Abstract
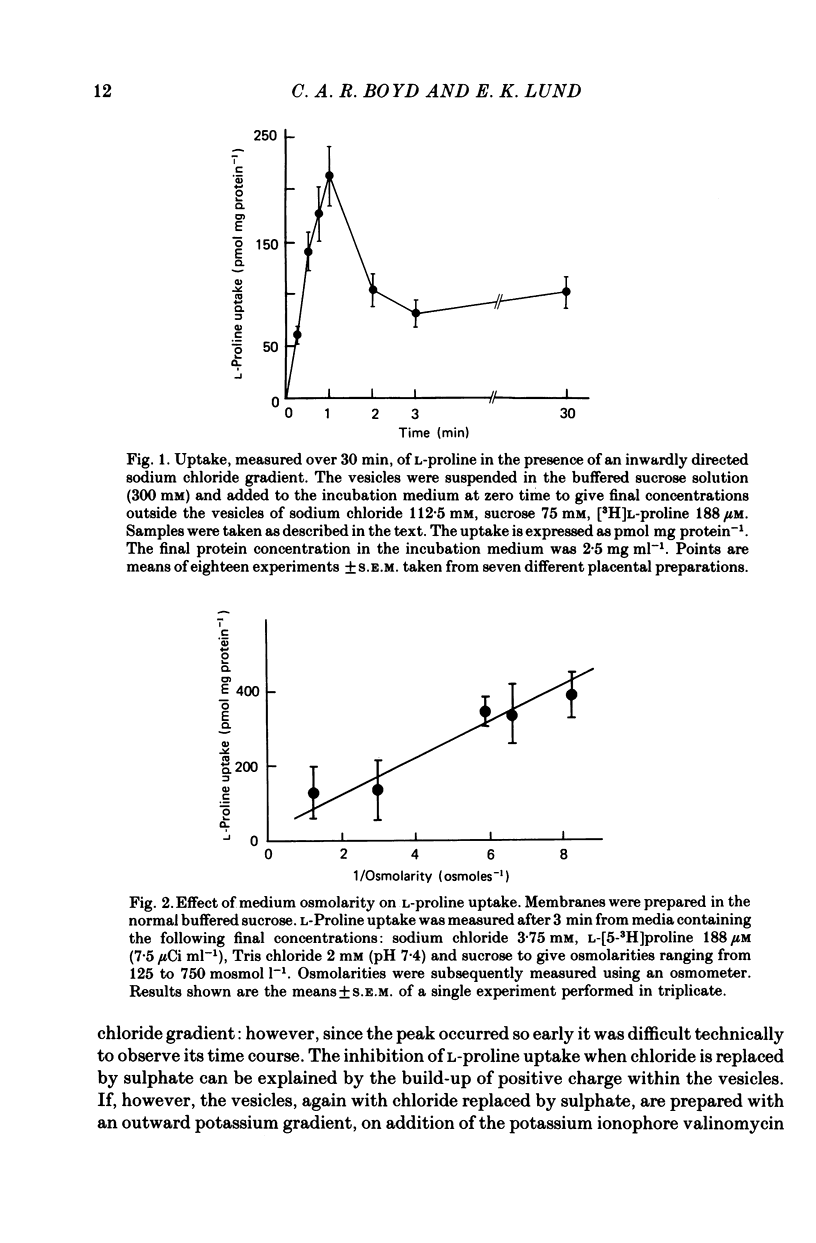
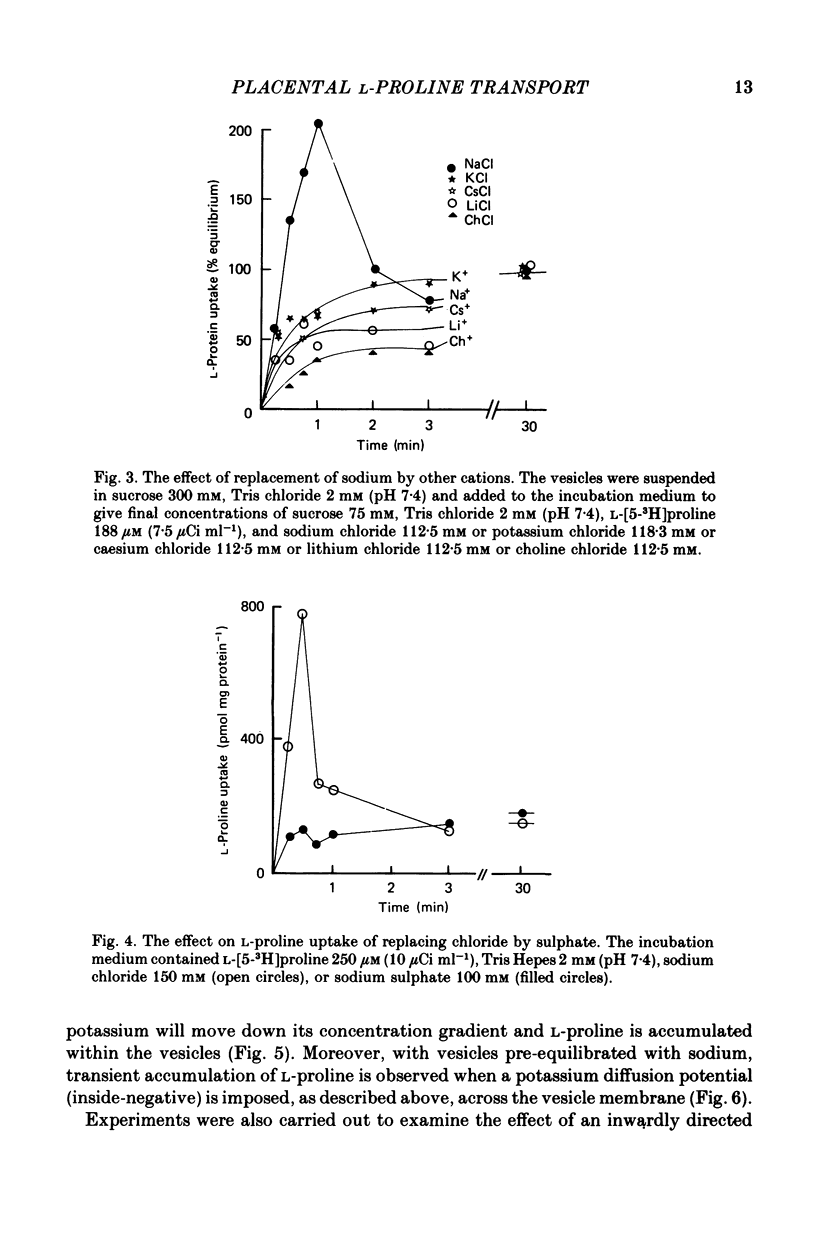
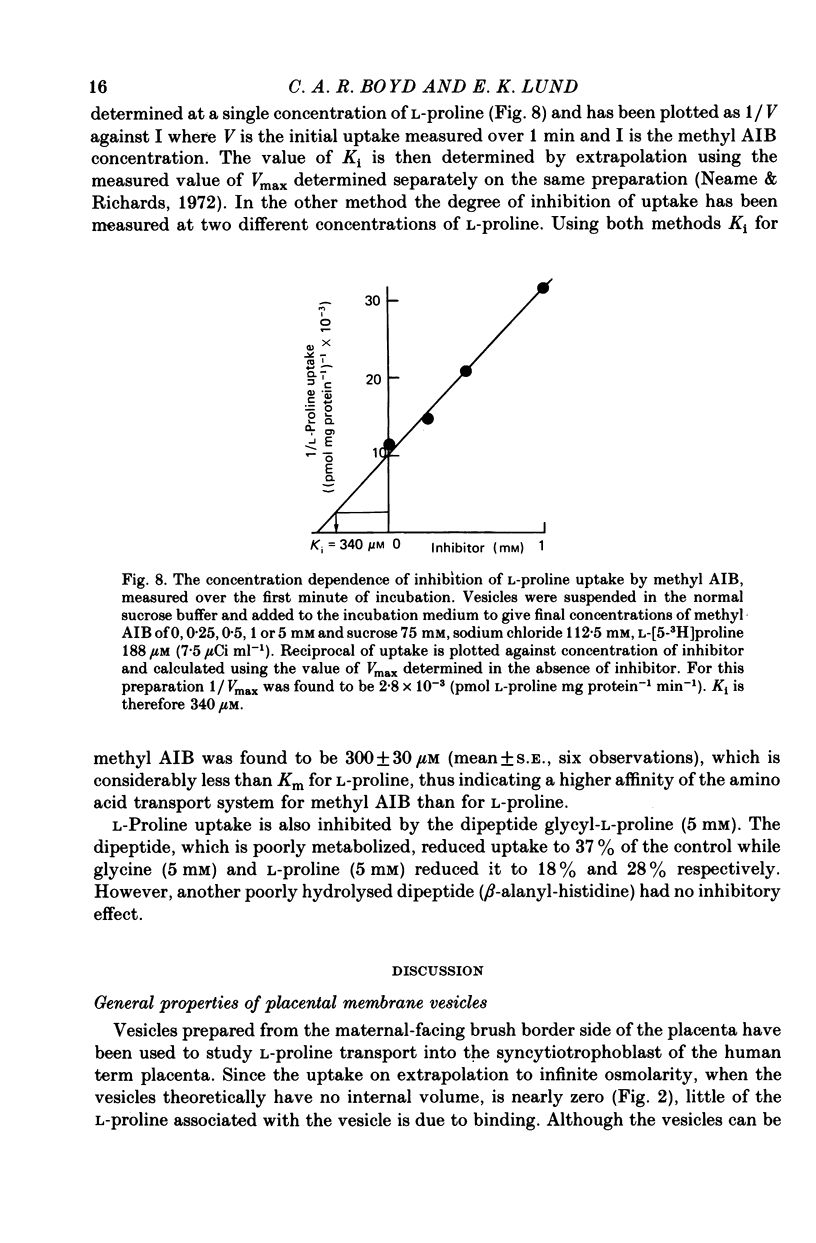
1. Brush border microvillous plasma membranes were prepared from syncytiotrophoblast of human term placenta by a method of differential centrifugation. 2. Such plasma membranes form closed, osmotically active, right-side-out vesicles into which L-proline (188 microM) is shown to be transported in a time-dependent manner. There is no detectable metabolism of L-proline within the vesicles during 30 min of incubation. 3. Transient accumulation of L-proline to levels of up to three times its equilibrium value occurs in the presence of an inward gradient of sodium chloride. The proline and sodium are shown to have reached electrochemical equilibrium by 30 min, at which stage there is about 100 pmol L-proline mg protein-1. 4. This transient accumulation is abolished by the prior equilibration of the sodium chloride gradient, or by the replacement of sodium by inwardly directed gradients of other cations. Entry of the amino acid into the vesicles is also shown to be influenced by