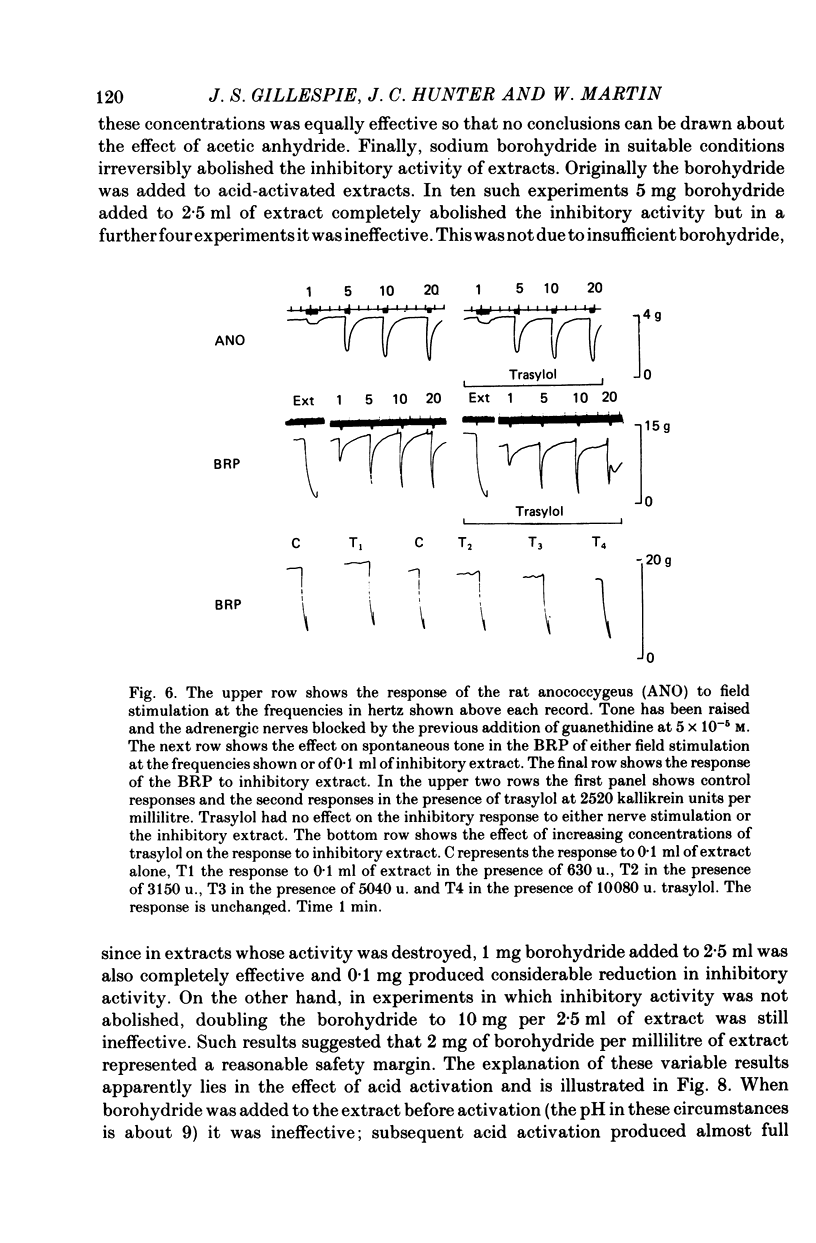
Abstract
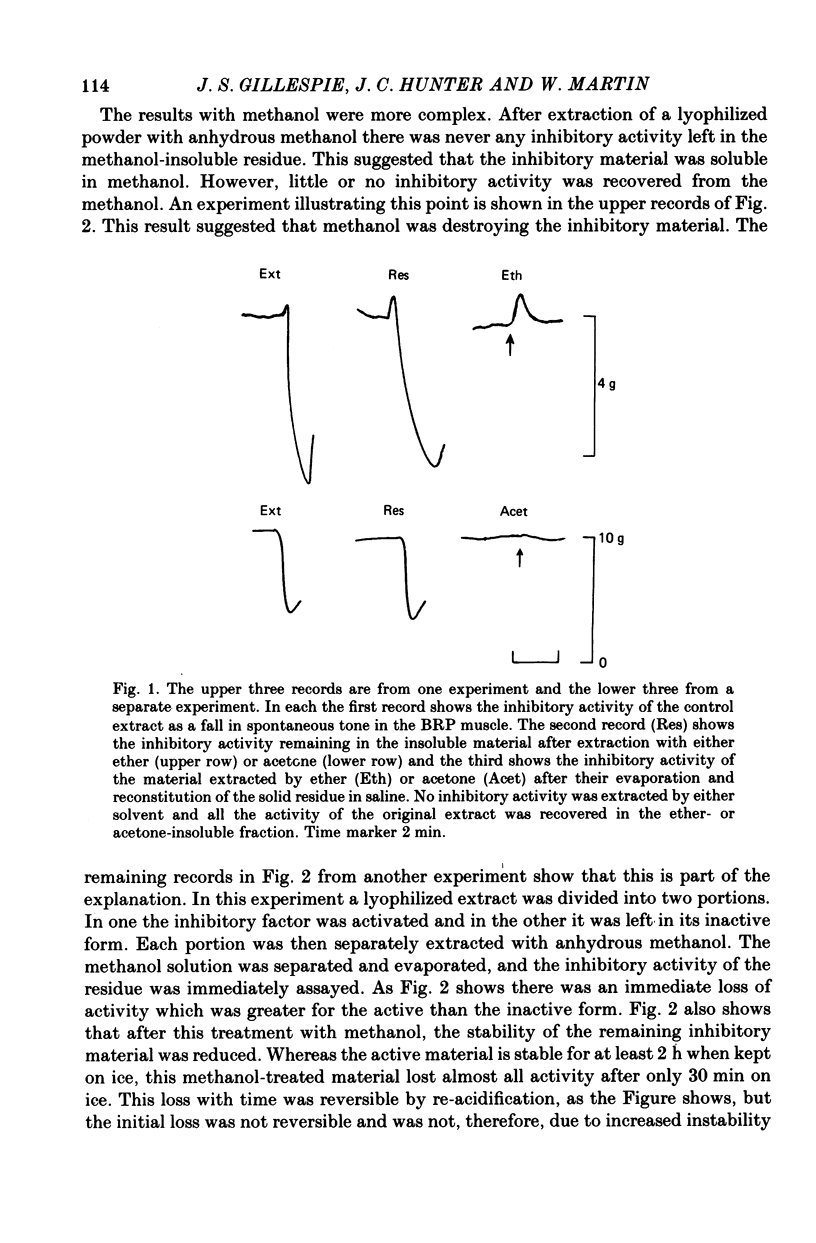
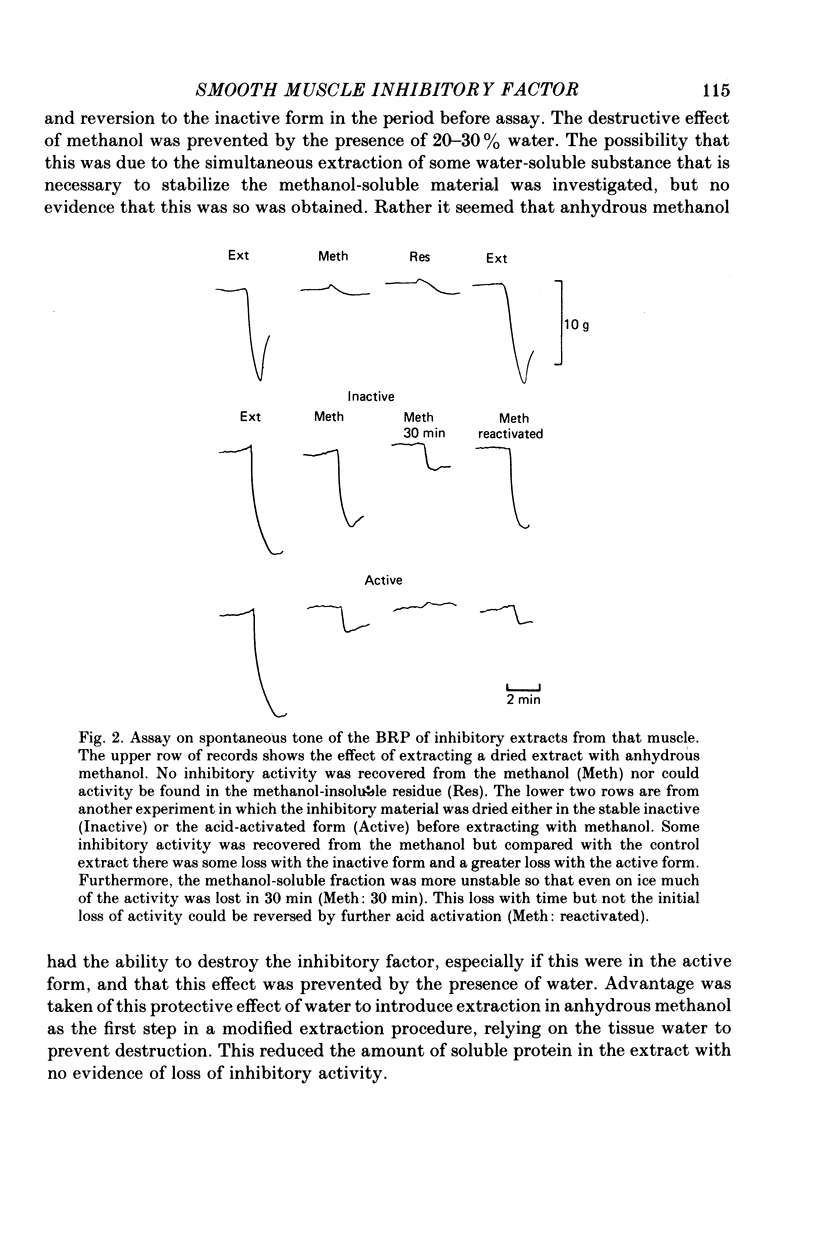
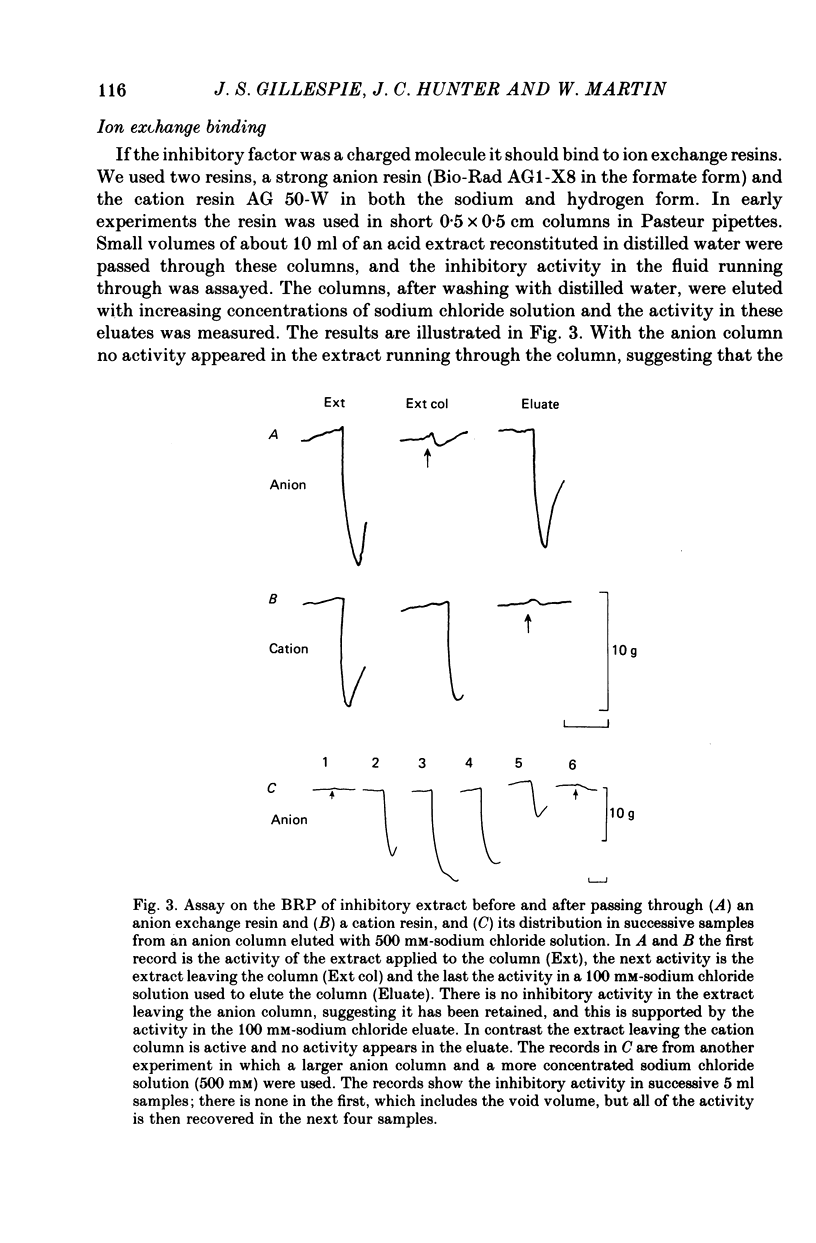
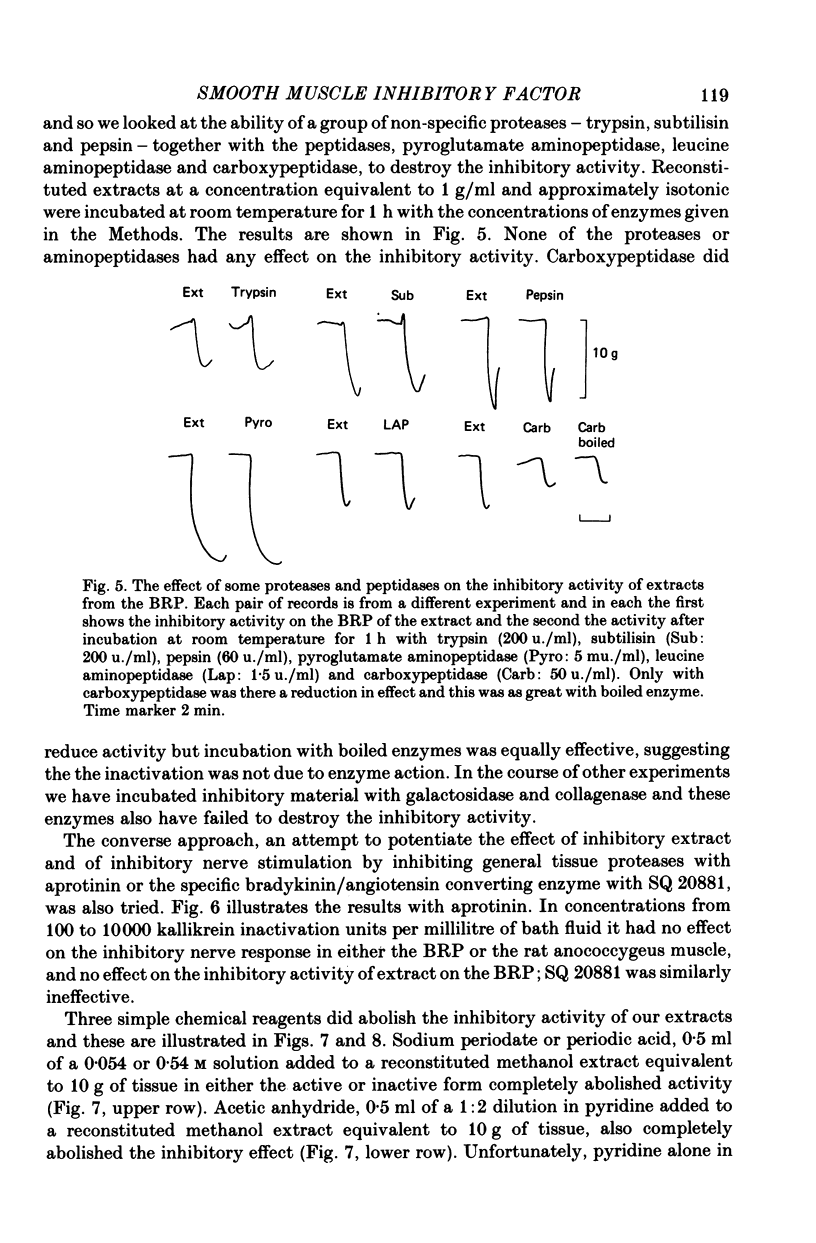
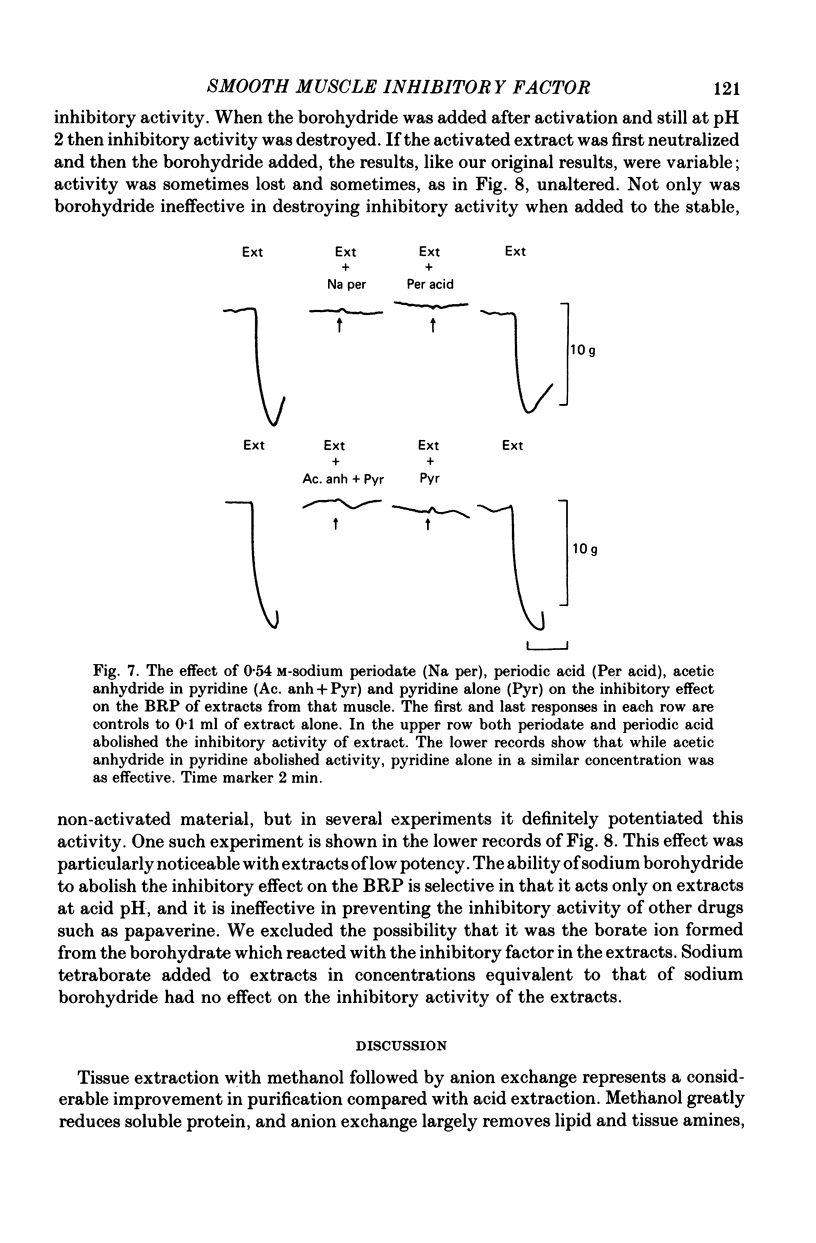
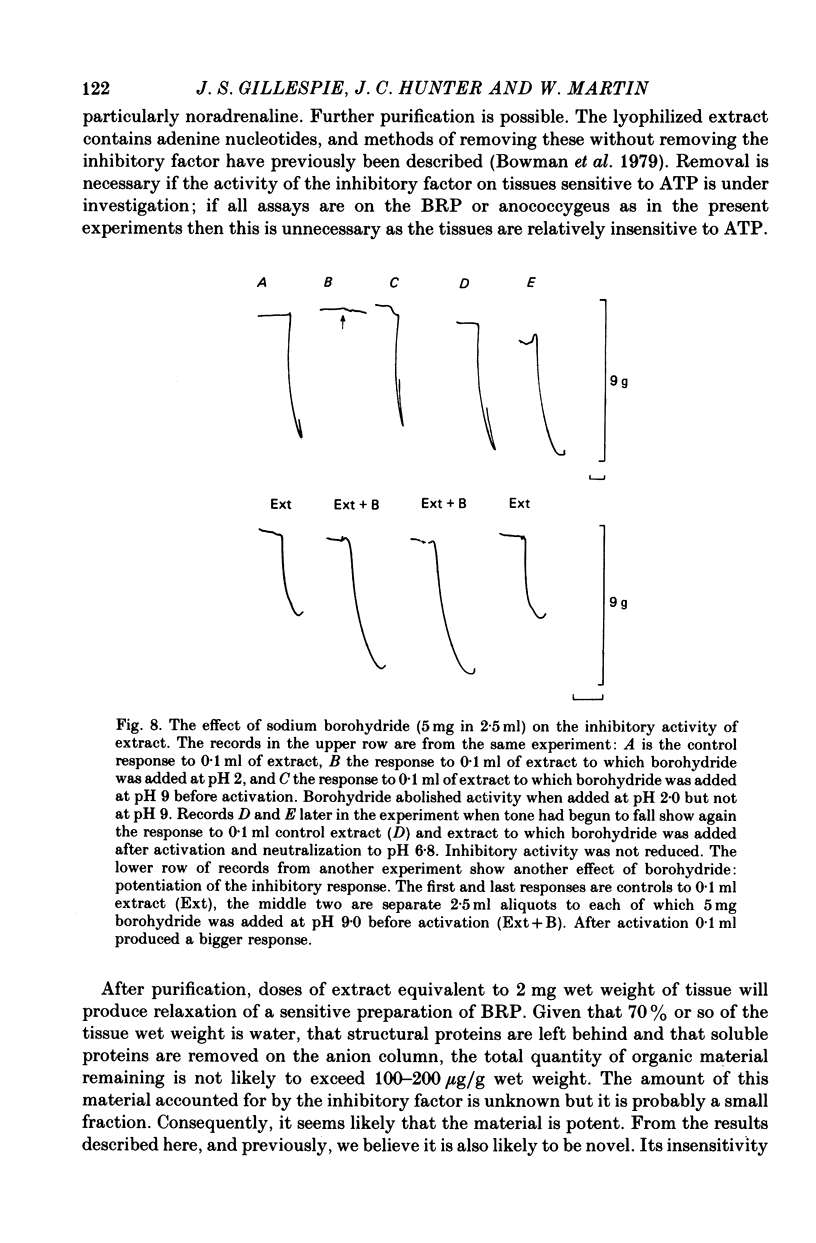
1. A method of extracting and partially purifying a smooth muscle inhibitory factor from the bovine retractor penis is described. This consists of extraction in methanol followed by adsorption on an anion exchange resin, elution from the resin with 500 mM-sodium chloride solution and, if necessary, removal of adenine nucleotides by adsorption on alumina. 2. The inhibitory factor exists in a stable pharmacologically inactive form and an unstable pharmacologically active form. Conversion to the active form is by a brief exposure to acid at pH 2.0. 