Abstract
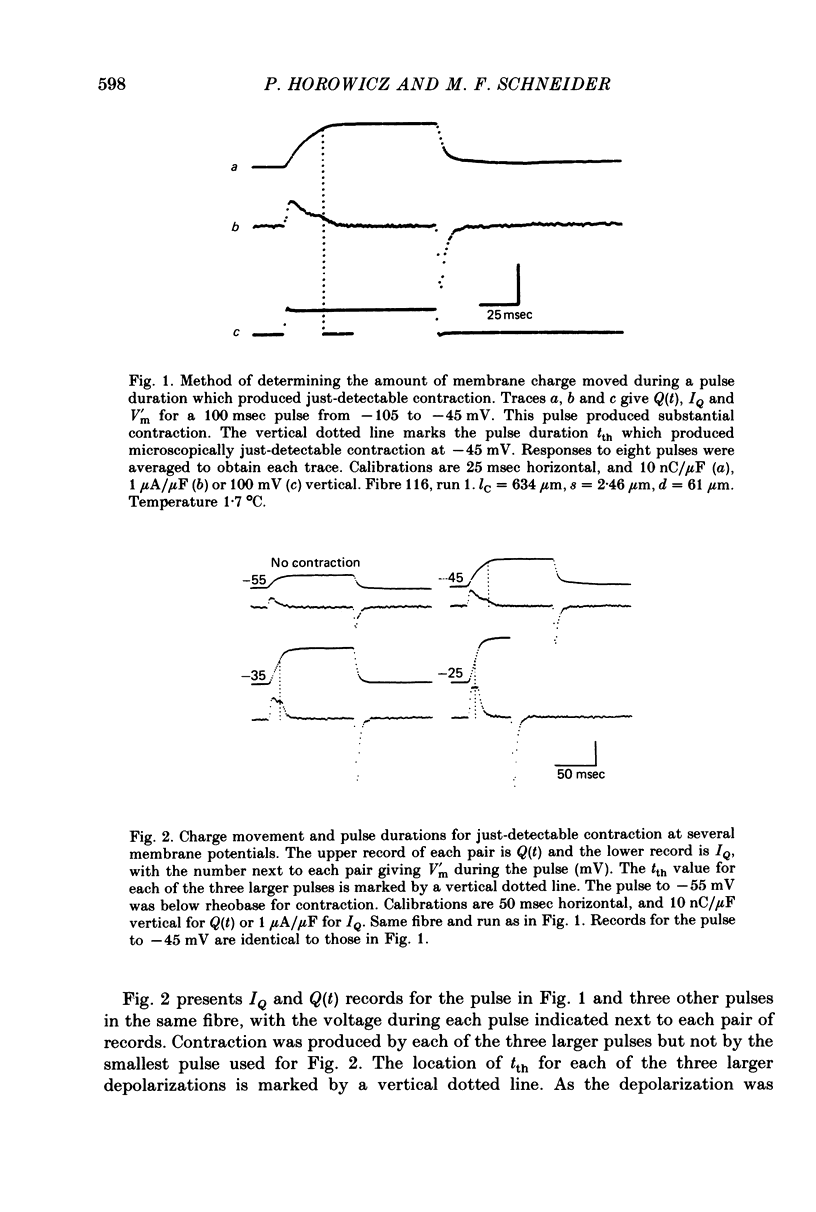
1. The current IQ due to membrane charge movement and the threshold pulse duration tth required to produce microscopically just-detectable contraction were determined for pulses to a variety of membrane potentials in tendon-terminated short segments of cut frog skeletal muscle fibres voltage-clamped using a single gap technique.
2. The time course Q(t) of membrane charge movement was calculated as the running integral of