the permeability of the anion in the medium and by an imposed potassium diffusion potential. L-Proline transport across the brush border membrane of human placental syncytiotrophoblast is thus a sodium-dependent, electrogenic process. 5. Studies of the transport processes indicate saturation at higher concentrations of L-proline with a 'Km' of about 1 mM; Vmax averaged about 2 nmol mg protein-1 min-1 varied considerably between preparations. 6. L-Proline (188 microM) transport is inhibited competitively by the presence of many amino acids and by the dipeptide glycyl-L-proline. The Ki for inhibition by methyl AIB is 300 microM. 7. These findings are discussed in relation to the mechanism of transplacental amino acid transfer.
Full text
PDF


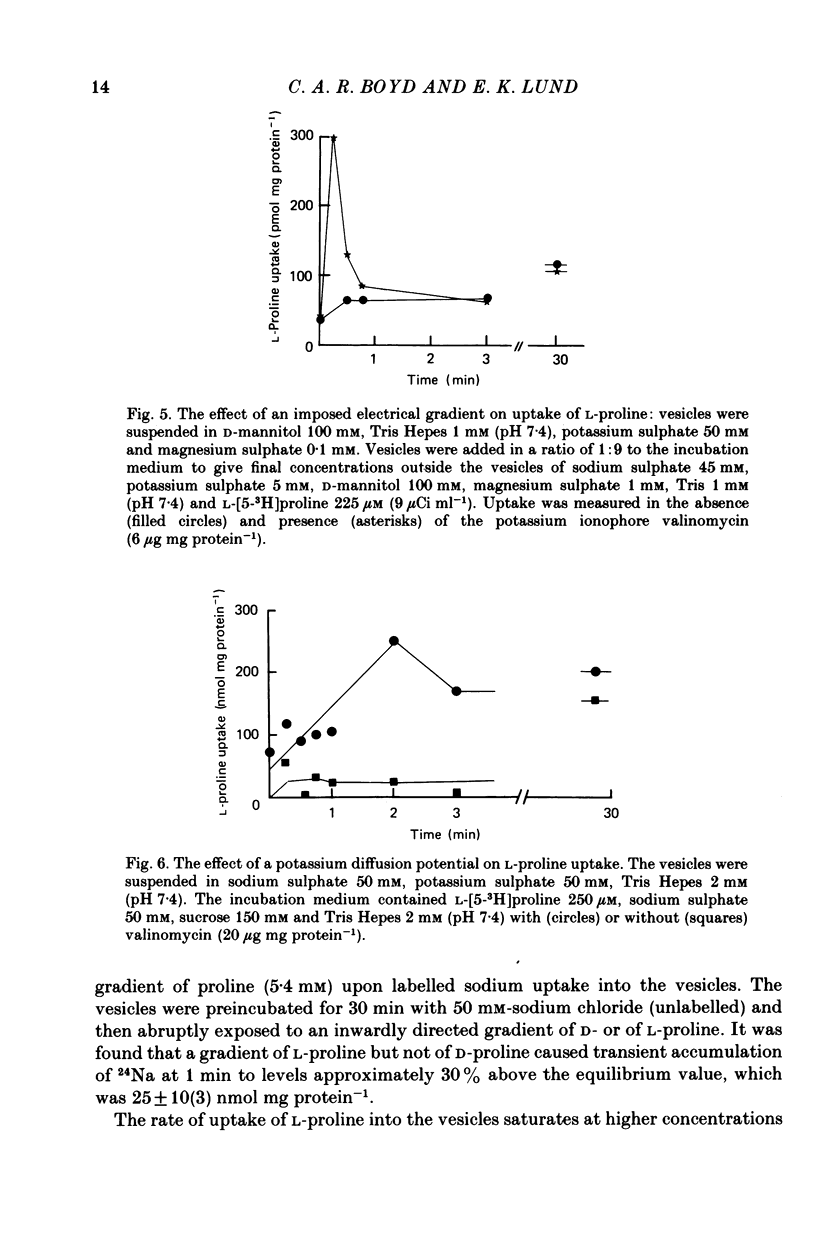
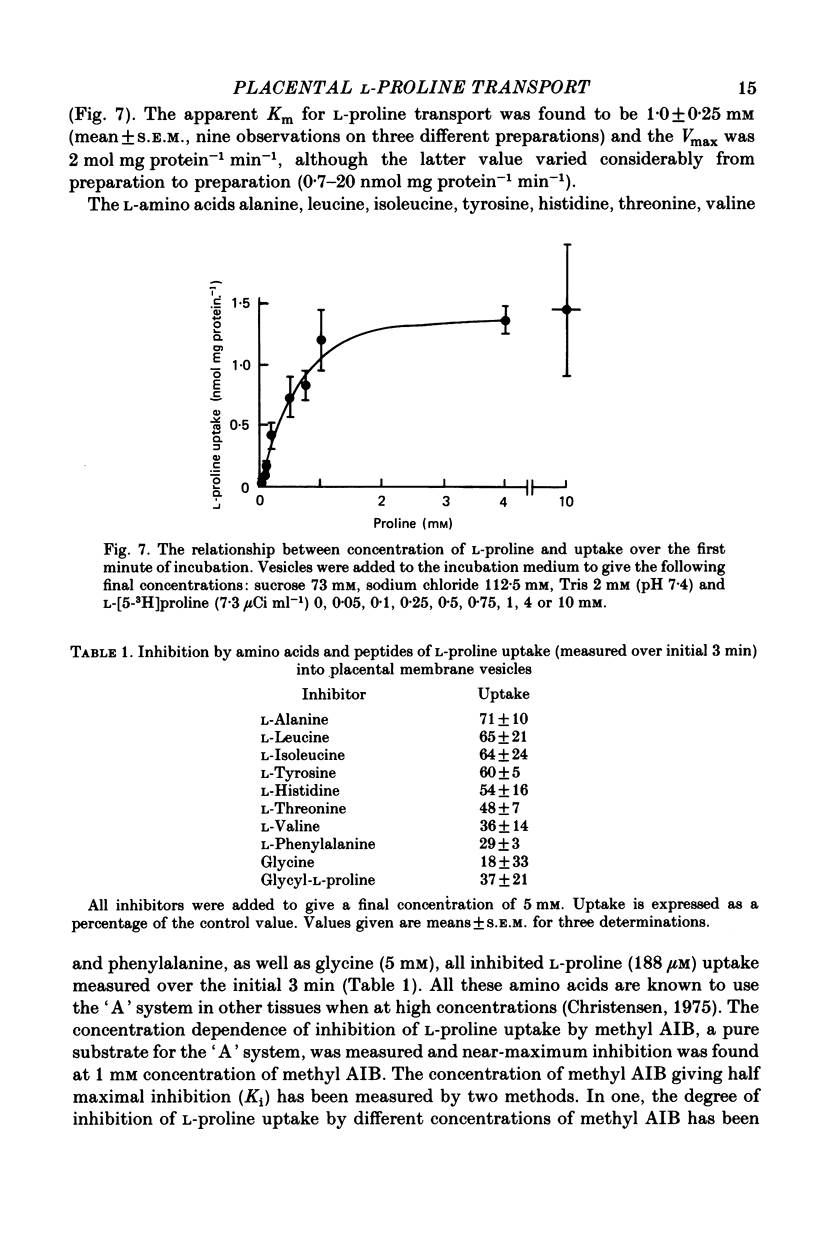



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyd C. A., Chipperfield A. R., Steele L. W. Separation of the microvillous (maternal) from the basal (fetal) plasma membrane of human term placenta: methods and physiological significance of marker enzyme distribution. J Dev Physiol. 1979 Oct;1(5):361–377. [PubMed] [Google Scholar]
