Abstract
Cultured fibroblasts possess a characteristic polarized phenotype manifested by an elongate cell body with an anterior lamella whose cell edge is divided into protrusion-forming and inactive zones. Disruption of the fibroblast microtubule cytoskeleton leads to an increase in Rho-dependent acto-myosin contractile activity and concomitant loss of structural polarity. The functional relationship of myosin-driven contractile activity to loss of fibroblast anterior–posterior polarity is unknown. To dissect the roles of microtubule assembly and of Rho-dependent contractility on structural polarization of cells, polarized fibroblasts and nonpolarized epitheliocytes were treated with the microtubule-depolymerizing drug, nocodazole, and/or the Rho kinase inhibitor, Y-27632. Fibroblasts incubated with Y-27632 increased their degree of polarization by developing a highly elongate cell body with multiple narrow processes extended from the edges of the cell. Treatment of fibroblasts with nocodazole, alone or in combination with Rho kinase inhibitor, produced discoid or polygonal cells having broad, flattened lamellae that did not form long lamellar extensions. Single cultured epitheliocytes of the IAR-2 line do not display anterior–posterior polarization. When treated with Y-27632, the cells acquired a polarized, elongate shape with narrow protrusions and wide lamellas. Nocodazole alone or in combination with Y-27632 did not change the discoid shape of epitheliocytes, however treatment with Y-27632 produced thinning of the lamellar cytoplasm. We conclude that microtubules provide the necessary framework for polarization of fibroblasts and epitheliocytes, whereas Rho-regulated contractility modulates the degree of polarization of fibroblasts and completely inhibits polarization in epitheliocytes.
Many cell types, from yeast to neurons, require the establishment and maintenance of a polarized structural organization or axis as an essential component for directed movements, morphogenesis, tissue development, and other activities. Structural asymmetry may be induced by an assortment of regulatory factors, for example extracellular cues such as chemotactic gradients (1) or intracellular gradients developed by spatial segregation of mRNA (2). Whereas the cues initiating the process of cell polarization are varied, there is typically an invariable dependence on the microtubule and/or the actin cytoskeleton for development and maintenance of cell structural polarity.
Asymmetries of myosin-driven contractile activity seem to be sufficient for polarization of cell types such as keratocytes, neutrophils, and lymphocytes, as well as for certain types of cell fragments (3, 4). However, in other cell types, such as cultured fibroblasts and neurons, microtubules are an essential structural element required for establishing cell polarity. Fibroblasts treated with specific microtubule-depolymerizing drugs, such as colcemide or nocodazole, lose their elongate shape and single anterior leading lamella characteristic of migrating cells (5, 6). Loss of microtubules leads to cells acquiring irregular polygonal shapes with multiple lamellas exhibiting protrusive activity located along the entire periphery of the cell. Accompanying microtubule depolymerization-induced loss of polarity is an increase in the number and thickness of stress fibers throughout the cytoplasm, suggestive of an increase in acto-myosin-dependent contractile activity. Increased isometric contractile activity was verified by measuring the pattern of wrinkles formed when cells grown on elastic substrates were treated with microtubule depolymerizing agents (7, 8).
Several recent reports have identified important crossroads that link microtubule dynamics and structure to specific interactions with signaling pathways operating through the Rho family of GTPases (9, 10). Interestingly, the intersection of Rho family-dependent signaling with the actin and microtubule cytoskeletal networks seems to converge on providing structural polarity to the cell (11, 12). The increase in stress fiber number and enhanced contractile activity upon depolymerization of microtubules is a consequence of activating RhoA (13, 14), possibly through release of GEF-H1 from previously assembled microtubules (12). Activation of Rho stimulates Rho kinase, leading to elevated levels of myosin light chain (MLC) phosphorylation through direct phosphorylation of MLC and inhibition of MLC phosphatase, thereby enhancing contractile activity of myosin-II within stress fibers (15).
