Abstract
1. Membrane potential fluctuations were measured in cells from mouse Islets of Langerhans identified as beta-cells by the characteristic pattern of electrical activity induced by 11 mM-D-glucose. 2. The membrane potential was controlled by adjusting the external potassium concentration, [K+]o, keeping the sum [Na+]o plus [K+]o constant. In the absence of glucose, when [K+]o is raised, the resulting depolarization is accompanied by a significant increase in voltage noise. 3 The amplitude and time course of the voltage noise were measured under various experimental conditions. The variance of the fluctuating voltage decreased monotonically along the depolarization induced by sudden increase in [K+]o, suggesting a monotonic reduction in the number of elementary events. 4. The frequency characteristics of the excess noise could be analysed as the sum of 1/f and 1/f2 components. While the 1/f component remained unaffected by the external application of 20mM-tetraethylammonium (TEA) and either 2 mM-Mn2+ or 2 mM-Co2+, the 1/f2 component was suppressed by both Mn2+ and Co2+. 5. The corner frequency, fc, of the 1/f2 component depended on membrane potential, which was adjusted by adjusting the [K+]o jump. These results support the idea that fc in these experiments is a measure of the channel relaxation. 6. Measurements of the input resistance in the frequency range from 0 to 25 Hz were used to obtain a rough estimate of the size of the channel conductance as 5 x 10(-12) omega (-1).
Full text
PDF

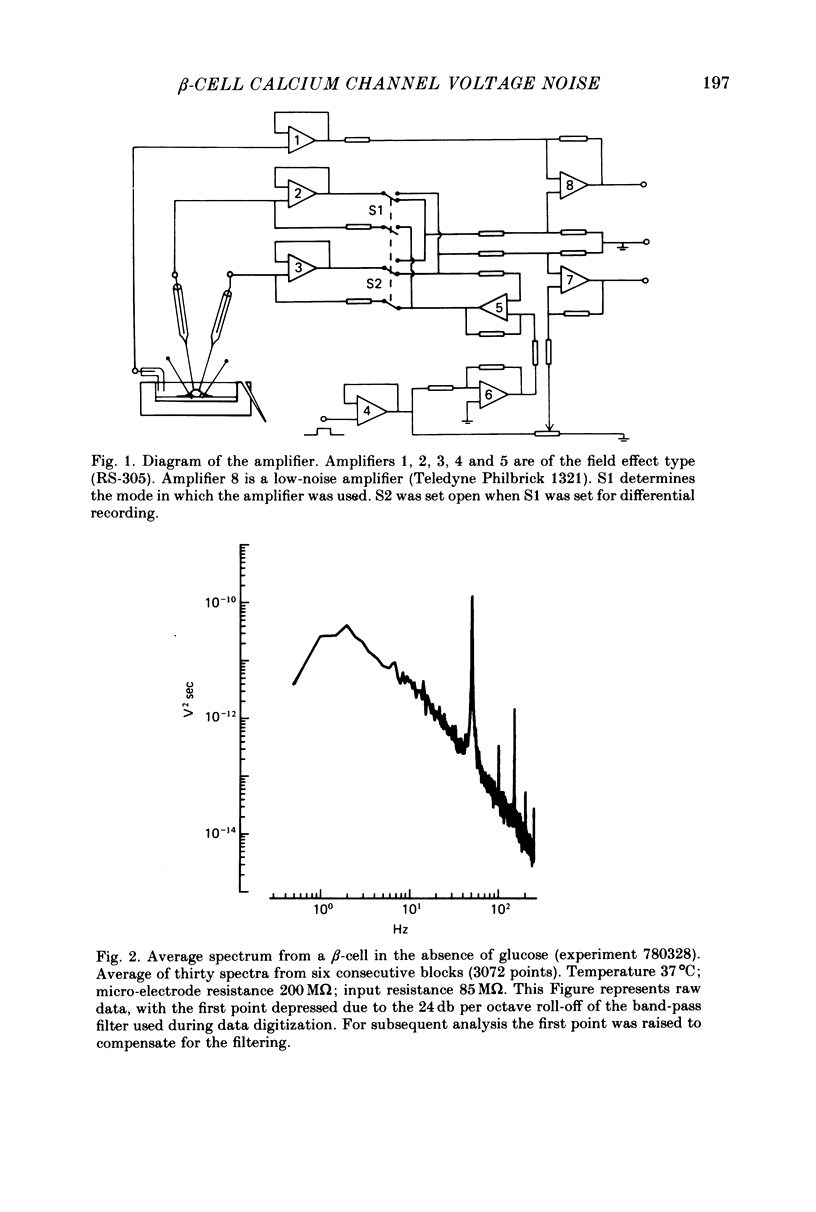

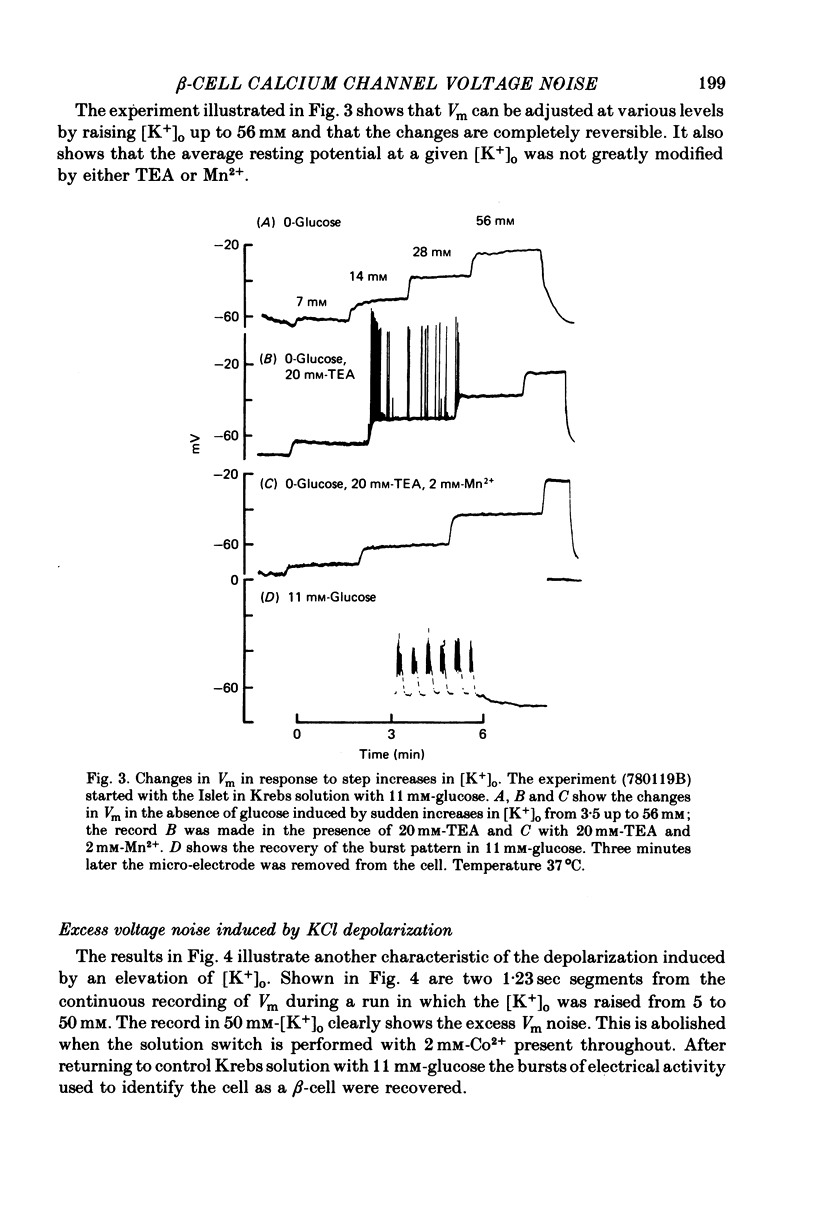
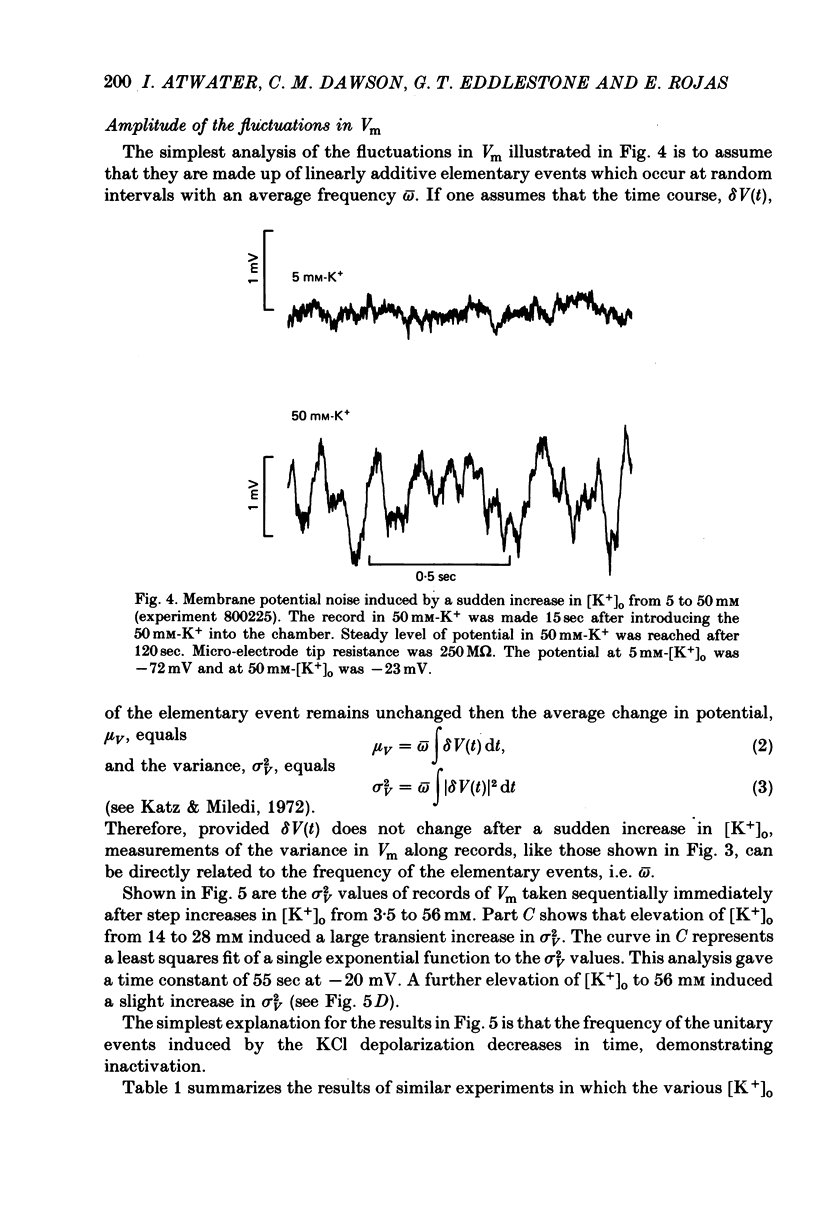
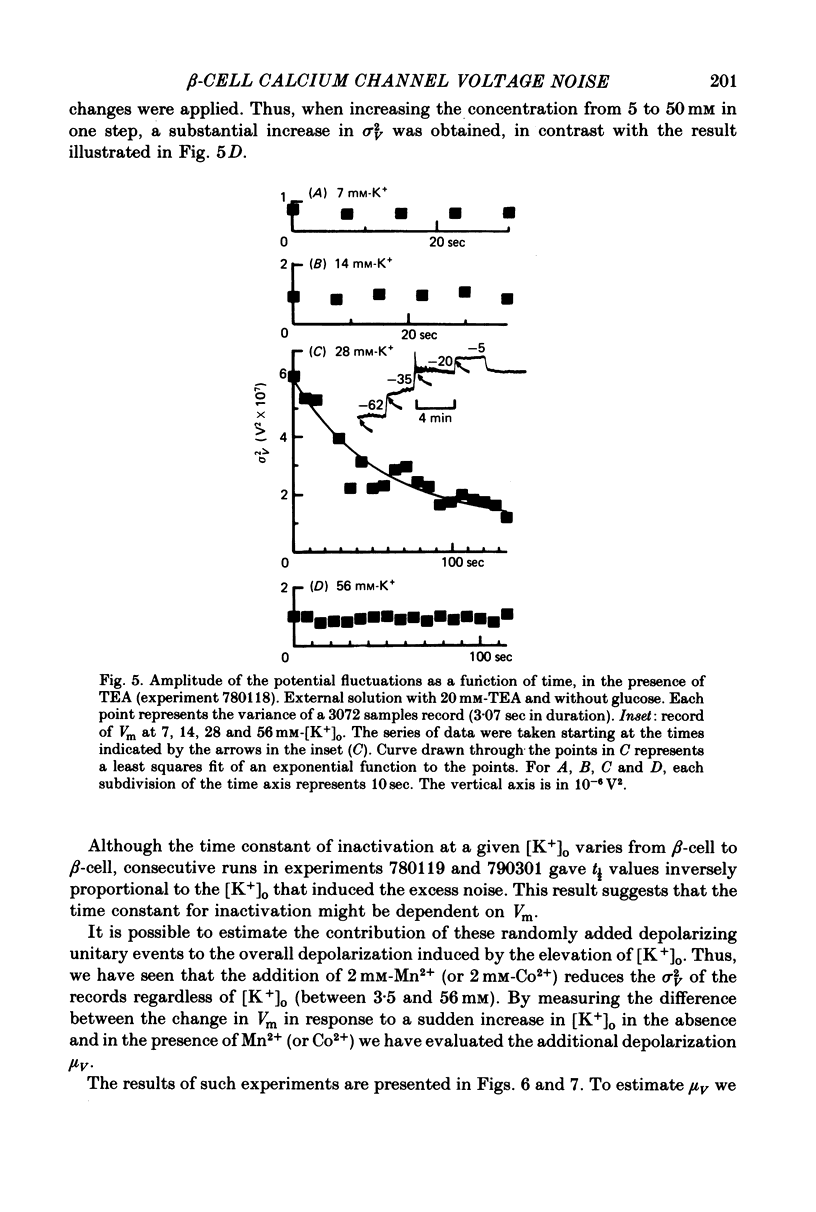
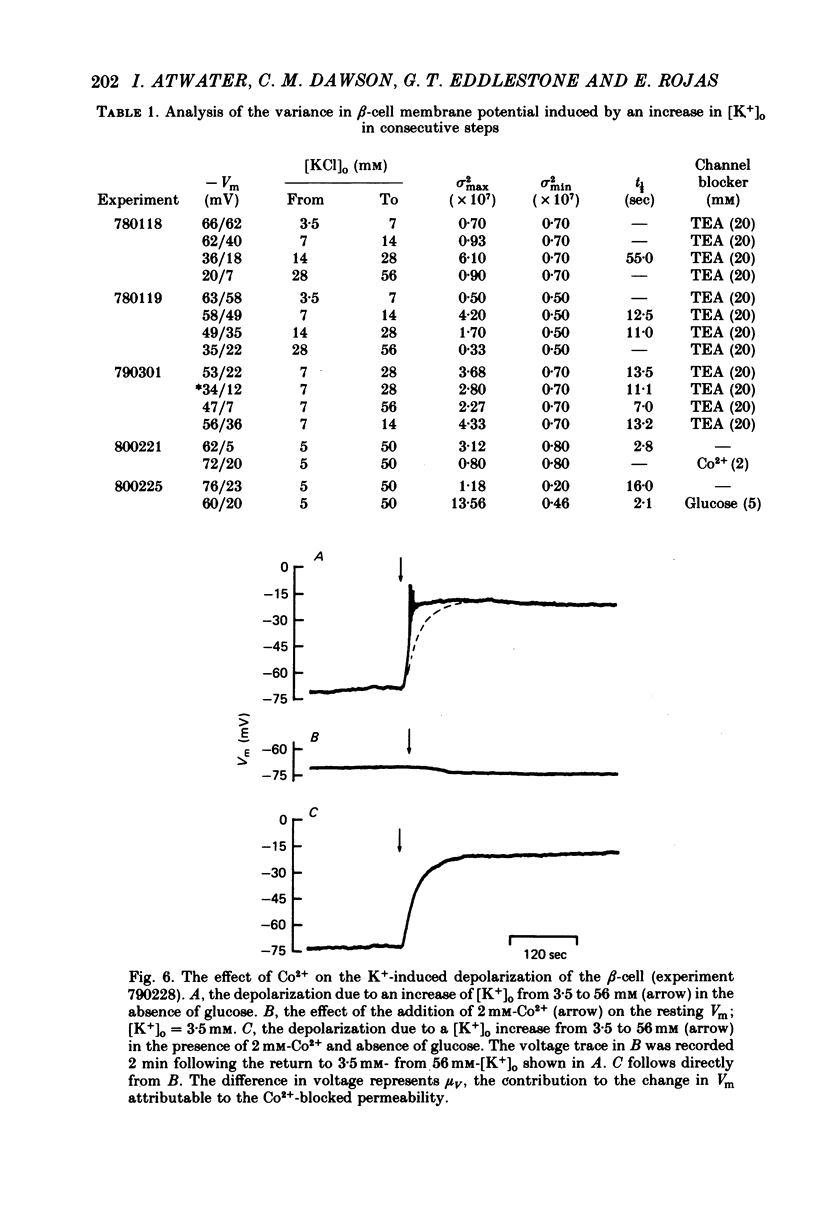
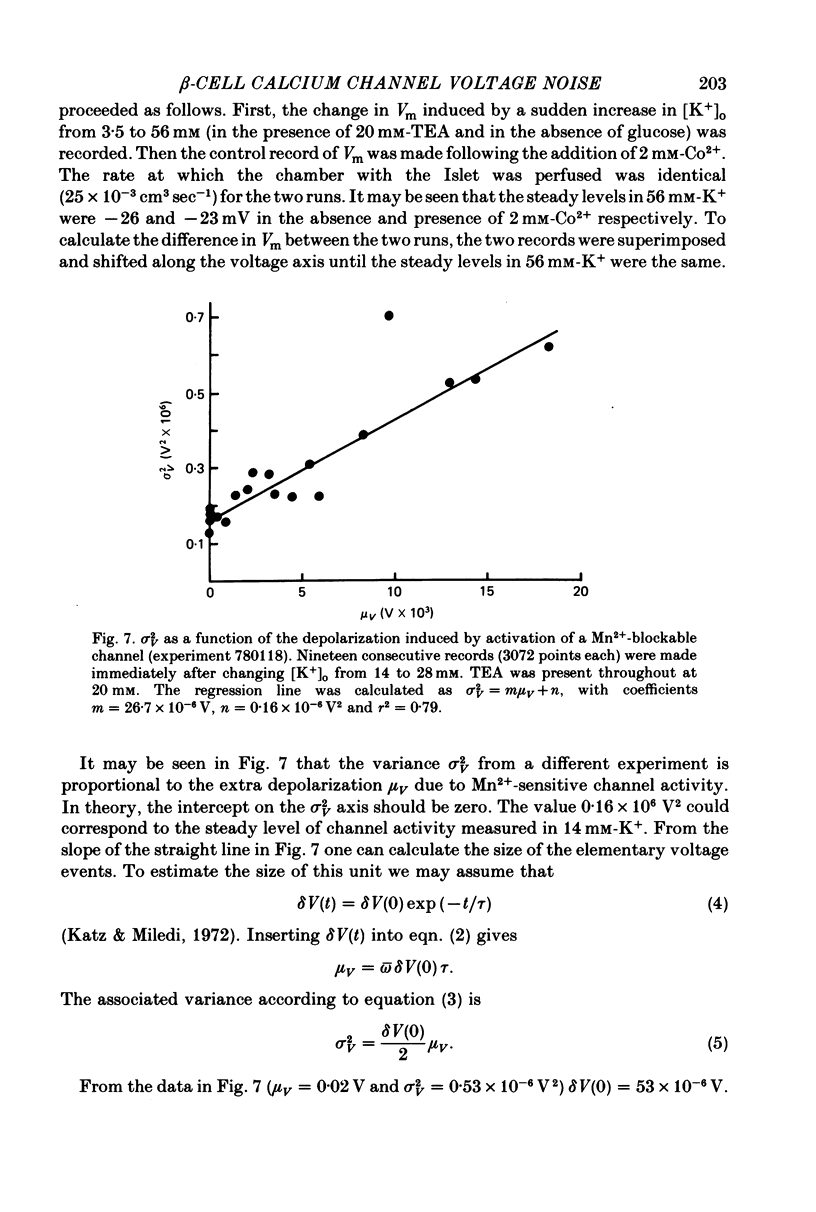

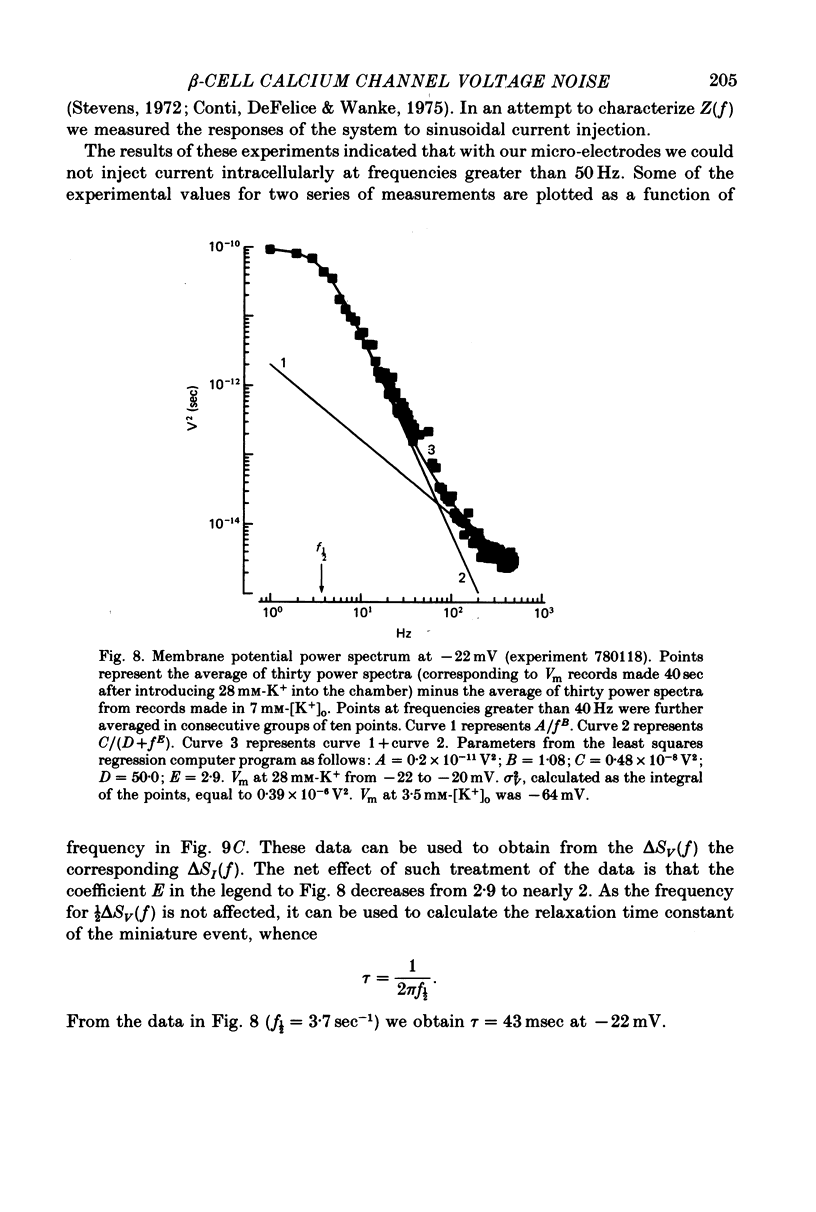
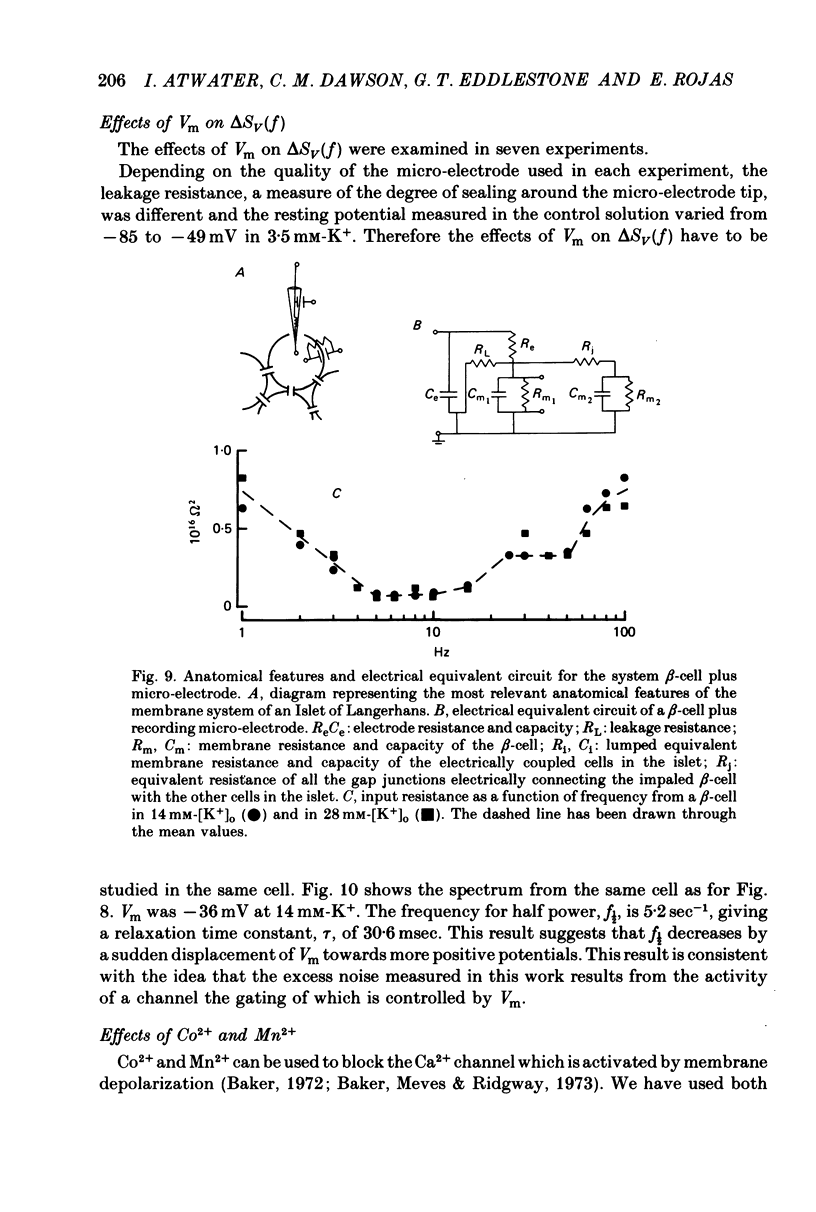




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albano J. D., Ekins R. P., Maritz G., Turner R. C. A sensitive, precise radioimmunoassay of serum insulin relying on charcoal separation of bound and free hormone moieties. Acta Endocrinol (Copenh) 1972 Jul;70(3):487–509. doi: 10.1530/acta.0.0700487. [DOI] [PubMed] [Google Scholar]
