Abstract
Study Objectives
To develop a practical pre-eastward flight treatment to advance circadian rhythms as much as possible but not misalign them with sleep.
Design
One group had their sleep schedule advanced by 1 hour per day and another by 2 hours per day.
Setting
Baseline at home, treatment in lab.
Participants
Young healthy adults (11 men, 15 women) between the ages of 22 and 36 years.
Interventions
Three days of a gradually advancing sleep schedule (1 or 2 hours per day) plus intermittent morning bright light (one-half hour ~5000 lux, one-half hour of < 60 lux) for 3.5 hours.
Measurements and Results
The dim light melatonin onset was assessed before and after the 3-day treatment. Subjects completed daily sleep logs and symptom questionnaires and wore wrist activity monitors.
The dim light melatonin onset advanced more in the 2-hours-per-day group than in the 1-hour-per-day group (median phase advances of 1.9 and 1.4 hours), but the difference between the means (1.8 and 1.5 hours) was not statistically significant. By the third treatment day, circadian rhythms were misaligned relative to the sleep schedule, and subjects had difficulty falling asleep in the 2-hours-per-day group, but this was not the case in the 1-hour-per-day group. Nevertheless, the 2-hours-per-day group did slightly better on the symptom questionnaires. In general, sleep disturbance and other side effects were small.
Conclusions
A gradually advancing sleep schedule with intermittent morning bright light can be used to advance circadian rhythms before eastward flight and, thus, theoretically, prevent or reduce subsequent jet lag. Given the morning light treatment used here, advancing the sleep schedule 2 hours per day is not better than advancing it 1 hour per day because it was too fast for the advance in circadian rhythms. A diagram is provided to help the traveler plan a preflight schedule.
Keywords: jet lag, circadian rhythms, bright light, sleep, phase shifts, melatonin, phase response curve, human, travel
Abbreviations: ANOVA, analysis of variance; DLMO, dim light melatonin onset; MANOVA, multiple analysis of variance; PRC, phase response curve; SOL, sleep-onset latency; SSS, Stanford Sleepiness Scale; Tmin, temperature minimum; TST, total sleep time
INTRODUCTION
RAPID TRANSMERIDIAN TRAVEL CAN PRODUCE JET LAG, WHICH IS CHARACTERIZED BY A CONSTELLATION OF SYMPTOMS, INCLUDING POOR NIGHTTIME SLEEP, INCREASED DAYTIME SLEEPINESS, DECREASED ALERTNESS, IMPAIRED PERFORMANCE, FATIGUE, IRRITABILITY, DEPRESSED MOOD, AND GASTROINTESTINAL DISTRESS.1–5 It is caused by a temporary misalignment between the endogenous circadian clock and the desired destination sleep/wake schedule. Thus, the symptoms of jet lag dissipate as the circadian clock gradually reentrains to the destination time.
The duration of jet lag depends on the number of time zones crossed and the direction of travel. Reentrainment is typically slower following eastward than westward flight,2,6 which can be explained by the fact that the free-running period (tau) of humans is slightly longer than 24 hours.7 To understand how this creates directional asymmetry, consider a person with a tau of 24.5 hours, which is the average for free-running blind people.8 If the circadian clock can phase shift by 1 hour per day, then it can assume a period of 23.5 hours (24.5 − 1.0 = 23.5), equivalent to a phase advance—relative to the 24-hour day—of 0.5 hour per day, or it can assume a period of 25.5 hours (24.5 + 1.0 = 25.5), equivalent to a phase delay of 1.5 hours per day. Thus, given these parameters, phase delays would proceed 3 times as fast as phase advances. Early estimates from actual flights are 92 minutes per day for delays (westward flights) and 57 minutes per day for advances (eastward flights).6 The rate of phase shift for an individual should depend on their tau and on the pattern of light exposure to which they are exposed (see below).
Directional asymmetry, with phase delays faster than phase advances, has been shown in more recent carefully controlled laboratory studies of the young9–11 as well as the old.12 In one of these studies of young subjects,10 the sleep/dark episode was shifted 12 hours and bright light was applied to hasten reentrainment. Given a 12-hour shift, circadian rhythms could either advance or delay to reentrain. The direction of phase shift was determined by the timing of the bright light. When the rhythms delayed, they shifted 9.6 hours in 4 days, but when they advanced, they only shifted 6.2 hours. In the study of older subjects,12 the sleep/dark episode was advanced or delayed by 6 hours, and the subjects were kept indoors and, thus, in relatively dim light. After 4 days of an advanced schedule, the rhythms had not yet begun to shift, but after 4 days of the delayed schedule, the rhythms had delayed about 3 hours. Thus, it is much more difficult to advance human circadian rhythms (as needed for an eastward flight) than to delay them, even in older people.
An additional problem with eastward flight is that circadian rhythms may phase delay instead of phase advance in order to reentrain.13–15 Such antidromic reentrainment (in the wrong direction) can be explained by the pattern of light exposure the traveler experiences after landing. For example, when landing after an eastward flight across 9 time zones, the temperature minimum (Tmin) of a typical traveler would occur at 2 pm in the new time zone (the equivalent of 5 am in the home time zone). For flights that land early in the morning (which are common), the traveler will be exposed to light (including outdoor light that is much more intense than indoor light) for several hours before the Tmin. Light before the Tmin, a common estimate for the crossover point in the human phase response curve (PRC) to light, phase delays circadian rhythms (see Figure 6 in Eastman and Martin10). A laboratory study of 9-hour phase advances and delays of sleep/dark showed that bright-light exposure at the wrong time can prevent the desired phase shift.9 Travelers often develop misconceptions about which procedures help them overcome jet lag. It is possible that the time of landing combined with the light exposure during the first few days after landing can explain most of the differences commonly attributed to other factors (such as diet or the amount of sleep obtained during the flight). Daan and Lewy16 first proposed scheduling exposure to, and avoidance of, daylight at specific times after landing in order to hasten reentrainment and reduce jet lag.
Figure 6.
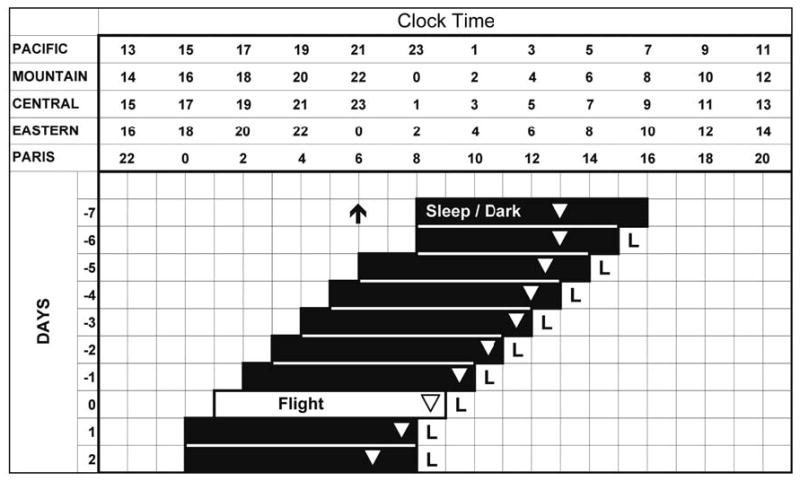
Sleep and light schedule to be used to advance circadian rhythms before eastward flight and thus reduce jet lag. The time axes show the 4 United States time zones and 1 European time zone. Day -7 shows a typical sleep schedule for someone living in the Pacific time zone, and the arrow and triangle show the typical time of the dim light melatonin onset (DLMO) and minimum temperature (Tmin) for that schedule. The Ls show the time for intermittent bright-light exposure (2–3 hours each morning). Wake time is advanced 1 hour per day. Fewer days of the advancing schedule are needed if the traveler starts out in a more-eastern time zone, or has an earlier sleep schedule, or wants to adopt a later sleep schedule in Europe. With this schedule, the traveler arrives in Europe already phase shifted to the new time zone. Even if only part of the schedule is done pre-flight, the time required for reentrainment after landing and the possibility of antidromic reentrainment will be reduced.