Considering the synergy that exists between the assembly dynamics of microtubules and myosin-II contractility via Rho signaling, a fundamental unanswered question emerges concerning the specific roles that microtubules and myosin contractility have in directing cell polarity. Is acto-myosin contractile activity required for loss of cell polarity upon depolymerization of microtubules or does the organization of microtubules determine a cell's polarity? To address these issues, we investigated the effect of microtubule depolymerization and myosin-II inhibition on the structural polarity of polarized fibroblasts and nonpolarized epithelial cells. Myosin-II inhibition was induced by treatment with Y-27632 [(R)-(+)-trans-N-(4-pyridyl)-4-(1-aminoethyl)cyclohexanecarboxamide⋅ 2HCl], a highly selective inhibitor of Rho kinase (16). It was found that depolymerization of microtubules was sufficient to induce loss of polarity in fibroblasts even in the absence of Rho kinase activity. By contrast, inhibition of Rho-dependent contractility in the presence of microtubules led to enhanced polarization of fibroblasts and the induction of polarization by nonpolarized epitheliocytes. Thus, contractile activity of the actin cytoskeleton seems to modulate the degree of cell polarization that would otherwise be driven by the assembly dynamics of the microtubule cytoskeleton.
Materials and Methods
Cell Culture.
Rat-1 fibroblasts (17) and IAR-2 liver epithelial cells (18) were cultured in DMEM supplemented with 10% FCS (Atlanta Biologicals, Norcross, GA) and antibiotics at 37°C and 5% CO2.
Drug Treatment and Video Observations.
Cultures of sparse cells were grown on glass coverslips for 20–24 h. Individual coverslips with plated cells were treated in the following ways: 25 or 50 μM Y-27632 (Calbiochem); 3 μM nocodazole (Sigma); 3 μM nocodazole and 50 μM Y-27632; or DMSO for controls. For video light microscopy, coverslips with plated cells were sealed onto glass slides, and observations were made using a microscope equipped with a temperature-controlled stage. For some experiments, cells were treated directly while in observation chambers to monitor the same cells before and after treatment. In other experiments, cells were cultured to confluency, and the monolayers were wounded and immediately treated with inhibitors. Time-lapse video differential interference contrast microscopy was performed using a Zeiss Axiophot microscope equipped with differential interference contrast optics (19).
Immunofluorescence Staining and Confocal Microscopy.
For simultaneous localization of actin and microtubules, cells were fixed in PBS containing 3.7% formaldehyde for 10 min, permeabilized for 1 min with 0.5% Triton X-100 in PBS, and stained with mouse anti-tubulin antibody IgG1 clone DM1α (Sigma, 1:50 dilution) and rhodamine–phalloidin (Molecular Probes, 1:50 dilution). For simultaneous localization of actin and focal contacts, cells were fixed and permeabilized as described above and stained with a 1:50 dilution of mouse anti-paxillin monoclonal IgG1 antibodies (BD Transduction Laboratories, Lexington, KY) and rhodamine–phalloidin. For actin and myosin-II labeling, cells were permeabilized and fixed as described (20) and subsequently stained with anti-nonmuscle myosin monoclonal antibodies M2–42 and rhodamine–phalloidin. For localization of vimentin and microtubules, cells were fixed for 5 min in methanol at −20°C and subsequently stained with mouse monoclonal anti-vimentin IgM antibodies clone VIM-13.2 (Sigma, 1:100 dilution) and with a rabbit peptide antibody specific for Tyr tubulin (W2, 1:100 dilution). Detyrosinated microtubule staining was performed as described (21) using a rabbit peptide antibody specific for Glu tubulin (SG, 1:100 dilution). W2 and SG were generous gifts from G. Gundersen (Columbia University), and M2–42 was kindly provided by T. Pollard (Yale University). All primary antibodies were detected using anti-mouse Alexa Fluor 488 or anti-rabbit Alexa Fluor 568-conjugated secondary IgG antibodies (Molecular Probes, 1:200 dilution). Fluorescence images were collected using the Bio-Rad MRC 1024 laser-scanning confocal microscope system of the Rutgers–Newark Imaging Facility.
Results
Effect of Rho Kinase Inhibition on Fibroblast Polarization.
Individual Rat-1 fibroblasts possess a prototypic shape of an elongate cell body, wide fanlike lamella, and often a short tail (Fig. 1A). The cells were motile with extensive protrusion and retraction of lamellipodia along the convex edge of the anterior lamella. Unattached lamellipodia were transformed into straight or curved ruffles 5–7 μm wide that moved centripetally across the upper surface of a lamella. Actin filaments and myosin-II were colocalized within numerous stress fibers whose ends were located at elongate focal contacts as determined by anti-paxillin antibody staining (data not shown).