IQ. The threshold charge Qth moved by pulses which produced just-detectable contraction was estimated as Q(tth).
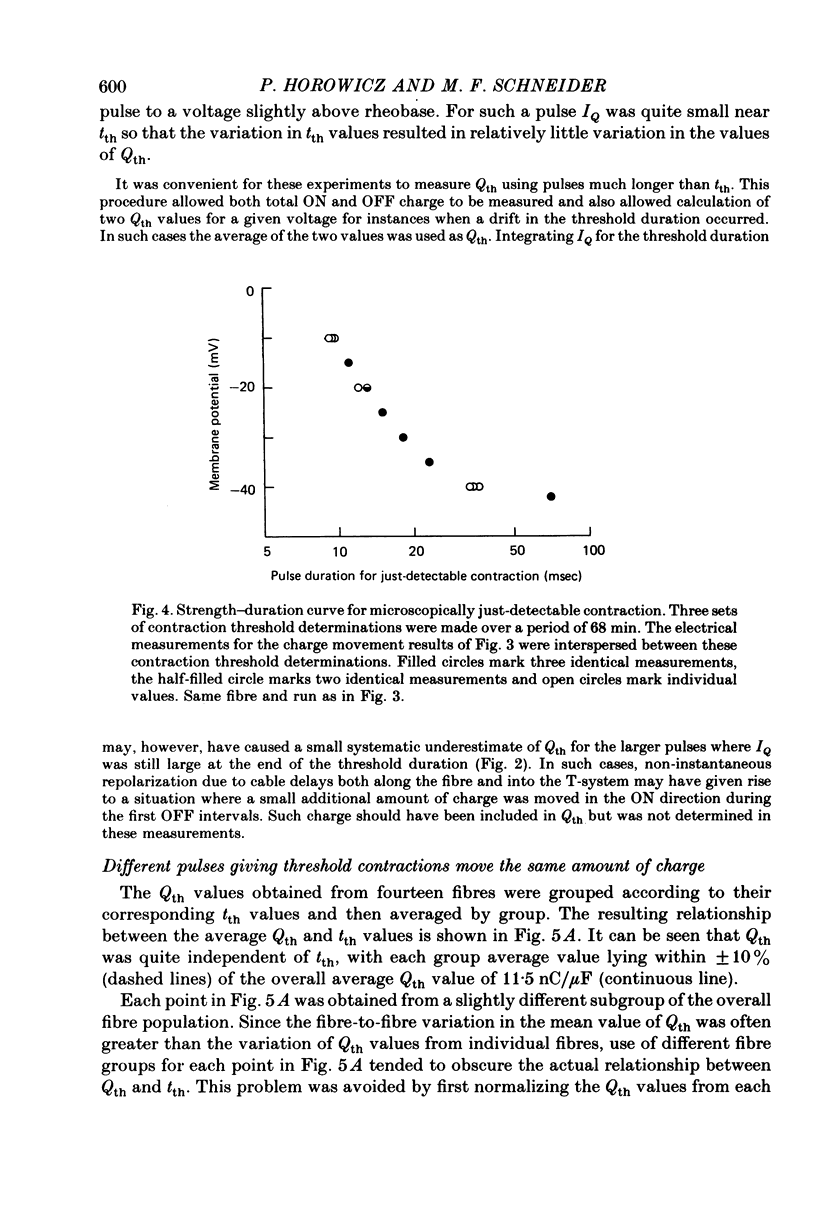
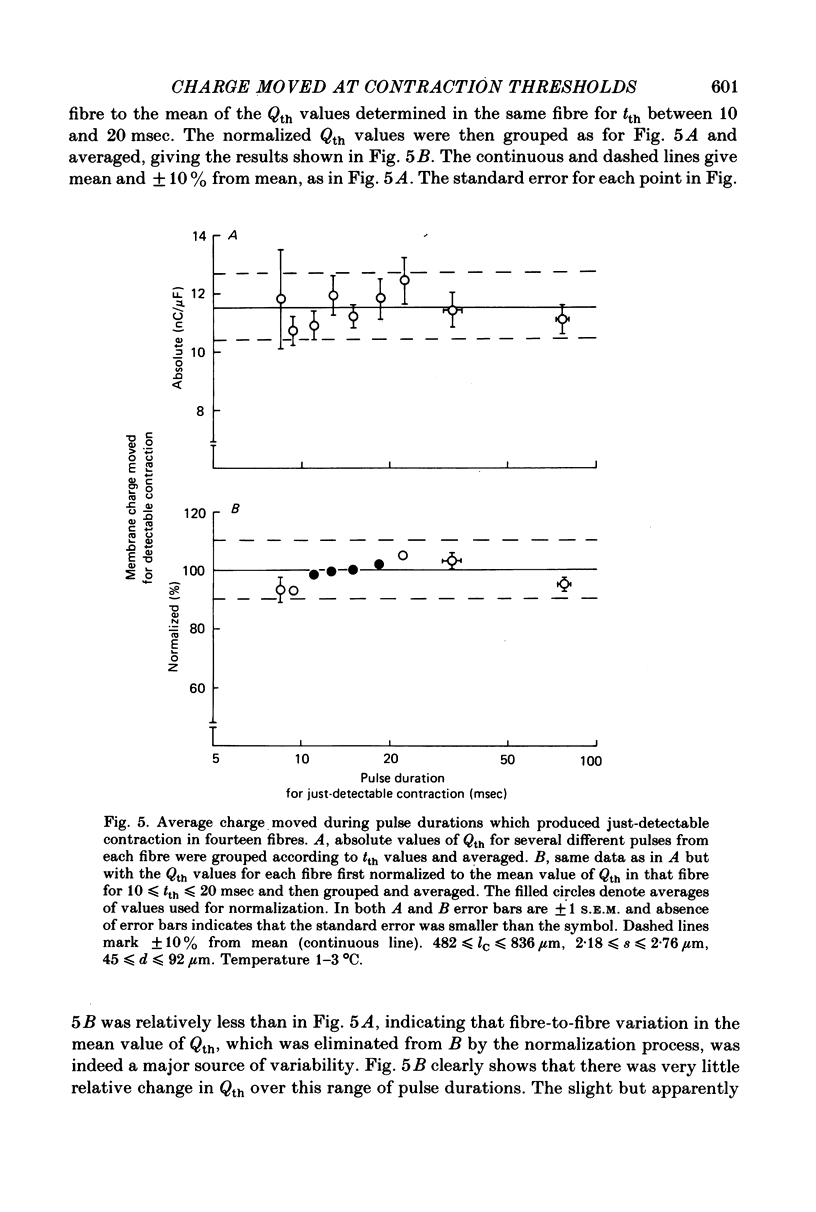
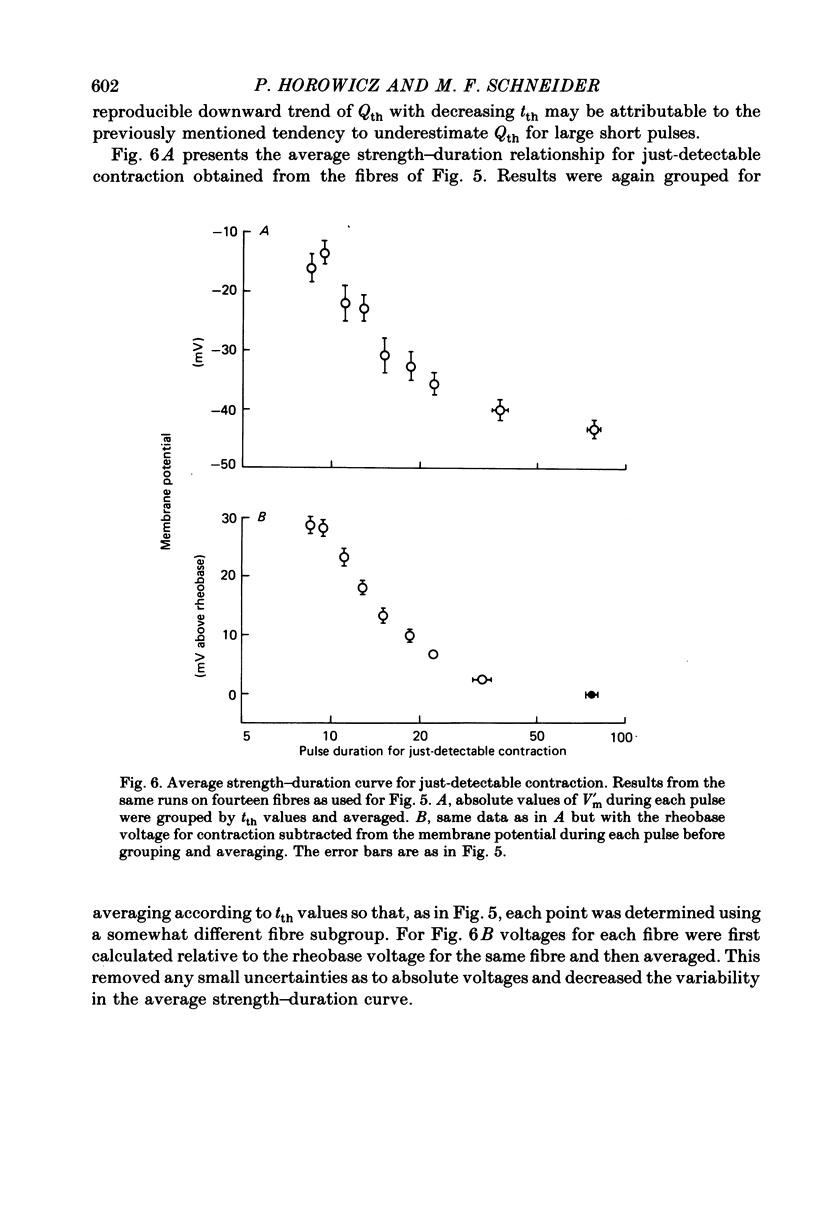
3. Qth was constant for pulses to potentials ranging from about -45 mV, the rheobase potential for contraction, to about -15 mV, where tth was about 9 msec. The mean Qth from fourteen fibres was 11.5 nC/μF, when the holding potential was about -100 mV.
