Abstract
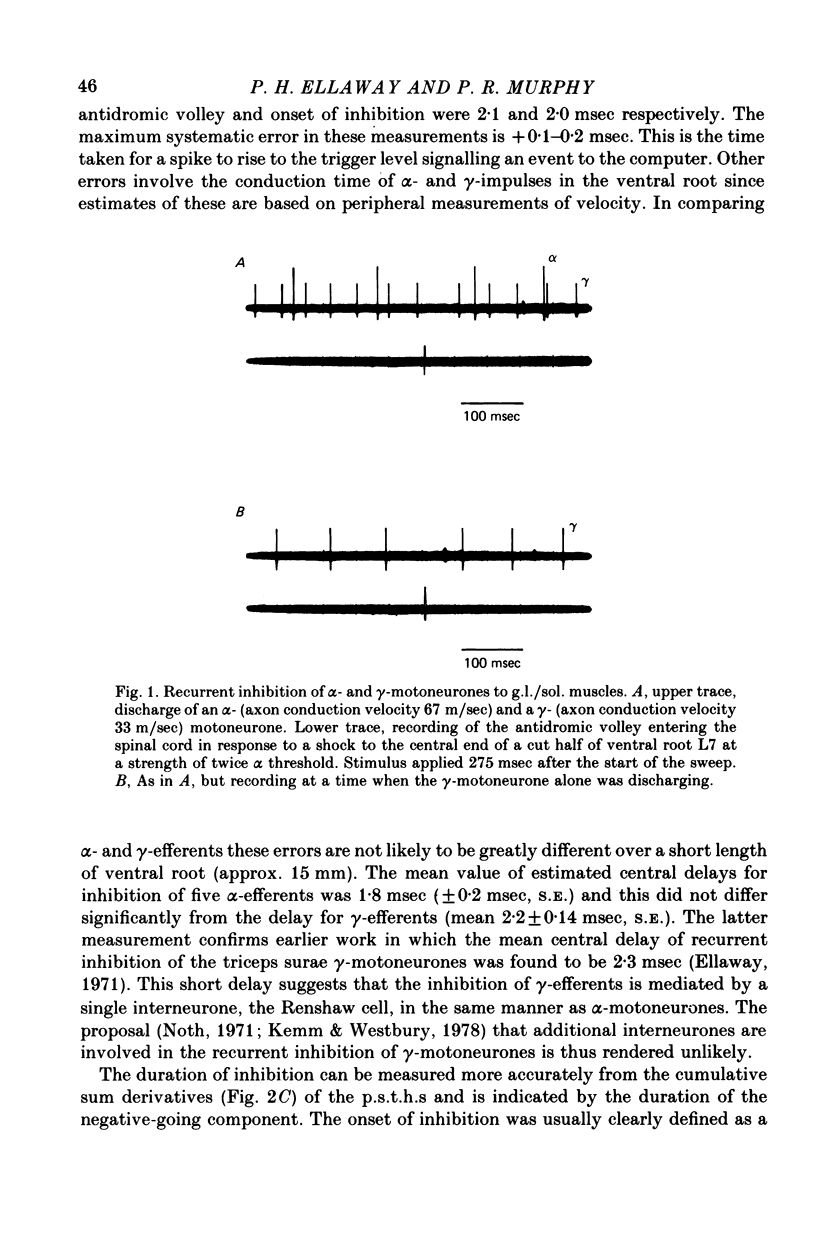
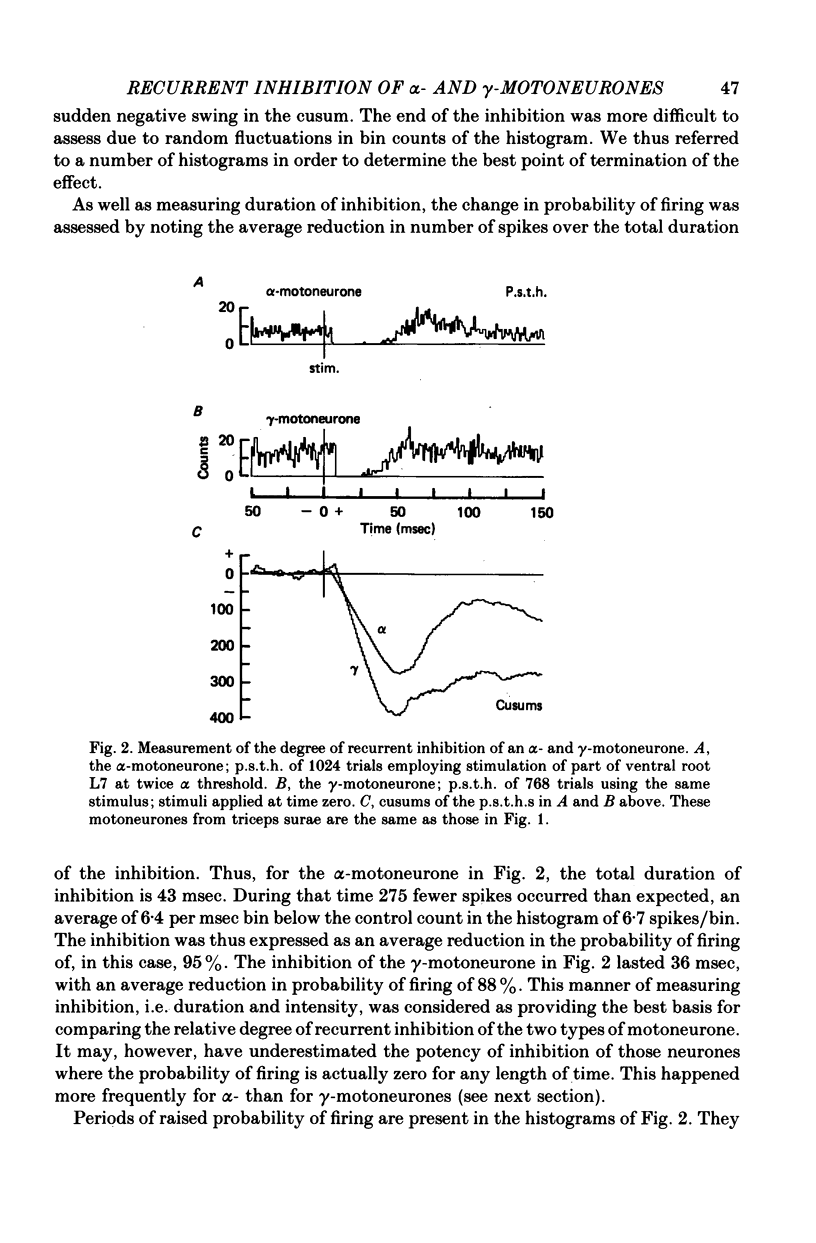
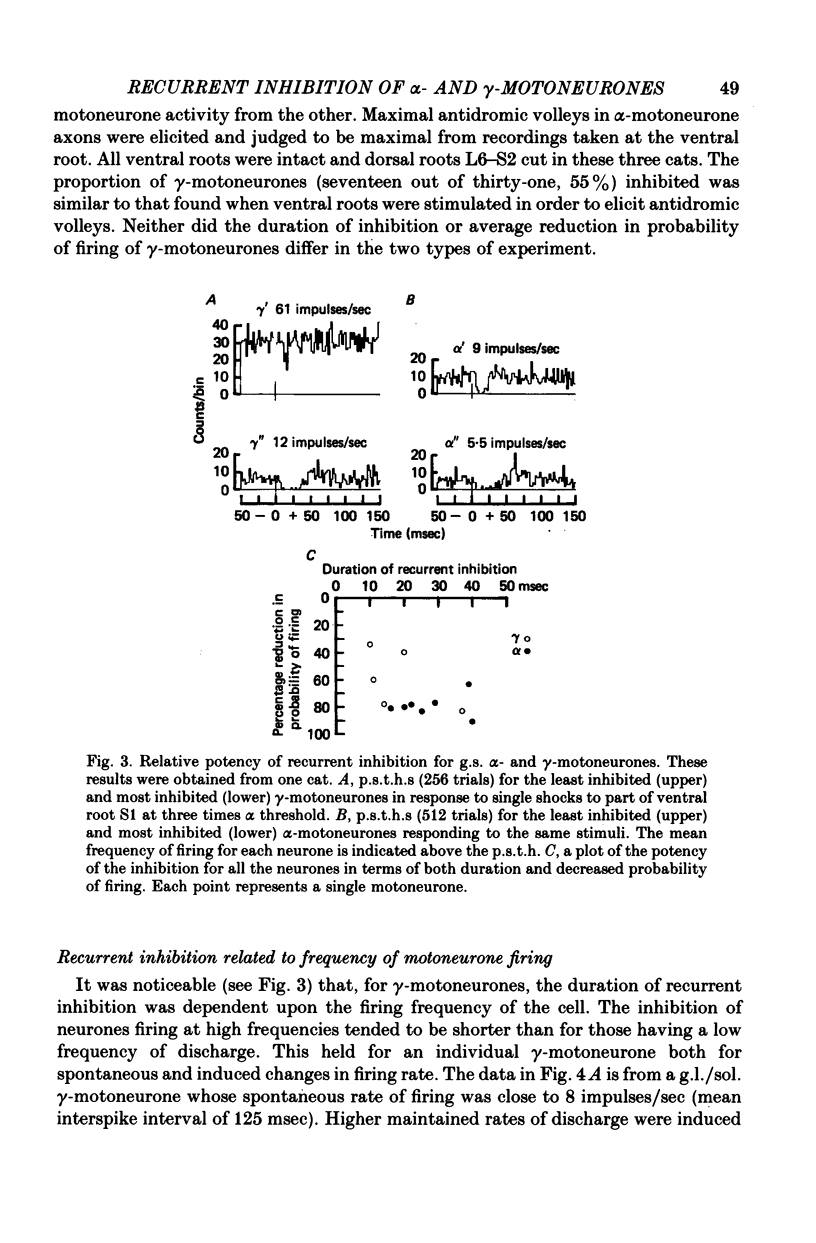
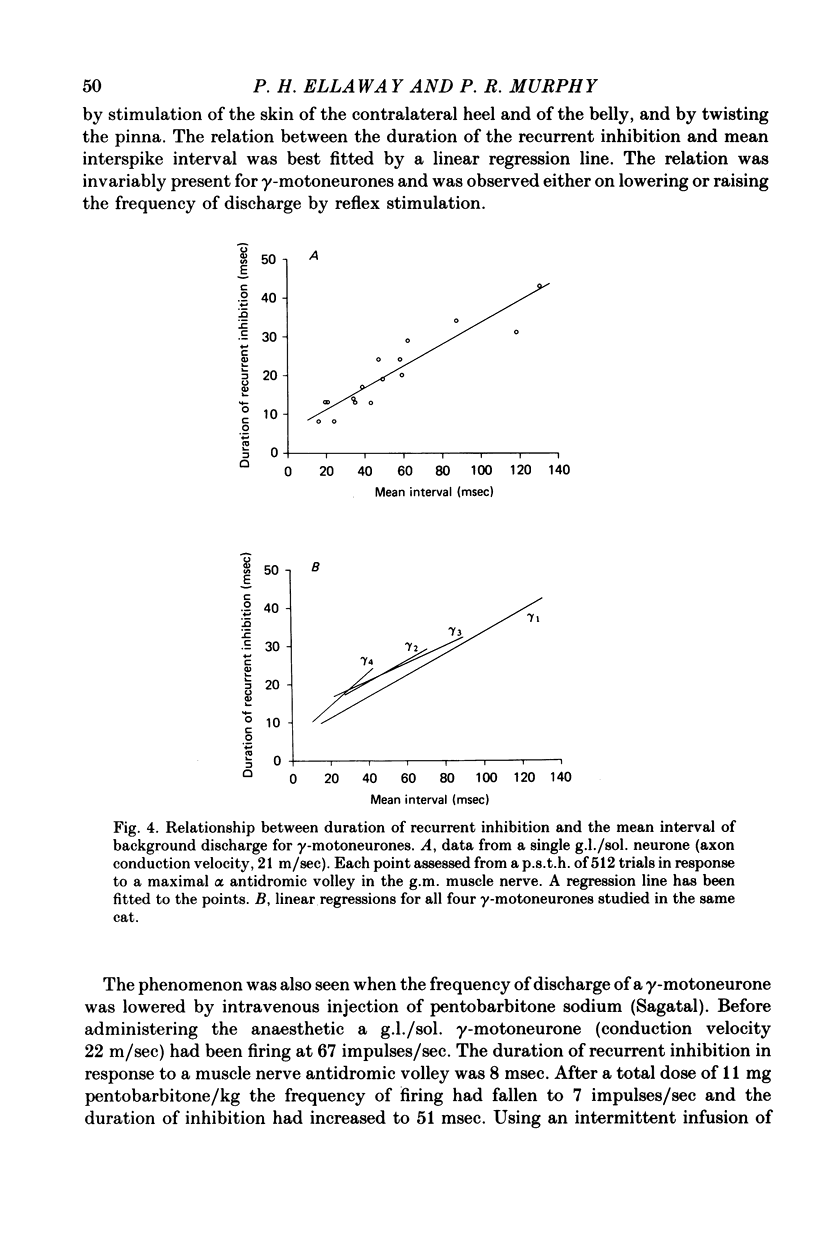
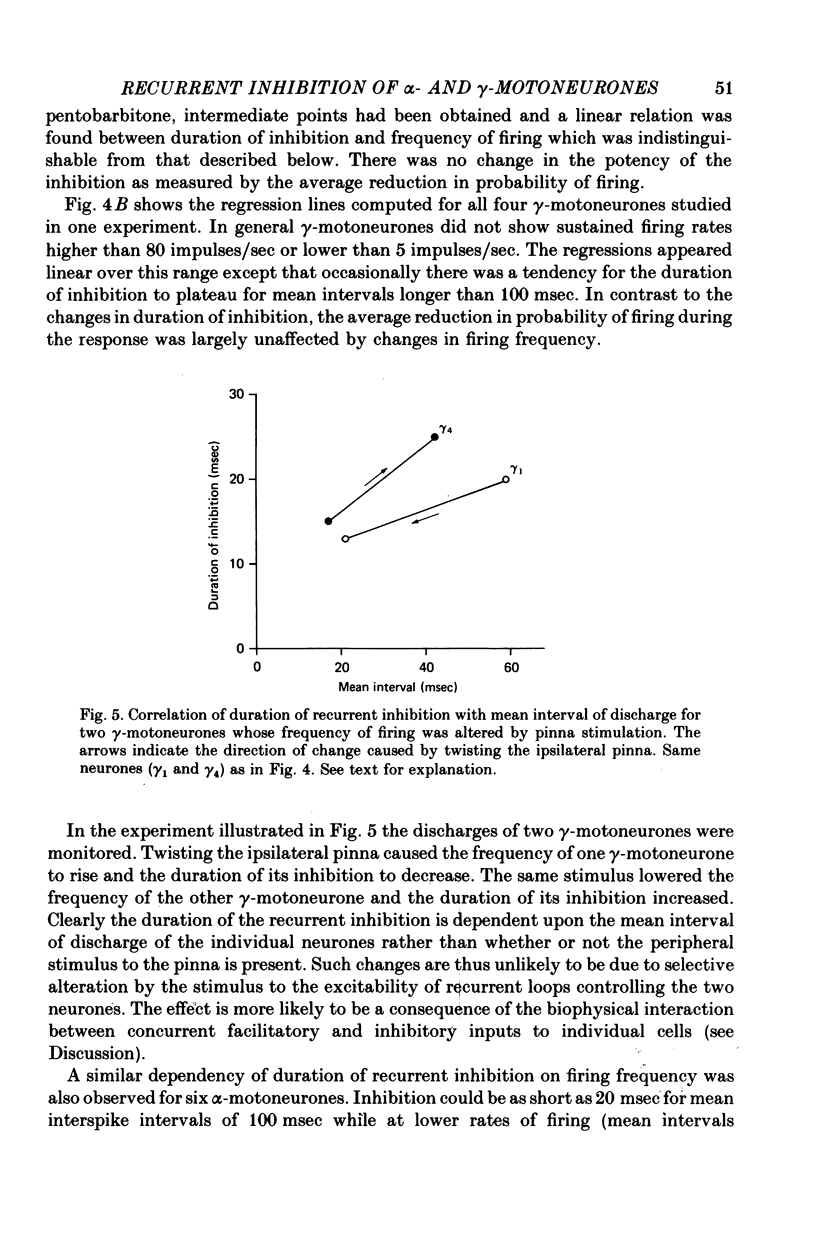
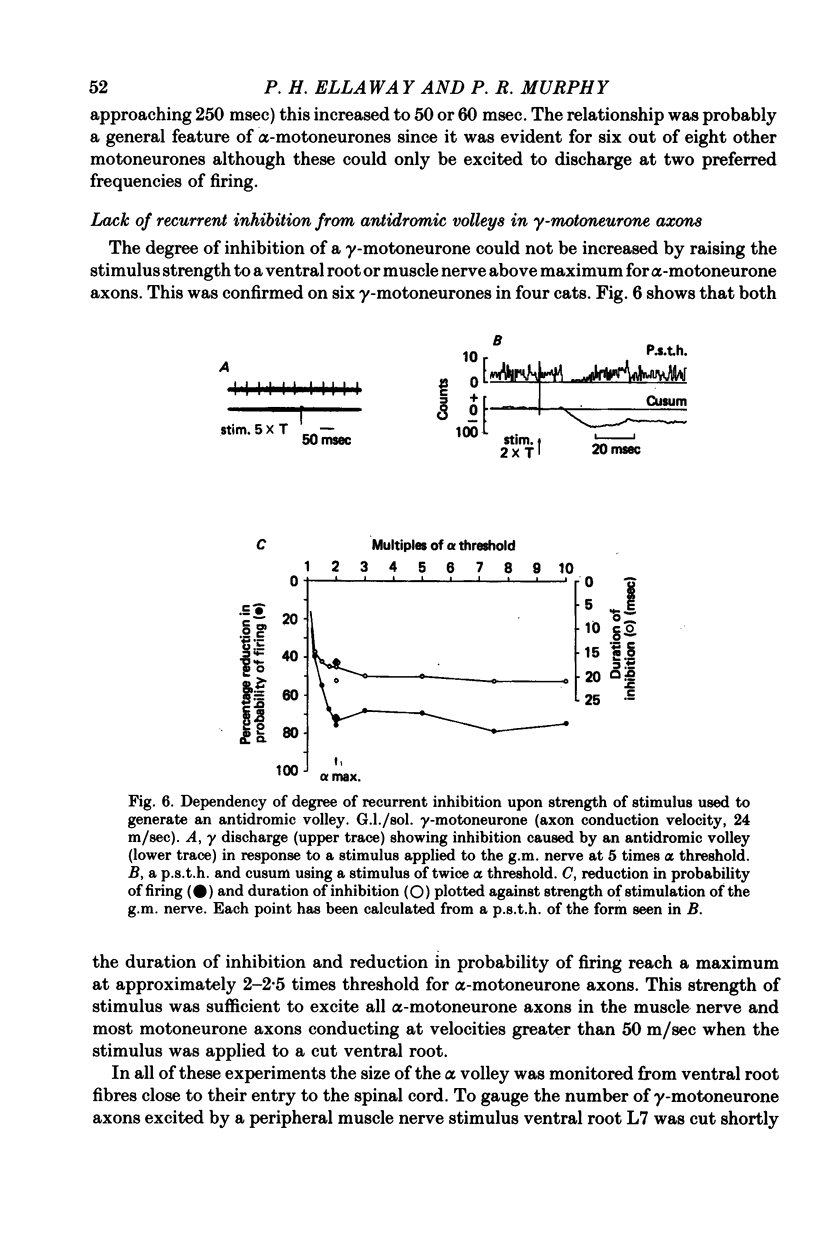
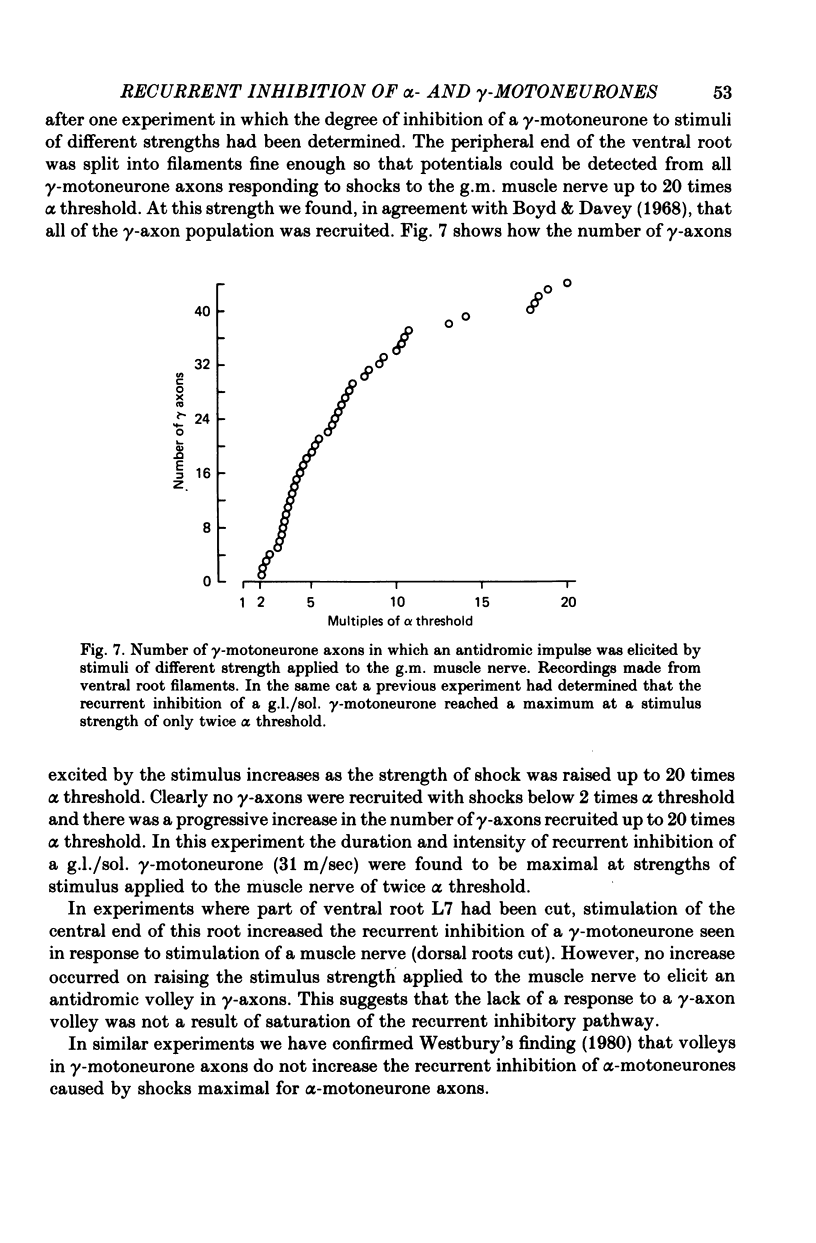
1. The degree of recurrent inhibition of tonically firing alpha- and gamma-motoneurones to triceps surae muscles was assessed in decerebrated cats by measuring the change in probability of firing caused by an antidromic volley in other motoneurone axons. 2. In nine cats 91% (thirty-one out of thirty-three) of alpha- and 54% (twenty-five out of forty-six) of gamma-motoneurones could be inhibited by antidromic volleys in alpha-motoneurone axons. 3. The degree of recurrent inhibition, expressed as the average reduction in probability of firing during the response, was typically in the range of 59-95% for alpha-motoneurones compared to 20-85% for gamma-motoneurones. 