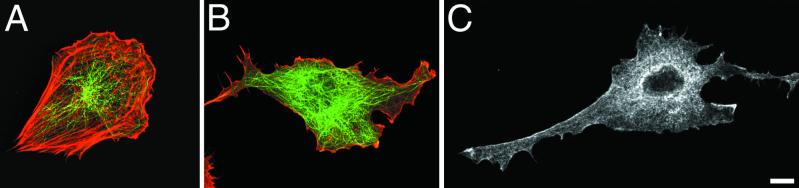
Fig 1.
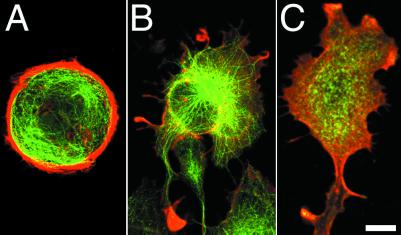
Effect of 50 μM Y-27632 on cell shape of the polarized Rat-1 fibroblasts. Untreated cells (A) possessed stereotypic wide anterior lamella and short tail. The anterior lamella of the same cell became more narrow and long after 58 min of exposure to Y-27632 (B) and collapsed into a long narrow process after 122 min (C). A new process extended from the right side of original lamella and its proximal section collapsed into a stable narrow process, whereas the distal part remained an active lamella (C). (Bar = 10 μm.)
After treatment for 1 h with 25 or 50 μM Y-27632 (16, 22, 23), fibroblasts started to lose their characteristic anterior–posterior morphology by becoming more elongate and multipolar (Fig. 1 B and C). The observed effect was fully reversible within 24 h after culturing cells in non-Y-27632-containing medium. Cells elongated by producing a massive protrusion 30–40 μm long and up to 10–15 μm wide from their anterior lamellas (Fig. 1B). These lamellar expansions appeared to be very flattened, often contained many bidirectionally moving organelles, and exhibited reduced levels of membrane ruffling. Subsequently, the expansion would collapse laterally and form large “stalk-like” extensions that possessed multiple branches that formed by localized lateral collapsing of the cytoplasm (Fig. 1C). Worthylake et al. (24) observed that inhibition of RhoA activity prevented monocyte tail retraction during migration, leading to an exceedingly long tail trailing the migrating monocyte. We also observed a similar stretching of trailing tails as fibroblasts tried to migrate into wounds created in monolayers. Real-time observations of nonmigrating cells in sparse cultures identified that long processes usually formed by elongation of posterior and anterior extensions rather than by stretching attributed to cell translocation.
Rho kinase inhibitor-treated fibroblasts no longer possessed numerous stress fibers as typically observed in untreated fibroblasts (compare Fig. 2 A and B). Rhodamine–phalloidin staining occurred along the entire outer edge of the cell, indicating the presence of a narrow rim of actin filaments at the cell edge (Fig. 2B). This type of staining pattern was typically observed at the edge of leading lamellas of untreated fibroblasts, an area rich in dynamic actin assembly. Myosin-II was diffusely localized throughout Y-27632-treated fibroblasts, and there was no detectable association with the actin filament rich outer edge of the cell (Fig. 2C), suggesting that treatment with inhibitor abolished myosin-II interaction with actin filaments. Microtubules radiated from the central region of the body to all parts of the periphery, and microtubules often projected into many of the small cytoplasmic extensions that emanated outward from the cell edge (Fig. 2B). This was in contrast to control untreated cells where microtubules tended not to enter cell protrusions but instead would turn and curve along the edge of the cell (compare Fig. 2 A and B; also see ref. 9). Long thin processes, including tails, contained compact bundles of microtubules and intermediate filaments (Fig. 3). Interestingly, anti-Glu-tubulin antibodies stained the microtubules in the processes, indicating the presence of a significant population of stable microtubules (Fig. 3B).
Fig 2.
Effect of 50 μM Y-27632 on organization of actin filaments, microtubules, and myosin-II distribution in Rat-1 fibroblasts. Untreated (A) and fibroblast incubated with Y-27632 for 1 h (B) were fixed and double stained for F-actin (red) and tubulin (green). Actin bundles were not detected, and actin-rich zones at the active edge of lamella were formed in Y-27632-treated fibroblasts (compare A and B). The narrow processes that formed were not enriched with actin filaments but did have microtubules (B). Cells incubated with Y-27632 for 5 h and stained for myosin-II exhibit diffuse staining throughout the cell body and in processes, and there is little evidence of colocalization of actin filaments with myosin-II (C). (Bar = 10 μm.)