- Atwater I., Beigelman P. M. Dynamic characteristics of electrical activity in pancreatic beta-cells. I. - Effects of calcium and magnesium removal. J Physiol (Paris) 1976 Nov;72(6):769–786. [PubMed] [Google Scholar]
- Atwater I., Dawson C. M., Ribalet B., Rojas E. Potassium permeability activated by intracellular calcium ion concentration in the pancreatic beta-cell. J Physiol. 1979 Mar;288:575–588. [PMC free article] [PubMed] [Google Scholar]
- Atwater I., Dawson C. M., Scott A., Eddlestone G., Rojas E. The nature of the oscillatory behaviour in electrical activity from pancreatic beta-cell. Horm Metab Res Suppl. 1980;Suppl 10:100–107. [PubMed] [Google Scholar]
- Atwater I., Eddlestone G. T., Ribalet B., Rojas E. Calcium channel voltage noise across the beta-cell membrane in Islet of Langerhans of the mouse [proceedings]. J Physiol. 1979 Jun;291:69P–70P. [PubMed] [Google Scholar]
- Atwater I., Ribalet B., Rojas E. Cyclic changes in potential and resistance of the beta-cell membrane induced by glucose in islets of Langerhans from mouse. J Physiol. 1978 May;278:117–139. doi: 10.1113/jphysiol.1978.sp012296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwater I., Ribalet B., Rojas E. Mouse pancreatic beta-cells: tetraethylammonium blockage of the potassium permeability increase induced by depolarization. J Physiol. 1979 Mar;288:561–574. [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Meves H., Ridgway E. B. Effects of manganese and other agents on the calcium uptake that follows depolarization of squid axons. J Physiol. 1973 Jun;231(3):511–526. doi: 10.1113/jphysiol.1973.sp010246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F. Transport and metabolism of calcium ions in nerve. Prog Biophys Mol Biol. 1972;24:177–223. doi: 10.1016/0079-6107(72)90007-7. [DOI] [PubMed] [Google Scholar]
- Begenisich T., Stevens C. F. How many conductance states do potassium channels have? Biophys J. 1975 Aug;15(8):843–846. doi: 10.1016/S0006-3495(75)85858-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boschero A. C., Kawazu S., Duncan G., Malaisse W. J. Effect of glucose on K+ handling by pancreatic islets. FEBS Lett. 1977 Nov 1;83(1):151–154. doi: 10.1016/0014-5793(77)80662-5. [DOI] [PubMed] [Google Scholar]
