Abstract
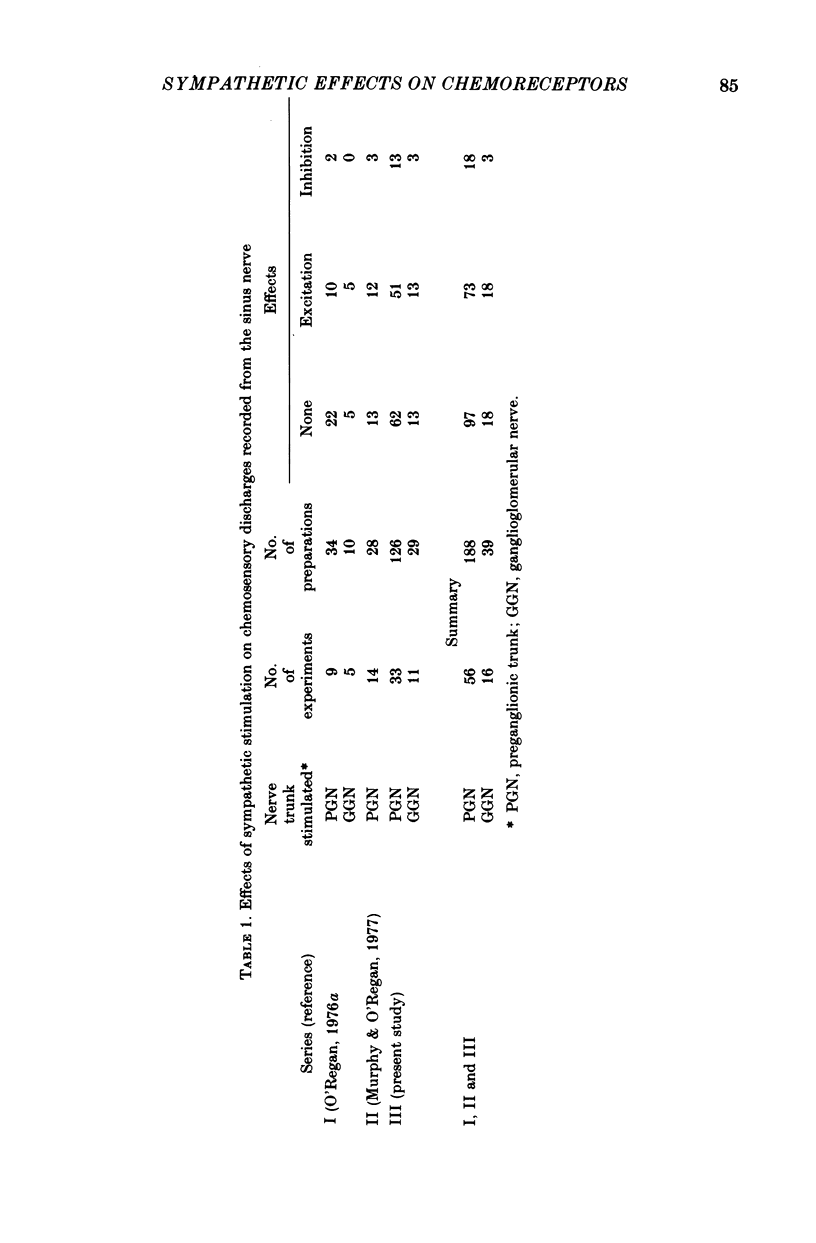
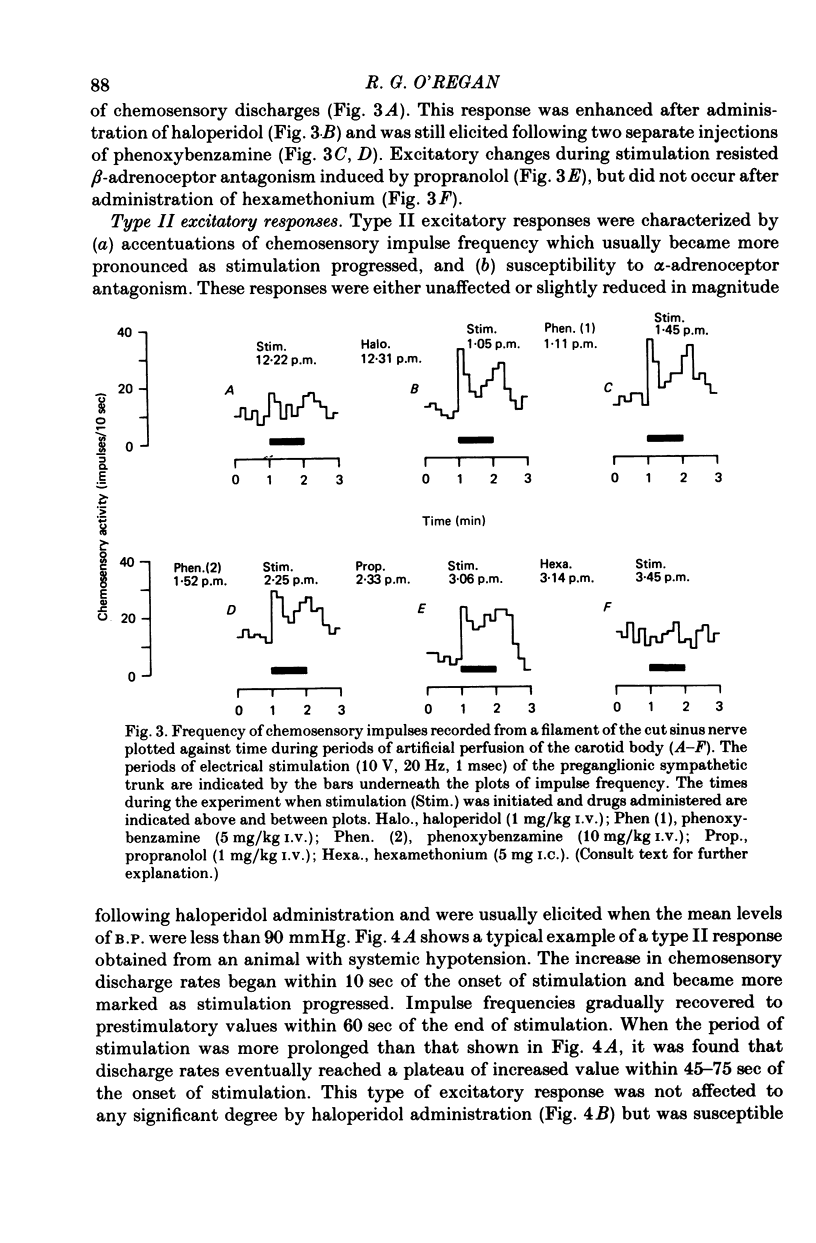
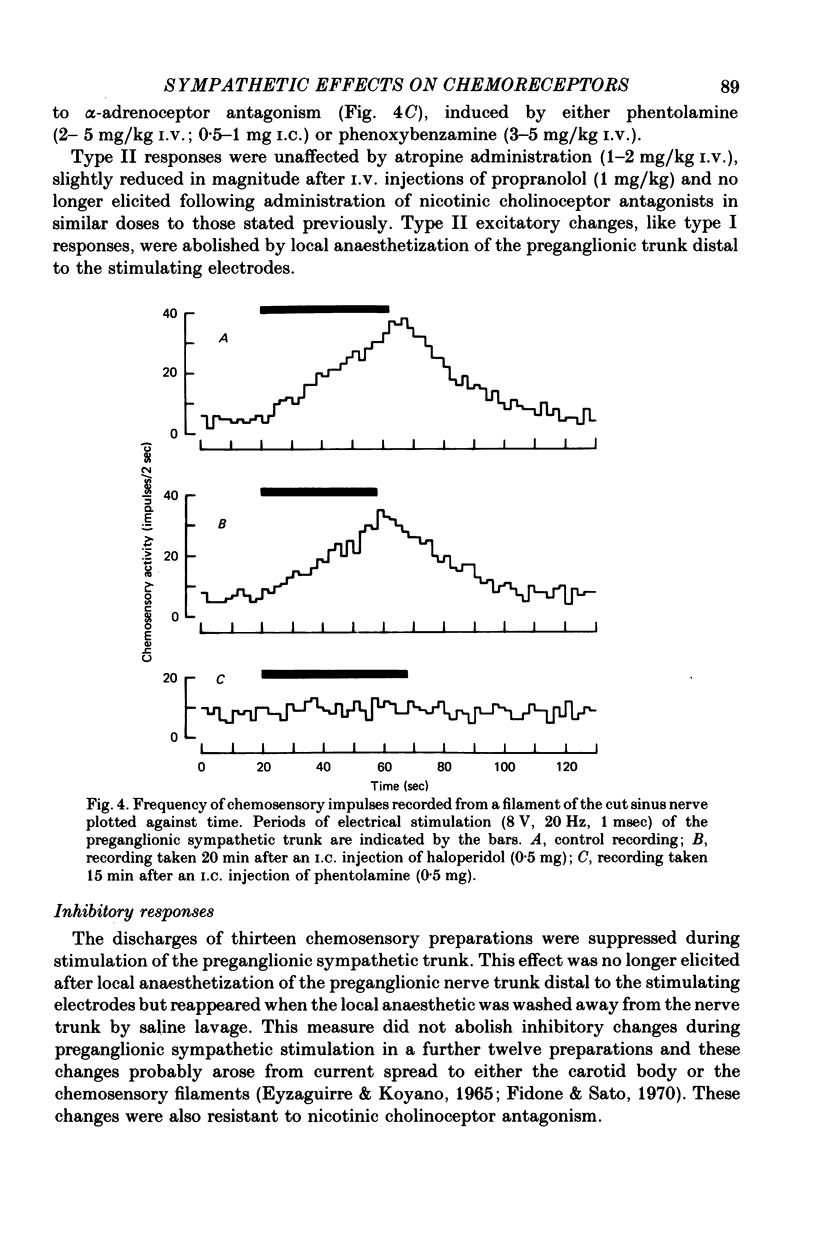
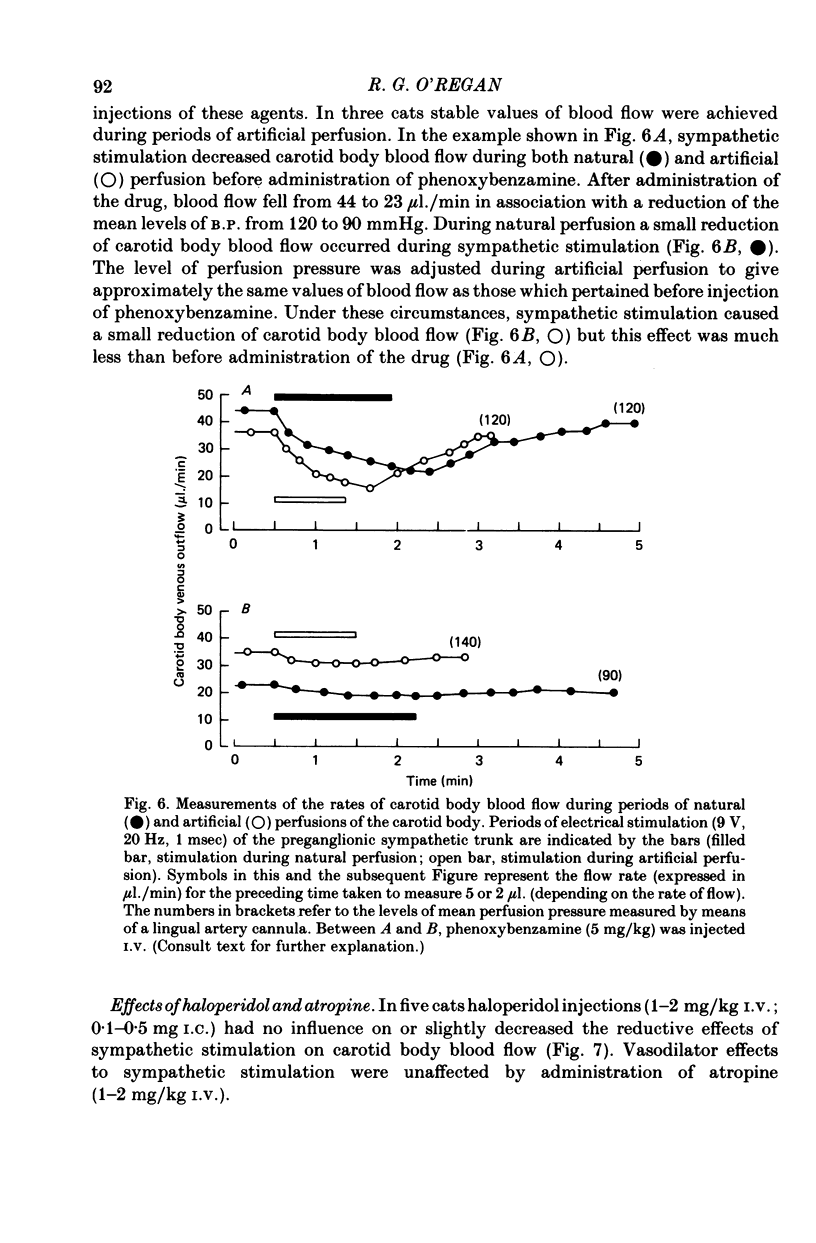
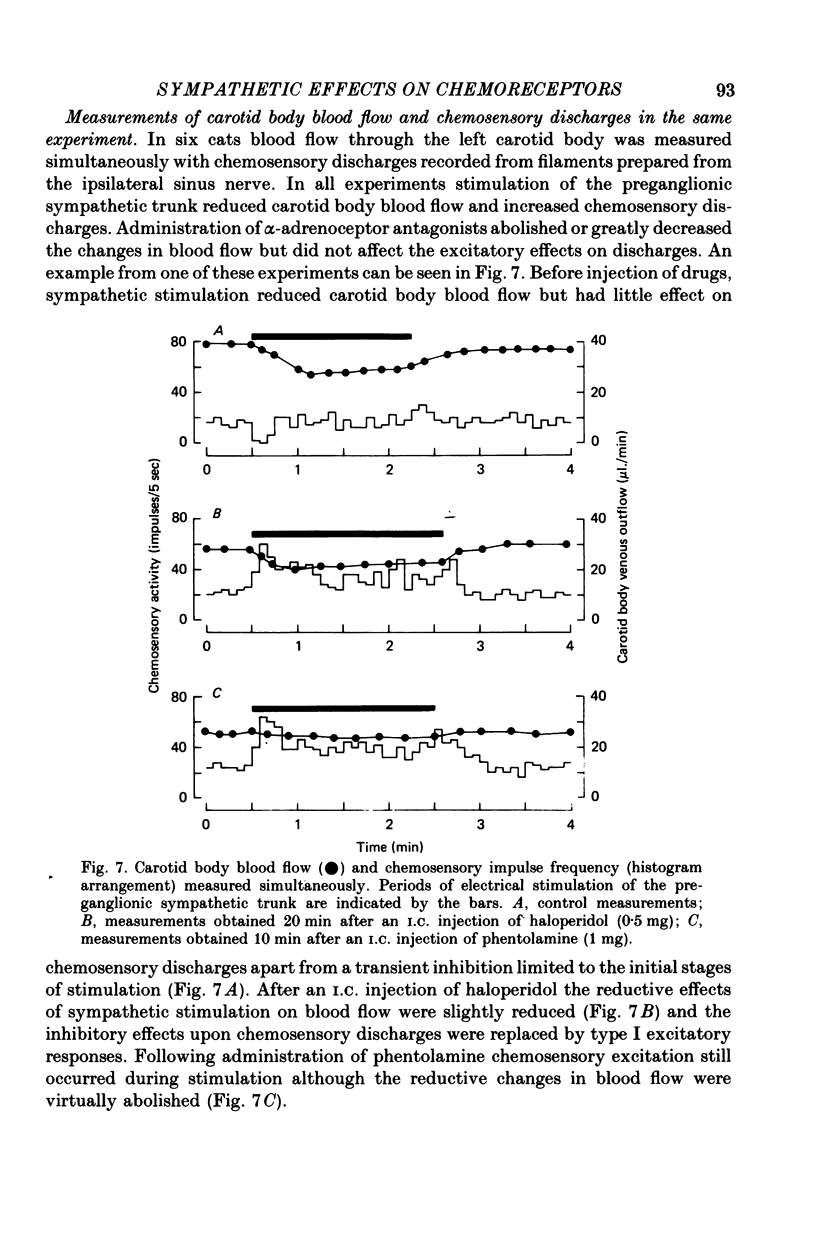
1. The effects of electrical stimulation of sympathetic nerves on sinus nerve chemosensory activity and carotid body blood flow were investigated in anaesthetized cats. 2. Two categories, designated as types I and II, of excitatory responses of chemosensory discharges to sympathetic stimulation were distinguished. Type I responses displayed elevations in impulse frequencies which were usually maximal in the initial 10-20 sec of stimulation, resisted alpha-adrenoceptor antagonism induced by phentolamine or phenoxybenzamine and were enhanced after administration of the dopamine antagonist, haloperidol. Type II responses showed increases in impulse frequencies which became more pronounced as stimulation progressed. These responses were susceptible to alpha-adrenoceptor blockade, were unaffected by haloperidol administration and were usually recorded during systemic hypotension. 3. Inhibitory changes due to activation of sympathetic fibres were recorded in 10% of chemosensory preparations. These effects were usually either abolished or replaced by type I excitatory responses after haloperidol administration. 4. Sympathetic stimulation caused reductions of carotid body blood flow during both natural and artificial perfusion of the organ. This effect was abolished or considerably attenuated by alpha-adrenoceptor antagonism and was unaffected by haloperidol administration. 