related processes to promote data quality (e.g., double data entry; range checks for data values). Reference to where details of data management procedures can be found, if not in the protocol | Appendix C |
| Statistical methods | 20a | Statistical methods for analysing primary and secondary outcomes. Reference to where other details of the statistical analysis plan can be found, if not in the protocol | 31–32 |
| 20b | Methods for any additional analyses (e.g., subgroup and adjusted analyses) | 31–32 | |
| 20c | Definition of analysis population relating to protocol non-adherence (e.g., as randomized analysis), and any statistical methods to handle missing data (e.g., multiple imputation) | 31–32 | |
| Methods: Monitoring | |||
| Data monitoring | 21a | Composition of data monitoring committee (DMC); summary of its role and reporting structure; statement of whether it is independent from the sponsor and competing interests; and reference to where further details about its charter can be found, if not in the protocol. Alternatively, an explanation of why a DMC is not needed | 33 |
| 21b | Description of any interim analyses and stopping guidelines, including who will have access to these interim results and make the final decision to terminate the trial | NA | |
| Harms | 22 | Plans for collecting, assessing, reporting, and managing solicited and spontaneously reported adverse events and other unintended effects of trial interventions or trial conduct | Appendix D |
| Auditing | 23 | Frequency and procedures for auditing trial conduct, if any, and whether the process will be independent from investigators and the sponsor | NA |
| Ethics and dissemination | |||
| Research ethics approval | 24 | Plans for seeking research ethics committee/institutional review board (REC/IRB) approval | 32 |
| Protocol amendments | 25 | Plans for communicating important protocol modifications (eg, changes to eligibility criteria, outcomes, analyses) to relevant parties (eg, investigators, REC/IRBs, trial participants, trial registries, journals, regulators) | 33 |
| Consent or assent | 26a | Who will obtain informed consent or assent from potential trial participants or authorized surrogates, and how (see Item 32) | 25 |
| 26b | Additional consent provisions for collection and use of participant data and biological specimens in ancillary studies, if applicable | NA | |
| Confidentiality | 27 | How personal information about potential and enrolled participants will be collected, shared, and maintained in order to protect confidentiality before, during, and after the trial | Appendix C |
| Declaration of interests | 28 | Financial and other competing interests for principal investigators for the overall trial and each study site | Title page |
| Access to data | 29 | Statement of who will have access to the final trial dataset, and disclosure of contractual agreements that limit such access for investigators | 32 |
| Ancillary and post-trial care | 30 | Provisions, if any, for ancillary and post-trial care, and for compensation to those who suffer harm from trial participation | NA |
| Dissemination policy | 31a | Plans for investigators and sponsor to communicate trial results to participants, healthcare professionals, the public, and other relevant groups (e.g., via publication, reporting in results databases, or other data sharing arrangements), including any publication restrictions | 33 |
| 31b | Authorship eligibility guidelines and any intended use of professional writers | Title page, 33 | |
| 31c | Plans, if any, for granting public access to the full protocol, participant-level dataset, and statistical code | 33–34 | |
| Appendices | |||
| Informed consent materials | 32 | Model consent form and other related documentation given to participants and authorized surrogates | Project website |
| Biological specimens | 33 | Plans for collection, laboratory evaluation, and storage of biological specimens for genetic or molecular analysis in the current trial and for future use in ancillary studies, if applicable | NA |
*It is strongly recommended that this checklist be read in conjunction with the SPIRIT 2013 Explanation & Elaboration for important clarification on the items. Amendments to the protocol should be tracked and dated. The SPIRIT checklist is copyrighted by the SPIRIT Group under the Creative Commons “Attribution-NonCommercial-NoDerivs 3.0 Unported” license
Appendix B
Proposed intervention outcome measures
Primary intervention outcome measure
The feasibility trial will evaluate the psychometric properties (sensitivity-to-change, coefficient-of-variance, floor- and ceiling-effect metrics, completion rates and loss of data due to participant refusal or invalid administration) of the proposed primary outcome measure:
BRIEF-P Global Executive Composite (GEC) at End-point or Follow-up (Primary carer report). Raw scores for each of the 5 contributing scales (Inhibit, Shift, Emotional Control, Working Memory, Plan/Organize) will be explored to provide further insight.