3. The inhibitory factor is insoluble in ether or acetone but soluble in methanol. Anhydrous methanol, however, irreversibly destroys pharmacological activity especially if the inhibitory factor is in the active form. This effect of methanol is prevented by the presence of 20-30-% water. 4. The inhibitory factor binds to an anion exchange resin but not to a cation exchange resin. It can be eluted from the resin by 500 mM-sodium chloride solution. 5. The molecular weight of the inhibitory factor, as judged by the ability to pass ultrafiltration membranes, is about 500. 6. Inhibitory activity is unaffected by the proteases trypsin, subtilisin or pepsin or by leucine aminopeptidase, pyroglutamate aminopeptidase or carboxypeptidase. The inhibitory effect of the extract and the inhibitory response to stimulation of the non-adrenergic, non-cholinergic nerves are also unaffected by the protease inhibitor, aprotinin. The active material, therefore, is unlikely to be a peptide. 7. Inhibitory activity is abolished by exposure of the extracts to periodic acid or sodium periodate. Acetic anhydride in pyridine also abolishes activity but the vehicle pyridine is also effective. 8. Sodium borohydride but not borate abolishes inhibitory activity when added to the acid-activated material at pH 2.0 but has no effect or may even potentiate activity if added to the stable inactive form at pH 9.0. When added to the acid-activated but neutralized material at pH 6.8 it usually abolishes inhibitory activity but occasionally has no effect. 