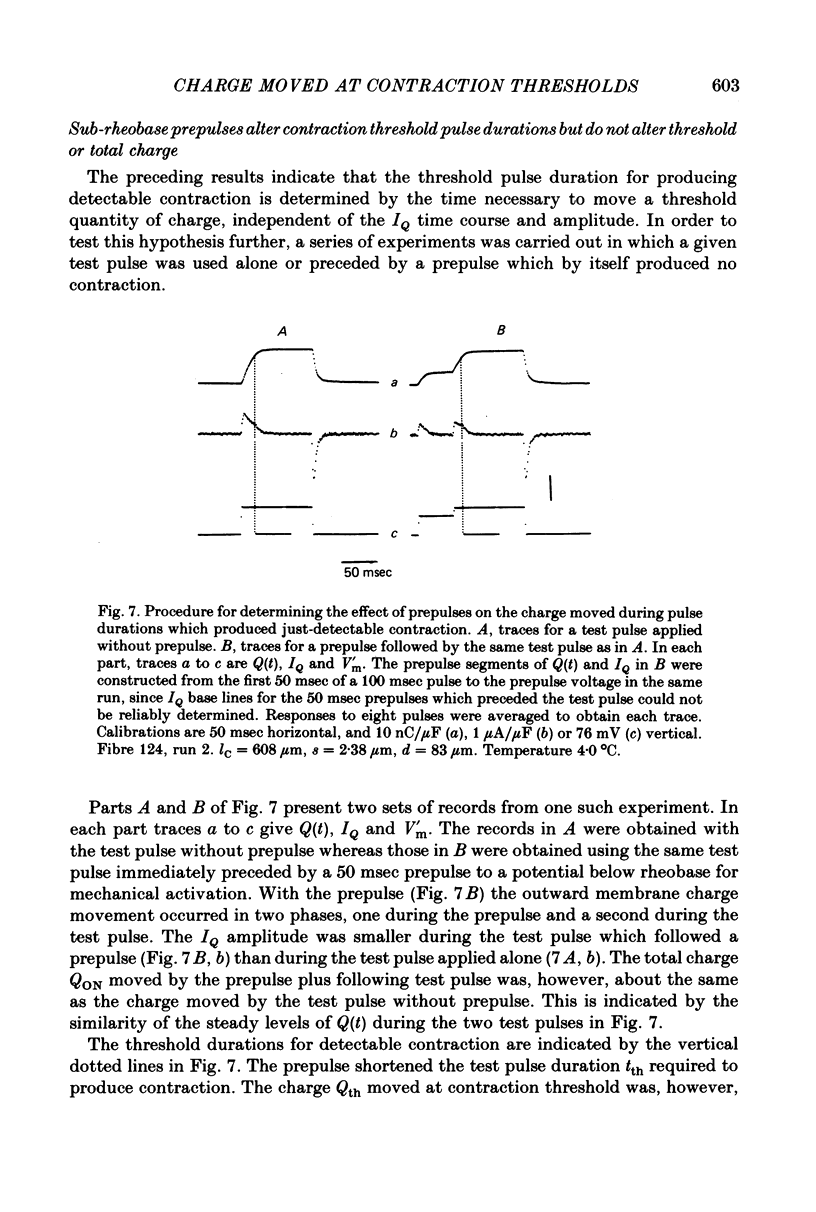
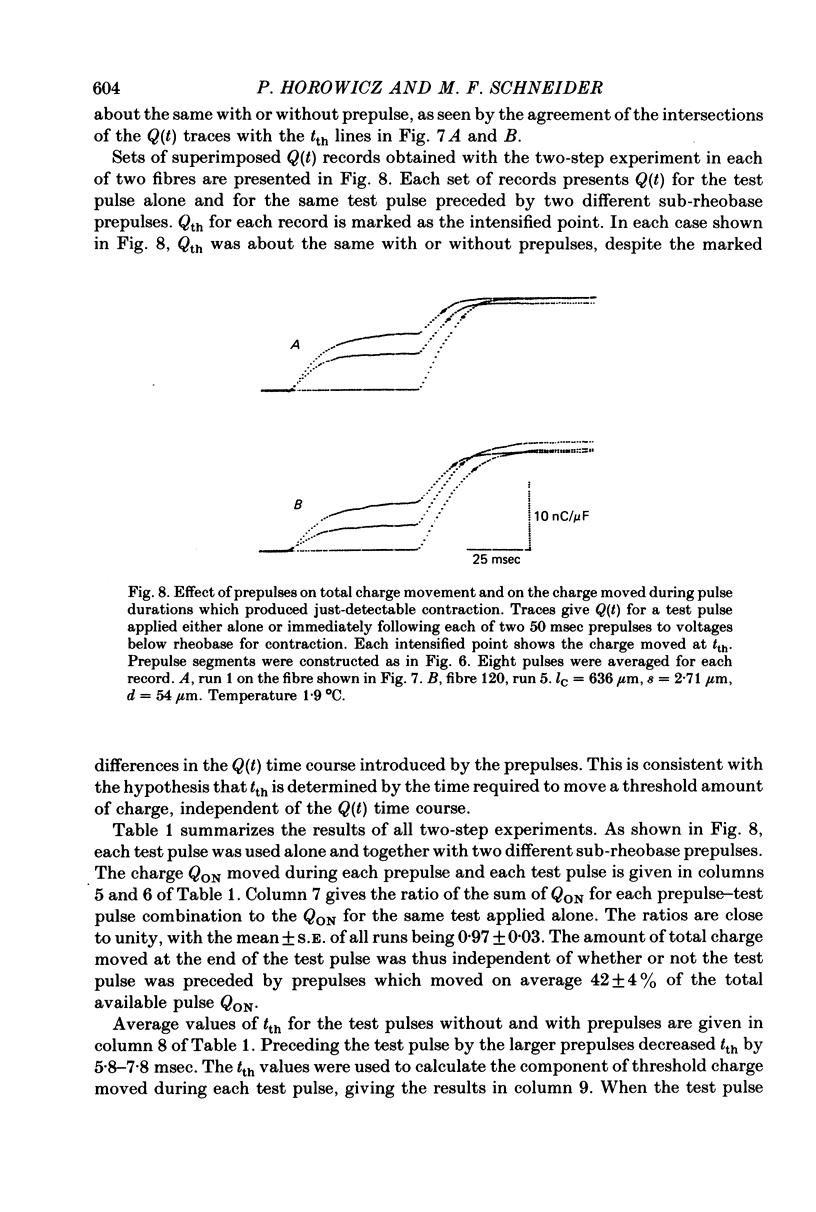
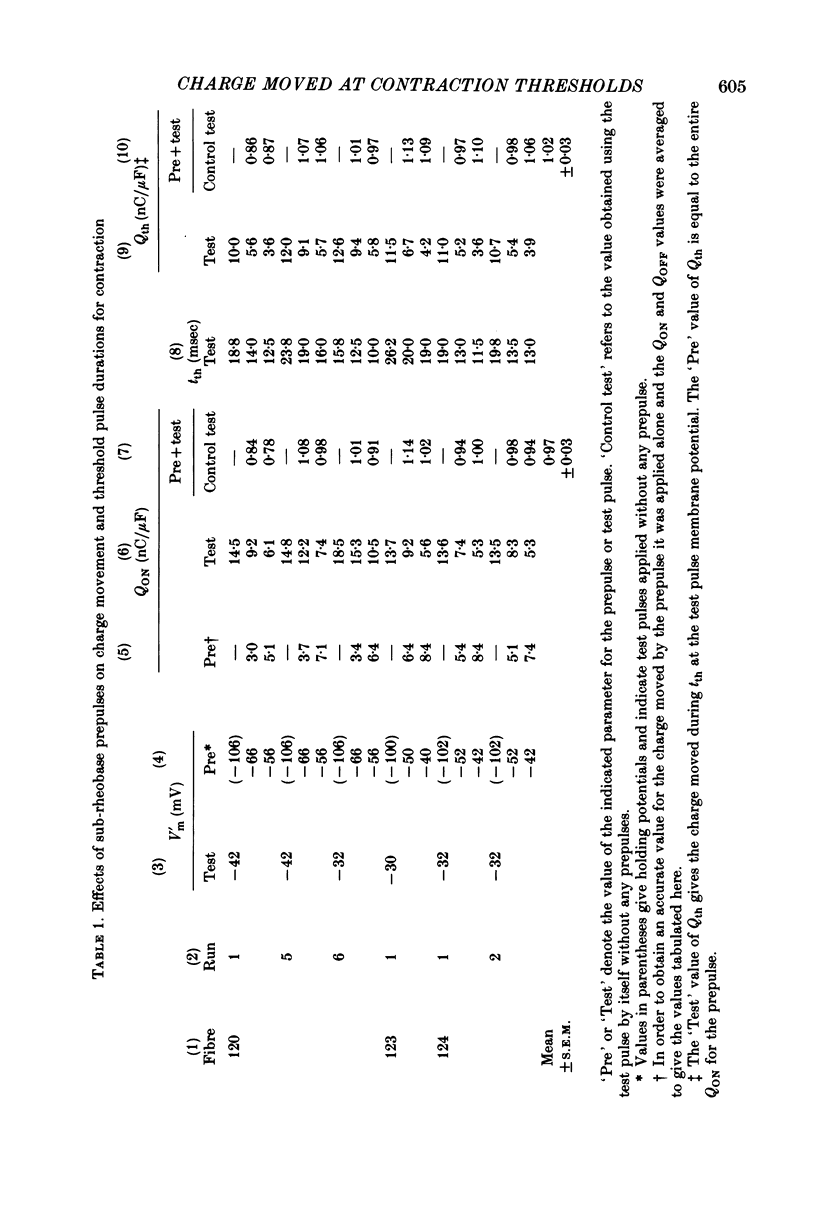
4. Prepulses of 50 msec which were themselves sub-rheobase for producing contraction decreased the tth for an immediately following test pulse. The total threshold charge moved during the prepulse and during tth of the test pulse was equal to Qth for the test pulse without prepulse.
