Abstract
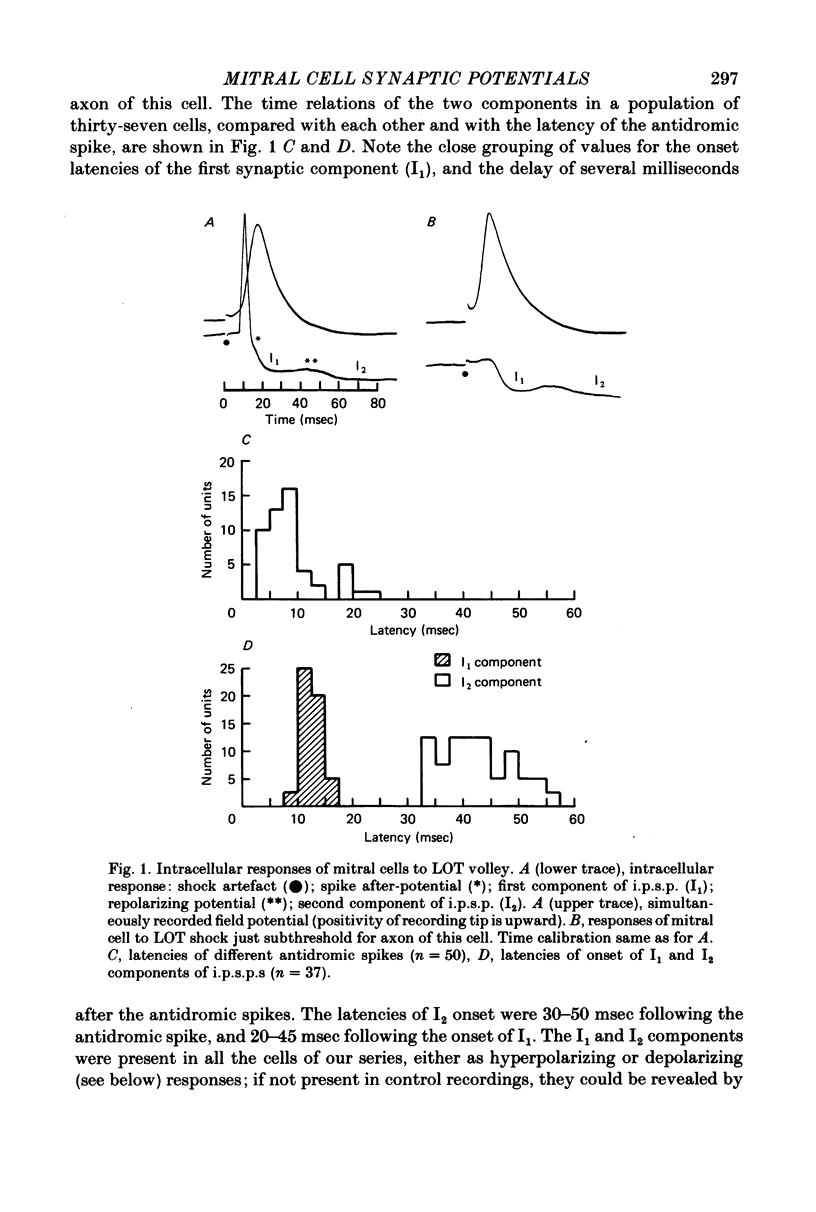
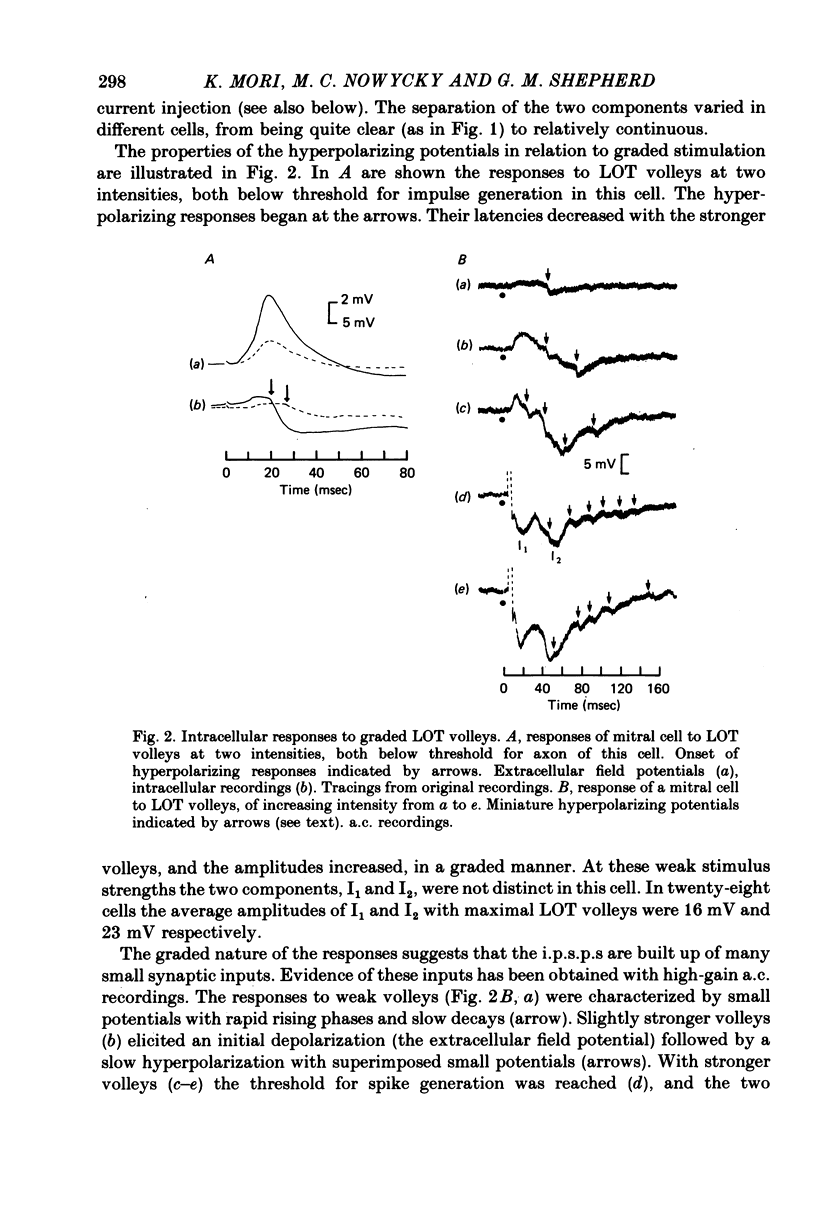
1. The synaptic responses of mitral cells have been analysed in intracellular recordings from the isolated olfactory bulb of the turtle. 2. The response of a mitral cell to a single volley in the lateral olfactory tract consisted of and antidromic impulse and a complex hyperpolarizing potential that had the properties of an inhibitory post-synaptic potential. The inhibitory response consisted of two successive components I1 and I2, followed by a prolonged hyperpolarization. 3. High-gain recordings revealed miniature hyperpolarizing potentials during the I1 and I2 responses. Both the miniature potentials and the I1 and I2 responses were increased in amplitude by depolarizing injected currents, and decreased and reversed in polarity by hyperpolarizing currents. The input conductance was increased during the I2 component. In some cells the I1 and I2 components, recorded with micropipettes filled with potassium acetate or potassium citrate, were depolarizing. 4 A single orthodromic volley in the olfactory nerves elicited a complex depolarizing-hyperpolarizing potential in mitral cells. The depolarization consisted of two successive components, E1 and E2. The hyperpolarization consisted of two successive components, I1 and I2, followed by a prolonged hyperpolarization. 5. The depolarizing components had the properties of excitatory post-synaptic potentials. They decreased in amplitude with depolarizing current injection and increased with hyperpolarizing injection. The hyperpolarizing components resembled the I1 and I2 components of the tract-evoked responses in their timing and properties. 6. It is postulated that the E1 component reflects the initial excitation by olfactory nerve terminals of the mitral cell dendritic tufts in the olfactory glomeruli. The I1 component is postulated to arise from dendrodendritic synaptic input mediated by interneurones, mainly granule cells. The E2 and I2 components are likely to arise mainly from intrinsic synaptic circuits within the olfactory bulb.