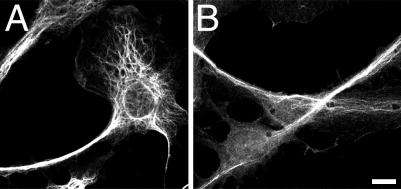
Fig 3.
Localization of intermediate filaments and Glu microtubules (B) in Rat-1 fibroblasts treated with Y-27632. Cells were treated for 5 h with 50 μM Y-27632 followed by fixation and staining for vimentin (A) and Glu-microtubules (B). Note the presence of compact bundles of intermediate filaments and dynamically stable Glu microtubules in the narrow processes. (Bar = 10 μm.)
Effect of Rho Kinase Inhibition and Microtubule Disassembly on Fibroblast Polarity.
Simultaneous treatment of fibroblasts with nocodazole and Y-27632 resulted in the loss of cell polarity. After 5 h, individual fibroblasts became discoid-shaped with wide, flattened lamellae surrounding a centrally localized nucleus. Protrusion and retraction of the cell edges were significantly reduced as compared with nontreated cells, and the most notable activity was the occasional formation of short lamellipodia that might form into a single ruffle. Cells appeared to lose their ability to extend large processes, and the discoid shape resulted from a generalized spreading of the cell leading to a loss of polarity. Cells located at the edge of wounds failed to migrate into wounds. Also noteworthy was the absence of refractile organelles in the highly flattened lamella, indicating the absence of microtubule-dependent organelle transport observed in lamellae of Y-27632-treated fibroblasts.
Similar to Y-27632-treated cells, nocodazole and Y-27632-treated fibroblasts lacked organized bundles of actin filaments; however, there was a small rim of rhodamine–phalloidin staining around the edge of the cell (data not shown; see Fig. 2B). Myosin-II was diffusely localized across the entire cell similar to Y-27632-treated cells (Fig. 2C). The intermediate filament network surrounded the nucleus (data not shown) upon disassembly of microtubules. Interestingly, cells treated with nocodazole and Y-27632 possessed a “serrated” edge of dotlike focal complexes around the entire periphery of the cell (Fig. 4B).
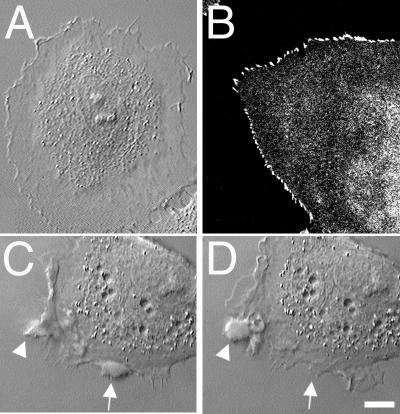
Fig 4.
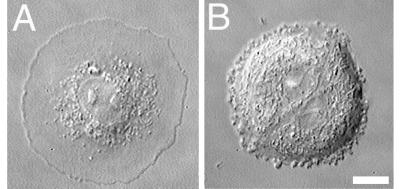
Effect of simultaneous treatment with nocodazole and Y-27632 on cell shape and distribution of focal complexes of polarized Rat-1 fibroblasts. Cells treated with nocodazole and Y-27632 for 5 h had discoid nonpolarized shape with flat ringlike lamella around the cell body (A). Small dotlike, serrated focal complexes were present at the edges of lamella as determined by staining with anti-paxillin antibodies (B). Cell treated with nocodazole alone for 5 h (C) formed irregularly shaped swellings at the cell edge. The observed swellings appeared to form by the fusion of multiple ruffles at the lamellipodial edge (C, see arrowhead). The swellings did not exhibit retrograde flow but remained at the cell edge, where they eventually dissolved back into the cytoplasm after a short period (see arrows in C and D). (Bar = 10 μm.)
As expected, fibroblasts treated with nocodazole possessed irregular polygonal shapes, lacked microtubules, contained numerous thick stress fibers, and formed an increased number of elongated focal adhesions (data not shown). Time-lapse differential interference contrast microscopy identified that the activity at the edges of cells lacking microtubules was different from that of nontreated, Y-27632-treated, or nocodazole/Y-27632- treated cells (Fig. 4 C and D). Lamellipodia formed along the cell edge would start to retract as ruffles, but the retraction soon stopped as multiple ruffles fused near the cell edge into a characteristic round or sausage-like swelling. The swellings remained at the cell edge without any centripetal movement and then they would eventually dissolve into a normal thin cytoplasm. Such swellings were enriched in actin filaments and myosin-II (data not shown).