Several publications, commercial computer programs, and devices are available to tell the traveler when to seek and when to avoid outdoor light in the days after landing in order to accelerate the entrainment of the circadian clock to the new time zone.17–19 They are based on the light PRC and prescribe outdoor light to coincide with the appropriate portion of the PRC and prescribe avoiding bright light that would coincide with the opposite portion of the PRC.20 However, they differ in (1) how they estimate the crossover point between delays and advances in the light PRC (eg, a set clock time, such as between 4 and 5 am on home time18 or a relative time such as 3 hours before habitual wake up time17); (2) how many hours per day they expect the circadian clock to advance or delay and therefore by how many hours per day to change the timing of light (eg, 3 hours per day for both advances and delays17 and 1.5 hours per day for advances and 2.0 hours per day for delays19); and (3) the point at which it is better to delay, rather than advance, the clock to adjust after an eastward flight, ie, the “critical shift”21 (eg, 8 or more time zones,19 10 or more18). This third item depends on the estimate of directional asymmetry given the suggested light exposure/avoidance pattern. For example, if you expect rhythms to delay 2 hours per day and advance 1 hour per day, then it will take the same time to adjust to an 8-hour eastward flight by delaying 16 hours as by advancing 8 hours (8 days in both cases). Aside from these uncertainties about how to schedule light exposure and light avoidance after landing, there is the problem of weather interfering with the desired light-dark pattern. Portable light devices and dark sunglasses have been tested, so far with little success.22,23
We have been exploring a different approach: phase advancing circadian rhythms at home before an eastward flight by gradually advancing the sleep schedule each day and exposing subjects to bright light immediately after waking. Arriving in the new time zone with circadian rhythms already somewhat phase advanced will reduce the risk of antidromic reentrainment. A large enough preflight advance could ensure that all postflight outdoor light exposure serves to facilitate rather than inhibit the phase advance. Theoretically, a large enough phase advance before flight, close to the number of time zones to be crossed, can completely eliminate jet lag; the traveler would arrive with circadian rhythms already set to the new time zone. Obviously, the greater the phase advance accomplished before flight, the less will be needed after landing. The goal of the preflight schedule is to advance the rhythms as much as possible, while keeping them reasonably aligned with the advancing sleep schedule. Otherwise, “jet lag” would be produced before the flight, which would defeat the purpose.
In our previous study,24 we measured the phase advance produced by 3 days of an advancing sleep schedule (advancing wake 1 hour per day). Subjects sat at a desk in front of a light box for 3.5 hours after waking each morning. There were 3 conditions: continuous bright light (~5000 lux), intermittent bright light (one-half hour ~5000 lux, one-half hour of < 60 lux), and dim indoor room light (< 60 lux). They produced average phase shifts of 2.1, 1.5, and 0.6 hours, respectively. Both the continuous and intermittent groups advanced significantly more than the dim light group but were not significantly different from each other. In addition, sleep measures from actigraphy and sleep logs and subjective jet-lag symptoms remained fairly constant throughout the treatments (ie, side effects were minimal).
The aim of the present study was to create a larger phase advance with a similar protocol, by increasing the daily advance of wake time from 1 hour per day to 2 hours per day. Since the intermittent morning-light condition from our previous study produced a comparable phase shift to continuous light and is more practical (and more like what actual travelers could do), we used intermittent light.
METHODS
Subjects
Fifteen healthy young adults participated in the 2-hours-per-day protocol and were compared to 11 healthy young adults who participated in the 1-hour-per-day protocol in the previous study.24 We matched the subjects in the 2-hours-per-day group to the subjects from the 1-hour-per-day group on prestudy sleep times, sex, age, and scores on the Morningness-Eveningness Questionnaire.25 The subjects in the 2-hours-per-day protocol (6 men, 9 women) were between the ages of 22 and 35 years (mean ± SD = 26.5 ± 3.7 years) and had scores on the Morningness-Eveningness Questionnaire of 52.1 ± 8.1; the subjects in the 1-hour-per-day group (5 men, 6 women) were between the ages of 22 and 36 years (26.7 ± 4.0 years) and had scores on the Morningness-Eveningness Questionnaire of 53.0 ± 8.4.
All subjects had normal body mass indexes (mean = 23.6 ± 3.5 kg/m2), were nonsmokers, did not consume large caffeine doses (> 500 mg/day), and reported no medical, psychiatric, or sleep disorders as assessed from a telephone interview, in-person interview, medical history, and several screening questionnaires (Minnesota Multiphasic Personality Inventory-2, Pittsburgh Sleep Quality Index,26 and part of a general health questionnaire27). Subjects were free from prescription medications, except for 5 women who were taking oral contraceptives. We excluded individuals who had worked a night shift during the 2 months prior to the start of the study or had traveled overseas in the month prior to the start of the study. The Rush University Medical Center Institutional Review Board approved the protocol, and subjects gave written informed consent prior to participation. Subjects were paid for their participation.
Protocol
This was a between-subjects design with 2 groups differing only in the amount of daily wake-time advance during the 3 treatment days: 2 hours per day versus 1 hour per day. Figure 1 shows a condensed version of the protocols normalized to a 7:00 am baseline wake time. For a more complete protocol diagram, see Figure 1 in Burgess et al.24 Each subject adhered to a strict baseline sleep schedule that matched to within 1 hour their self-reported typical sleep onset and wake times, as confirmed with sleep logs, completed for at least a week before the start of the study. The fixed sleep schedule was designed to stabilize circadian phase and ensure that the subjects were not sleep deprived before the treatment began. The earliest scheduled baseline bedtime (lights out) was 10:00 pm and the latest was 1:00 am. The earliest scheduled wake time was 6:00 am and the latest was 8:00 \. The time allotted for sleep was between 7 and 8 hours. Subjects with matching sleep schedules were run in pairs.
Figure 1.
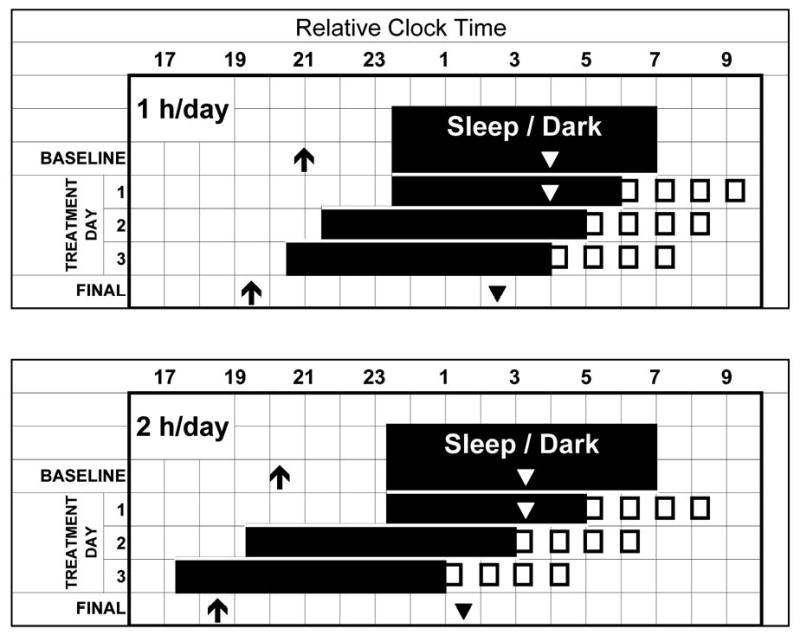
Protocol for 1-hour-per-day (top) and 2-hours-per-day (bottom) groups. All elements are normalized to a 7:00 am wake time. Arrows represent the time of the average dim light melatonin onsets (DLMO) determined from the baseline and final phase assessments. Triangles represent the estimated times of the temperature minima (7 hours after the DLMO). The first 2 rows represent the average sleep/dark times during baseline. There was no sleep/dark episode during the phase assessments; the arrow and triangle from the baseline phase assessment (which was conducted a few days earlier) are drawn on the last baseline day to illustrate the phase relationships between these circadian phase markers and sleep. Open boxes represent the time of the 0.5-hour bright-light pulses each morning.