5. Items 3 and 4 above indicate that tth is determined by the time required to move a set amount of intramembrane charge, independent of the kinetics of the charge movement.
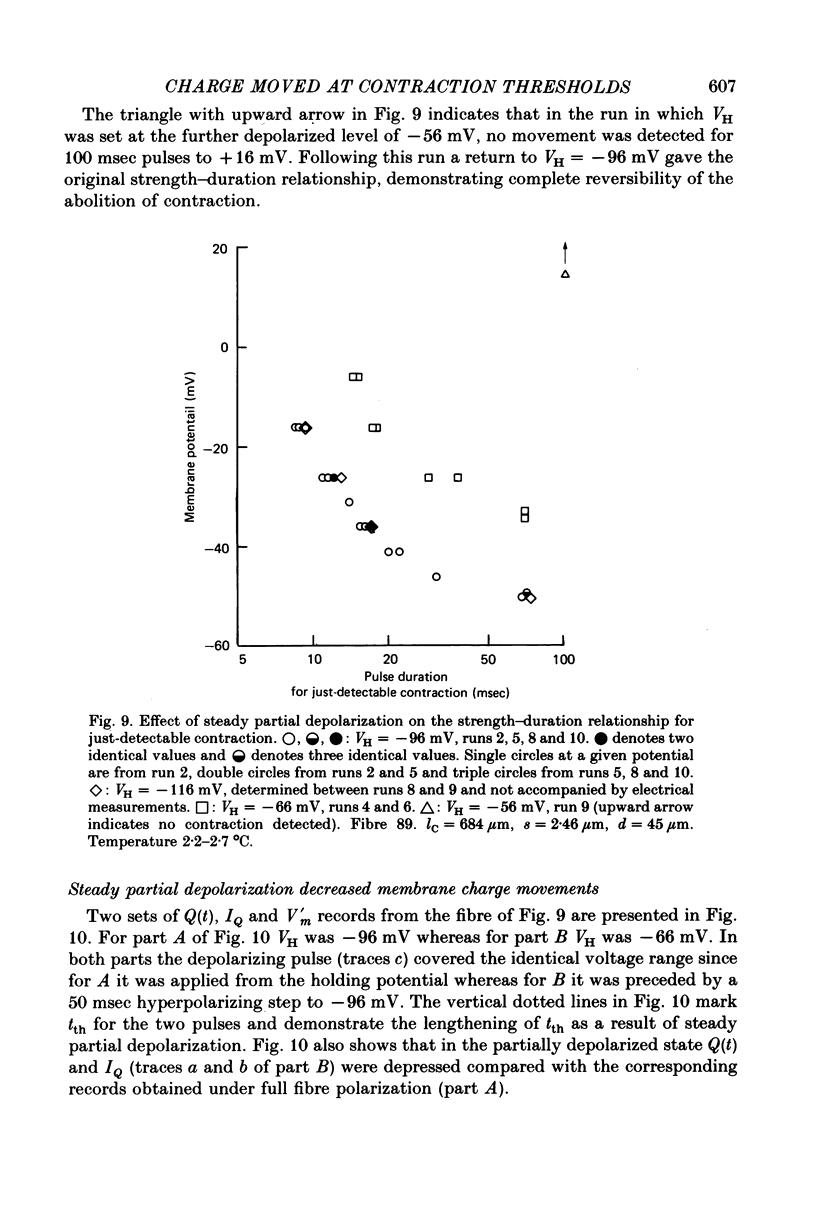
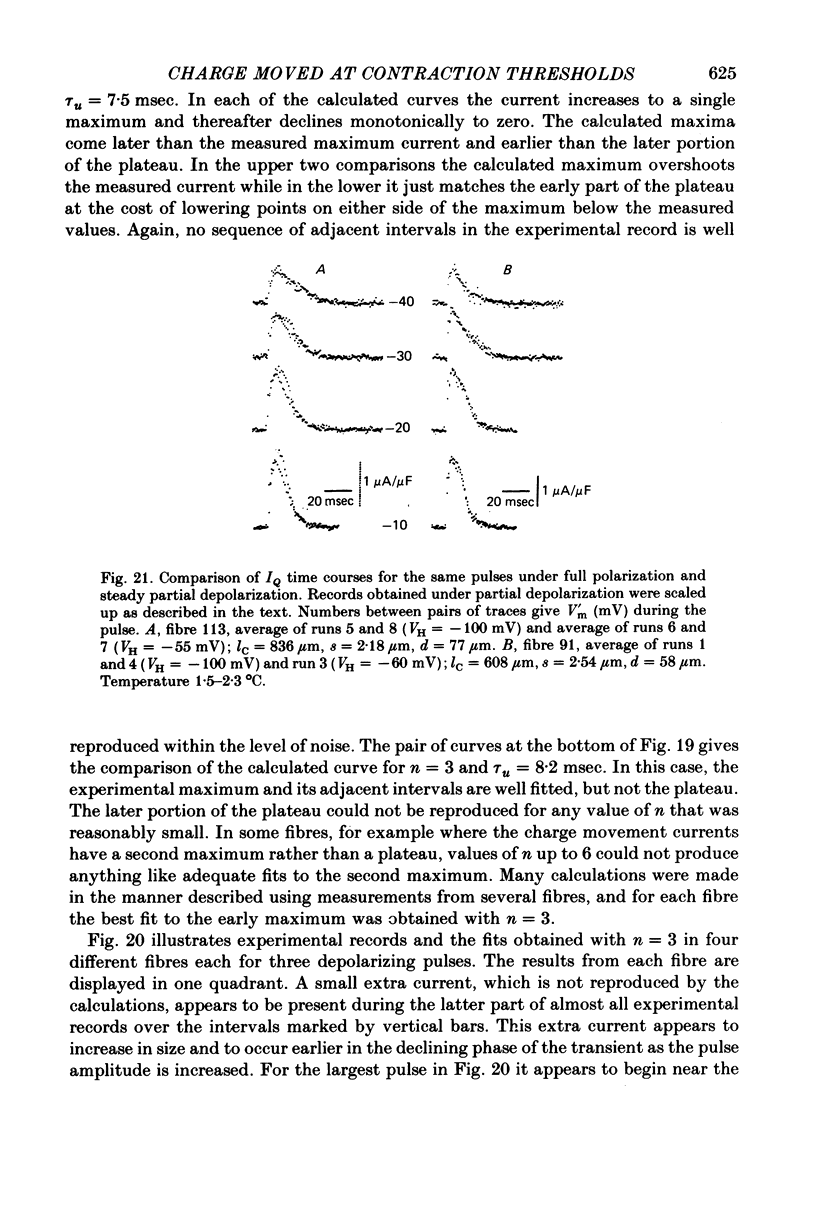
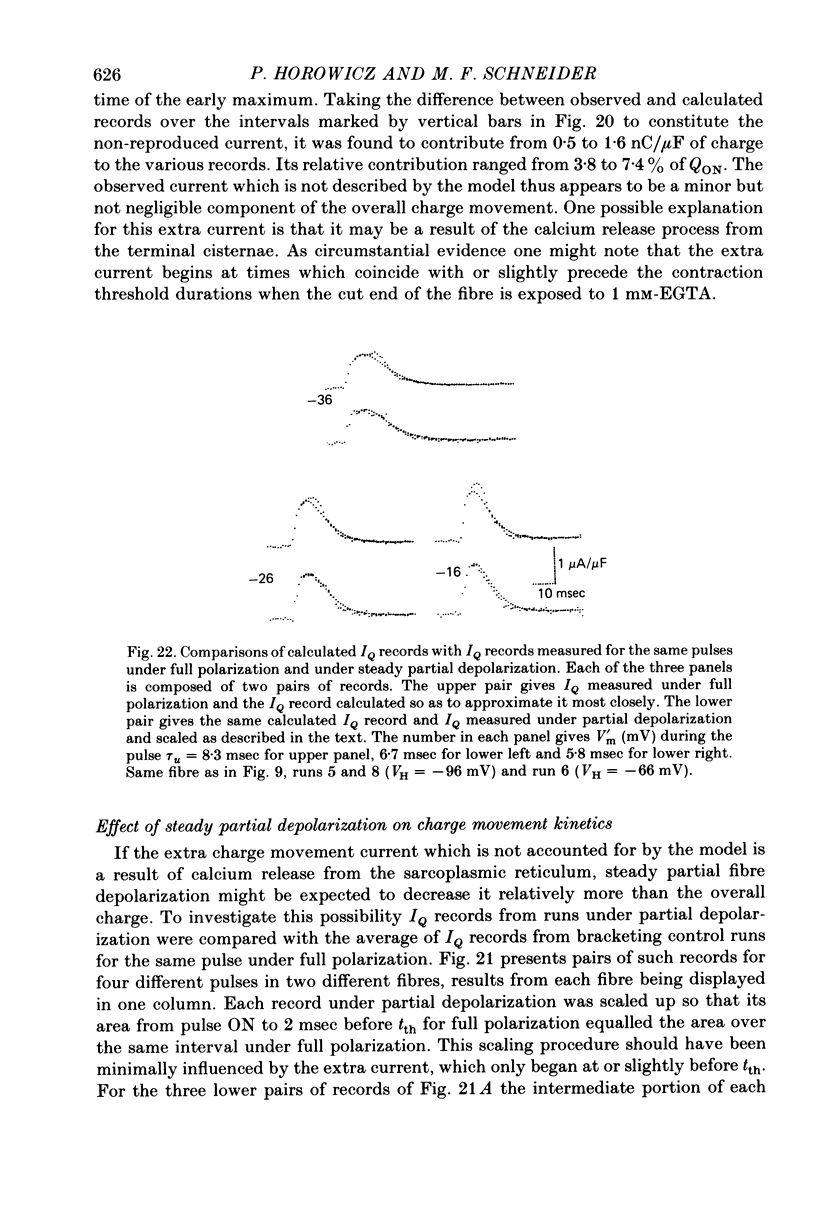
6. Steady partial fibre depolarization to between -70 and -55 mV increased tth at all membrane potentials and elevated the rheobase potential for contraction. Slight further steady depolarization totally eliminated contraction.