- Boyd C. A., Lund E. K. Neutral amino acid transport into microvillus membrane vesicles prepared from human placentae [proceedings]. J Physiol. 1979 Jun;291:29P–29P. [PubMed] [Google Scholar]
- Carstensen M., Leichtweibb J. P., Schröder H. Zellpotentiale in der Placenta des Menschen. Arch Gynakol. 1973;215(3):299–303. doi: 10.1007/BF00672813. [DOI] [PubMed] [Google Scholar]
- Christensen H. N., Handlogten M. E. Cellular uptake of lithium via amino acid transport system A. Biochim Biophys Acta. 1978 Oct 4;512(3):598–602. doi: 10.1016/0005-2736(78)90169-4. [DOI] [PubMed] [Google Scholar]
- Enders R. H., Judd R. M., Donohue T. M., Smith C. H. Placental amino acid uptake. III. Transport systems for neutral amino acids. Am J Physiol. 1976 Mar;230(3):706–710. doi: 10.1152/ajplegacy.1976.230.3.706. [DOI] [PubMed] [Google Scholar]
- Evers J., Murer H., Kinne R. Phenylalanine uptake in isolated renal brush border vesicles. Biochim Biophys Acta. 1976 Apr 5;426(4):598–615. doi: 10.1016/0005-2736(76)90124-3. [DOI] [PubMed] [Google Scholar]