- Brown A. M., Akaike N., Lee K. S. The calcium conductance of neurons. Ann N Y Acad Sci. 1978 Apr 28;307:330–344. doi: 10.1111/j.1749-6632.1978.tb41960.x. [DOI] [PubMed] [Google Scholar]
- Conti F., De Felice L. J., Wanke E. Potassium and sodium ion current noise in the membrane of the squid giant axon. J Physiol. 1975 Jun;248(1):45–82. doi: 10.1113/jphysiol.1975.sp010962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean P. M., Matthews E. K. Electrical activity in pancreatic islet cells. Nature. 1968 Jul 27;219(5152):389–390. doi: 10.1038/219389a0. [DOI] [PubMed] [Google Scholar]
- Dean P. M., Matthews E. K. Electrical activity in pancreatic islet cells: effect of ions. J Physiol. 1970 Sep;210(2):265–275. doi: 10.1113/jphysiol.1970.sp009208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean P. M., Matthews E. K. Glucose-induced electrical activity in pancreatic islet cells. J Physiol. 1970 Sep;210(2):255–264. doi: 10.1113/jphysiol.1970.sp009207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodsky G. M., Bennett L. L. Cation requirements for insulin secretion in the isolated perfused pancreas. Diabetes. 1966 Dec;15(12):910–913. doi: 10.2337/diab.15.12.910. [DOI] [PubMed] [Google Scholar]