Subjects slept at home according to their assigned baseline sleep schedules for the first 6 days of the study. On day 7, they underwent the baseline phase assessment beginning 5 to 6 hours before and ending 3 hours after their scheduled baseline bedtimes. Subjects then slept at the lab and were awakened at their baseline wake times. On days 8 to 10, subjects returned to sleeping at home according to their baseline sleep schedules. Days 11 to 13 were the 3 treatment days. On day 14, subjects underwent the final phase assessment beginning 9 hours before and ending 3 hours after their scheduled baseline bedtimes for the 1-hour-per-day group and beginning 12 hours before and ending at their scheduled baseline bedtimes for the 2-hours-per-day group.
On treatment day 1, time in bed was reduced by 1 hour in the 1-hour-per-day group and 2 hours in the 2-hours-per-day group because wake time but not bedtime was earlier. Time in bed returned to baseline durations on treatment days 2 and 3. Napping was permitted during the 10 days of baseline but only during a 6-hour period centered 12 hours from the midpoint of the nocturnal sleep episodes. Sleep (dark) at this time should not phase shift circadian rhythms because neither afternoon sleep/dark episodes28 nor afternoon bright-light episodes29 shift rhythms.
Phase-Advancing Treatment
On the 3 treatment days, subjects entered the lab at least 2 hours before bedtime. They slept in individual, dark, temperature-controlled bedrooms. They received intermittent bright-light exposure in the morning: four 0.5-hour pulses separated by 0.5 hours in ordinary room light. The bright light was produced by a single light box (54 × 54 cm screen size, Enviro-Med, Vancouver, Wash) placed on a desk, about 40 cm in front of the subjects’ eyes. Each light box had a diffuser screen and contained four 54-cm long, 40-watt fluorescent horizontal lamps (Philips PL-L 40 W/41/RS/IS, 4100 K). Subjects were permitted to eat and read during the light sessions. The light intensity was 3030 to 9680 lux depending on the angle of gaze (mean intensity 5075 ± 1075 lux, as measured periodically with an Extech 401025 Light Meter, Waltham, Mass). We measured the irradiance after the study with an International Light Model IL1400 (Radiometer, Newburyport, Mass) and SEL – 033/F/W detector placed at the typical distance from the light box (40 cm) in the typical angle of gaze. The irradiance (400–1064 nm) was about 2100 μW/cm2. Using a SCS490 sharp-cut filter, we calculated that the irradiance from the blue part of the spectrum (400 to 490 nm) was 580 μW/cm2. Each bedroom had 1 ceiling light fixture containing 3 cool-white fluorescent lamps, which was controlled by a dimmer switch. The ambient room light in between the light pulses was produced by this ceiling fixture and was less than 60 lux.
Phase Assessments
During the phase assessments, subjects remained awake in dim light (< 10 lux), as verified at the level of the subjects’ eyes and in the direction of gaze, with a Minolta TL-1 light meter (Ramsey, NJ). The subjects sat in recliners and only stood up for trips to the nearby washroom. The washroom and hall to the washroom were also dimly lit (< 10 lux). Washroom trips were discouraged during the 10 minutes before each saliva sample. Subjects gave a saliva sample every 30 minutes using Salivettes (Sarstedt, Newton, NC). To avoid contamination of the samples, caffeine, chocolate, bananas, and lipstick were not allowed in the 5 hours before or during the phase assessments. Toothpaste and mouthwash were not allowed during the phase assessments. Small snacks and fluids were permitted, except in the 10 minutes before each sample, and if subjects consumed food or drink, they were required to rinse and brush their teeth with water (while sitting) 10 minutes before each sample. Saliva samples were centrifuged immediately after collection and frozen. The samples were later shipped to Pharmasan Labs (Osceola, Wisc) in dry ice to be radioimmunoassayed for melatonin. All samples from an individual subject were assayed in the same batch. The sensitivity of the assay was 0.7 pg/mL, and intraassay and interassay coefficients of variabilities were 12.1% and 13.2%, respectively.
Measures of Sleep and Other Procedures
Bedtimes, sleep-onset times, wake times of greater than 5 minutes during sleep, final morning awakening times, and naps were reported by subjects using daily sleep logs. Subjects wore actigraphy monitors (Actiwatch 64, Mini-Mitter, Bend, Ore) on their nondominant wrists, which allowed us to estimate sleep parameters and monitor compliance. Subjects also wore a photosensor around their necks as a medallion (Actiwatch-L, Mini-Mitter). During their sleep/dark episodes, subjects were instructed to lie in bed in the dark even if they could not sleep. Subjects were required to call the lab voice mail (time and date of call was recorded) each day before turning out their lights at night and when they woke in the morning. Every 1 to 3 days, subjects came to the lab where the data from their wrist actigraph, photosensor, and sleep logs were examined and compared in their presence. This procedure helped us train subjects to keep accurate sleep logs. Eighteen subjects began the 2-hours-per-day protocol but 3 did not complete it: 1 for noncompliance, 1 due to poor baseline sleep, and 1 because of a family emergency.
Subjects were only permitted to consume caffeine (up to 300 mg) in the first 3 hours after their wake time and up to 2 standard drinks of alcohol per day on days 1 to 4, after which alcohol was not permitted. Subjects were Breathalyzed® on nights they came to the lab. Nonsteroidal anti-inflammatory drugs were permitted on days 1 to 3 but thereafter were not allowed, as these drugs suppress melatonin.30
Symptom Questionnaires
Subjects completed symptom questionnaires every day of the study. The “How Are You Feeling Right Now?” questionnaire was completed 5 times per day (prompted by timers): about 15 minutes after scheduled wake time, 4 hours after wake time, in the middle of the waking episode, 4 hours before bedtime, and about 15 minutes before bedtime. This questionnaire consisted of the Stanford Sleepiness Scale (SSS),31 where 1 of 7 descriptors is circled from (1) Feeling active and vital; alert; wide awake to (7) Almost in reverie; sleep onset soon; must struggle to remain awake. There were also 6 questions relating to physical fatigue, mental fatigue, sadness, anxiety, irritability, and gastrointestinal problems. Subjects reported their symptoms by circling a number from 1 “very little” to 10 “very much.”
Subjects also completed the Columbia Jet Lag Scale 32 just before bedtime. It consists of 9 items relating to sleepiness, fatigue, daytime alertness, concentration, lethargy, light-headed-ness, weakness, clumsiness, and memory. Subjects indicated how much these symptoms bothered them during that day by circling a number from 0 “not at all” to 4 “extremely.” The items on each day were summed to yield an overall jet-lag score, with a possible range of 0 to 36. Due to a mistake in our instructions to research assistants, the treatment day 3 Columbia Jet Lag Scale questionnaire for the 2-hours-per-day subjects was administered at the end of the final phase assessment, at the time of baseline bedtime, instead of about 8 hours earlier, 2 hours before the previous day’s questionnaire. The treatment day 3 questionnaire for the 1-hour-per-day subjects was administered 1 hour earlier than the previous day’s questionnaire, as planned.
Data Analysis
Circadian Phase
The threshold used to determine the dim light melatonin onset (DLMO) was based on the Kennaway method.33 The threshold was equal to the mean + 2 SDs of the first 5 values from the final phase assessment, which were low daytime values. The DLMO was the point in time when the melatonin concentration exceeded and remained above the threshold for at least 2 points and was determined with linear interpolation. For each subject, the same threshold was used for both phase assessments. We added 7 hours to each DLMO to estimate the Tmin, based on the results of several studies.34–37
Sleep Parameters
Sleep parameters from the last 5 days before the baseline phase assessment were averaged to produce baseline values and were compared to treatment days 1, 2, and 3. Total sleep time (TST) on each day was the sleep time during the nighttime sleep episode, not including naps.
Sleep times were estimated from wrist monitors using the Actiware-Sleep 3.1 analysis program (Mini-Mitter). We analyzed the time from 1 hour before to 1 hour after the subjects’ self-reported bed and wake times. The analysis program was set to highest sensitivity for detecting movement, which may underestimate sleep as any movement during sleep is considered wake. The program provides objective estimates of TST, sleep onset latency (SOL), and sleep efficiency (equal to TST/time in bed).
