Abstract
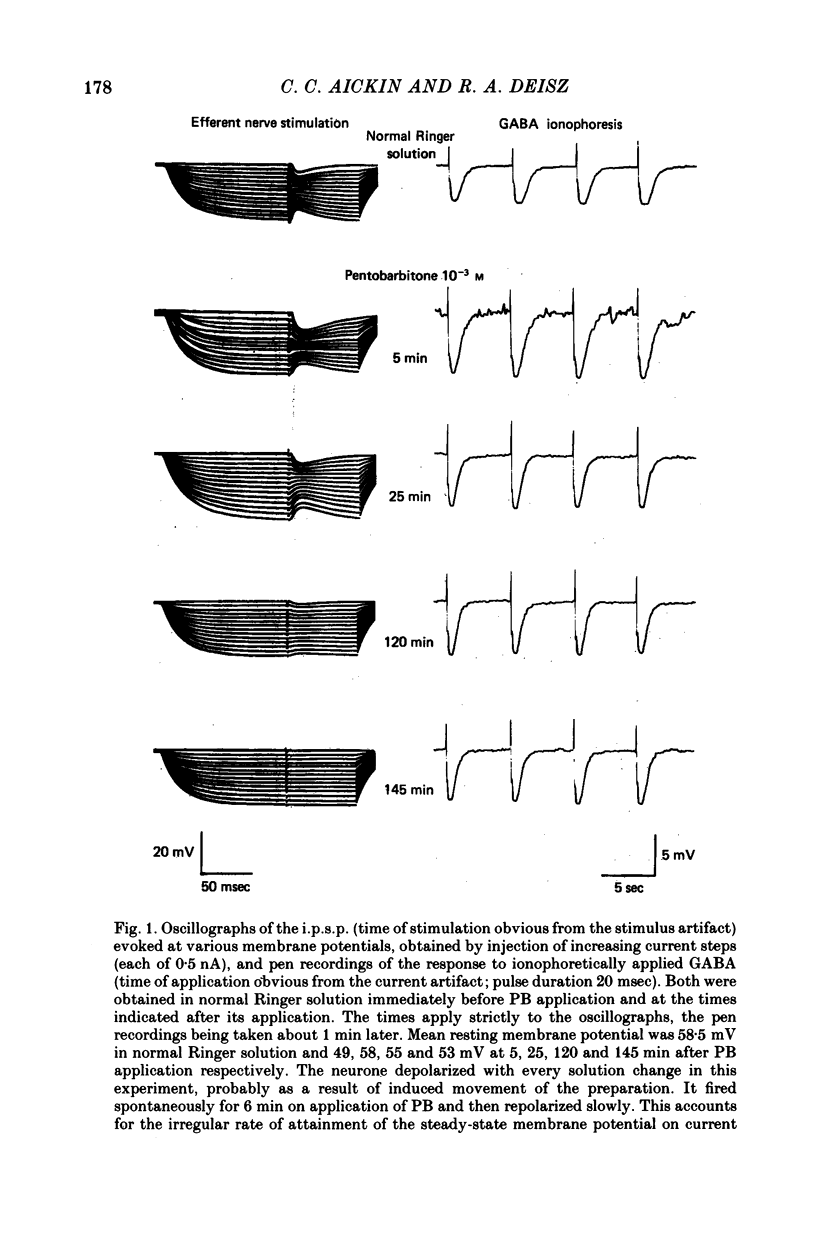
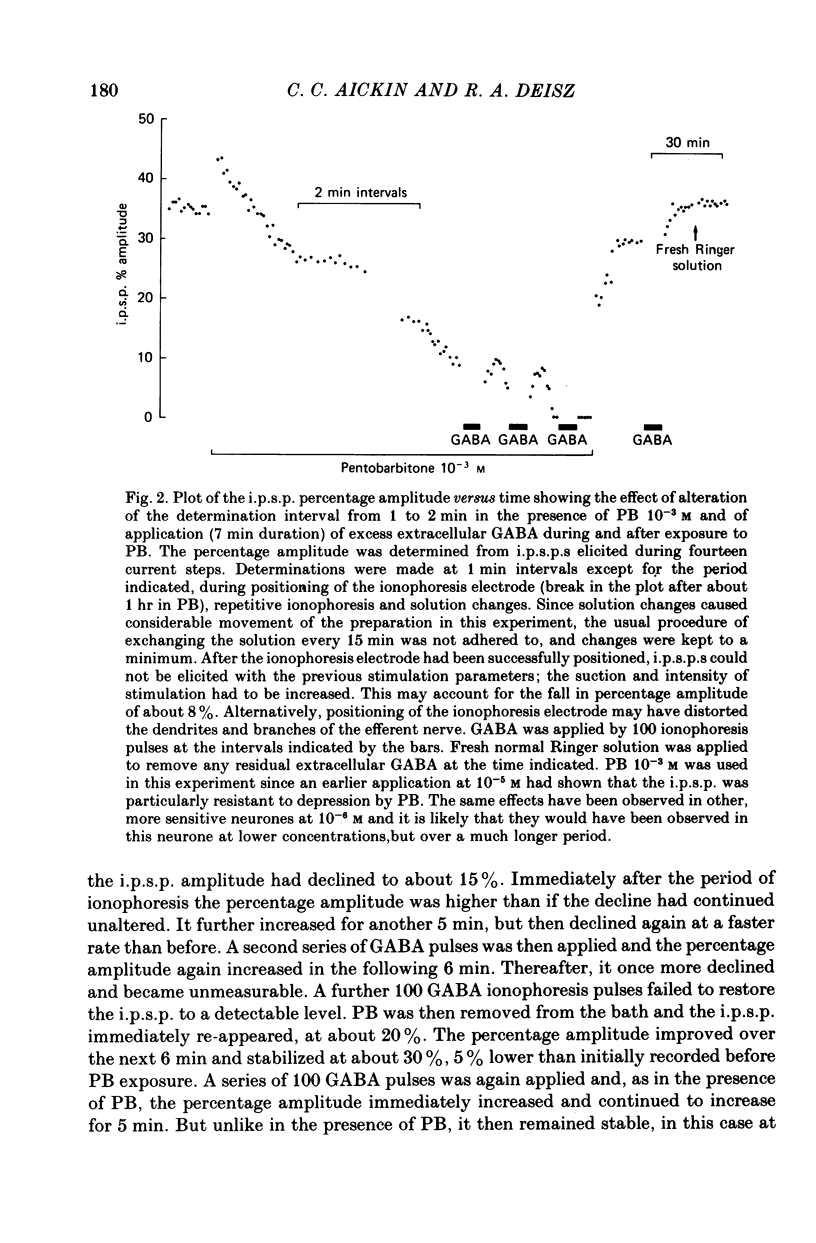
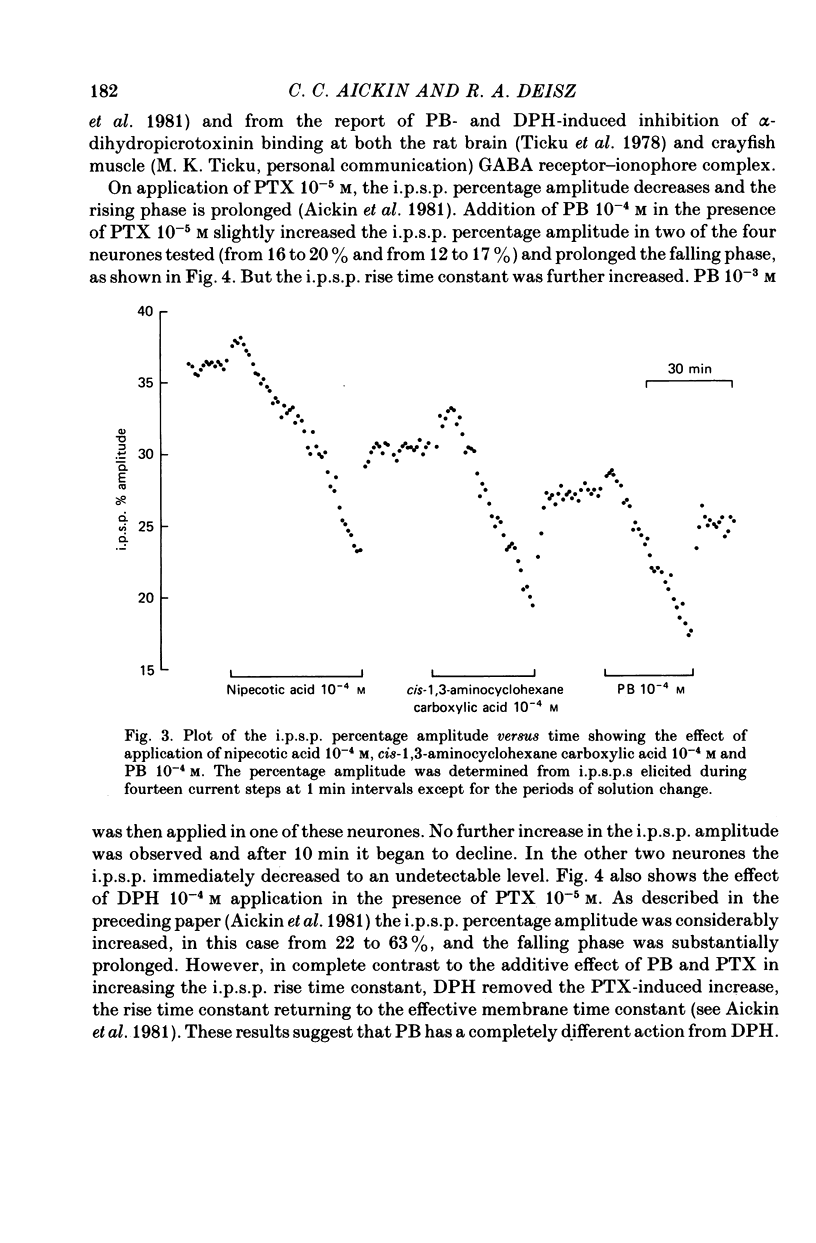
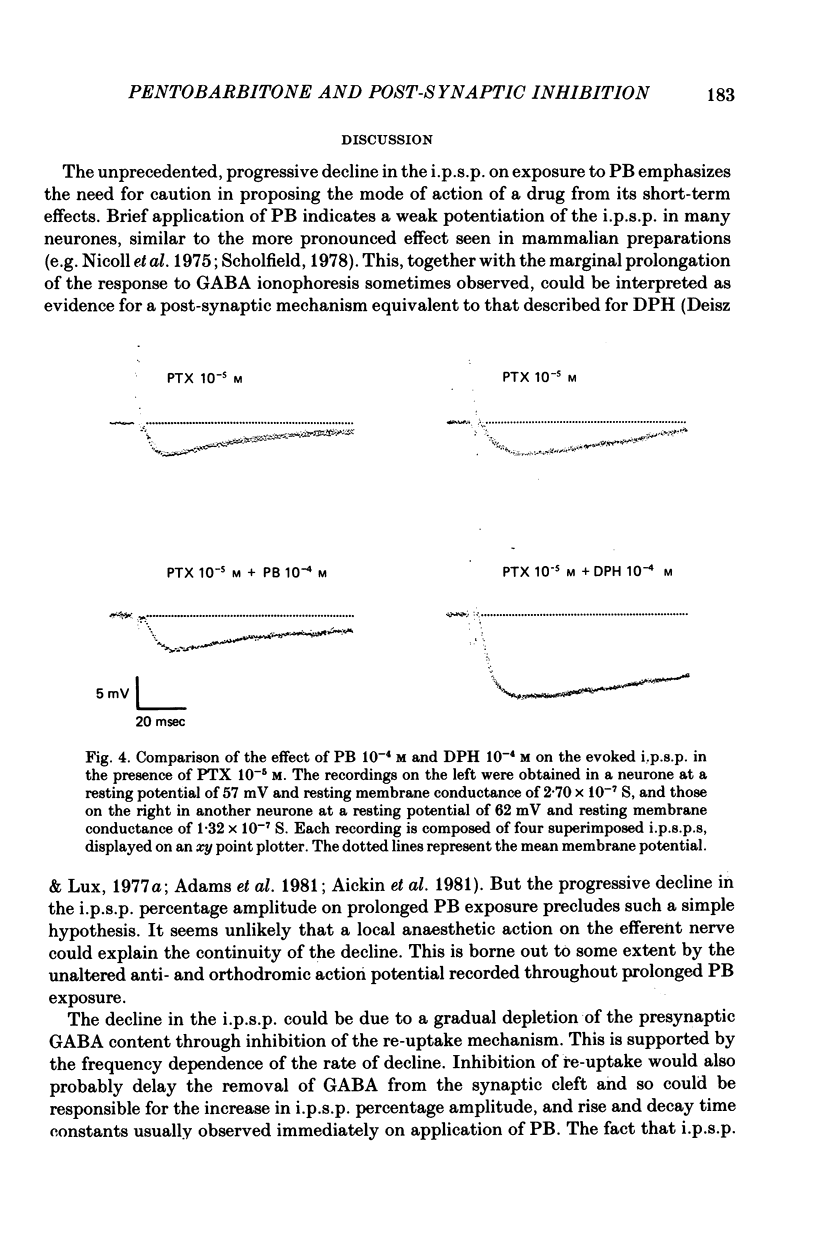
1. The effect of pentobarbitone (PB) on GABA-ergic inhibition was investigated in the isolated crayfish stretch receptor. The soma of the slowly adapting neurone was impaled with two micro-electrodes to give an accurate determination of membrane conductances. 2. Application of PB in concentrations from 10(-6) to 10(-3) M increased the rise time constant of the inhibitory post-synaptic potential (i.p.s.p.). The i.p.s.p. percentage amplitude and decay time constant were also increased in eight out of twelve neurones. On prolonged exposure, the percentage amplitude declined at a rate dependent upon the dose and the frequency of stimulation until the i.p.s.p. became undetectable. 3. The response to ionophoretically applied GABA remained essentially unaltered in the presence of PB, but the falling phase was prolonged by up to 8% in four of the ten neurones tested. Resting membrane conductance, i.p.s.p. driving force (i.p.s.p. reversal potential minus resting membrane potential), and parameters of the anti- and orthodromic action potential were not significantly affected. 4. Removal of PB after prolonged exposure usually caused an immediate increase in i.p.s.p. percentage amplitude but the i.p.s.p. rising phase remained slowed. 5. Application of excess extracellular GABA only affected the i.p.s.p. percentage amplitude after it had been reduced by PB. It transiently increased the attenuated i.p.s.p. percentage amplitude in the presence of PB, and after the removal of PB permanently increased the amplitude to its original value. 