9. These results suggest the smooth muscle inhibitory factor in these extracts is potent and probably novel. It does not appear to be a peptide or a lipid but may contain a carbohydrate as part of the molecule. Its possible physiological role is discussed.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bargmann W., Lindner E., Andres K. H. Uber Synapsen an endokrinen Epithelzellen und die Definition sekretorischer Neurone. Untersuchungen am Zwischenlappen der Katzenhypophyse. Z Zellforsch Mikrosk Anat. 1967;77(2):282–298. [PubMed] [Google Scholar]
- Baumgarten H. G., Holstein A. F., Owman C. Auerbach's plexus of mammals and man: electron microscopic identification of three different types of neuronal processes in myenteric ganglia of the large intestine from rhesus monkeys, guinea-pigs and man. Z Zellforsch Mikrosk Anat. 1970;106(3):376–397. doi: 10.1007/BF00335780. [DOI] [PubMed] [Google Scholar]
- Bowman A., Gillespie J. S., Martin W. The inhibitory material in extracts from the bovine retractor penis muscle is not an adenine nucleotide. Br J Pharmacol. 1979 Nov;67(3):327–328. doi: 10.1111/j.1476-5381.1979.tb08683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Purinergic nerves. Pharmacol Rev. 1972 Sep;24(3):509–581. [PubMed] [Google Scholar]
- Eränkö O., Klinge E., Sjöstrand N. O. Different types of synaptic vesicles in axons of the retractor penis muscle of the bull. Experientia. 1976 Oct 15;32(10):1335–1337. doi: 10.1007/BF01953125. [DOI] [PubMed] [Google Scholar]