5. Possible mechanisms which could account for the influences of sympathetic stimulation on chemoreceptor activity and carotid body blood flow are discussed. It is concluded that inhibitory and type I excitatory responses probably arise from activation of sympathetic fibres with non-vascular terminations within the carotid body. Type II excitatory responses are most likely due to blood flow changes.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acker H., O'Regan R. G. The effects of stimulation of autonomic nerves on carotid body blood flow in the cat. J Physiol. 1981 Jun;315:99–110. doi: 10.1113/jphysiol.1981.sp013735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmonte C., Eyzaguirre C. Efferent influences on carotid body chemoreceptors. J Neurophysiol. 1974 Nov;37(6):1131–1143. doi: 10.1152/jn.1974.37.6.1131. [DOI] [PubMed] [Google Scholar]
- Biscoe T. J., Silver A. The distribution of cholinesterases in the cat carotid body. J Physiol. 1966 Mar;183(2):501–512. doi: 10.1113/jphysiol.1966.sp007880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black A. M., Comroe J. H., Jr, Jacobs L. Species difference in carotid body response of cat and dog to dopamine and serotonin. Am J Physiol. 1972 Nov;223(5):1097–1102. doi: 10.1152/ajplegacy.1972.223.5.1097. [DOI] [PubMed] [Google Scholar]