4. The duration of recurrent inhibition was 20-50 msec for alpha-motoneurones and 5-40 msec for gamma-motoneurones. The duration was dependent upon the frequency of firing of a neurone, being shorter at high frequencies than at low frequencies. When alpha- and gamma-motoneurones had similar frequencies of discharge the durations of their recurrent inhibition were comparable. 5. Raising the strength of electrical stimulation to elicit an antidromic volley in gamma- as well as alpha-motoneurone axons never produced or increased recurrent inhibition in either type of motoneurone. 6. The quantitative differences in recurrent inhibition of alpha- and gamma-motoneurones are discussed in relation to the control of firing frequency.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown M. C., Lawrence D. G., Matthews P. B. Antidromic inhibition of presumed fusimotor neurones by repetitive stimulation of the ventral root in the decerebrate cat. Experientia. 1968 Dec 15;24(12):1210–1212. doi: 10.1007/BF02146625. [DOI] [PubMed] [Google Scholar]
- COOMBS J. S., ECCLES J. C., FATT P. The inhibitory suppression of reflex discharges from motoneurones. J Physiol. 1955 Nov 28;130(2):396–413. doi: 10.1113/jphysiol.1955.sp005414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullheim S., Ulfhake B. Observations on the morphology of intracellularly stained gamma-motoneurons in relation to their axon conduction velocity. Neurosci Lett. 1979 Jun;13(1):47–50. doi: 10.1016/0304-3940(79)90073-9. [DOI] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES D. M., FATT P. Pharmacological investigations on a central synapse operated by acetylcholine. J Physiol. 1956 Jan 27;131(1):154–169. doi: 10.1113/jphysiol.1956.sp005452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., FATT P., KOKETSU K. Cholinergic and inhibitory synapses in a pathway from motor-axon collaterals to motoneurones. J Physiol. 1954 Dec 10;126(3):524–562. doi: 10.1113/jphysiol.1954.sp005226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellaway P. H. An application of cumulative sum technique (cusums) to neurophysiology [proceedings]. J Physiol. 1977 Feb;265(1):1P–2P. [PMC free article] [PubMed] [Google Scholar]