We also determined TST, SOL, and sleep efficiency from the sleep logs. TST equaled sleep duration minus awakenings of > 5 minutes. SOL was the time between bedtime, recorded each night, and sleep-onset time, estimated the next morning.
Questionnaires
Each item on the 5 “How Are You Feeling Right Now” questionnaires completed each day was averaged into 1 value per day. For this questionnaire and for the Columbia Jet Lag Scale, the 5 baseline days were averaged together so they could be compared to each of the 3 treatment days. For this analysis, we used the last 5 complete baseline days that did not include hours spent in the baseline phase assessment. In our previous analysis,24 we used the last 5 baseline days in which the last day extended into the baseline phase assessment.
Statistical Analysis
The phase advance in the DLMO (from baseline to final phase assessment) in the 2 groups (1-hour-per-day and 2-hours-per-day) was compared with a t test. The sleep parameters (from wrist actigraphy and sleep logs) were analyzed with a 2-way multivariate analyses of variance (MANOVA) with within-subjects factor SECTION OF STUDY (baseline, treatment days 1, 2, and 3) and 1 between-subjects factor GROUP (1-hour-per-day and 2-hours-per-day). When significant, Greenhouse-Geisser corrected degrees of freedom were used for univariate analyses, although the original degrees of freedom are reported. Similarly, the SSS and Columbia Jet Lag score were each analyzed with 2-way analyses of variance (ANOVA) (SECTION OF STUDY × GROUP), and the remaining 6 How Are You Feeling Right Now items were analyzed with a 2-way MANOVA (SECTION OF STUDY × GROUP). Any significant interaction in these ANOVA indicates that changes in the variable of interest from baseline to treatment days 1, 2, and 3 were different between the 2 groups. A significant interaction was explored with Winer simple main effects analyses to compare the groups during each section of the study. Data are presented as means ± SDs unless otherwise specified.
RESULTS
Circadian Phase
Figure 2 and Table 1 (top row) show that the average phase advance in the 2-hours-per-day group was slightly larger than in the 1-hour-per-day group, but this difference was not statistically significant. Figure 3 shows the phase advances of the individual subjects. The median phase advance was 1.4 hours in the 1-hour-per-day group and 1.9 hours in the 2-hours-per-day group. By the final phase assessment, the mean Tmin had advanced to 4.5 hours before the baseline wake time in the 1-hour-per-day group and 5.5 hours before baseline wake in the 2-hours-per-day group (Figure 1).
Figure 2.
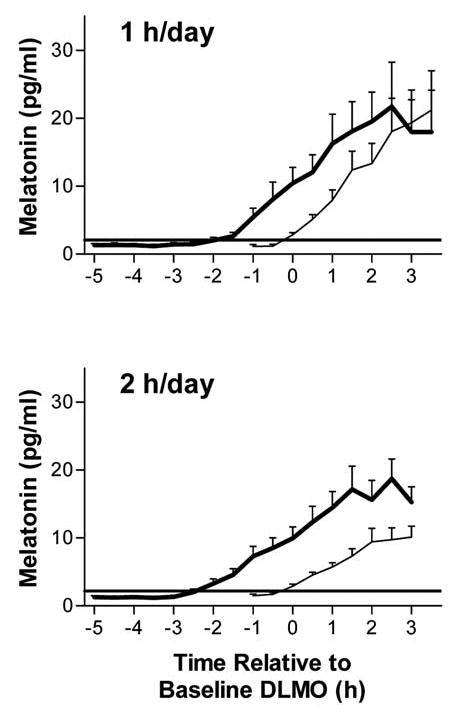
Melatonin profiles for the 1-hour-per-day (n = 11) and 2-hours-per-day (n = 15) groups. Error bars represent SEM. In each graph, the lighter line shows the mean profile during the baseline phase assessment, and the bold line shows the profile during the final phase assessment. Horizontal lines indicate the average dim light melatonin onset (DLMO) threshold for each group. The mean melatonin profiles were constructed referencing each subject’s data to the time of his or her DLMO from the baseline phase assessment.
Table 1.
Circadian Phase and Scheduled Sleep Times
| Phase-Advance Group | ||||
|---|---|---|---|---|
| 1 hour per day | 2 hours per day | |||
| Mean | SD (hours) | Mean | SD (hours) | |
| Phase advance of DLMO, hours | 1.5 | 0.8 | 1.8 | 0.5 |
| Baseline | ||||
| DLMO | 8:37 pm | 1.1 | 8:03 pm | 1.4 |
| Bedtime | 11:11 pm | 1.0 | 11:08 pm | 0.8 |
| Wake time | 6:38 am | 0.8 | 6:44 am | 0.7 |
| Estimated Tmin* | 3:37 am | 1.1 | 3:03 am | 1.4 |
| Tmin to wake interval, hours | 3.0 | 1.2 | 3.7 | 1.2 |
| Treatment day 1 | ||||
| Tmin to wake interval, hours | 2.0 | 1.2 | 1.7 | 1.2 |
| Treatment day 3 | ||||
| Bedtime | 8:11 pm | 1.0 | 5:08 pm | 0.8 |
| Wake time | 3:38 am | 0.8 | 00:44 am | 0.7 |
| Estimated Tmin† | 2:36 am | 0.9 | 1:51 am | 1.7 |
| Tmin to wake interval, hours | 1.0 | 1.1 | −1.1‡ | 1.4 |
| Final | ||||
| DLMO | 7:06 pm | 0.9 | 6:15 pm | 1.7 |
| Estimated Tmin* | 2:06 am | 0.9 | 1:15 am | 1.7 |
Calculated by adding 7 hours to the time of the dim light melatonin onset (DLMO).
Calculated with linear interpolation between the baseline and final temperature minimum (Tmin).
Negative number indicates that the estimated Tmin occurred after wake up time.
Figure 3.
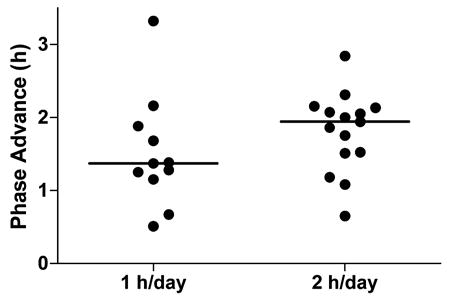
Phase advances in dim light melatonin onset for all subjects. Horizontal lines represent the medians.
As planned by matching the 2 groups, there were no differences in sleep schedules between the groups at baseline (Table 1). The scheduled time in bed was 7.5 ± 0.5 hours for the 1-hour-per-day group and 7.6 ± 0.5 hours for the 2-hours-per-day group. There was also no difference in baseline circadian phase (DLMO) between the groups (Table 1). The interval between the baseline Tmin and baseline wake was slightly longer in the 2-hours-per-day group (Table 1 and Figure 1), but this difference was not statistically significant. If the baseline Tmin to wake intervals were exactly the same in both groups, then the interval between the Tmin and the start of the bright light on treatment day 1 would be exactly 1 hour shorter in the 2-hours-per-day group because these subjects were awakened 1 hour earlier than the other subjects for their bright-light treatment. However, because of the slight difference in the baseline Tmin to wake intervals, the Tmin to wake interval on treatment day 1 was only 0.3 hours shorter in the 2-hours-per-day group (Table 1). Thus, bright-light treatment started at similar circadian phases in both groups. We estimated the time of the DLMO and the Tmin on each treatment day by calculating a linear advance from treatment day 1 to the final phase assessment (eg, from the triangle on treatment day 1 to the triangle on the final phase assessment day in Figure 1). The bright light on treatment day 3 started 1 hour after the Tmin for the 1-hour-per-day group but about 1 hour before the Tmin for the 2-hours-per-day group (treatment day 3, Tmin to wake interval, Table 1).