- DE BURGH DALY M., LAMBERTSEN C. J., SCHWEITZER A. Observations on the volume of blood flow and oxygen utilization of the carotid body in the cat. J Physiol. 1954 Jul 28;125(1):67–89. doi: 10.1113/jphysiol.1954.sp005143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EYZAGUIRRE C., LEWIN J. The effect of sympathetic stimulation on carotid nerve activity. J Physiol. 1961 Dec;159:251–267. doi: 10.1113/jphysiol.1961.sp006806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EYZAGUIRRE C., UCHIZONO K. Observations on the fibre content of nerves reaching the carotid body of the cat. J Physiol. 1961 Dec;159:268–281. doi: 10.1113/jphysiol.1961.sp006807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyzaguirre C., Koyano H. Effects of electrical stimulation on the frequency of chemoreceptor discharges. J Physiol. 1965 Jun;178(3):438–462. doi: 10.1113/jphysiol.1965.sp007636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLOYD W. F., NEIL E. The influence of the sympathetic innervation of the carotid bifurcation on chemoceptor and baroceptor activity in the cat. Arch Int Pharmacodyn Ther. 1952 Sep 1;91(1-2):230–239. [PubMed] [Google Scholar]
- Fidone S. J., Sato A. Efferent inhibition and antidromic depression of chemoreceptor A-fibers from the cat carotid body. Brain Res. 1970 Aug 27;22(2):181–193. doi: 10.1016/0006-8993(70)90003-x. [DOI] [PubMed] [Google Scholar]
- Knoche H., Kienecker E. W. Sympathetic innervation of the carotid bifurcation in the rabbit and cat: blood vessels, carotid body and carotid sinus. A fluorescence and electron microscopic study. Cell Tissue Res. 1977 Oct 21;184(1):103–112. doi: 10.1007/BF00220530. [DOI] [PubMed] [Google Scholar]