- Ellaway P. H. Cumulative sum technique and its application to the analysis of peristimulus time histograms. Electroencephalogr Clin Neurophysiol. 1978 Aug;45(2):302–304. doi: 10.1016/0013-4694(78)90017-2. [DOI] [PubMed] [Google Scholar]
- Ellaway P. H., Pascoe J. E. Discharges of semitendinosus fusimotor neurones in the decerebrated and spinalized rabbit. J Physiol. 1965 Nov;181(1):200–213. doi: 10.1113/jphysiol.1965.sp007755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellaway P. H. Recurrent inhibition of fusimotor neurones exhibiting background discharges in the decerebrate and the spinal cat. J Physiol. 1971 Jul;216(2):419–439. doi: 10.1113/jphysiol.1971.sp009533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellaway P. H. The variability in discharge of fusimotor neurones in the decerebrate cat. Exp Brain Res. 1972;14(2):105–117. doi: 10.1007/BF00234794. [DOI] [PubMed] [Google Scholar]
- Ellaway P. H., Trott J. R. Autogenetic reflex action on to gamma motoneurones by stretch of triceps surae in the decerebrated cat. J Physiol. 1978 Mar;276:49–66. doi: 10.1113/jphysiol.1978.sp012219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromm C., Haase J., Noth J. Length-dependent autogenetic inhibition of extensor gamma-motoneurones in the decerebrate cat. Pflugers Arch. 1974;346(3):251–262. doi: 10.1007/BF00595711. [DOI] [PubMed] [Google Scholar]
- Fromm C., Noth J. Reflex responses of gamma motoneurones to vibration of the muscle they innervate. J Physiol. 1976 Mar;256(1):117–136. doi: 10.1113/jphysiol.1976.sp011315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRANIT R., PASCOE J. E., STEG G. The behaviour of tonic alpha and gamma motoneurones during stimulation of recurrent collaterals. J Physiol. 1957 Oct 30;138(3):381–400. doi: 10.1113/jphysiol.1957.sp005857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granit R., Haase J., Rutledge L. T. Recurrent inhibition in relation to frequency of firing and limitation of discharge rate of extensor motoneurones. J Physiol. 