Sleep
The sleep-parameters MANOVA showed a statistically significant section-of-study by group interaction (F21,204 = 6.50, P < .001). TST from sleep logs (Figure 4, top panel) in both groups was less during treatment day 1, when time in bed was truncated by the protocol, but then returned to close to baseline levels. There was a significant main effect of section of study (F3,72 = 161.29, P < .001) and a significant section-of-study by group interaction (F3,72 = 13.01, P < .001) but no main effect of group. The simple main-effects analysis showed that TST was significantly greater for the 1-hour-per-day group on treatment days 1 and 3 (F1,72 = 47.11, P < .001; F1,72 = 6.58, P < .05, respectively). TST from actigraphy followed a similar pattern to the sleep-log data, except all the means were about 1 hour less (recall the program was set to the highest sensitivity), and, on treatment day 3, the means for both groups were similar to baseline. There was a significant main effect of section of study (F3,72 = 77.46, P < .001) and a significant section-of-study by group interaction (F3,72 = 8.30, P < .001) but no main effect of group. The simple main-effects analysis for actigraphy showed that TST was significantly greater for the 1-hour-per-day group on treatment day 1 (F1,72 = 22.48, P < .001).
Figure 4.
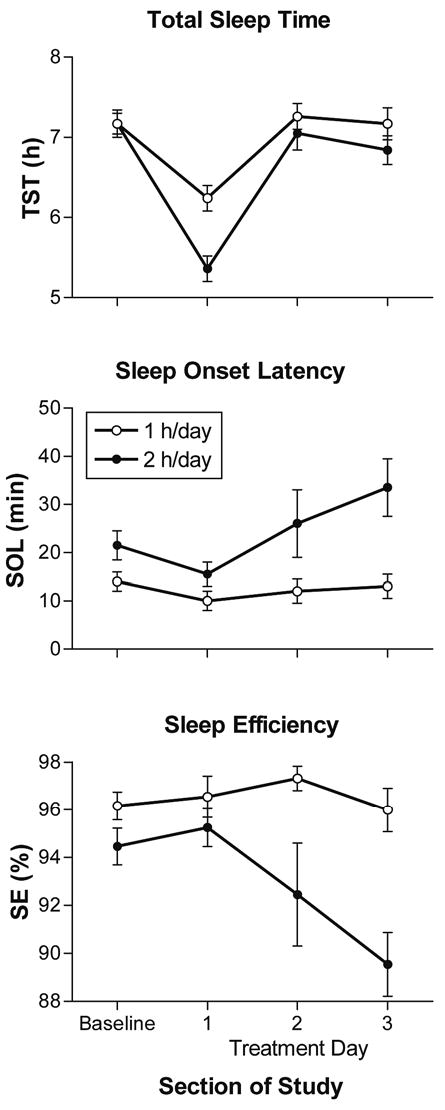
Sleep parameters from sleep logs during baseline (days 2–6) and treatment days for the 1-hour-per-day (open circles) and 2-hours-per-day (closed circles) groups. Values are mean ± SEM.
SOL from sleep logs (Figure 4, middle panel) remained similar to baseline in the 1-hour-per-day group but increased slightly on treatment days 2 and 3 in the 2-hours-per-day group. There was a significant main effect of group (F1,24 = 7.27, P < .05) and a nonsignificant trend in section of study (F3,72 = 2.98, P = .056) but no interaction. SOL from actigraphy showed a similar pattern, with the largest differences between the groups on treatment days 2 and 3, but there were no significant main effects of section of study or group, and there was no interaction.
Sleep efficiency from sleep logs (Figure 4, bottom panel) remained similar to baseline in the 1-hour-per-day group but decreased a little during treatment days 2 and 3 in the 2-hours-per-day group. There was a significant main effect of group (F1,24 = 9.64, P < .01) and of section of study (F3,72 = 3.69, P < .05), as well as a marginally significant section-of-study by group inter-action (F3,72 = 3.07, P = .053). The simple main-effects analysis showed that sleep efficiency was significantly greater for the 1-hour-per-day group on treatment days 2 and 3 (F1,72 = 8.26, P < .01; F1,72 = 14.48, P < .001, respectively). Sleep efficiency from actigraphy remained close to baseline in the 1-hour-per-day group. In the 2-hours-per-day group, it increased slightly on treatment day 1 and decreased thereafter reaching the baseline level on treatment day 3. However, there were no significant main effects of section of study or group, and there was no interaction.
Symptom Questionnaires
Figure 5 shows that the SSS score in the 1-hour-per-day group increased slightly from baseline through treatment day 2 and remained constant on treatment day 3. In the 2-hours-per-day group, it remained close to baseline throughout the treatment. There was a significant main effect of section of study (F3,72 = 5.38, P = .006) and a significant section-of-study by group interaction (F3,72 = 3.50, P = .032) but no significant main effect of group. The simple main-effects analysis showed that sleepiness was significantly greater for the 1-hour-per-day group on treatment days 2 and 3 (F1,72 = 7.57, P < .01; F1,72 = 10.43, P < .01, respectively). In general, subjects’ ratings on the additional 6 items from the “How Are You Feeling Right Now?” questionnaire showed little change from baseline through the treatment days. No mean scores were above 4 (the scales were from 1 to 10), and the MANOVA was not significant.
Figure 5.
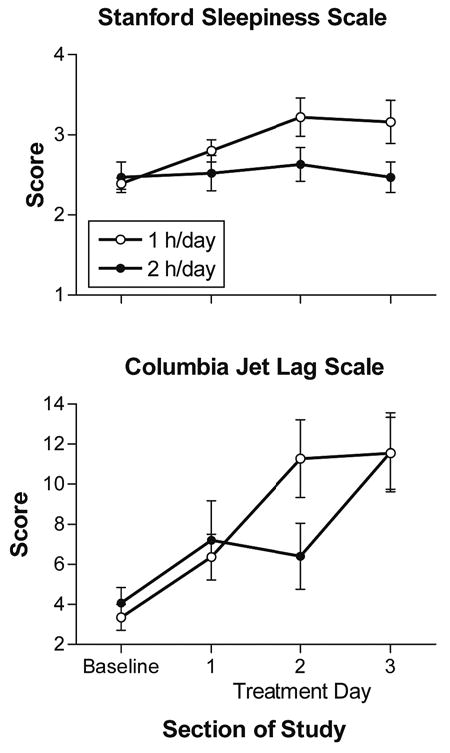
Stanford Sleepiness Scale and Columbia Jet Lag scale scores for the 1-hour-per-day (open circles) and 2-hours-per-day (closed circles) groups. Values are mean ± SEM.
Figure 5 shows that the Columbia Jet Lag score in the 1-hour-per-day group increased gradually from baseline through the 3 treatment days. In the 2-hours-per-day group, it declined slightly on treatment day 2 and then increased on treatment day 3. There was a significant main effect of section of study (F3,72 = 18.44, P < .001) and a significant section-of-study by group interaction (F3,72 = 3.12, P = .037) but no significant main effect of group. The simple main-effects analysis showed that the score for the 1-hour-per-day group was significantly greater on treatment day 2 (F1,72 = 8.97, P < .01).
DISCUSSION
We tested schedules of gradually advancing sleep/dark and morning intermittent bright light designed to phase advance circadian rhythms before an eastward flight and thus reduce jet lag. The protocol in which wake time was advanced 2 hours per day produced a slightly larger phase advance than the 1 in which wake was advanced 1 hour per day (medians of 1.9 vs 1.4 hours and means of 1.8 vs 1.5 hours), but the mean difference was not statistically significant. One reason that the advance was not much larger in the 2-hours-per-day group could be that the first morning light treatment began at similar circadian phases in both groups. It is possible that a slightly larger phase advance would have been produced if the light treatment began an hour earlier, closer to the crossover point on the light PRC, as planned.