- Kobayashi S. An autoradiographic study of the mouse carotid body using tritiated leucine, dopa, dopamine and ATP with special reference to the chief cell as a paraneuron. Arch Histol Jpn. 1976 Nov;39(5):295–317. doi: 10.1679/aohc1950.39.295. [DOI] [PubMed] [Google Scholar]
- Kondo H. Innervation of the carotid body of the adult rat. A serial ultrathin section analysis. Cell Tissue Res. 1976 Oct 1;173(1):1–15. doi: 10.1007/BF00219262. [DOI] [PubMed] [Google Scholar]
- Korkala O., Eränkö O., Partanen S., Eränkö L., Hervonen A. Histochemically demonstrable increase in the catecholamine content of the carotid body in adult rats treated with methylprednisolone or hydrocortisone. Histochem J. 1973 Sep;5(5):479–485. doi: 10.1007/BF01012005. [DOI] [PubMed] [Google Scholar]
- Llados F., Zapata P. Effects of dopamine analogues and antagonists on carotid body chemosensors in situ. J Physiol. 1978 Jan;274:487–499. doi: 10.1113/jphysiol.1978.sp012162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg J. M., Hökfelt T., Fahrenkrug J., Nilsson G., Terenius L. Peptides in the cat carotid body (glomus caroticum): VIP-, enkephalin-, and substance P-like immunoreactivity. Acta Physiol Scand. 1979 Nov;107(3):279–281. doi: 10.1111/j.1748-1716.1979.tb06475.x. [DOI] [PubMed] [Google Scholar]
- McCloskey D. I. Mechanisms of autonomic control of carotid chemoreceptor activity. Respir Physiol. 1975 Oct;25(1):53–61. doi: 10.1016/0034-5687(75)90050-x. [DOI] [PubMed] [Google Scholar]