Effects of Rho Kinase Inhibition on Epithelial Cell Polarity.
As described previously (21), individual IAR-2 cells are discoid shaped, and lamellipodia extend and retract along the free edge of the cell. Within 1 h of exposure to Y-27632, cells started to change their shape, most notably developing several wide processes extending 10–15 μm in length (Fig. 5). As observed with fibroblasts, flattened lamellar extensions appeared to result from continuous expansion of the cell edge without any countering retraction. The thin expanded lamellas contained numerous bidirectionally moving organelles. Expansion of the edge was even observed along the boundary of cell–cell contact, an area that is typically quiescent with regard to protrusion–retraction. Along the cell–cell contact, each cell would expand its edge, thereby increasing the surface area of contact, placing the cells into a “cellular embrace.” Even after prolonged exposure (up to 20 h), processes continued to elongate, and individual cells could develop numerous processes that were heterogeneous in shape (Fig. 5 C and D). Stalk-like processes were formed by lateral collapse of extensions as described for fibroblasts (see above). Often, epitheliocytes developed highly elongated bodies with wide, flattened lamellas containing moving organelles and active edges exhibiting protrusion.
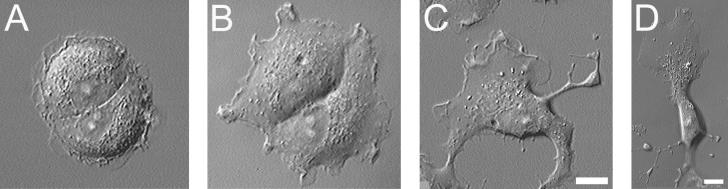
Fig 5.
Effect of Y-27632 on the shape of nonpolarized IAR-2 epitheliocytes. An untreated group of two epitheliocytes (A) began to extend multiple processes from their edges after 40 min of treatment with 50 μM Y-27632 (B). After 120 min of exposure to Y-27632, cells acquired highly polarized shapes with wide lamellas and multiple narrow processes (C and D). (Bar = 10 μm.)
Changes to the organization of the actin and microtubule cytoskeleton in epithelial cells followed along the general lines observed for fibroblasts. Treatment with Y-27632 led to the complete disassembly of the marginal bundle of actin filaments that encircles individual epithelial cells (Fig. 6). There were no longer any detectable stress fibers as determined by staining cells for actin filaments or myosin-II. Actin filaments were present at the leading edges of cells, indicating that actin assembly continued to occur at the edge and was probably responsible for driving lamellar expansion. Microtubules were spread throughout the cells; however, unlike nontreated cells, individual microtubules were now able to radiate outward to the very edge of the cell. Myosin-II was diffusely localized throughout the cytoplasm, and there did not appear to be colocalization with the actin filaments present at the cell edge (Fig. 6C).
Fig 6.
Localization of actin filaments, microtubules, and myosin-II in IAR-2 epitheliocytes treated with Y-27632. Cells were double-stained for actin (red) and tubulin (green) (A and B). Control cells not treated with inhibitor possessed thick marginal bundles of actin filaments that isolated microtubules from the edge (A). Upon treatment with Y-27632, marginal bundles disassembled, and microtubules radiated out to the cell edges and into processes (B). Double-labeling cells incubated with Y-27632 for actin (red) and nonmuscle myosin-II (green) established the absence of myosin-II or actin filament containing stress fibers (C). Actin filaments were present at the edges of cells; however, myosin-II tended to be diffusely localized throughout the cell (C). (Bar = 10 μm.)
Effects of Rho Kinase Inhibition and Microtubule Depolymerization on Epithelial Cell Polarity.
Depolymerization of microtubules and inhibition of Rho kinase resulted in dramatic spreading of epithelial cells to the point where they formed round cells that looked like fried eggs (Fig. 7A). The lamellar cytoplasm was highly flattened, and there was no bidirectional movement of organelles within the lamella. Essentially, the overall morphology of the epithelial cell became indistinguishable from fibroblasts that were treated with nocodazole and Y-27632 (compare Fig. 4A with Fig. 7A). Although not quantitated, the edge of epithelial cells appeared to be smoother, indicating less overall protrusive activity at the edge of epithelial cells as compared with fibroblasts when treated with both reagents. This difference in the quality of the free edge may be a reflection of inherent differences in the actin filament assembly dynamics between the two cell types.
