Until about 12 years ago, almost all experimental work with antigens capable of stimulating T cells had been performed with proteins and peptides, as well as haptenated proteins and peptides. By far most of the work had been performed with mice that had been immunized and examined for responses in assays for T cell proliferation. Pure carbohydrates were found to be incapable of major histocompatibility complex (MHC) binding and T cell stimulation (1, 2). However, within the last 10 years it has become evident that both CD4+ and CD8+ T cells can recognize glycopeptides carrying mono- and disaccharides in a MHC-restricted manner provided the glycan group is attached to the peptide at suitable positions. In such glycopeptides, the primed T cells recognize the glycan structure with high fidelity (see below). The question of T cell recognition of glycopeptides may be important in the immune defense against microorganisms, because many microbial antigens are in fact glycosylated. T cell recognition of glycans may also play an important role in the immune defence against tumors, because one of the most consistent traits of a cancer cell is an abnormal glycosylation of the proteins of the malignant cell (3). In this issue of PNAS, Bäcklund et al. (4) provide evidence that T cell recognition of protein glycans may be crucial also for T cell responses to autoantigens in the course of autoimmune diseases. Below we will describe and discuss the general rules for MHC class II restricted T cell recognition of glycans, the fate of glycoprotein glycans during antigen processing, and the role of antigen glycosylation in tolerance to autoantigens and tumor antigens.
T cell recognition of glycans may be crucial for T cell responses to autoantigens.
A sine qua non for a compound to be able to stimulate an antigen-specific T helper cell response is that it can bind to an appropriate MHC class II molecule and obtain presentation to T helper cells. The MHC class II molecule functions as a receptor capable of binding 10–25 residue long peptide fragments of antigens with a broad specificity and transporting them to the surface of the antigen-presenting cell (APC) for presentation to T cells. In an immunogenic glycopeptide antigen, the peptide provides the binding motif that enables the glycopeptide to bind to the MHC molecule, and the glycan group provides an important part of the structure that constitutes the epitope, i.e., the structure that is recognized by the T cell through its T cell receptor (TCR). Eukaryote protein glycosylation may be N-linked to asparagine or O-linked to serine, threonine, or hydroxy-lysines. Studies of glycopeptide recognition by T cells have shown that a glycan group located outside the peptide binding core of the MHC class II molecule will not be specifically recognized, though it may change the overall conformation of the MHC bound peptide and in this way indirectly influence the structure of the MHC-bound T cell epitope (5, 6). In contrast, a glycan group positioned within the MHC binding core may influence the T cell recognition in several ways. If the glycan is linked to an amino acid functioning as an MHC anchor residue the glycopeptide may be incapable of MHC binding and thus become nonimmunogenic (2, 7). However, if the glycan is linked to an amino acid pointing away from the binding site of the MHC molecule, the binding is maintained, and in antigens with the glycan attached to central residue within the MHC core, the glycan becomes the dominant structure in the epitope, which is recognized with very high fidelity by T cells specific for the particular glycopeptide (7–11).
Glycopeptides with simple sugars have been suitable for studies of the antigen fine specificity of glycopeptide-specific T cells. Most common monosaccharides differ from one another by the orientation of hydroxy groups. Progress in technology has made it relatively easy to synthesize glycopeptides with the same amino acid sequence, but with closely related sugar groups differing only in the orientation of the hydrophilic hydroxy groups attached to the same glycosylation site. Experiments with such sugar-variant glycopeptides have revealed the glycan group as an integrated part of the T cell epitope that may be recognized with high specificity. One example is a large collection of glycopeptide-specific T cell hybridomas that were unable of recognizing a glycopeptide identical with the cognate glycopeptide except for the orientation of a single hydroxy group (9). The glycan specificity is reflected in the overall amino acid composition of the central parts of the TCR's complementarity determining region (CDR3) of glycopeptide-specific T cell hybridomas and clones. Conserved amino acid motifs and dominance of small polar amino acids, which are frequently found in antibodies and other glycan-recognizing proteins, have been identified within αβTCR CDR3 regions of glycopeptide-specific T cells (12–14). In addition, amino acids that are flanking the glycan group and are oriented away from the binding cleft of the MHC molecule are recognized. The crystal structure of MHC class I/glycopeptide complexes also show that glycans can be accommodated by the TCR (15, 16). However, not all peptide-attached glycans can elicit a T cell response (17). It appears that αβT cells are unable to recognize large and highly complex glycan structures. A possible molecular explanation for this may be that the central CDR3 region of the TCR cannot accommodate very large glycans, while other parts of the receptor at the same time interact with the α-helices of the presenting MHC molecule. Studies of the immunogenicity of linear and branched sugars of varying length demonstrated that the αβ TCR may recognize extensions of glycan groups consisting of up to three or four sugars, whereas even larger glycan structures lead to loss of MHC class II restricted T cell recognition (16–18). Such large glycans may activate MHC unrestricted γδT cells, which recognize the glycan antigen regardless of whether it is associated with the APC through an MHC binding peptide or through a lipid tail.