- Gibbins I. L., Haller C. J. Ultrastructural identification of non-adrenergic, non-cholinergic nerves in the rat anococcygeus muscle. Cell Tissue Res. 1979 Aug;200(2):257–271. doi: 10.1007/BF00236418. [DOI] [PubMed] [Google Scholar]
- Gillespie J. S., Martin W. A smooth muscle inhibitory material extracted from the bovine retractor penis and rat anococcygeus muscles [proceedings]. J Physiol. 1978 Jul;280:45P–46P. [PubMed] [Google Scholar]
- Gillespie J. S., Martin W. A smooth muscle inhibitory material from the bovine retractor penis and rat anococcygeus muscles. J Physiol. 1980 Dec;309:55–64. doi: 10.1113/jphysiol.1980.sp013493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J. S., McKnight A. T. The actions of some vasoactive polypeptides and their antagonists on the anococcygeus muscle. Br J Pharmacol. 1978 Feb;62(2):267–274. doi: 10.1111/j.1476-5381.1978.tb08455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson L. I., Fahrenkrug J., Schaffalitzky De Muckadell O., Sundler F., Håkanson R., Rehfeld J. R. Localization of vasoactive intestinal polypeptide (VIP) to central and peripheral neurons. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3197–3200. doi: 10.1073/pnas.73.9.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris H. R., Piper P. J., Taylor G. W., Tippins J. R. Comparative studies on immunologically and non-immunologically produced slow-reacting substances from man, guinea-pig and rat. Br J Pharmacol. 1979 Oct;67(2):179–184. doi: 10.1111/j.1476-5381.1979.tb08664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