It is also possible that a different pattern of morning light could have produced a larger advance. In our previous study in which sleep was advanced 1 hour per day,24 continuous bright light produced a slightly larger advance compared to intermittent bright light (means of 2.1 vs 1.5 hours), although again this difference was not statistically significant. Nevertheless, if we had used longer light pulses, or, at the extreme, 1 continuous light exposure, it is possible that we could have produced a slightly larger phase advance. It is also conceivable that more-frequent and shorter pulses, eg, 15 minutes of bright light alternating with 15 minutes of dim light rather than 30 minutes of bright light and 30 minutes of dim light, could increase the advance, since the beginning of each pulse exerts the most effect.38,39
Increasing the intensity of the bright light might have increased the magnitude of the phase shift, but subjects sat fairly close to the light box and received high-intensity light, about 5000 lux. Increasing the intensity much more could be aversive. However, given the recent discovery that the human circadian system is most sensitive to short wavelength (blue) light of about 460 nm,40,41 and that phase advances are larger with blue light,42,43 it is possible that we could produce a larger phase advance with bluer lamps, without increasing subjective brightness. The spectral-power plots of the cool-white 4100 K lamps used in the present study had large peaks around 550 nm (green) and 610 nm (orange). Much smaller peaks were at about 410 (purple), 430 (indigo), and 490 (blue/green). According to our light-meter readings, subjects were exposed to only about 500 μW/cm2 in the blue region (400–490 nm), whereas they received about 1500 mW/cm2 in the other part of the spectrum (490–1064 nm).
Another way to increase the phase advance might be to have subjects wear dark sunglasses whenever they go outside in order to increase their sensitivity to the artificial morning light produced by the light box.44 In the present study, subjects were not required to wear sunglasses, but they were required to note on daily light logs if and when they wore them. Only 1 of the 15 subjects wore sunglasses, and this was only during the first 3 baseline days.
Circadian rhythms shift gradually to reentrain to a shift of the light-dark cycle and the size of the daily phase shift decreases as the circadian rhythms approach complete reentrainment.6 This can be seen in our data after a 12-hour shift of sleep/dark (Figure 1 in Eastman and Martin10 and Figure 4 in Eastman45). However, the phase shifts appear linear (ie, equal shifts per day) during the first 3 to 4 days. Similarly, other studies, to be described below, did not find a consistent change in the rate of phase shift, at least in the first few days.11,46 Therefore, in the present study, we assumed a daily linear advance, and thus the mean phase shifts produced with the 1-hour-per-day and 2-hours-per-day protocols with intermittent morning light were 30 and 36 minutes per day, respectively. In our previous study,24 the 1-hour-per-day protocol with continuous morning light produced advances of 42 minutes per day. We do not know the maximum phase advances that can be produced given these gradually advancing schedules. Furthermore, there is a limit to how fast human circadian rhythms can phase advance, even with more radical shifts of sleep. Perhaps the largest advances would be expected after real eastward flights because of the simultaneous advance of all possible social and physical zeitgebers and the large abrupt advance of the sleep episode exposing the most sensitive part of the light PRC to blue skies. Recall that the classic estimate from 1975 for advances after eastward flight was 57 minutes per day.6 Of course, it is possible that some of the subjects in some of the studies in that review were exposed to outdoor light primarily at the wrong time, which would inhibit the advance, and that larger advances can be produced.
There is a dearth of more recent studies with good circadian phase markers that can be used to calculate the phase advance after eastward flights. In 1 study,15 subjects flew 11 time zones east, and the melatonin profile was assessed before and after the flight. However, 7 of the 8 subjects reentrained by delaying instead of advancing. In a similar study by this group,13 6 subjects flew 8 time zones east. The melatonin rhythm of 1 subject delayed instead of advancing, and the rhythm of another did not shift at all. The melatonin profiles of these 2 subjects peaked inappropriately in the middle of the day, even after 5 days in the new time zone. The other 4 subjects phase advanced 66 minutes per day, which is close to the classic estimate of 57 minutes per day. Still, judging from the fact that one third of the subjects did not phase advance, there was probably a lot of natural light at times that inhibited rather than facilitated phase advances.
Perhaps more tightly controlled laboratory studies can tell us how fast human circadian rhythms can advance. In several studies, bright light was used to hasten reentrainment to an abrupt shift of the sleep schedule. None of these studies reported the phase advances in terms of minutes per day, so we calculated it based on the number of days between circadian phase assessments. In 1 of our studies, there was a 12-hour shift of sleep/dark and 4 days of 6-hour bright light (~5000 lux). When most of the bright light occurred after the baseline Tmin, the rhythms advanced 6.2 hours in 4 days, or 93 minutes per day.10 In another study from our lab,9 there was a 9-hour advance of sleep/dark and a moving bright-light pattern (3 hours of ~5000 lux/day). The first bright-light pulse started soon after the Tmin and was advanced 1 hour per day. This pattern was designed to coincide with the advance portion of the PRC and keep up with the gradual advance of the circadian clock and, thus, its PRC. The phase advance was 6.7 hours averaged over postshift days 5 to 8. For the sake of discussion, we can assume that the 6.7-hour phase advance corresponds to day 6.5, yielding a daily phase advance of 62 minutes per day. Honma et al46 used bright light to facilitate entrainment to an 8-hour advance of sleep/dark. The bright-light pulses (3 hours of 4000-6000 lux) were applied in a stepwise manner, advancing 3 hours per day. Two of the 9 subjects phase delayed rather than advanced, which can be attributed to the bright-light pulses advancing too fast and soon coinciding with the delay portion of the PRC. The melatonin rhythms of the remaining 7 subjects advanced between 36 to 72 minutes per day.
Shanahan et al11 studied the daily course of reentrainment to an abrupt phase advance of the sleep/dark schedule with 3 days of 5 hours of bright light (~9500 lux) aimed so that 1 hour occurred before and 4 hours occurred after the baseline Tmin. Unfortunately, the protocol was not set up to produce the same phase advance of the sleep/dark schedule in all subjects, but a figure shows that a typical advance shift was about 8 hours. The rhythms advanced 1.5 hours after 2 advanced sleep/dark episodes and 1 bright-light “pulse” (corresponding to the second day after a flight), 1.0 hour over the next day, and 1.9 hours over the next. Thus, the rhythms advanced 45 to 114 minutes per day. This 3-pulse protocol, with both advances and delays of the sleep/dark episode was used to generate a light PRC.47 Phase shifts of as much as 12 hours were produced, which were probably delays. With phase markers assessed 5 days apart, it can be difficult to distinguish between advances and delays. However, maximum phase advances clustered at approximately 8 to 9 hours, which, given a linear shift, means daily phase advances of about 96 to 108 minutes per day. A 1-pulse protocol, with a longer bright-light “pulse” of 6.7 hours and 2 shifted sleep/dark episodes showed maximum phase advances of about 2 hours.48 Phase markers were assessed 3 days apart, so the daily advance was at least 40 minutes, or slightly more if most of the phase advance was completed within 2 days.
We can also consider the lab study of Boivin and James.49 The sleep/dark episode was advanced 5 hours and room light (6 hours of ~400 lux) was used in a gradually advancing pattern similar to what Mitchell et al9 used to facilitate entrainment to a 9-hour advance of sleep/dark. Although 400 lux is much less intense than the light used in the other studies mentioned above, it was the most intense light the subjects saw during their 2-week stay in the lab. Otherwise, the subjects were in very dim light of approximately 4 lux or in complete darkness during the 8-hour sleep episodes. Since the circadian system responds to the relative rather than absolute intensity of light,50,51 the 400-lux light should be very effective for facilitating phase shifts. Phase markers taken 10 days apart showed a phase advance of 5.4 hours or 32 minutes per day. Of course, it is possible that larger phase advances occurred in the first few days; however, there were no other phase assessments.
Thus, the 7 lab studies reviewed above, all with an abrupt advance of the sleep/dark episode, which is what happens to most people after eastward flight, suggests that human circadian rhythms can be pushed to advance more than the classic estimate of about 1 hour per day.6 However, all the estimates are less than 2 hour per day. Clearly, more research is necessary to determine the uppermost limit of daily phase advances in human circadian rhythms. More importantly, for practical purposes, we need to determine the maximum phase advance possible with a gradually advancing sleep/dark episode rather than the abrupt advance of sleep/dark used in the studies reviewed above. In those protocols, the abrupt shift of sleep resulted in a drastic misalignment of circadian rhythms with the sleep/wake schedule. Such misalignment causes the symptoms of jet lag, and, therefore, we need to keep circadian rhythms reasonably well aligned with sleep during a preflight treatment or we will be causing the same syndrome we are trying to prevent.