- HALES C. N., RANDLE P. J. Immunoassay of insulin with insulin-antibody precipitate. Biochem J. 1963 Jul;88:137–146. doi: 10.1042/bj0880137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. Currents carried by sodium and potassium ions through the membrane of the giant axon of Loligo. J Physiol. 1952 Apr;116(4):449–472. doi: 10.1113/jphysiol.1952.sp004717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales C. N., Milner R. D. The role of sodium and potassium in insulin secretion from rabbit pancreas. J Physiol. 1968 Feb;194(3):725–743. doi: 10.1113/jphysiol.1968.sp008433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henquin J. C. D-glucose inhibits potassium efflux from pancreatic islet cells. Nature. 1978 Jan 19;271(5642):271–273. doi: 10.1038/271271a0. [DOI] [PubMed] [Google Scholar]
- Henquin J. C., Lambert A. E. Cationic environment and dynamics of insulin secretion. II. Effect of a high concentration of potassium. Diabetes. 1974 Dec;23(12):933–942. doi: 10.2337/diab.23.12.933. [DOI] [PubMed] [Google Scholar]
- Henquin J. C., Lambert A. E. Cobalt inhibition of insulin secretion and calcium uptake by isolated rat islets. Am J Physiol. 1975 Jun;228(6):1669–1677. doi: 10.1152/ajplegacy.1975.228.6.1669. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The statistical nature of the acetycholine potential and its molecular components. J Physiol. 1972 Aug;224(3):665–699. doi: 10.1113/jphysiol.1972.sp009918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacy P. E., Kostianovsky M. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes. 1967 Jan;16(1):35–39. doi: 10.2337/diab.16.1.35. [DOI] [PubMed] [Google Scholar]
- Mattews E. K., Sakamoto Y. Pancreatic islet cells: electrogenic and electrodiffusional control of membrane potential. J Physiol. 1975 Mar;246(2):439–457. doi: 10.1113/jphysiol.1975.sp010898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews E. K., Sakamoto Y. Electrical characteristics of pancreatic islet cells. J Physiol. 