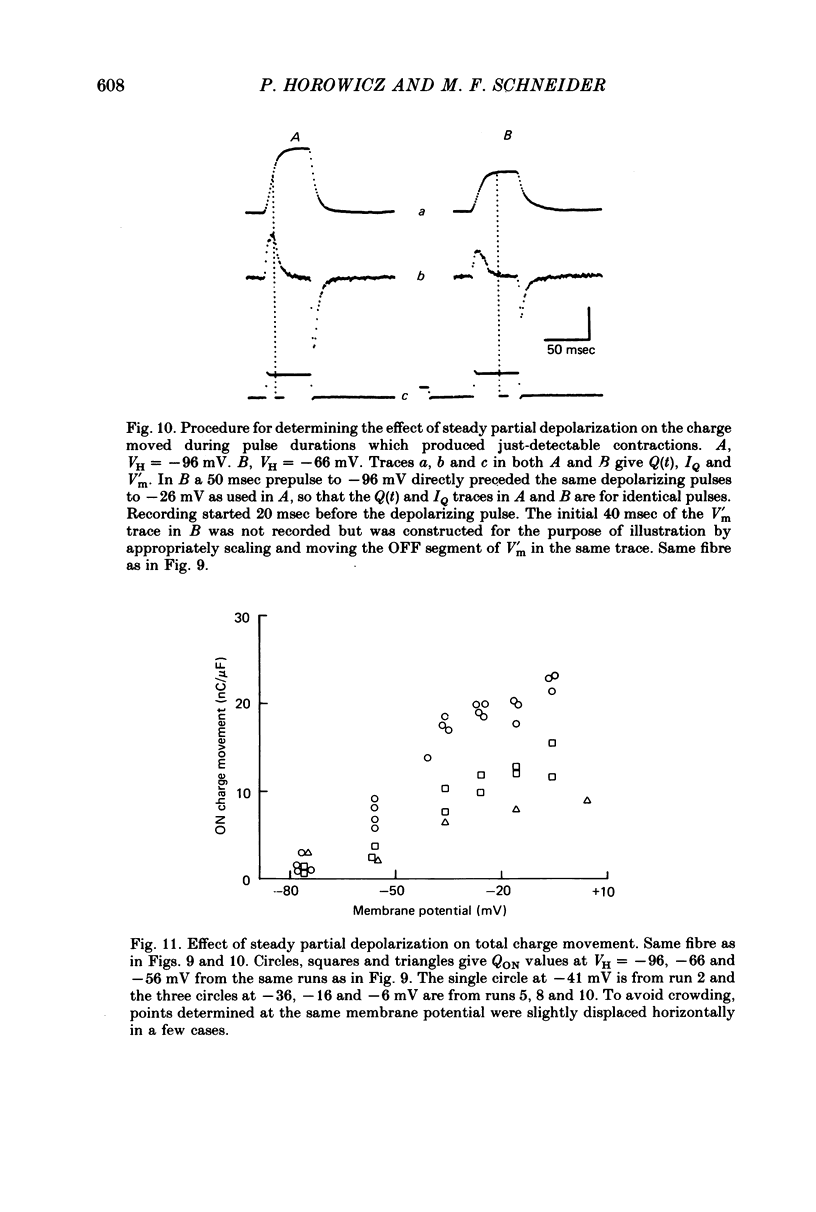
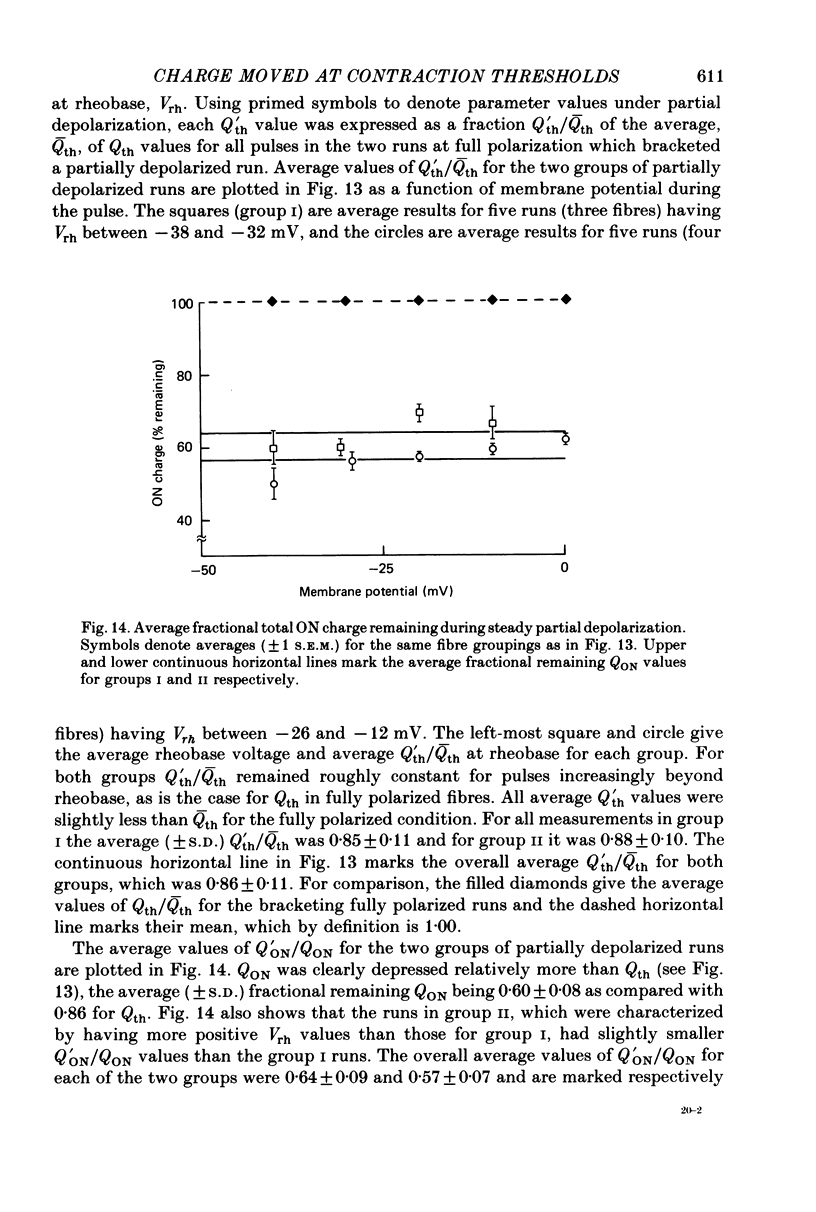
7. Steady partial depolarization decreased the total ON charge movement QON by about the same factor for pulses to all potentials tested.
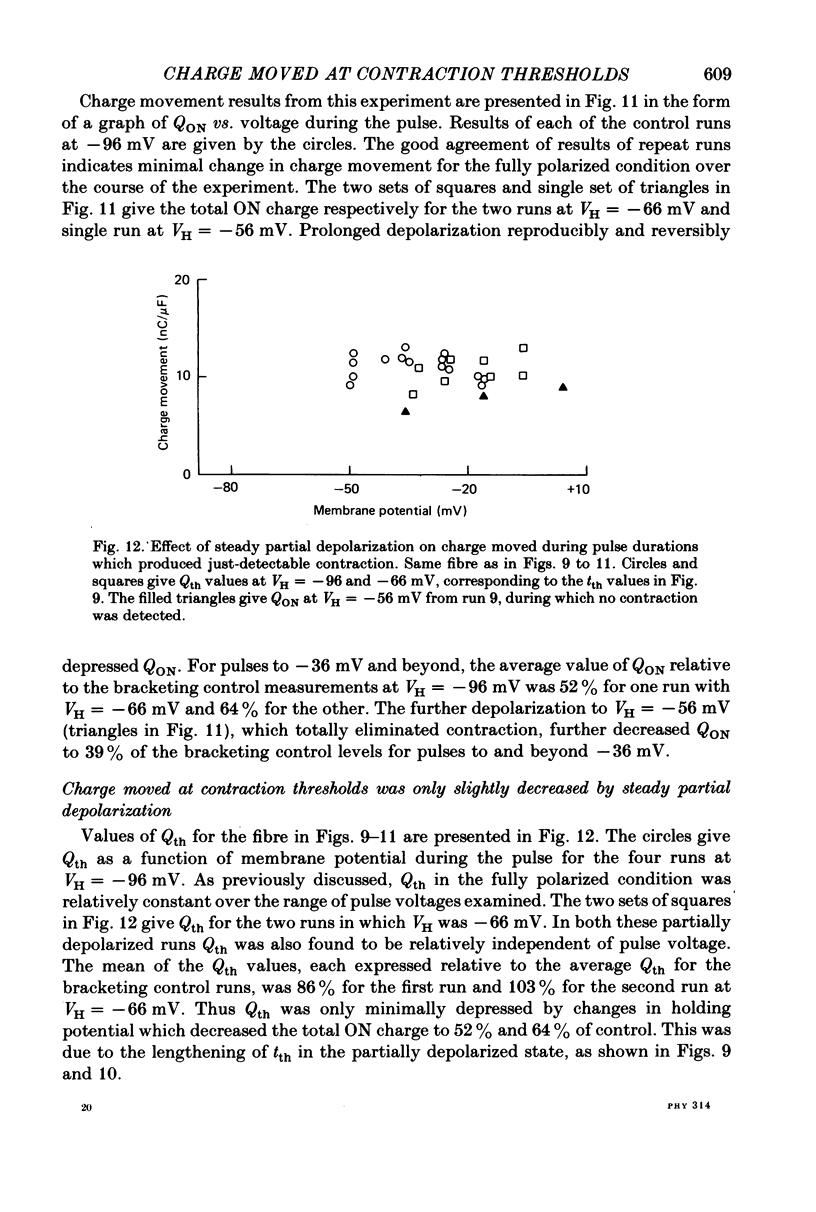
8. Qth for partially depolarized but still-contracting fibres remained approximately independent of membrane potential from rheobase to about 0 mV but was slightly less than Qth for the same fibres when fully polarized.