Secondary intervention outcome measures
BRIEF-P GEC at Follow-up (Secondary carer report)
Early Executive Functions Questionnaire Cognitive Executive Functions and Regulation score (Primary carer report) at End-point
Executive Function score(s) at 36 months: Performance scores and observer ratings from Executive Function behavioral measures (see Table SM 1) will be considered at the task level if correlations with performance or observer scores are below.3, or as part of a composite score (formed by averaging z-scores for all tasks correlating at.3 or above).
Success Score on the Problem-Solving Box task at Follow-up. Generativity, Persistence and Perseveration scores on the task will also be explored to provide further insight.
SDQ total difficulties score at Follow-up. Scores for each of the 5 contributing scales (Emotional symptoms, Conduct problems, Hyperactivity/Inattention, Peer relationship problems and Prosocial behavior) will be explored to provide further insight.
PROMIS Early Childhood Parent Report: Engagement—Persistence; Self-Regulation—Flexibility; and Self-Regulation—Frustration Tolerance scales at Follow-up.
Table 4.
Behavioral measures of Executive Function
| Task | Task type |
|---|---|
| Delayed Alternation | Touchscreen |
| Early Childhood Inhibitory Touchscreen Task | Touchscreen |
| Hide and Seek | Touchscreen |
| Bubble Conflict | Touchscreen |
| Shape box | Tabletop |
| Reverse categorisation | Tabletop |
| Prohibition task | Tabletop |
| Gift delay | Tabletop |
| Go/No-Go | Touchscreen |
| Mr Ant | Touchscreen |
| Card Sort | Touchscreen |
| Spin the pots | Tabletop |
Health economic evaluation
There is a lack of established health-related quality of life measures for the target population age in general and for neurodivergent toddlers in particular, therefore the plan for economic evaluation will be reviewed in the light of any new recommendations. Preliminary plans are to collect data which, in a definitive trial would enable us to explore the incremental cost-effectiveness of the START intervention compared to usual practice by:
Calculating the mean cost-per-case per trial arm for the intervention and control group, and rates of significance of difference between arms.
Conducting a cost-effectiveness analysis of the intervention based on the primary outcome measure (BRIEF-P) and quality-adjusted life-years (QALYs) using Child Health Utility index 9D (CHU-9D) scores computed from SDQ scores using a published algorithm.
Appendix C
Data management and quality assurance plan
Participants’ name and contact information will be stored in a password-protected spreadsheet (the keyfile) stored on a secure University-approved server. Access will be granted only to the core research team and facilitator team. A participant ID will be generated at enrollment (i.e., only for eligible participants), at which point a Case Report Form will be created on a secure research database hosted by the University. Contact information for parents who expressed an interest in the study but were ineligible or did not choose to proceed to the full study will be deleted from the keyfile within 2 weeks of the decision being communicated and the corresponding participant ID will be marked as inactive.
Paper records of consent will be collected at the Baseline assessment and 36-month assessments. These will be separated from questionnaires and stored in a separate filing cabinet for a minimum of three years after publication of the primary feasibility trial study is published.
Questionnaire data will either be entered directly into the research database via online form by parent-participants (or the research team if the questionnaire is completed over the phone), with auto-validation checks (e.g., for missing data and impossible answers) or collected on paper forms, stored in a filing cabinet then inputted at regular intervals (minimum 6-monthly) into the research database. Twenty percent of paper questionnaires will be double entered and instances of disagreement will be reviewed and rectified. If agreement between data enterers falls below 80%, double entry will be increased to 50%. Paper copies of forms will be stored for the duration of the project and shredded during project close down.
Performance scores for tasks completed at the assessment visits will either be collected via touchscreen device, or recorded on a spreadsheet (e.g. for table top tasks). Data will be backed up to the University-approved cloud server after the session. Clinical and standardized assessment scores (e.g. ADOS, Mullen) will be recorded on the relevant paper forms, which will be stored in a filing cabinet. Within 1 month of assessment completion, summary scores for subdomains will be entered into the research database, with double entry of 20% of forms. Instances of disagreement will be reviewed and rectified. If agreement between data enterers falls below 80%, double entry will be increased to 50%. Paper copies of forms will be stored for the duration of the project and shredded during project close down.