6. Nipecotic acid and cis-1,3-aminocyclohexane carboxylic acid, inhibitors of GABA re-uptake, slightly increased the i.p.s.p. percentage amplitude, and prolonged the falling phase but did not affect the rising phase. The percentage amplitude declined on prolonged exposure. 7. We conclude that PB has no electrophysiologically demonstrable post-synaptic action in the crayfish stretch receptor neurone, but it inhibits the presynaptic release and re-uptake of GABA.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aickin C. C., Deisz R. A., Lux H. D. On the action of the anticonvulsant 5,5-diphenylhydantoin and the convulsant picrotoxin in crayfish stretch receptor. J Physiol. 1981 Jun;315:157–173. doi: 10.1113/jphysiol.1981.sp013739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aickin C. C., Deisz R. A. Pentobarbitone affects post-synaptic inhibition by presynaptic mechanisms [proceedings]. J Physiol. 1979 Feb;287:27P–28P. [PubMed] [Google Scholar]
- Barker J. L., Gainer H. Pentobarbital: selective depression of excitatory postsynaptic potentials. Science. 1973 Nov 16;182(4113):720–722. doi: 10.1126/science.182.4113.720. [DOI] [PubMed] [Google Scholar]
- Barker J. L., McBurney R. N. Phenobarbitone modulation of postsynaptic GABA receptor function on cultured mammalian neurons. Proc R Soc Lond B Biol Sci. 1979 Dec 31;206(1164):319–327. doi: 10.1098/rspb.1979.0108. [DOI] [PubMed] [Google Scholar]
- Barker J. L., Ransom B. R. Pentobarbitone pharmacology of mammalian central neurones grown in tissue culture. J Physiol. 1978 Jul;280:355–372. doi: 10.1113/jphysiol.1978.sp012388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer B. Effect of pentobarbitone on the spontaneous efflux of gamma-amino acids from rabbit retina. Brain Res. 1979 Mar 16;163(2):307–317. doi: 10.1016/0006-8993(79)90358-5. [DOI] [PubMed] [Google Scholar]