In the current study, in the 2-hours-per-day protocol, sleep was advanced so much faster than the circadian rhythms that, by the third treatment day, on average, subjects were going to bed before their DLMO and waking up before their Tmin (Figure 1). People usually go to bed about 2 to 3 hours after their DLMO52,53 and wake up a few hours after their Tmin.54 Thus, we forced subjects to go to bed at too early a circadian phase, which can account for their increased SOL and decreased sleep efficiencies (Figure 4). We expected the 2-hours-per-day subjects to feel very sleepy in the morning, especially on the second and third treatment day, since they had to wake up close to or before their Tmin. However, SSS ratings in the morning, about 15 minutes after waking, were similar across the 3 treatment days and similar to those obtained during baseline (data not shown). Perhaps subjects did not feel sleepy because they were sitting in front of the light box during the morning rating, which has an alerting effect.55 This suggests that advancing the sleep schedule slightly faster than the rhythms will not create a large problem in the morning. The greater concern, as mentioned above, is with difficulty falling asleep at night.
The SSS scores of the 2-hours-per-day subjects did not increase during the 3 treatment days (Figure 5). There were slight increases at some times of day that were counteracted by decreased sleepiness at bedtime (the 5 daily ratings were averaged). However, this decreased sleepiness became a problem in that it contributed to the difficulty falling asleep. The SSS ratings of the 1-hour-per-day subjects increased slightly, especially by the second and third treatment days (Figure 5). This was due to an increase in sleepiness at all 5 timepoints across the day. We are puzzled by the fact that the 2-hours-per-day subjects did not experience a similar increase in sleepiness, especially during the first 4 time points of the day. The Columbia Jet Lag Scale showed fewer symptoms for the 2-hours-per-day subjects than the 1-hour-per-day subjects during treatment day 2. It is possible that the values on treatment day 3 would also be lower if the questionnaire had been given earlier in the day, as had been originally planned. More research, preferably with larger sample sizes, will be necessary to sort this out.
In any case, the increase in SSS and Columbia scale ratings during the 3 days of treatment were slight. The means SSS scores were not higher than about 3, and a 3 out of 7 corresponds to “relaxed, awake, not at full alertness, responsive.” The highest scores on the Columbia scale were small compared to the largest possible score of 36 and correspond to an average item score of 1.3 out of a possible score of 0 to 4. Thus, we can say that neither the 1-hour nor the 2-hour protocols were associated with a large increase in symptoms. Therefore, importantly, we did not produce severe jet lag before the projected flight.
Although the ideal preflight schedule and light parameters await further research, there is no reason that people cannot use a system similar to that described here, with the commercial light boxes currently available, to prepare for eastward travel. Based on the results from the current study, we recommend that sleep should be advanced by 1 hour per day but not 2 hours per day, as this may make falling asleep at night difficult. The morning light treatment does not need to be the strict schedule of 0.5 hours by the light box and 0.5 hours away used in this study. Rather, the person should feel free to get up from the light box if necessary. However, he or she should try to sit in front of the light box as soon as possible after waking for a consolidated period, since phase advances are larger when light occurs closer to the Tmin (see PRC, Figure 6 in Eastman and Martin10). Thus, showers and other morning activities should be postponed until after this initial bout in front of the light box.
Figure 6 shows a schedule that can be used to customize a pre-flight plan. The Tmin advances 0.5 hours per day after each of the first 3 morning light treatments, because that is the size of the advance we produced with 3 days of advancing wake 1 hour per day combined with intermittent morning bright light. The advances on subsequent days are predicted to be larger, at 1 hour per day, because the morning light occurs closer to the Tmin, although this assumption needs to be empirically verified. It may be possible to produce even larger daily phase advances, so that fewer days of preflight treatment would be needed, by using blue-enriched morning light and/or combining afternoon melatonin with morning light. According to the 0.5-mg melatonin PRC56 (see Figure 1 in Burgess et al57), the maximum phase advance will be produced by melatonin taken about 3 hours before the DLMO or about 5 hours before bedtime. We are currently testing by how much melatonin can enhance the advance in circadian rhythms that occurs with an advancing sleep schedule and morning bright light. When following such an advancing schedule, if wake time occurs after sunrise, it would be better to go outside if possible, rather than use a light box for the morning light treatment, because outdoor light is much more intense and contains more blue light than commercial light boxes. When bedtime occurs earlier than sunset, the bedroom should be made very dark, or eye masks could be worn, to avoid inappropriate exposure to evening light that could phase delay the circadian clock.
The largest inconvenience in following a gradually advancing schedule such as Figure 6 is that the early bedtimes may cause people to miss out on evening social and family activities. In addition, meals should be advanced concurrently with sleep so that they continue to be eaten at an appropriate circadian phase, and this could further reduce social interactions. Thus, the importance of a few nights of social contacts has to be balanced with the importance of optimal sleep and alertness for several days after landing. Some people will also find sitting near a light box in the morning inconvenient. However, the intermittent light schedule makes it possible to do various activities in between the bright-light pulses (such as showering and dressing). Furthermore, many activities can be done while sitting near a light box, such as eating or working on a computer. People who have desk jobs and who work at home or who can bring a light box to work will find the morning light treatment relatively easy to fit into their schedule. In any case, after a few days, the light treatment would not interfere with the individual’s usual morning activities because, by the second or third day of treatment (day -5 or -4 in Figure 6), the light exposure will end at the time the individual was waking up before treatment began (ie, on day -7).
This type of preflight schedule would be the easiest (take the fewest days) in the case of an “early bird” on an early sleep schedule living on the east coast, especially if he or she wanted to be on a later schedule while in the new time zone. It would be the most difficult (take the most days) for a “night-owl” on a late sleep schedule living on the west coast, especially if he or she wanted to be on an early sleep schedule in the new time zone. In that case, a better preflight plan might be to follow a gradually delaying schedule (we suggest 2 hours per day) and use bright light in the 2 to 3 hours before bed in order to delay the circadian clock to match the new time zone.
There are a number of benefits to adopting a preflight treatment to reduce jet lag as opposed to waiting and only trying to minimize jet lag upon arrival in the new time zone by trying to control light exposure in order to hasten adaptation. Obviously, the weather and commitments (such as business meetings) could prevent the traveler from getting outdoor light exposure at the desired time. Similarly, the traveler might have to be in bright places, or even outside, when light should be avoided. Even the darkest sunglasses do not block light completely. On the other hand, even a partial preflight adjustment that makes the traveler arrive with rhythms already somewhat advanced will reduce the risk of antidromic reentrainment. Preadaptation to the destination time zone would be beneficial to all travelers, including business people, the military, and people on vacation. As the travelers would experience little or no jet lag, they would be able to carry out tasks, such as attending business meetings or performing military operations, soon after their arrival. Therefore, there would be a reduction in the number of days that would be “wasted” in the new time zone due to the travelers suffering from jet lag and not being able to perform to their full potential.
Acknowledgments
This work was supported by a grant to C. Eastman: NINR R01 NR07667. We thank Clara Lee, Natalie Stroupe, Barbara Trzop, and Christine Tseng for their assistance with data collection. We thank Enviro-Med, Vancouver, Wash, USA, for donating the light boxes. We thank Victoria Revell, PhD, for helping to revise the discussion.
Footnotes
Disclosure Statement
This is not an industry supported study. Dr. Fogg has received financial support from the Hartford Foundation. Drs. Eastman, Gazda, Burgess, and Crowley have indicated no financial conflicts of interest.
References
- 1.Graeber RC. Alterations in performance following rapid trans-meridian flight. In: Brown FM, Graeber RC, eds. Rhythmic Aspects of Behavior. Mahwah, NJ: Lawrence Erlbaum Associates; 1982:173–212.