Assessment visits will be video recorded for the purposes of offline behavioral coding and validation of touchscreen and table-top task scores. Recordings will be able on an encrypted device and then backed up after the session to the secure University-approved cloud server and an encrypted external hard drive (which will be stored in a locked office), then deleted from the device. All primary variables generated from coded videos will be checked for research reliability with at least one other coder using standards appropriate for that variable. Videos will be stored until all planned coding is complete and the primary feasibility trial study and any related mechanism studies published. Intervention sessions will be recorded using an encrypted device and uploaded to the University-approved cloud server after the session. Recordings will be coded for fidelity from the audio recordings and will not be transcribed. Recordings will be stored until fidelity ratings are complete and the primary feasibility trial study published, at which point they will be deleted.
Data stored on the research database will be exported at regular intervals (minimum 6 monthly) to the University-approved server. If consent for long-term storage is granted, participant contact information will be kept for up to 30 years to allow for the possibility of contacting participants to collect follow-up data in the future. If this is not granted, participant contact information will be deleted at project close-down. Research data will be kept for 30 years to allow for follow-up studies—with the exception of paper forms, audio recordings, and video recordings as outlined above. During the project close down phase, de-identified research data (excluding paper forms, audio and video recordings) will be deposited on the Oxford University Research Archive (or its equivalent) but will be embargoed by default. The descriptive record will be public. De-identified research data for participants who have agreed to data sharing beyond the core research team will be uploaded to the OSF platform (or its equivalent).
Appendix D
START Feasibility RCT- Adverse Events Standard Operating Procedure
Applicable to
All START research team and intervention team members.
Purpose
The purpose of this standard operating procedure (SOP) is to outline the process for recording adverse events. This is to ensure participant safety, but also to enable the study team to gather information consistently across all participants.
Scope
The SOP covers the gathering of information relating to adverse events (AE) and Serious Adverse Events (SAE): see definitions below. It also covers recording of adverse events where there is a safety concern for participants as well as anything relevant to the continuation of the study.
Expected AEs include, but are not limited to:
Increased child refusal to go to Early Childhood Education and Care (ECEC) (i.e. nursery/childminder) or community activities.
Exclusion from ECEC or community activities.
Deterioration in child behavior (including deliberate self-harm) or wellbeing.
Deterioration in parent behaviour or wellbeing (including deliberate self-harm).
Parental physical chastisement.
Increased family discord.
Breakdown in family structure.
Social work involvement or child protection concerns.
Physical injury to participants that may be caused by (1) another child’s actions, (2) a trip or fall, or 3) from inappropriate use of resources outside of guidance and intended purpose (such as ingesting glue or small parts)
Serious ill health of participants (e.g., contracting a serious infection that is not widely circulating in the community such as measles)
Responsibilities
It is the responsibility of the START delivery team to record any AE disclosed or observed during START sessions, or during interim discussions with parent participants (see procedure below) and to report those adverse events to the Process Evaluation team, who will escalate to the PI/Gaia Scerif as appropriate
It is the responsibility of the START Research Team to monitor for any AE as part of the 31-, 36- and 37-month assessments (see procedure below) and to report those adverse events to the PI
It is the responsibility of the PI to assess whether the AE is serious and whether the sponsor should be notified. The PI will advise on what information needs to be shared with the Study Steering Committee, and when, and whether the Multi-Agency Safeguarding Hub referral process should be initiated.
Procedure for monitoring for adverse events during intervention sessions, or during interim discussions with parent participants (START Intervention team members).
Facilitators of the START sessions should be alert for evidence or disclosure of an AE, both during the sessions and in any interim discussions (e.g. calls to check for attendance)
If an AE is detected, this should be reported to the Process evaluation team (via email or telephone call as appropriate) who will complete the adverse event (AE) Reporting Form (digital copy), and the Adverse Event ID log (saved separately) and immediately email the study PI who will report to the Sponsor/Study Steering Committee if necessary.