- Blaustein M. P., Ector A. C. Barbiturate inhibition of calcium uptake by depolarized nerve terminals in vitro. Mol Pharmacol. 1975 May;11(3):369–378. [PubMed] [Google Scholar]
- Bowery N. G., Dray A. Barbiturate reversal of amino acid antagonism produced by convulsant agents. Nature. 1976 Nov 18;264(5583):276–278. doi: 10.1038/264276a0. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Constanti A. Interaction of pentobarbitone and gamma-aminobutyric acid on mammalian sympathetic ganglion cells. Br J Pharmacol. 1978 May;63(1):217–224. doi: 10.1111/j.1476-5381.1978.tb07791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Galvan M. Influence of neuroglial transport on the action of gamma-aminobutyric acid on mammalian ganglion cells. Br J Pharmacol. 1977 Feb;59(2):373–378. doi: 10.1111/j.1476-5381.1977.tb07502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman-Riese D., Cutler R. W. Inhibition of gamma-aminobutyric acid release from rat cerebral cortex slices by barbiturate anesthesia. Neurochem Res. 1978 Aug;3(4):423–429. doi: 10.1007/BF00966324. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Lodge D. Effect of pentobarbitone on the inhibition of spinal interneurones in the cat by glycine and GABA [proceedings]. J Physiol. 1977 Oct;272(1):48P–49P. [PubMed] [Google Scholar]
- Cutler R. W., Markowitz D., Dudzinski D. S. The effect of barbiturates on (3H)GABA transport in rat cerebral cortex slices. Brain Res. 1974 Dec 6;81(2):189–197. doi: 10.1016/0006-8993(74)90935-4. [DOI] [PubMed] [Google Scholar]
- Deisz R. A., Aickin C. C., Lux H. D. Decrease of inhibitory driving force in crayfish stretch reception: a mechanism of the convulsant action of penicillin. Neurosci Lett. 1979 Mar;11(3):347–352. doi: 10.1016/0304-3940(79)90020-x. [DOI] [PubMed] [Google Scholar]