1975 Mar;246(2):421–437. doi: 10.1113/jphysiol.1975.sp010897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner H. P., Atwater I. J. The kinetics of electrical activity of beta cells in response to a "square wave" stimulation with glucose or glibenclamide. Horm Metab Res. 1976 Jan;8(1):11–16. doi: 10.1055/s-0028-1093685. [DOI] [PubMed] [Google Scholar]
- Orci L. A portrait of the pancreatic B-cell. The Minkowski Award Lecture delivered on July 19, 1973, during the 8th Congress of the International Diabetes Federation, held in Brussels, Belgium. Diabetologia. 1974 Jun;10(3):163–187. doi: 10.1007/BF00423031. [DOI] [PubMed] [Google Scholar]
- Rojas E. Gating mechanism for the activation of the sodium conductance in nerve membranes. Cold Spring Harb Symp Quant Biol. 1976;40:305–320. doi: 10.1101/sqb.1976.040.01.031. [DOI] [PubMed] [Google Scholar]
- Sehlin J., Taljedal I. B. Glucose-induced decrease in Rb+ permeability in pancreatic beta cells. Nature. 1975 Feb 20;253(5493):635–636. doi: 10.1038/253635a0. [DOI] [PubMed] [Google Scholar]
- Stevens C. F. Inferences about membrane properties from electrical noise measurements. Biophys J. 1972 Aug;12(8):1028–1047. doi: 10.1016/S0006-3495(72)86141-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