If throughout the duration of the study, a member of the study team or facilitator team becomes concerned regarding the wellbeing or safety of a study participant or their child, study staff must follow study and site-specific protocols for dealing with harm (e.g., Safeguarding procedures).
Procedure for monitoring for adverse events as part of the 31-, 36-, and 36-month assessments (Research Team members)
- The team member responsible for collecting/reviewing that data-point should monitor the below for evidence of an AE:
- The Study leaver questionnaire free text fields: report of an AE (VH)
- The Active intervention weekly questionnaire free text fields: report of an AE (VH)
- The Recent Life Events questionnaire (31 month questionnaire): any of the following items are endorsed :
-
i.Have you had a serious illness or been seriously injured?
-
ii.Has one of your child been seriously ill or injured?
-
iii.Have you separated from your partner?
-
i.
- Qualitative interviews at 37 months: report of an AE
If an AE is detected, this should be reported on an Adverse Event (AE) Reporting Form (digital copy). The Adverse Event (AE) Reporting Form should also be immediately emailed to the PI who will report to the Sponsor/Study Steering Committee if necessary.
If throughout the duration of the study, a member of the study team or facilitator team becomes concerned regarding the wellbeing or safety of a study participant or their child, study staff must follow study and site-specific protocols for dealing with harm.
Note that at a global level, the research team will monitor for group level increases in:
Parental mental health problems at 36 months: PHQ-9 scores above the clinical threshold of concern and/or GAD-7 scores above the clinical threshold of concern
Child emotional-behavioural problems at 36 months: Strengths and Difficulties Questionnaire emotional problems score and/or conduct problems scores in the very high band
If found, these would be reported as possible negative outcomes of the START intervention.
Forms or templates to be used
START Adverse Events Reporting Form 1.1
Coding guidelines
If there are multiple categorizations relevant to an adverse events (for example, if the child begins to refuse to go to school and presents with severe tantrums on school mornings), this should be recorded as two events.
Death of child, parent or other immediate family member as a result of a medical illness should be coded as a ‘significant medical issue’ (m) resulting in death. If death is the result of an accident or other non-medical cause, the death should be coded as ‘death in immediate family ’ (j). Immediate family members are defined as those who live with the child (including grandparents), or immediate family members who live elsewhere (e.g., a second parent living in another household, older siblings). If someone with a strong attachment to the child becomes ill or dies who does not meet this criteria this could be coded as ‘other personal/family issue’ (l) and specified.
Any queries with completion and categorisation of the adverse events form should be discussed with the PI.
Standard definitions (drawing on NIHR guidelines derived from the EU Directive 2001/20/EC and The Medicines for Human Use (Clinical Trials) Regulations 2004)
Adverse event (AE) Any untoward medical occurrence in a patient or clinical trial subject administered a medicinal product which does not necessarily have a causal relationship with this treatment.
Serious adverse event (SAE) Any AE, which results in death, is life-threatening, requires hospitalisation or prolonged hospitalisation, causes persistent or significant disability or incapacity, or consists of a congenital abnormality or birth defect. Important medical events that may not be immediately life-threatening or result in death or hospitalisation but may jeopardize the patient or may require intervention to prevent one of the other outcomes listed in the definition above should also be considered serious.
For the START feasibility RCT, the treatment is psychological in nature, not pharmacological. Medical adverse events will be recorded as standard. Other adverse events may relate to child and family wellbeing. All events should be noted, regardless of whether they unrelated to the START research or programme.
Appendix E
World Health Organization Trial Registration Data Set.
Table 5.