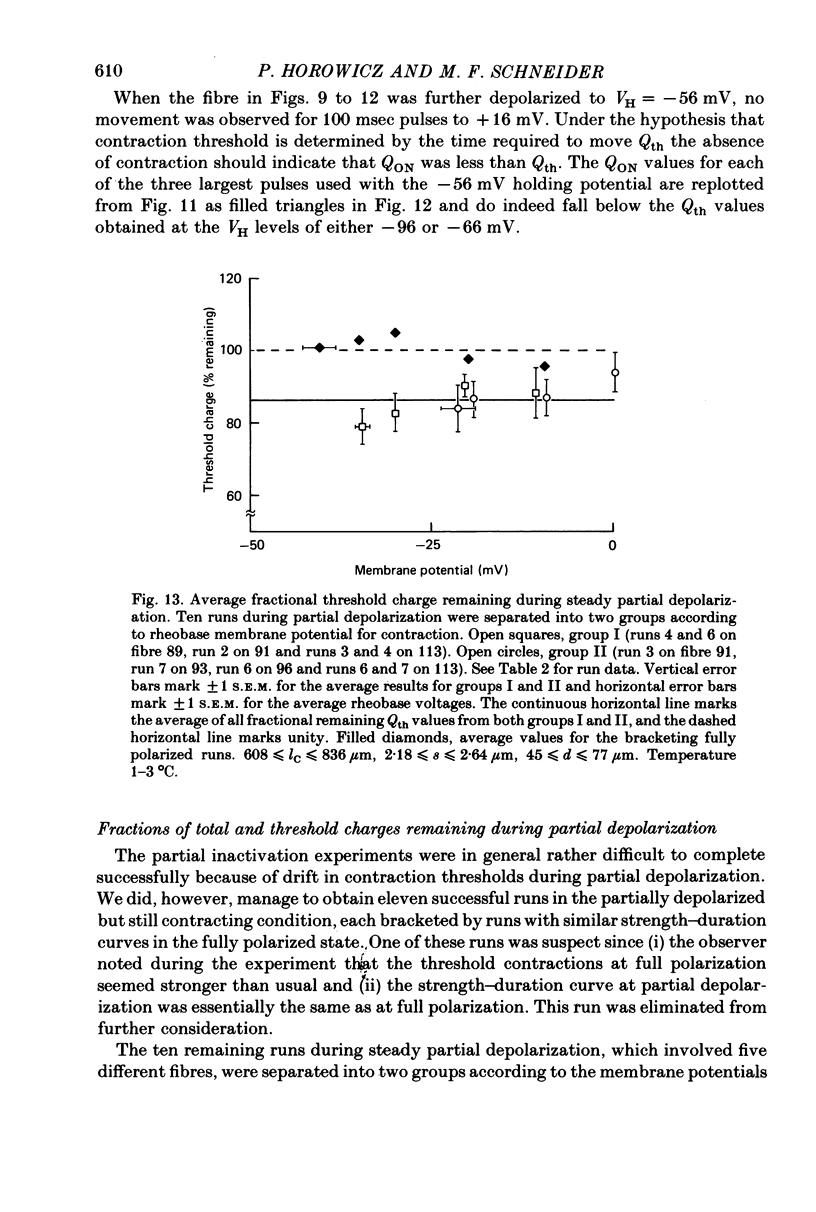
9. Steady partial depolarizations which reduced the mean (±s.d.) ON charge movement QON to 60 ± 8% of its value under full polarization reduced Qth to 86 ± 11% of its full polarization value (n = 10). These steady partial depolarizations produced no change in the linear capacitance measured with hyperpolarizing pulses.
10. Contraction was completely abolished by steady partial depolarizations which reduced QON to 41% of its value under full polarization (mean of three runs). The maximum value of QON was then 77% of the Qth value for the same fibres under full polarization.
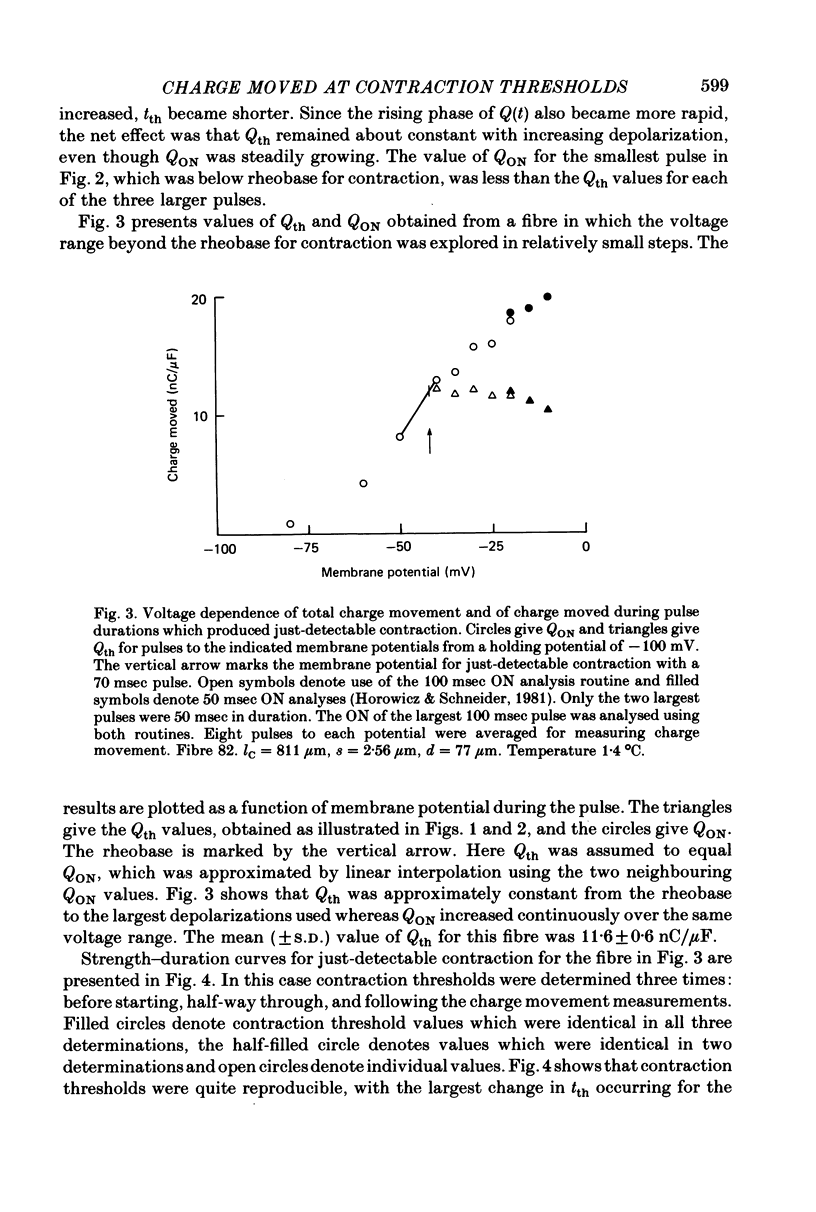
11. A prolonged tail, a shoulder, a second rising phase or an early relatively high flat segment were successively evident in the IQ records as the depolarizing pulse was successively increased to and beyond the rheobase potential for contraction. It was found that tth either coincided with or occurred slightly later than the start of such tails, shoulders or second rising phases.
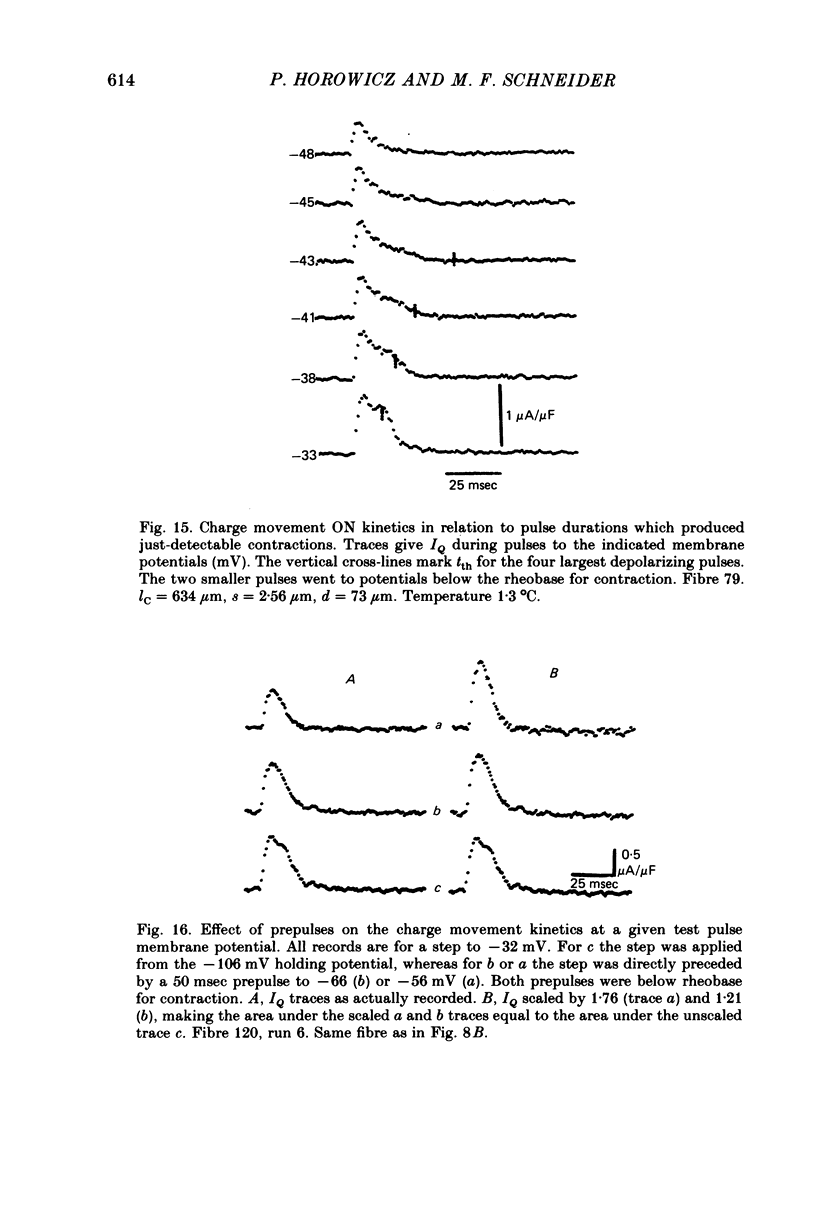