- ECCLES J. C., SCHMIDT R., WILLIS W. D. PHARMACOLOGICAL STUDIES ON PRESYNAPTIC INHIBITION. J Physiol. 1963 Oct;168:500–530. doi: 10.1113/jphysiol.1963.sp007205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. H. Potentiation of the effects of GABA by pentobarbitone. Brain Res. 1979 Jul 27;171(1):113–120. doi: 10.1016/0006-8993(79)90736-4. [DOI] [PubMed] [Google Scholar]
- Gottesfeld Z., Kelly J. S., Renaud L. P. The in vivo neuropharmacology of amino-oxyacetic acid in the cerebral cortex of the cat. Brain Res. 1972 Jul 20;42(2):319–335. doi: 10.1016/0006-8993(72)90534-3. [DOI] [PubMed] [Google Scholar]
- Hajós F., Csillag A., Kálmán M. The effect of pentobarbital, chloralhydrate, ether and protoveratrine on the distribution of synaptic vesicles in rat cortical synaptosomes. Exp Brain Res. 1978 Sep 15;33(1):91–99. doi: 10.1007/BF00238797. [DOI] [PubMed] [Google Scholar]
- Haycock J. W., Levy W. B., Cotman C. W. Pentobarbital depression of stimulus-secretion coupling in brain--selective inhibition of depolarization-induced calcium-dependent release. Biochem Pharmacol. 1977 Jan 15;26(2):159–161. doi: 10.1016/0006-2952(77)90389-6. [DOI] [PubMed] [Google Scholar]
- Jessell T. M., Richards C. D. Barbiturate potentiation of hippocampal i.p.s.p.s is not mediated by blockade of GABA uptake [proceedings]. J Physiol. 1977 Jul;269(1):42P–44P. [PubMed] [Google Scholar]
- Johnston G. A., Krogsgaard-Larsen P., Stephanson A. Betel nut constituents as inhibitors of gamma-aminobutyric acid uptake. Nature. 1975 Dec 18;258(5536):627–628. doi: 10.1038/258627a0. [DOI] [PubMed] [Google Scholar]
- Jones D. G., Devon R. M. An ultrastructural study into the effects of pentobarbitone on synaptic organization. Brain Res. 1978 May 19;147(1):47–63. doi: 10.1016/0006-8993(78)90771-0. [DOI] [PubMed] [Google Scholar]