World Health Organization Trial Registration Data Set
| Data category | Information |
|---|---|
| Primary registry and trial identifying number | ISRCTN99820028 |
| Date of registration in primary registry | 30/01/2023 |
| Secondary identifying numbers | NA |
| Source(s) of monetary or material support | NIHR and Castang Foundation |
| Primary sponsor | University of Oxford |
| Secondary sponsor(s) | NA |
| Contact for public queries | Dr Alexandra Hendry |
| Contact for scientific queries | Dr Alexandra Hendry |
| Public title | Supporting Toddlers with a family connection to autism or ADHD to develop strong Attention, Regulation and Thinking skills (START) |
| Scientific title | Supporting Toddlers with a family connection to autism or ADHD to develop strong Attention, Regulation and Thinking skills (START) programme Feasibility RCT |
| Countries of recruitment | UK |
| Health condition(s) or problem(s) studied | Executive function difficulties, autism, ADHD |
| Intervention(s) |
Active group: START programme and usual practice Control group: usual practice |
| Key inclusion and exclusion criteria |
Inclusion criteria for the child-participant are: • has either (1) at least 1 first-degree relative with a community diagnosis (as reported by the parent) OR (2) at least 1 first-degree relative with presumed autism who scores above the clinical threshold on an autism screening measure OR (3) at least 1 first-degree relative with presumed ADHD who scores above the clinical threshold on an ADHD screening measure OR 4) has been identified by their parent, or health or Early Years practitioner as showing high autistic traits. • is aged 22 months or less at the time of the eligibility screen, and older than 18 months at the time of the final scheduled intervention start date. Exclusion criteria for the child-participant are: • has significant uncorrected visual or hearing problems. • has a known genetic condition associated with developmental delay. • is currently placed in a 24-h residential placement, or a foster placement due to end before the child reaches 36months of age. • any parent in their family has already participated in the START trial. • they, or any parent in their family has participated in a research trial involving a parenting intervention or other EF-related intervention within 3 months of the baseline assessment, or during the course of the START trial. Inclusion criteria for the parent-participant are: • is aged 18 years or over. • provides solo or joint childcare for the child-participant for at least 1 day per week. • has at least conversational-level spoken English. • has capacity to attend 12 weekly 1-hour intervention sessions. |
| Study type |
Interventional Allocation: randomized Intervention model: parallel assignment Masking: single blind (investigator, outcomes assessor) |
| Date of first enrolment | February 2023 |
| Target sample size | 60 |
| Recruitment status | Recruiting |
| Primary outcome(s) | Parent-reported Executive Function difficulties (BRIEF-P) |
| Key secondary outcomes |
Secondary carer report of Executive Function difficulties (BRIEF-P) Parent-reported Executive Function skills (EEFQ) Experimenter assessment of Executive Function skills Parent-reported psycho-social difficulties (SDQ) Parent-reported Persistence, Flexibility, and Frustration tolerance (PROMIS Early Childhood Parent Report) |
Authors’ contributions
T Charman: supervision, writing—review and editing. L Constable: methodology, writing—review and editing. A Hendry: conceptualization, methodology, investigation, resources, data curation, project administration, writing—original draft, writing—review and editing, visualization, project administration, funding acquisition. J Hudson: methodology, writing—review and editing. V Hulks: investigation, data curation, project administration. S Mathers: supervision, writing—review and editing. M Nosyk: investigation, data curation, project administration. S Rhodes: supervision, writing—review and editing. G Scerif: supervision, writing—review and editing.
Funding
NIHR Oxford Biomedical Research Centre,National Institute for Health and Care Research,NIHR300880,Alexandra Hendry
Data availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Declarations
Ethics approval and consent to participate
Ethical approval for this study has been granted from University of Oxford Medical Sciences Interdivisional Research Ethics Committee, reference number R67115.
Consent for publication
Not applicable.