Full text
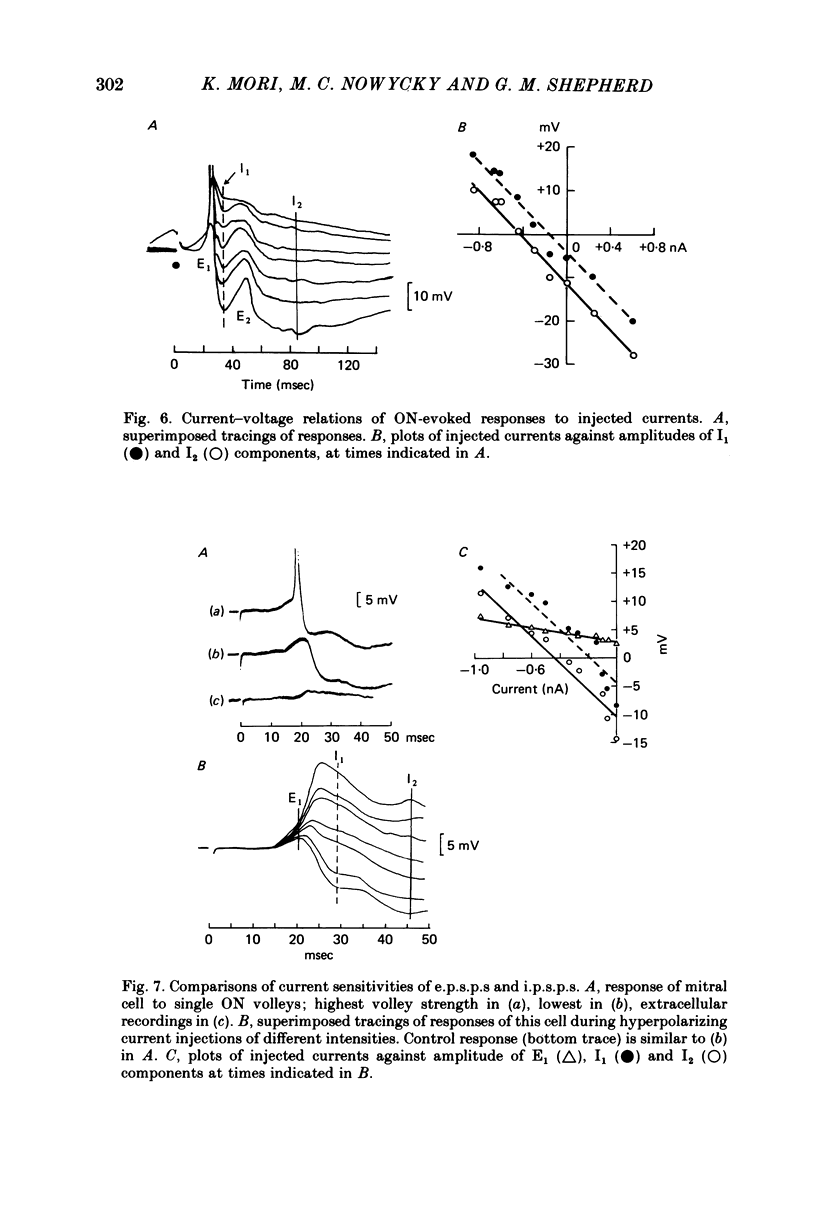
PDF



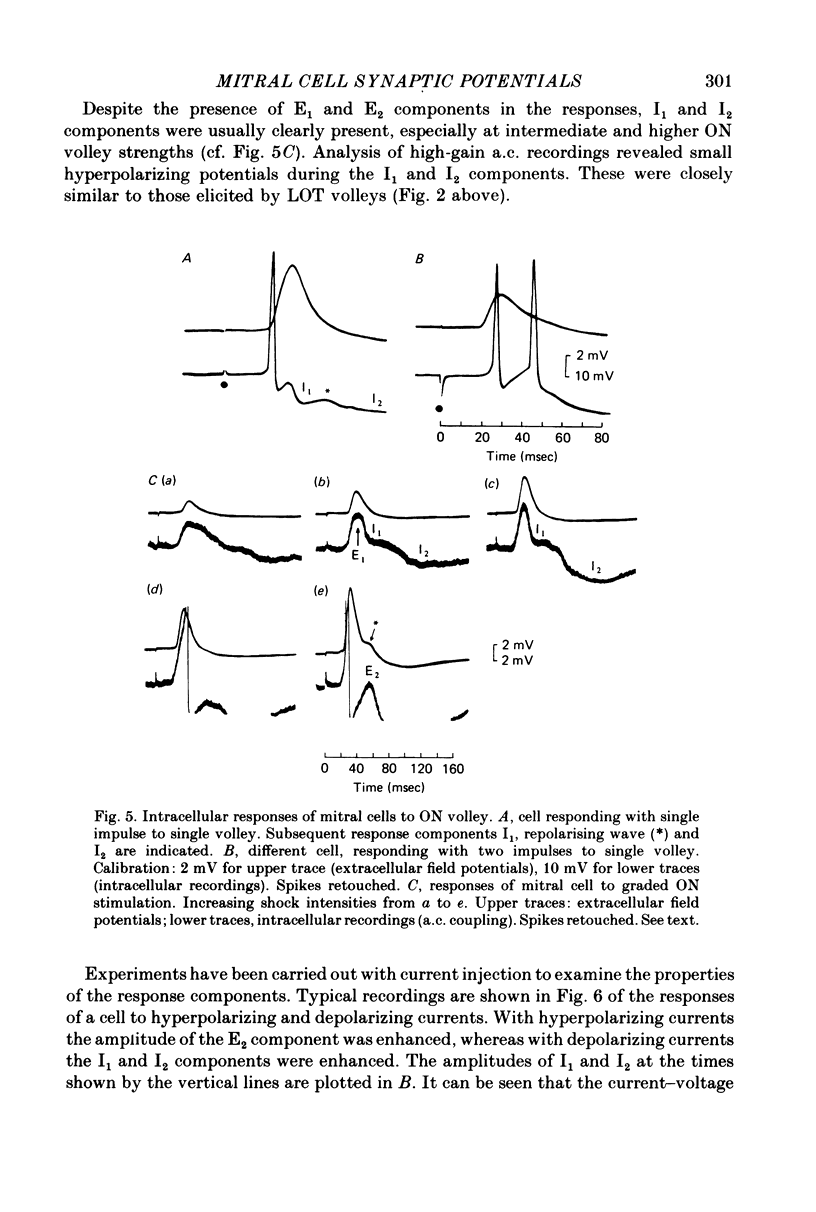





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Eccles J. C., Llinás R., Sasaki K. Intracellularly recorded responses of the cerebellar Purkinje cells. Exp Brain Res. 1966;1(2):161–183. doi: 10.1007/BF00236869. [DOI] [PubMed] [Google Scholar]
- Getchell T. V., Shepherd G. M. Short-axon cells in the olfactory bulb: dendrodendritic synaptic interactions. J Physiol. 1975 Oct;251(2):523–548. doi: 10.1113/jphysiol.1975.sp011106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getchell T. V., Shepherd G. M. Synaptic actions on mitral and tufted cells elicited by olfactory nerve volleys in the rabbit. J Physiol. 1975 Oct;251(2):497–522. doi: 10.1113/jphysiol.1975.sp011105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackowski A., Parnavelas J. G., Lieberman A. R. The reciprocal synapse in the external plexiform layer of the mammalian olfactory bulb. Brain Res. 1978 Dec 22;159(1):17–28. doi: 10.1016/0006-8993(78)90106-3. [DOI] [PubMed] [Google Scholar]
- Jahr C. E., Nicoll R. A. Dendrodendritic inhibition: demonstration with intracellular recording. Science. 1980 Mar 28;207(4438):1473–1475. doi: 10.1126/science.7361098. [DOI] [PubMed] [Google Scholar]
- Kauer J. S., Shepherd G. M. Analysis of the onset phase of olfactory bulb unit responses to odour pulses in the salamander. J Physiol. 1977 Nov;272(2):495–516. doi: 10.1113/jphysiol.1977.sp012056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLennan H. The pharmacology of inhibition of mitral cells in the olfactory bulb. Brain Res. 1971 Jun 18;29(2):177–184. doi: 10.1016/0006-8993(71)90026-6. [DOI] [PubMed] [Google Scholar]