- McQueen D. S. Effects of substance P on carotid chemoreceptor activity in the cat. J Physiol. 1980 May;302:31–47. doi: 10.1113/jphysiol.1980.sp013228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills E., Smith P. G., Slotkin T. A., Breese G. Role of carotid body catecholamines in chemoreceptor function. Neuroscience. 1978;3(12):1137–1146. doi: 10.1016/0306-4522(78)90134-3. [DOI] [PubMed] [Google Scholar]
- Neil E., O'Regan R. G. The effects of electrical stimulation of the distal end of the cut sinus and aortic nerves on peripheral arterial chemoreceptor activity in the cat. J Physiol. 1971 May;215(1):15–32. doi: 10.1113/jphysiol.1971.sp009455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Regan R. G. Carotid chemoreceptor responses to sympathetic excitation [proceedings]. J Physiol. 1976 Dec;263(2):267P–268P. [PubMed] [Google Scholar]
- Purves M. J. The effect of hypoxia, hypercapnia and hypotension upon carotid body blood flow and oxygen consumption in the cat. J Physiol. 1970 Aug;209(2):395–416. doi: 10.1113/jphysiol.1970.sp009171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves M. J. The role of the cervical sympathetic nerve in the regulation of oxygen consumption of the carotid body of the cat. J Physiol. 1970 Aug;209(2):417–431. doi: 10.1113/jphysiol.1970.sp009172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson S. R., Aminoff M. J., Jaffe R. A., Vidruk E. H. Analysis of inhibitory effect of dopamine on carotid body chemoreceptors in cats. Am J Physiol. 1976 Jun;230(6):1494–1498. doi: 10.1152/ajplegacy.1976.230.6.1494. [DOI] [PubMed] [Google Scholar]