Fig 7.
Video-differential interference contrast micrographs of epithelial IAR-2 cells treated with nocodazole and Y-27632 or with nocodazole. Cell treated with 3 μM nocodazole and 50 μM Y-27632 for 5 h became highly flattened and discoid shaped (A). Note the absence of organelles within the flattened lamella. Group of two rounded cells treated with nocodazole for 5 h formed small swellings at the edges (B). (Bar = 10 μm.)
Whereas epithelial cells treated with nocodazole retained their discoid shape, there was a noticeable difference in the dynamic activity at the cell edges. Most cells continued to extend and retract lamellipodia; however, this process led to formation of small “swellings” that formed from fusion of localized ruffles (Fig. 7B). The swellings remained at the edge of the cell, did not migrate centripetally, and would eventually dissolve back into the cytoplasm.
Discussion
Structural Polarization of Fibroblasts Depends on the Integrity of Microtubules and Not on Rho Kinase-Dependent Myosin-II Contractility.
Available published data would suggest the hypothesis that the loss of polarization in fibroblasts after disassembly of microtubules may be driven by the pronounced increase in Rho kinase-dependent myosin-II contraction of the actin cytoskeleton (7, 8). To test this hypothesis, we examined the effects of Rho kinase inhibition on polarization of fibroblasts treated with the microtubule poison, nocodazole. We found that the degree of polarization of fibroblasts was not dependent upon Rho kinase-activated myosin-II contractile activity. Cells with intact microtubule networks retained their polarized elongated shape and protrusive activity at cell edges after Y-27632-induced loss of stress fibers and decreased contractility. In fact, cells treated with Rho inhibitor were often more elongate and produced multiple extensions as compared to control fibroblasts. When cells were treated simultaneously with nocodazole and Y-27632, they acquired nearly discoid or polygonal nonpolarized shape. Thus, loss of structural polarity by depolymerization of microtubules is independent of Rho-stimulated formation of stress fibers and increased myosin-II contractile activity.
What is the mechanism of control of fibroblast polarity by the microtubule cytoskeleton? Polarization may be based on localized stimulation of actin polymerization by the plus-ends of dynamic microtubules that reach out into the periphery of the cell. The growth of microtubules out to the cell edge could lead to positioning or activation of Rac1 (25), which then stimulates a burst of actin assembly and formation of ruffles and lamellipodia (26). It has been proposed that retrograde flow of the actin cytoskeleton pushes the microtubules away from the cell edge, which would produce an effective countering action that limits the degree of actin filament assembly (27, 28). When fibroblasts are treated with Rho kinase inhibitor, normal retrograde flow may be affected, and microtubules could continuously probe the cell edge leading to over-stimulation of actin filament polymerization leading to massive expansion of lamellae. An alternative possibility would be that cargo needed for actin filament assembly is transported along microtubules to the cell edge (29).
Another important component of structural reorganization of cells treated with Y-27632 was the formation of long thick stalks and shorter thin sticks that formed by narrowing of a previously flattened lamella. Microtubules were located inside each long slender process, and in the case of the thick stalks, there seemed to be an enrichment of Glu-tubulin. It is possible that inactivation of Rho kinase leads to a destabilization of the cortical actin cytoskeleton, which is now missing a key binding protein, myosin-II. This destabilization of the actin network coupled to microtubule-initiated disassembly of focal contacts (30) located at cell edges could result in a lateral collapse of the lamella about bundles of microtubules and intermediate filaments. This suggestion remains to be tested.
Regardless of the presence of Y-27632, cells with depolymerized microtubules did not form tails or any other long extensions, and so microtubules are essential for elongation of these processes. Also, the degree of contractility must play a role in the control of process elongation. This was shown by comparison of extensions formed by nocodazole-treated cells in the presence or absence of Y-27632. When treated with nocodazole and Rho inhibitor, cells only formed very flat and short lamellipodia. In contrast, treatment with nocodazole alone induced protrusions having profoundly different shapes and dynamics. The short rare lamellipodia rapidly retracted and were transformed into actin and myosin-II-rich swellings of cytoplasm at the cell's edge. It seems probable that these peculiar formations were a result of increased Rho-dependent contractility and decreased stimulation of actin filament assembly caused by depolymerization of microtubules. Because contractility would predominate over polymerization (extension), high contractility might lead to formation of monstrous local swellings resulting from retraction of ruffles.