Competing interests
AH is a programme developer for the START programme, but does not receive funds from its administration. TC has served as a paid consultant to F. Hoffmann-La Roche Ltd. and Servier; and has received royalties from Sage Publications and Guilford Publications.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hendry A, Jones EJH, Charman T. Executive function in the first three years of life: Precursors, predictors and patterns. Dev Rev. 2016;42:1–33. 10.1016/j.dr.2016.06.005. [Google Scholar]
- 2.Diamond A. Executive functions. Annu Rev Psychol. 2013;64(64):135–68. 10.1146/annurev-psych-113011-143750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Snyder HR, Miyake A, Hankin BL. Advancing understanding of executive function impairments and psychopathology: bridging the gap between clinical and cognitive approaches. Front Psychol. 2015;6. 10.3389/fpsyg.2015.00728. [DOI] [PMC free article] [PubMed]
- 4.Wallace GL, et al. Assessment and treatment of executive function impairments in autism spectrum disorder: an update. Int Rev Res Dev Disabil. 2016;51:85–122. [Google Scholar]
- 5.de Vries M, Geurts HM. Influence of autism traits and executive functioning on quality of life in children with an autism spectrum disorder. J Autism Dev Disord. 2015;45:2734–43. 10.1007/s10803-015-2438-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dijkhuis RR, Ziermans TB, Van Rijn S, Staal WG, Swaab H. Self-regulation and quality of life in high-functioning young adults with autism. Autism. 2017;21:896–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eaton C, et al. The prevalence of attention deficit/hyperactivity disorder symptoms in children and adolescents with autism spectrum disorder without intellectual disability: a systematic review. J Atten Disord. 2023;27:1360–76. 10.1177/10870547231177466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghirardi L, et al. The familial co-aggregation of ASD and ADHD: a register-based cohort study. Mol Psychiatry. 2018;23:257–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller M, et al. Sibling Recurrence risk and cross-aggregation of attention-deficit/hyperactivity disorder and autism spectrum disorder. JAMA Pediatr. 2019;173:147–52. 10.1001/jamapediatrics.2018.4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Visser JC, Rommelse NNJ, Greven CU, Buitelaar JK. Autism spectrum disorder and attention-deficit/hyperactivity disorder in early childhood: A review of unique and shared characteristics and developmental antecedents. Neurosci Biobehav Rev. 2016;65:229–63. 10.1016/j.neubiorev.2016.03.019. [DOI] [PubMed] [Google Scholar]
- 11.Rommelse NNJ, Franke B, Geurts HM, Hartman CA, Buitelaar JK. Shared heritability of attention-deficit/hyperactivity disorder and autism spectrum disorder. Eur Child Adolesc Psychiatry. 2010;19:281–95. 10.1007/s00787-010-0092-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Craig F, et al. A review of executive function deficits in autism spectrum disorder and attention-deficit/hyperactivity disorder. Journal of Neuropsychiatric Disease and Treatment. 2016;12:1191–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Otterman DL, et al. Executive functioning and neurodevelopmental disorders in early childhood: a prospective population-based study. Child Adolesc Psychiatry Ment Health. 2019;13:38. 10.1186/s13034-019-0299-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bazelmans T, Scerif G, Holmboe K, Gonzalez-Gomez N, Hendry A. Prevalence of family history of autism and ADHD varies with recruitment approach and socio-economic status OSF (preprint). 2023. [DOI] [PMC free article] [PubMed]
- 15.Cusack J, Sterry R. Your questions: shaping future autism research. London: Autistica; 2016. [Google Scholar]
- 16.Morris C, et al. Setting research priorities to improve the health of children and young people with neurodisability: a British Academy of Childhood Disability-James Lind Alliance Research Priority Setting Partnership. BMJ Open. 2015;5: e006233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kassai R, Futo J, Demetrovics Z, Takacs ZK. A meta-analysis of the experimental evidence on the near-and far-transfer effects among children’s executive function skills. Psychol Bull. 2019;145:165. [DOI] [PubMed] [Google Scholar]
- 18.Valcan DS, Davis H, Pino-Pasternak D. Parental behaviours predicting early childhood executive functions: a meta-analysis. Educ Psychol Rev. 2018;30:607–49. [Google Scholar]
- 19.Hendry A, Hulks V, Murphy S, Radford H, Smith S, Charman T, Mathers S, Rhodes S, Scerif G. Learning from the community: iterative co-production of a programme to support the development of attention, regulation and thinking skills in toddlers at elevated likelihood of autism or ADHD. Res Involv Engagem. 2025;11(1):7. [DOI] [PMC free article] [PubMed]
- 20.Arain M, Campbell MJ, Cooper CL, Lancaster GA. What is a pilot or feasibility study? A review of current practice and editorial policy. BMC Med Res Methodol. 2010;10:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gioia GA, Espy KA, Isquith PK. Behavior rating inventory of executive function–preschool version: professional manual. Psychological Assessment Resources. 2003.
- 22.Hendry A, Holmboe K. Evidence of predictive associations of parent-reported Executive Functions from infancy to preschool age. 2025.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.