- Hammerman M. R., Sacktor B. Transport of amino acids in renal brush border membrane vesicles. Uptake of L-proline. J Biol Chem. 1977 Jan 25;252(2):591–595. [PubMed] [Google Scholar]
- Jones C. J., Fox H. An ultrahistochemical study of the distribution of acid and alkaline phosphatases in placentae from normal and complicated pregnancies. J Pathol. 1976 Mar;118(3):143–151. doi: 10.1002/path.1711180303. [DOI] [PubMed] [Google Scholar]
- Kessler M., Acuto O., Storelli C., Murer H., Müller M., Semenza G. A modified procedure for the rapid preparation of efficiently transporting vesicles from small intestinal brush border membranes. Their use in investigating some properties of D-glucose and choline transport systems. Biochim Biophys Acta. 1978 Jan 4;506(1):136–154. doi: 10.1016/0005-2736(78)90440-6. [DOI] [PubMed] [Google Scholar]
- OXENDER D. L., CHRISTENSEN H. N. DISTINCT MEDIATING SYSTEMS FOR THE TRANSPORT OF NEUTRAL AMINO ACIDS BY THE EHRLICH CELL. J Biol Chem. 1963 Nov;238:3686–3699. [PubMed] [Google Scholar]
- Philipps A. F., Holzman I. R., Teng C., Battaglia F. C. Tissue concentrations of free amino acids in term human placentas. Am J Obstet Gynecol. 1978 Aug 15;131(8):881–887. doi: 10.1016/s0002-9378(16)33136-2. [DOI] [PubMed] [Google Scholar]
- Reynolds M. L., Young M. The transfer of free alpha-amino nitrogen across the placental membrane in the guinea-pig. J Physiol. 1971 May;214(3):583–597. doi: 10.1113/jphysiol.1971.sp009450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzycki S. M., Kelley L. K., Smith C. H. Placental amino acid uptake. IV. Transport microvillous membrane vesicles. Am J Physiol. 1978 Jan;234(1):C27–C35. doi: 10.1152/ajpcell.1978.234.1.C27. [DOI] [PubMed] [Google Scholar]
- Smith C. H., Nelson D. M., King B. F., Donohue T. M., Ruzycki S., Kelley L. K. Characterization of a microvillous membrane preparation from human placental syncytiotrophoblast: a morphologic, biochemical, and physiologic, study. Am J Obstet Gynecol. 1977 May 15;128(2):190–196. doi: 10.1016/0002-9378(77)90686-x. [DOI] [PubMed] [Google Scholar]
- Smith N. C., Brush M. G., Luckett S. Preparation of human placental villous surface membrane. Nature. 1974 Nov 22;252(5481):302–303. doi: 10.1038/252302b0. [DOI] [PubMed] [Google Scholar]