Inhibition of Rho-Dependent Contractility Induces Polarization of Epitheliocytes.
Inhibition of contractility within epitheliocytes in the presence of intact microtubules induces even more striking effects on epithelial cells than on similarly treated fibroblasts. Nonpolarized epithelial cells would elongate, form polarized lamellas, and develop long processes. The effects of Rho-kinase inhibitor on epitheliocytes and on fibroblasts are qualitatively similar despite the considerable differences that exist in their initial phenotypes.
These data indicate that high Rho-dependent contractility may be essential for the maintenance of the nonpolarized, discoid-shaped, epithelial phenotype. The exact mechanisms preventing polarization of untreated epithelial cells remain unknown; however, the absence of polarization may be related to the inability of microtubules to reach the edge of the cell (10). It is possible that the marginal bundles of actin filaments that rim the cell serve to limit the outward growth of microtubules to the cell edge and thus prevent formation of long protrusions. Marginal bundles have been shown to move centripetally away from the free cell edge (31), which would move microtubules backward and prevent localized stimulation of actin filament assembly that could lead to polarization. Possibly, in epithelial cells, when the marginal bundle is destroyed in the presence of Rho-kinase inhibitor, microtubules are able to grow to the cell edge and induce formation of localized protrusions.
Conclusion
The experiments presented in this paper show that the loss of cell polarity and increase of Rho-dependent contractility are two independent consequences of drug-induced depolymerization of microtubules. Whereas microtubules control polarization of cells and elongation of processes, contractility modulates polarization of fibroblasts and completely inhibits polarization of epitheliocytes. Previous authors have shown that the inhibition of Rho and Rho kinase induces formation of long processes in other cell types: prostate carcinoma cells (32) and monocytes (24); however, the role of microtubules in the formation of these processes was not examined. As already discussed, the functional interactions of the microtubule cytoskeleton and myosin-dependent contraction may play differing roles in determining the polarization of various types of cells. Together, these data suggest that Rho-dependent variations of contractility can have profound consequences on the phenotype of the cell. This phenomenon may be critical in epithelio-mesenchymal transformations induced by different agents (33), in the formation of axonal processes by neuroblasts and neurons, as well as in morphological transformations occurring in the course of multistage carcinogenesis (32).
Acknowledgments
We thank Drs. G. Gundersen (Columbia University) and T. Pollard (Yale University) for providing antibodies. Funding for this study was provided by a Russian Foundation of Basic Investigations Grant and Ludwig Foundation Grant (to J.M.V.), by a gracious gift from Mrs. Harold Kaplan to the Research Exchange Program at Rutgers–Newark, and by a Johnson & Johnson Discovery Award (to E.M.B.).
References
- 1.Weiner O. D. (2002) Curr. Opin. Cell Biol. 14, 196-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shestakova E. A., Singer, R. H. & Condeelis, J. (2001) Proc. Natl. Acad. Sci. USA 98, 7045-7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Euteneuer U. & Schliwa, M. (1992) J. Cell Biol. 116, 1157-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verkhovsky A. B., Svitkina, T. M. & Borisy, G. G. (1999) Curr. Biol. 9, 11-20. [DOI] [PubMed] [Google Scholar]
- 5.Vasiliev J. M., Gelfand, I. M., Domnina, L. V., Ivanova, O. Y., Komm, S. G. & Olshevskaja, L. V. (1970) J. Embryol. Exp. Morphol. 24, 625-640. [PubMed] [Google Scholar]