- Jones G. P., Neal M. J. Selective inhibition of neuronal GABA uptake by cis-1,3-aminocyclohexane carboxylic acid. Nature. 1976 Nov 18;264(5583):281–284. doi: 10.1038/264281a0. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The binding of acetylcholine to receptors and its removal from the synaptic cleft. J Physiol. 1973 Jun;231(3):549–574. doi: 10.1113/jphysiol.1973.sp010248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson M. D., Major M. A. The effect of hexobarbital on the duration of the recurrent IPSP in cat motoneurons. Brain Res. 1970 Jul 14;21(2):309–311. doi: 10.1016/0006-8993(70)90377-x. [DOI] [PubMed] [Google Scholar]
- Macdonald R. L., Barker J. L. Different actions of anticonvulsant and anesthetic barbiturates revealed by use of cultured mammalian neurons. Science. 1978 May 19;200(4343):775–777. doi: 10.1126/science.205953. [DOI] [PubMed] [Google Scholar]
- Mitchell P. R., Martin I. L. Is GABA release modulated by presynaptic receptors? Nature. 1978 Aug 31;274(5674):904–905. doi: 10.1038/274904a0. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A., Eccles J. C., Oshima T., Rubia F. Prolongation of hippocampal inhibitory postsynaptic potentials by barbiturates. Nature. 1975 Dec 18;258(5536):625–627. doi: 10.1038/258625a0. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A., Iwamoto E. T. Action of pentobarbital on sympathetic ganglion cells. J Neurophysiol. 1978 Jul;41(4):977–986. doi: 10.1152/jn.1978.41.4.977. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A. Pentobarbital: action on frog motoneurons. Brain Res. 1975 Oct 10;96(1):119–123. doi: 10.1016/0006-8993(75)90582-x. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A. The effects of anaesthetics on synaptic excitation and inhibition in the olfactory bulb. J Physiol. 