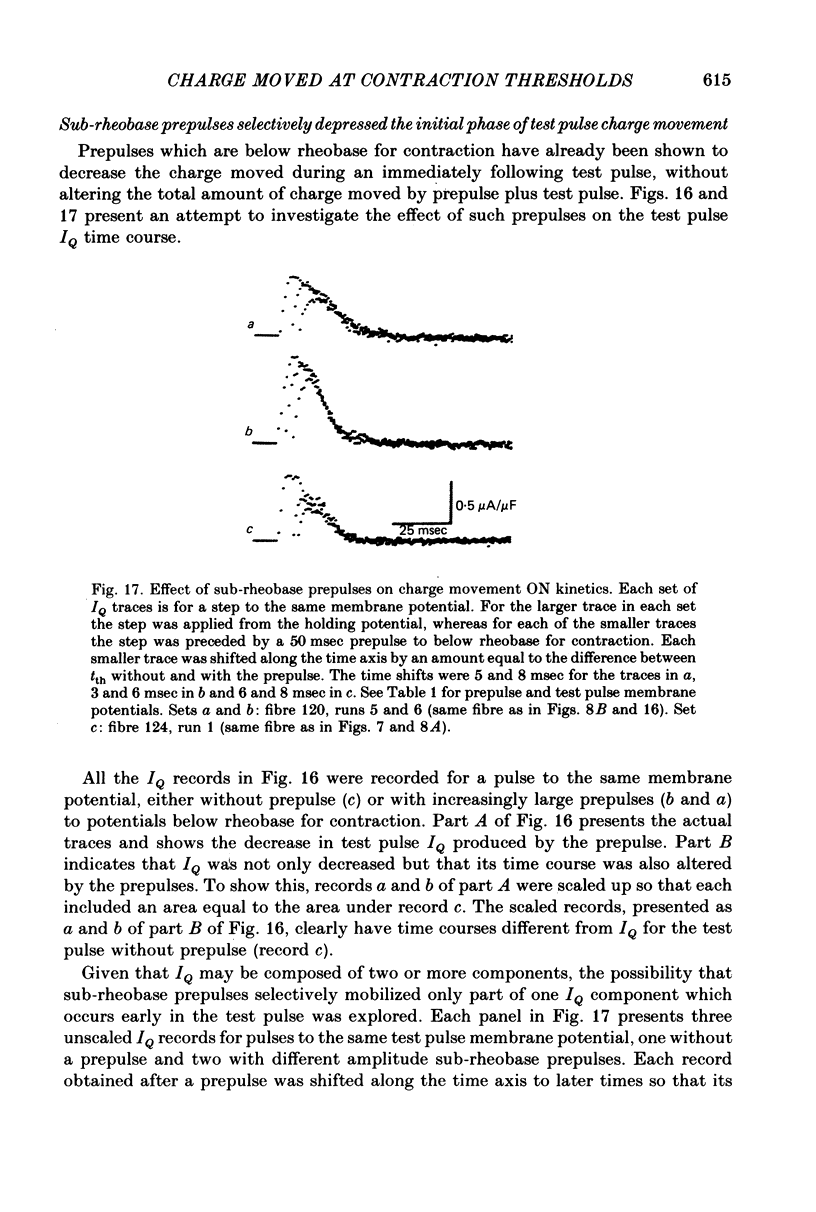
12. When test pulse IQ records with and without immediately preceding sub-rheobase prepulses were shifted in time so that their tth times coincided, the record with prepulse coincided with the later part of IQ without prepulse. This indicates that sub-rheobase prepulses moved the initial portion of the IQ that occurs during the test pulse alone, whereas they did not alter the latter portion of the test pulse IQ.
13. A model was developed which accounts for charge movement's voltage dependence and kinetics and for the relationship between charge movement and just-detectable contraction in both the fully polarized and partially depolarized states.
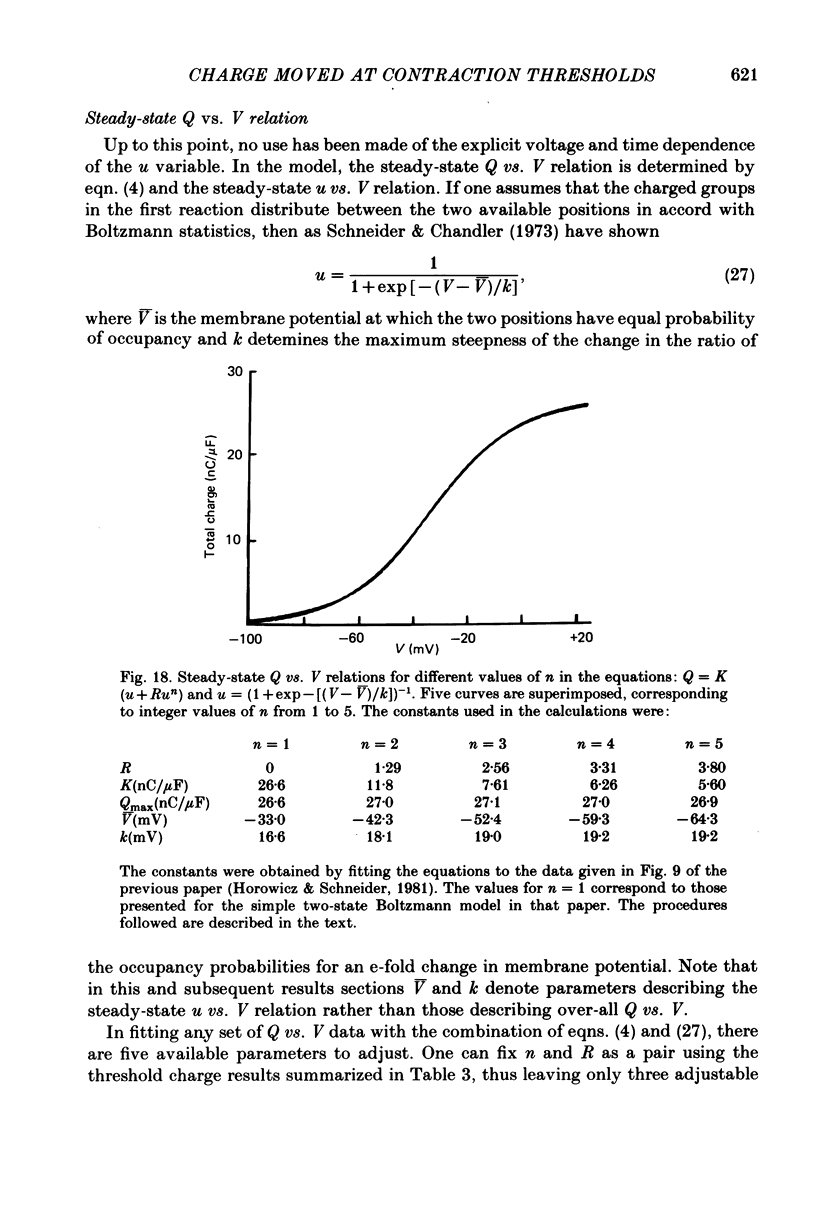
14. The model proposes that Q be composed of two components. Component A is due to the voltage and time-dependent movement of charges between two sites located within the membrane and separated by a single energy barrier. Component B is instantaneously proportional to an integer power n of the fraction of component A charges which have crossed the barrier.