- 6.Ivanova O. Y., Margolis, L. B. & Vasiliev, J. M. (1976) Exp. Cell Res. 101, 207-219. [DOI] [PubMed] [Google Scholar]
- 7.Harris A. K. (1999) Biochem. Soc. Symp. 65, 315-341. [PubMed] [Google Scholar]
- 8.Danowski B. A. (1989) J. Cell Sci. 93, 255-266. [DOI] [PubMed] [Google Scholar]
- 9.Wittmann T. & Waterman-Storer, C. M. (2001) J. Cell Sci. 114, 3795-3803. [DOI] [PubMed] [Google Scholar]
- 10.Gundersen G. G. (2002) Nat. Rev. Mol. Cell Biol. 3, 296-304. [DOI] [PubMed] [Google Scholar]
- 11.Palazzo A. F., Cook, T. A., Alberts, A. S. & Gundersen, G. G. (2001) Nat. Cell Biol. 3, 723-729. [DOI] [PubMed] [Google Scholar]
- 12.Krendel M., Zenke, F. T. & Bokoch, G. M. (2002) Nat. Cell Biol. 4, 294-301. [DOI] [PubMed] [Google Scholar]
- 13.Enomoto T. (1996) Cell Struct. Funct. 21, 317-326. [DOI] [PubMed] [Google Scholar]
- 14.Liu B. P., Chrzanowska-Wodnicka, M. & Burridge, K. (1998) Cell Adhes. Commun. 5, 249-255. [DOI] [PubMed] [Google Scholar]
- 15.Kjoller L. & Hall, A. (1999) Exp. Cell Res. 253, 166-179. [DOI] [PubMed] [Google Scholar]
- 16.Uehata M., Ishizaki, T., Satoh, H., Ono, T., Kawahara, T., Morishita, T., Tamakawa, H., Yamagami, K., Inui, J., Maekawa, M. & Narumiya, S. (1997) Nature (London) 389, 990-994. [DOI] [PubMed] [Google Scholar]
- 17.Steinberg B., Pollack, R., Topp, W. & Botchan, M. (1978) Cell 13, 19-32. [DOI] [PubMed] [Google Scholar]
- 18.Montesano R., Saint Vincent, L., Drevon, C. & Tomatis, L. (1975) Int. J. Cancer 16, 550-558. [DOI] [PubMed] [Google Scholar]
- 19.Gloushankova N. A., Alieva, N. A., Krendel, M. F., Bonder, E. M., Feder, H. H., Vasiliev, J. M. & Gelfand, I. M. (1997) Proc. Natl. Acad. Sci. USA 94, 879-883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gloushankova N. A., Krendel, M. F., Alieva, N. O., Bonder, E. M., Feder, H. H., Vasiliev, J. M. & Gelfand, I. M. (1998) Proc. Natl. Acad. Sci. USA 95, 4362-4367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Omelchenko T., Fetisova, E., Ivanova, O., Bonder, E. M., Feder, H., Vasiliev, J. M. & Gelfand, I. M. (2001) Proc. Natl. Acad. Sci. USA 98, 8632-8637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rottner K., Hall, A. & Small, J. V. (1999) Curr. Biol. 9, 640-648. [DOI] [PubMed] [Google Scholar]
- 23.Pletjushkina O. J., Rajfur, Z., Pomorski, P., Oliver, T. N., Vasiliev, J. M. & Jacobson, K. A. (2001) Cell Motil. Cytoskeleton 48, 235-244. [DOI] [PubMed] [Google Scholar]
- 24.Worthylake R. A., Lemoine, S., Watson, J. M. & Burridge, K. (2001) J. Cell Biol. 154, 147-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kraynov V. S., Chamberlain, C., Bokoch, G. M., Schwartz, M. A., Slabaugh, S. & Hahn, K. M. (2000) Science 290, 333-337. [DOI] [PubMed] [Google Scholar]
- 26.Waterman-Storer C. M., Worthylake, R. A., Liu, B. P., Burridge, K. & Salmon, E. D. (1999) Nat. Cell Biol. 1, 45-50. [DOI] [PubMed] [Google Scholar]
- 27.Waterman-Storer C. M. & Salmon, E. (1999) Curr. Opin. Cell Biol. 11, 61-67. [DOI] [PubMed] [Google Scholar]
- 28.Yvon A. M. & Wadsworth, P. (2000) J. Cell Biol. 151, 1003-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodionov V. I., Hope, A. J., Svitkina, T. M. & Borisy, G. G. (1998) Curr. Biol. 8, 165-168. [DOI] [PubMed] [Google Scholar]
- 30.Kaverina I., Krylyshkina, O. & Small, J. V. (2002) Int. J. Biochem. Cell Biol. 34, 746-761. [DOI] [PubMed] [Google Scholar]
- 31.Krendel M. F. & Bonder, E. M. (1999) Cell Motil. Cytoskeleton 43, 296-309. [DOI] [PubMed] [Google Scholar]
- 32.Somlyo A. V., Bradshaw, D., Ramos, S., Murphy, C., Myers, C. E. & Somlyo, A. P. (2000) Biochem. Biophys. Res. Commun. 269, 652-659. [DOI] [PubMed] [Google Scholar]
- 33.Hay E. D. (1995) Acta Anat. 154, 8-20. [DOI] [PubMed] [Google Scholar]