1960 Dec;154(2):308–328. doi: 10.1113/jphysiol.1960.sp006581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillner S. The influence of DOPA on the static and the dynamic fusimotor activity to the triceps surae of the spinal cat. Acta Physiol Scand. 1969 Dec;77(4):490–509. doi: 10.1111/j.1748-1716.1969.tb04592.x. [DOI] [PubMed] [Google Scholar]
- Gustafsson B., Lipski J. Do gamma-motoneurons lack a long-lasting afterhyperpolarization? Brain Res. 1979 Aug 24;172(2):349–353. doi: 10.1016/0006-8993(79)90545-6. [DOI] [PubMed] [Google Scholar]
- HENATSCH H. D., SCHULTE F. J. Reflexerregung und Eigenhemmung tonischer und phasischer Alpha-Motoneurone während chemischer Dauererregung der Muskelspindeln. Pflugers Arch. 1958;268(2):134–147. doi: 10.1007/BF00386085. [DOI] [PubMed] [Google Scholar]
- Haase J., Vogel B. Direkte und indirekte Wirkungen supraspinaler Reizungen auf Renshaw-Zellen. Pflugers Arch. 1971;325(4):334–346. doi: 10.1007/BF00592174. [DOI] [PubMed] [Google Scholar]
- Henneman E., Somjen G., Carpenter D. O. Excitability and inhibitability of motoneurons of different sizes. J Neurophysiol. 1965 May;28(3):599–620. doi: 10.1152/jn.1965.28.3.599. [DOI] [PubMed] [Google Scholar]
- Hultborn H., Jankowska E., Lindström S. Recurrent inhibition from motor axon collaterals of transmission in the Ia inhibitory pathway to motoneurones. J Physiol. 1971 Jul;215(3):591–612. doi: 10.1113/jphysiol.1971.sp009487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H., Lindström S., Wigström H. On the function of recurrent inhibition in the spinal cord. Exp Brain Res. 1979 Oct;37(2):399–403. doi: 10.1007/BF00237722. [DOI] [PubMed] [Google Scholar]
- Kato M., Fukushima K. Effect of differential blocking of motor axons on antidromic activation of Renshaw cells in the cat. Exp Brain Res. 1974;20(2):135–143. doi: 10.1007/BF00234008. [DOI] [PubMed] [Google Scholar]
- Kemm R. E., Westbury D. R. Some properties of spinal gamma-motoneurones in the cat, determined by micro-electrode recording. J Physiol. 1978 Sep;282:59–71. doi: 10.1113/jphysiol.1978.sp012448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAINTAL A. S. Functional analysis of group III afferent fibres of mammalian muscles. J Physiol. 1960 Jul;152:250–270. doi: 10.1113/jphysiol.1960.sp006486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryall R. W., Piercey M. F. Excitation and inhibition of Renshaw cells by impulses in peripheral afferent nerve fibers. J Neurophysiol. 1971 Mar;34(2):242–251. doi: 10.1152/jn.1971.34.2.242. [DOI] [PubMed] [Google Scholar]
- Ryall R. W. Renshaw cell mediated inhibition of Renshaw cells: patterns of excitation and inhibition from impulses in motor axon collaterals. J Neurophysiol. 1970 Mar;33(2):257–270. doi: 10.1152/jn.1970.33.2.257. [DOI] [PubMed] [Google Scholar]
- WILSON V. J., BURGESS P. R. Disinhibition in the cat spinal cord. J Neurophysiol. 1962 May;25:392–404. doi: 10.1152/jn.1962.25.3.392. [DOI] [PubMed] [Google Scholar]
- Westbury D. R. Lack of a contribution from gamma motoneurone axons to Renshaw inhibition in the cap spinal cord. Brain Res. 1980 Mar 17;186(1):217–221. doi: 10.1016/0006-8993(80)90269-3. [DOI] [PubMed] [Google Scholar]