Antigen processing and presentation is a thoroughly studied area in immunology and our knowledge of the mechanisms whereby peptides are generated for MHC class II and class I molecules is amazing. However, a less investigated area is the effect of protein glycosylation on the processing of antigen in APCs, where a glycan can interfere with the proteolytic fragmentation of the large glycoprotein antigen and influence the pattern of T cell epitopes that are created. Glycoprotein antigens are ingested by APCs by endocytosis and transported in the endocytic pathway from the cell surface toward the lysosomal compartments of the cell. During the transport, proteolytic enzymes with low pH optimum are added to the endosome and the pH decreases progressively, leading to activation of the proteolytic enzymes. The enzymes, which include endoproteases and exoproteases of many different substrate specificities, attack and fragment the antigen into peptides. Suitable peptides bind to empty MHC class II molecules, which are accumulating within the acidic compartments and the peptides are from that point on protected against further proteolysis (19). Finally, the MHC-peptide complexes are transported to the cell surface and presented to the T cell system.
Some observations pointing to the fate of glycans during antigen processing have been made with a mouse hemoglobin-derived decaglycopeptide O-glycosylated with α-d-GalNAc on a central threonine. Mice immunized with this glycopeptide gave a proliferative T cell response to the cognant glycopeptide, but they also responded to the unglycosylated decapeptide (7). This was not a simple cross-response from some glycopeptide specific clones. That became clear when T cell hybridomas were raised against the glycopeptide and analyzed for antigen-fine specificity (9). The majority of the raised T cell hybridomas responded to the glycopeptide but not to the peptide, and a few hybridomas responded to the peptide but not to the glycopeptide. No hybridoma responded to both. The presence of T cell clones only recognizing the unglycosylated form of the glycopeptide used for the immunization strongly indicates that deglycosylation of some of the antigen molecules had occurred during the priming phase of the immune response. The significance of deglycosylation may be quite different for various antigens, but should be considered. In the present study by Bäcklund et al. (4), some of the important glycopeptides obviously survive antigen processing with the glycan intact.
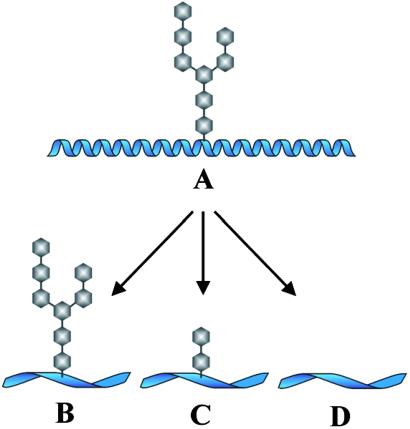
Studies by Chicz et al. (20, 21) have shown that in natural glycoprotein processing some glycan groups remain attached to the antigen fragments bound onto MHC class II molecules. These workers eluted material bound onto the human HLA-DR and HLA-DQ molecules and identified some of them as glycopeptides containing N-linked GlcNAc residues. So far, however, N-linked carbohydrates have not been identified been identified on material eluted from MHC class I molecules. The majority of the MHC class I binding peptides are derived from cytosolic proteins that have been targeted by ubiquitinylation and degraded by the proteasome. The reason why these protein fragments are devoid of sugars may be that all sugars are removed by a cytosolic N-glycanase before the cytosolic protein interacts with the proteasome. By contrast, elution of peptides from MHC class I molecules have revealed that around 0.1% of all class I bound peptides carry small O-linked N-acetylglucosamine (O-βGlcNAc) residues (22). This finding is in agreement with the fact that glycosylation of serine and threonine residues on nuclear and cytosolic proteins by O-βGlcNAc are abundant in all multicellular eukaryotes. In Bäcklund et al.'s paper in this issue of PNAS (4) the authors demonstrate that glycans not only remain attached to the peptide backbone of the large glycoprotein antigen, collagen II (CII), during the processing in the APC, but such glycans are apparently also presented to the T cell system and stimulate specific CD4+ T helper cells. T cells with specificity for glycosylated epitopes of CII can be detected both in mice immunized with human CII and in patients suffering from rheumatoid arthritis, a disease in which CII is implicated as an important autoantigen. One evident implication of the alternate ways of glycan processing is that a glycan-containing T cell epitope may be converted and presented as several variants. This in turn diversifies the immune response, and it has practical consequences for the design of glycopeptide-based vaccines. Fig. 1 illustrates the alternative possibilities for the processing of glycoproteins.