- Sampson S. R. Mechanism of efferent inhibition of carotid body chemoreceptors in the cat. Brain Res. 1972 Oct 13;45(1):266–270. doi: 10.1016/0006-8993(72)90236-3. [DOI] [PubMed] [Google Scholar]
- Verna A. Ulstrastructure of the carotid body in the mammals. Int Rev Cytol. 1979;60:271–330. doi: 10.1016/s0074-7696(08)61265-6. [DOI] [PubMed] [Google Scholar]
- Vázquez-Nin G. H., Costero I., Echeverría O. M., Aguilar R., Barroso-Moguel R. Innervation of the carotid body. An experimental quantitative study. Acta Anat (Basel) 1978;102(1):12–28. doi: 10.1159/000145613. [DOI] [PubMed] [Google Scholar]
- Wharton J., Polak J. M., Pearse A. G., McGregor G. P., Bryant M. G., Bloom S. R., Emson P. C., Bisgard G. E., Will J. A. Enkephalin-, VIP- and substance P-like immunoreactivity in the carotid body. Nature. 1980 Mar 20;284(5753):269–271. doi: 10.1038/284269a0. [DOI] [PubMed] [Google Scholar]
- Zapata P. Effects of dopamine on carotid chemo- and baroreceptors in vitro. J Physiol. 1975 Jan;244(1):235–251. doi: 10.1113/jphysiol.1975.sp010794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapata P., Hess A., Bliss E. L., Eyzaguirre C. Chemical, electron microscopic and physiological observations on the role of catecholamines in the carotid body. Brain Res. 1969 Jul;14(2):473–496. doi: 10.1016/0006-8993(69)90123-1. [DOI] [PubMed] [Google Scholar]