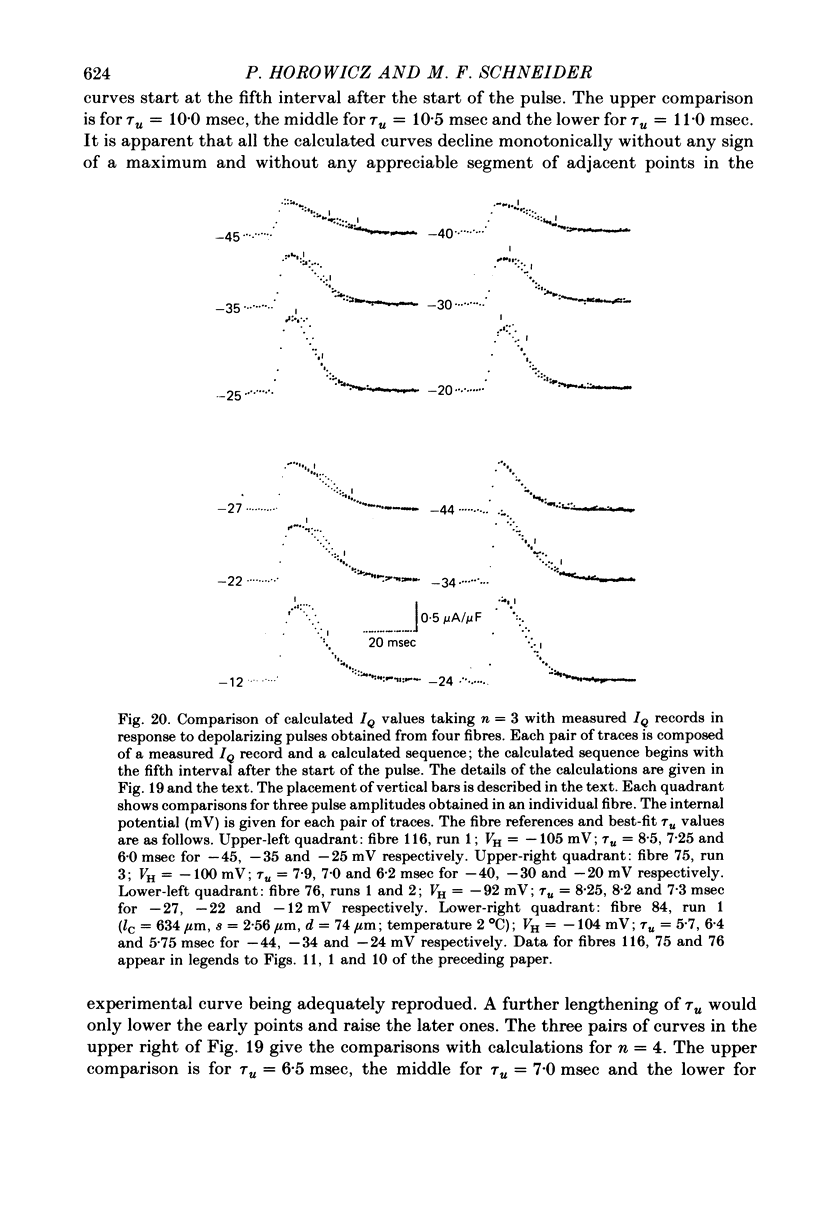
15. The IQ time courses were best approximated using n = 3, with which both the relatively early and late portions of the experimental IQ time courses could be reproduced. The best theoretical records obtained with n = 3 still passed below the shoulders, second rising phases and later parts of the early constant phases in the various experimental IQ records. Theoretical records did fit accurately the IQ time courses observed under steady partial fibre depolarization. The relatively small current not reproduced by the model may be an electrical accompaniment of the activation of calcium release or the elevation of internal free calcium levels in the space between the transverse tubules (T-tubules) and the sarcoplasmic reticulum.
Full text
PDF











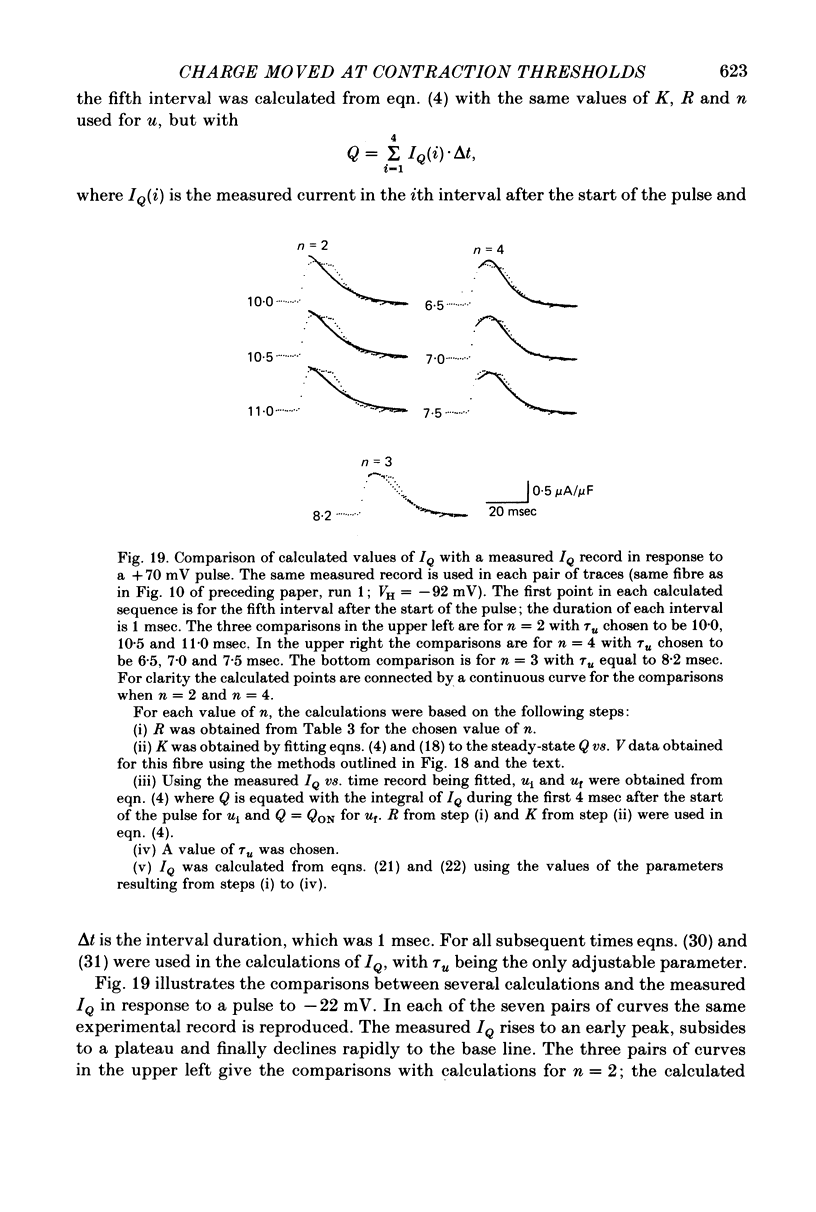






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian R. H., Almers W. Charge movement in the membrane of striated muscle. J Physiol. 1976 Jan;254(2):339–360. doi: 10.1113/jphysiol.1976.sp011235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H., Chandler W. K., Hodgkin A. L. Voltage clamp experiments in striated muscle fibres. J Physiol. 1970 Jul;208(3):607–644. doi: 10.1113/jphysiol.1970.sp009139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H., Chandler W. K., Rakowski R. F. Charge movement and mechanical repriming in skeletal muscle. J Physiol. 1976 Jan;254(2):361–388. doi: 10.1113/jphysiol.1976.sp011236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H. Charge movement in the membrane of striated muscle. Annu Rev Biophys Bioeng. 1978;7:85–112. doi: 10.1146/annurev.bb.07.060178.000505. [DOI] [PubMed] [Google Scholar]
- Adrian R. H., Peres A. R. A gating signal for the potassium channel? Nature. 1977 Jun 30;267(5614):800–804. doi: 10.1038/267800a0. [DOI] [PubMed] [Google Scholar]
- Adrian R. H., Peres A. Charge movement and membrane capacity in frog muscle. J Physiol. 1979 Apr;289:83–97. doi: 10.1113/jphysiol.1979.sp012726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almers W., Best P. M. Effects of tetracaine on displacement currents and contraction of frog skeletal muscle. J Physiol. 1976 Nov;262(3):583–611. doi: 10.1113/jphysiol.1976.sp011611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almers W. Observations on intramembrane charge movements in skeletal muscle. Philos Trans R Soc Lond B Biol Sci. 1975 Jun 10;270(908):507–513. doi: 10.1098/rstb.1975.0027. [DOI] [PubMed] [Google Scholar]
- Almers W. Some dielectric properties of muscle membrane and their possible importance for excitation-contraction coupling. Ann N Y Acad Sci. 1975 Dec 30;264:278–292. doi: 10.1111/j.1749-6632.1975.tb31489.x. [DOI] [PubMed] [Google Scholar]
- Chandler W. K., Rakowski R. F., Schneider M. F. Effects of glycerol treatment and maintained depolarization on charge movement in skeletal muscle. J Physiol. 1976 Jan;254(2):285–316. doi: 10.1113/jphysiol.1976.sp011233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. Potassium contractures in single muscle fibres. J Physiol. 1960 Sep;153:386–403. doi: 10.1113/jphysiol.1960.sp006541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowicz P., Schneider M. F. Membrane charge movement in contracting and non-contracting skeletal muscle fibres. J Physiol. 1981 May;314:565–593. doi: 10.1113/jphysiol.1981.sp013725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovács L., Ríos E., Schneider M. F. Calcium transients and intramembrane charge movement in skeletal muscle fibres. Nature. 1979 May 31;279(5712):391–396. doi: 10.1038/279391a0. [DOI] [PubMed] [Google Scholar]
- Rakowski R. F. Reprimed charge movement in skeletal muscle fibres. J Physiol. 1978 Aug;281:339–358. doi: 10.1113/jphysiol.1978.sp012426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M. F., Chandler W. K. Voltage dependent charge movement of skeletal muscle: a possible step in excitation-contraction coupling. Nature. 1973 Mar 23;242(5395):244–246. doi: 10.1038/242244a0. [DOI] [PubMed] [Google Scholar]