Figure 1.
Processing of glycoproteins in APCs. (A) The intact glycoprotein has been taken up by the APC and is transported through the endocytic pathway. (B–D) The peptide fragments after alternate glycan processing. In all three, the peptide is in extended conformation. (B) The complex glycan group has survived the processing and is left intact on the peptide fragment. (C) Only some of the glycan has survived bound to the glycosylated segment. (D) The glycan has been removed entirely.
The question of antigen glycosylation is central to the work presented by Bäcklund et al. (4) and seems to be relevant to the breaking of immunological tolerance to CII. CII is an important autoantigen in patients with rheumatoid arthritis, and immunization of some mouse strains with CII triggers an inflammatory arthritis widely regarded as a model for rheumatoid arthritis. By genetic manipulation, Bäcklund et al. created mice equipped with human HLA-DR molecules and capable of giving HLA-DR4-restricted T cell responses. Into these mice they then introduced the gene for human CII. The expected outcome is that such mice will develop immunological tolerance to CII. The mice indeed failed to give T cell responses after appropriate immunization, but it turned out that the tolerance was incomplete. The crucial arthritogenic T cell epitope on CII is located on the sequence 261–278. In this epitope there are two hydroxylysines that are glycosylated on some CII molecules but not on others. Backlund et al. discovered that the CII-immunized mice were almost completely tolerant to the unglycosylated epitope variant but not to the glycosylated epitope variant. They also showed that patients with severe rheumatoid arthritis predominantly recognized the glycosylated CII epitope. Here, it is of great importance that the epitope actually is shown to be present in patients with rheumatoid arthritis. However, the complementary absence or reduced presence in mature individuals without rheumatoid arthritis could have been demonstrated to give this observation more significance.
Thus, although the T cell repertoire in patients with rheumatoid arthritis seems to have been purged of clones with specificity toward the unglycosylated variants of the arthritogenic CII epitope, some clones with specificity toward the glycosylated variant have escaped thymic deletion and are fully functional. This leaves one wondering whether tolerance mechanisms may be less efficient for T cell clones with specificity for glycosylated epitopes. Although such clones may be damaging for the patients with rheumatoid arthritis they may play a beneficial role in patients with cancer. Many tumors express glycosylated tumor antigens. One such tumor-associated antigen is the MUC1 molecule, which is heavily O-glycosylated with Tn (α-d-GalNAc) and T (β-d-Gal(1–3)α-d-GalNAc). Might some of the antitumor T cell responses observed in patients with breast cancer be directed against MUC1 epitopes containing the Tn or T glycans?
Footnotes
See companion article on page 9960.