1972 Jun;223(3):803–814. doi: 10.1113/jphysiol.1972.sp009875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck E. J., Miller A. L., Lester B. R. Pentobarbital and synaptic high-affinity receptive sites for gamma-aminobutyric acid. Brain Res Bull. 1976 Nov-Dec;1(6):595–597. doi: 10.1016/0361-9230(76)90087-3. [DOI] [PubMed] [Google Scholar]
- Proctor W. R., Weakly J. N. A comparison of the presynaptic and post-synaptic actions of pentobarbitone and phenobarbitone in the neuromuscular junction of the frog. J Physiol. 1976 Jun;258(1):257–268. doi: 10.1113/jphysiol.1976.sp011418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards C. D. On the mechanism of barbiturate anaesthesia. J Physiol. 1972 Dec;227(3):749–767. doi: 10.1113/jphysiol.1972.sp010057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholfield C. N. A barbiturate induced intensification of the inhibitory potential in slices of guinea-pig olfactory cortex. J Physiol. 1978 Feb;275:559–566. doi: 10.1113/jphysiol.1978.sp012208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit P., Akert K., Sandri C., Livingston R. B., Moor H. Dynamic ultrastructure of presynaptic membranes at nerve terminals in the spinal cord of rats. Anesthetized and unanesthetized preparations compared. Brain Res. 1972 Dec 24;48:11–26. doi: 10.1016/0006-8993(72)90168-0. [DOI] [PubMed] [Google Scholar]
- Ticku M. K., Ban M., Olsen R. W. Binding of [3H]alpha-dihydropicrotoxinin, a gamma-aminobutyric acid synaptic antagonist, to rat brain membranes. Mol Pharmacol. 1978 May;14(3):391–402. [PubMed] [Google Scholar]
- Ticku M. K., Olsen R. W. Interaction of barbiturates with dihydropicrotoxinin binding sites related to the GABA receptor-ionophore system. Life Sci. 1978 May 8;22(18):1643–1651. doi: 10.1016/0024-3205(78)90061-9. [DOI] [PubMed] [Google Scholar]