- Mori K., Nowycky M. C., Shepherd G. M. Analysis of a long-duration inhibitory potential in mitral cells in the isolated turtle olfactory bulb. J Physiol. 1981 May;314:311–320. doi: 10.1113/jphysiol.1981.sp013709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori K., Nowycky M. C., Shepherd G. M. Electrophysiological analysis of mitral cells in the isolated turtle olfactory bulb. J Physiol. 1981 May;314:281–294. doi: 10.1113/jphysiol.1981.sp013707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori K., Shepherd G. M. Synaptic excitation and long-lasting inhibition of mitral cells in the in vitro turtle olfactory bulb. Brain Res. 1979 Aug 17;172(1):155–159. doi: 10.1016/0006-8993(79)90904-1. [DOI] [PubMed] [Google Scholar]
- Mori K., Takagi S. F. Activation and inhibition of olfactory bulb neurones by anterior commissure volleys in the rabbit. J Physiol. 1978 Jun;279:589–604. doi: 10.1113/jphysiol.1978.sp012363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori K., Takagi S. F. An intracellular study of dendrodendritic inhibitory synapses on mitral cells in the rabbit olfactory bulb. J Physiol. 1978 Jun;279:569–588. doi: 10.1113/jphysiol.1978.sp012362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori K., Takagi S. F. Spike generation in the mitral cell dendrite of the rabbit olfactory bulb. Brain Res. 1975 Dec 26;100(3):685–689. doi: 10.1016/0006-8993(75)90170-5. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A. Inhibitory mechanisms in the rabbit olfactory bulb: dendrodendritic mechanisms. Brain Res. 1969 Jun;14(1):157–172. doi: 10.1016/0006-8993(69)90037-7. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A. Pharmacological evidence for GABA as the transmitter in granule cell inhibition in the olfactory bulb. Brain Res. 1971 Dec 10;35(1):137–149. doi: 10.1016/0006-8993(71)90600-7. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A. Recurrent excitation of secondary olfactory neurons: a possible mechanism for signal amplification. Science. 1971 Feb 26;171(3973):824–826. doi: 10.1126/science.171.3973.824. [DOI] [PubMed] [Google Scholar]