References
- 1.Harding C V, Roof R W, Allen P M, Unanue E R. Proc Natl Acad Sci USA. 1991;88:2740–2744. doi: 10.1073/pnas.88.7.2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ishioka G Y, Lamont A G, Thomson D, Bulbow N, Gaeta F C, Sette A, Grey H M. J Immunol. 1992;148:2446–2451. [PubMed] [Google Scholar]
- 3.Hakomori S. Adv Cancer Res. 1989;52:257–331. doi: 10.1016/s0065-230x(08)60215-8. [DOI] [PubMed] [Google Scholar]
- 4.Bäcklund J, Carlsen S, Höger T, Holm B, Fugger L, Kihlberg J, Burkhardt H, Holmdahl R. Proc Natl Acad Sci USA. 2002;99:9960–9965. doi: 10.1073/pnas.132254199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harding C V, Kihlberg J, Elofsson M, Magnusson G, Unanue E R. J Immunol. 1992;151:2419–2425. [PubMed] [Google Scholar]
- 6.Mouritsen S, Meldal M, Christiansen-Brams I, Elsner H, Werdelin O. Eur J Immunol. 1994;24:1066–1072. doi: 10.1002/eji.1830240509. [DOI] [PubMed] [Google Scholar]
- 7.Jensen T, Galli-Stampino L, Mouritsen S, Frische K, Peters S, Meldal M, Werdelin O. Eur J Immunol. 1996;26:1342–1349. doi: 10.1002/eji.1830260625. [DOI] [PubMed] [Google Scholar]
- 8.Deck B, Elofsson M, Kihlberg J, Unanue E R. J Immunol. 1995;155:1074–1078. [PubMed] [Google Scholar]
- 9.Jensen T, Hansen P, Galli-Stampino L, Mouritsen S, Frische K, Meinjohanns E, Meldal M, Werdelin O. J Immunol. 1997;158:3769–3778. [PubMed] [Google Scholar]
- 10.Deck M B, Sjolin P, Unanue E R, Kihlberg J. J Immunol. 1999;162:4740–4744. [PubMed] [Google Scholar]
- 11.Haurum J S, Arsequell G, Lellouch A, Wong S Y, Dwek R A, McMichael A J, Elliott T. J Exp Med. 1994;180:739–744. doi: 10.1084/jem.180.2.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corthay A, Bäcklund L, Broddefalk J, Michaëlsson E, Goldschmidt T J, Kihlberg J, Holmdahl R. Eur J Immunol. 1998;28:2580–2590. doi: 10.1002/(SICI)1521-4141(199808)28:08<2580::AID-IMMU2580>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 13.Jensen T, Hansen P, Faurskov Nielsen A, Meldal M, Komba S, Werdelin O. Eur J Immunol. 1999;29:2759–2768. doi: 10.1002/(SICI)1521-4141(199909)29:09<2759::AID-IMMU2759>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 14.Haurum J S, Tan L, Arsequell G, Frodsham P, Lellouch A C, Moss P A, Dwek R A, McMichael A J, Elliott T. Eur J Immunol. 1995;25:3270–3276. doi: 10.1002/eji.1830251211. [DOI] [PubMed] [Google Scholar]
- 15.Glithero A, Tormo J, Haurum J S, Arsequell G, Valencia G, Edwards J, Springer S, Townsend A, Pao Y L, Wormald M, Dwek R A, Jones E Y, Elliott T. Immunity. 1999;10:63–74. doi: 10.1016/s1074-7613(00)80007-2. [DOI] [PubMed] [Google Scholar]
- 16.Speir J A, Abdel-Motal U M, Jondal M, Wilson I A. Immunity. 1999;10:51–61. doi: 10.1016/s1074-7613(00)80006-0. [DOI] [PubMed] [Google Scholar]
- 17.Galli-Stampino L, Frische K, Meinjohanns E, Meldal M, Jensen T, Werdelin O, Mouritsen S. Cancer Res. 1997;57:3214–3222. [PubMed] [Google Scholar]
- 18.Abdel-Motal U M, Berg L, Rosen A, Bengtsson M, Thorpe C J, Kihlberg J, Dahmen J, Magnusson G, Karlsson K A, Jondal M. Eur J Immunol. 1996;26:544–551. doi: 10.1002/eji.1830260307. [DOI] [PubMed] [Google Scholar]
- 19.Mouritsen S, Meldal M, Werdelin O, Hansen A S, Buus S. J Immunol. 1992;149:1987–1993. [PubMed] [Google Scholar]
- 20.Chicz R M, Urban R G, Gorga J C, Vignali D A, Lane W S, Strominger J L. J Exp Med. 1993;178:27–47. doi: 10.1084/jem.178.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chicz R M, Lane W S, Robinson R A, Trucco M, Strominger J L, Gorga J C. Int Immunol. 1994;6:1639–1649. doi: 10.1093/intimm/6.11.1639. [DOI] [PubMed] [Google Scholar]
- 22.Haurum J S, Bjerring Højer I, Arsequell G, Neisig A, Valencia G, Zeuthen J, Neefjes J, Elliott T. J Exp Med. 1999;190:145–150. doi: 10.1084/jem.190.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]