- PHILLIPS C. G., POWELL T. P., SHEPHERD G. M. RESPONSES OF MITRAL CELLS TO STIMULATION OF THE LATERAL OLFACTORY TRACT IN THE RABBIT. J Physiol. 1963 Aug;168:65–88. doi: 10.1113/jphysiol.1963.sp007178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinching A. J., Powell T. P. The neuropil of the glomeruli of the olfactory bulb. J Cell Sci. 1971 Sep;9(2):347–377. doi: 10.1242/jcs.9.2.347. [DOI] [PubMed] [Google Scholar]
- Price J. L., Powell T. P. The synaptology of the granule cells of the olfactory bulb. J Cell Sci. 1970 Jul;7(1):125–155. doi: 10.1242/jcs.7.1.125. [DOI] [PubMed] [Google Scholar]
- Rall W., Shepherd G. M., Reese T. S., Brightman M. W. Dendrodendritic synaptic pathway for inhibition in the olfactory bulb. Exp Neurol. 1966 Jan;14(1):44–56. doi: 10.1016/0014-4886(66)90023-9. [DOI] [PubMed] [Google Scholar]
- Rall W., Shepherd G. M. Theoretical reconstruction of field potentials and dendrodendritic synaptic interactions in olfactory bulb. J Neurophysiol. 1968 Nov;31(6):884–915. doi: 10.1152/jn.1968.31.6.884. [DOI] [PubMed] [Google Scholar]
- Ribak C. E., Vaughn J. E., Saito K., Barber R., Roberts E. Glutamate decarboxylase localization in neurons of the olfactory bulb. Brain Res. 1977 Apr 22;126(1):1–18. doi: 10.1016/0006-8993(77)90211-6. [DOI] [PubMed] [Google Scholar]
- Shepherd G. M. Physiological evidence for dendrodendritic synaptic interactions in the rabbit's olfactory glomerulus. Brain Res. 1971 Sep 10;32(1):212–217. doi: 10.1016/0006-8993(71)90168-5. [DOI] [PubMed] [Google Scholar]
- White E. L. Synaptic organization in the olfactory glomerulus of the mouse. Brain Res. 1972 Feb 11;37(1):69–80. doi: 10.1016/0006-8993(72)90346-0. [DOI] [PubMed] [Google Scholar]
- White E. L. Synaptic organization of the mammalian olfactory glomerulus: new findings including an intraspecific variation. Brain Res. 1973 Oct 12;60(2):299–313. doi: 10.1016/0006-8993(73)90792-0. [DOI] [PubMed] [Google Scholar]
- YAMAMOTO C., YAMAMOTO T., IWAMA K. The inhibitory systems in the olfactory bulb studied by intracellular recording. J Neurophysiol. 1963 May;26:403–415. doi: 10.1152/jn.1963.26.3.403. [DOI] [PubMed] [Google Scholar]