- 2.Boulos Z, Campbell SS, Lewy AJ, Terman M, Dijk D-J, Eastman CI. Light treatment for sleep disorders: consensus report. VII. Jet lag. J Biol Rhythms. 1995;10:167–76. doi: 10.1177/074873049501000209. [DOI] [PubMed] [Google Scholar]
- 3.Haimov I, Arendt J. The prevention and treatment of jet lag. Sleep Med Rev. 1999;3:229–40. doi: 10.1016/s1087-0792(99)90004-7. [DOI] [PubMed] [Google Scholar]
- 4.Waterhouse J, Edwards B, Nevill A, et al. Do subjective symptoms predict our perception of jet-lag? Ergonomics. 2000;43:1514–27. doi: 10.1080/001401300750003943. [DOI] [PubMed] [Google Scholar]
- 5.Cho K, Ennaceur A, Cole JC, Suh CK. Chronic jet lag produces cognitive deficits. J Neurosci. 2000;20:1–5. doi: 10.1523/JNEUROSCI.20-06-j0005.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aschoff J, Hoffmann K, Pohl H, Wever R. Re-entrainment of circadian rhythms after phase shifts of the zeitgeber. Chronobiologia. 1975;2:23–78. [PubMed] [Google Scholar]
- 7.Wever RA. The Circadian System of Man. New York-Heidelberg-Berlin: Springer-Verlag; 1979.
- 8.Sack RL, Lewy AJ, Blood ML, Keith LD, Nakagawa H. Circadian rhythm abnormalities in totally blind people: Incidence and clinical significance. J Clin Endocrinol Metab. 1992;75:127–34. doi: 10.1210/jcem.75.1.1619000. [DOI] [PubMed] [Google Scholar]
- 9.Mitchell PJ, Hoese EK, Liu L, Fogg LF, Eastman CI. Conflicting bright light exposure during night shifts impedes circadian adaptation. J Biol Rhythms. 1997;12:5–15. doi: 10.1177/074873049701200103. [DOI] [PubMed] [Google Scholar]
- 10.Eastman CI, Martin SK. How to use light and dark to produce circadian adaptation to night shift work. Ann Med. 1999;31:87–98. doi: 10.3109/07853899908998783. [DOI] [PubMed] [Google Scholar]
- 11.Shanahan TL, Kronauer RE, Duffy JF, Williams GH, Czeisler CA. Melatonin rhythm observed throughout a three-cycle bright-light stimulus designed to reset the human circadian pacemaker. J Biol Rhythms. 1999;14:237–53. doi: 10.1177/074873099129000560. [DOI] [PubMed] [Google Scholar]
- 12.Monk TH, Buysse DJ, Carrier J, Kupfer DJ. Inducing jet-lag in older people: Directional asymmetry. J Sleep Res. 2000;9:101–6. doi: 10.1046/j.1365-2869.2000.00184.x. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi T, Sasaki M, Itoh H, et al. Re-entrainment of circadian rhythm of plasma melatonin on an 8-h eastward flight. Psychiatry Clin Neurosci. 1999;53:257–60. doi: 10.1046/j.1440-1819.1999.00537.x. [DOI] [PubMed] [Google Scholar]
- 14.Gundel A, Spencer MB. A circadian oscillator model based on empirical data. J Biol Rhythms. 1999;14:516–23. doi: 10.1177/074873099129000849. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi T, Sasaki M, Itoh H, et al. Re-entrainment of the circadian rhythms of plasma melatonin in an 11-h eastward bound flight. Psychiatry Clin Neurosci. 2001;55:275–6. doi: 10.1046/j.1440-1819.2001.00857.x. [DOI] [PubMed] [Google Scholar]
- 16.Daan S, Lewy AJ. Scheduled exposure to daylight: a potential strategy to reduce “jet lag” following transmeridian flight. Psychopharmacol Bull. 1984;20:566–568. [PubMed] [Google Scholar]
- 17.Houpt TA, Boulos Z, Moore-Ede MC. Midnightsun: software for determining light exposure and phase-shifting schedules during global travel. Physiol Behav. 1996;59:561–8. doi: 10.1016/0031-9384(95)02111-6. [DOI] [PubMed] [Google Scholar]
- 18.Waterhouse J, Reilly T, Atkinson G. Jet-lag. Lancet. 1997;350:1611–6. doi: 10.1016/S0140-6736(97)07569-7. [DOI] [PubMed] [Google Scholar]
- 19.Burgess HJ, Eastman CI. Prevention of jet lag. Physicians’ Information and Education Resource (PIER) 2003; American College of Physicians - American Society of Internal Medicine: Available at http://pier.acponline.org/index.html Accessed on September 10, 2004.
- 20.Boulos Z. Bright light treatment for jet lag and shift work. In: Lam RW, eds. Seasonal Affective Disorder and Beyond Light Treatment for SAD and Non-SAD conditions. Washington DC: American Psychiatric Press, Inc; 1998:253–87.
- 21.Gundel A, Wegmann HM. Transition between advance and delay responses to eastbound transmeridian flights. Chronobiol Int. 1989;6:147–56. doi: 10.3109/07420528909064625. [DOI] [PubMed] [Google Scholar]
- 22.Boulos Z, Macchi MM, Sturchler MP, et al. Light visor treatment for jet lag after westward travel across six time zones. Aviat Space Environ Med. 2002;73:953–63. [PubMed] [Google Scholar]
- 23.Suhner AG, Murphy PJ, Savage HC, Steffen R, Campbell SS. Failure to alleviate jet lag with timed light/dark exposure. Sleep. 2001;24:A190. [Google Scholar]
- 24.Burgess HJ, Crowley SJ, Gazda CJ, Fogg LF, Eastman CI. Preflight adjustment to eastward travel: 3 days of advancing sleep with and without morning bright light. J Biol Rhythms. 2003;18:318–28. doi: 10.1177/0748730403253585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horne JA, Östberg O. Self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- 26.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 27.Tasto DL, Colligan MJ, Skjei EW, Polly SJ. Health Consequences of Shift Work. Cincinnati: NIOSH Publication #78–154; 1978.
- 28.Buxton OM, L’Hermite-Baleriaux M, Turek FW, Van Cauter E. Daytime naps in darkness phase shift the human circadian rhythms of melatonin and thyrotropin secretion. Am J Physiol Regul Integr Comp Physiol. 2000;278:R373–82. doi: 10.1152/ajpregu.2000.278.2.R373. [DOI] [PubMed] [Google Scholar]
- 29.Dumont M, Carrier J. Daytime sleep propensity after moderate circadian phase shifts induced with bright light exposure. Sleep. 1997;20:11–7. doi: 10.1093/sleep/20.1.11. [DOI] [PubMed] [Google Scholar]
- 30.Murphy PJ, Myers BL, Badia P. Nonsteroidal anti-inflammatory drugs alter body temperature and suppress melatonin in humans. Physiol Behav. 1996;59:133–9. doi: 10.1016/0031-9384(95)02036-5. [DOI] [PubMed] [Google Scholar]
- 31.Hoddes E, Zarcone V, Smythe H, Phillips R, Dement WC. Quantification of sleepiness: A new approach. Psychophysiology. 1973;10:431–6. doi: 10.1111/j.1469-8986.1973.tb00801.x. [DOI] [PubMed] [Google Scholar]
- 32.Spitzer RL, Terman M, et al. Jet lag: clinical features, validation of a new syndrome-specific scale, and lack of response to melatonin in a randomized, double-blind trial. Am J Psychiatry. 1999;156:1392–6. doi: 10.1176/ajp.156.9.1392. [DOI] [PubMed] [Google Scholar]
- 33.Voultsios A, Kennaway DJ, Dawson D. Salivary melatonin as a circadian phase marker: validation and comparison to plasma melatonin. J Biol Rhythms. 1997;12:457–66. doi: 10.1177/074873049701200507. [DOI] [PubMed] [Google Scholar]
- 34.Cagnacci A, Soldani R, Laughlin GA, Yen SSC. Modification of circadian body temperature rhythm during the luteal menstrual phase: role of melatonin. J Appl Physiol. 1996;80:25–9. doi: 10.1152/jappl.1996.80.1.25. [DOI] [PubMed] [Google Scholar]
- 35.Brown EN, Choe Y, Shanahan TL, Czeisler CA. A mathematical model of diurnal variations in human plasma melatonin levels. Am J Physiol. 1997;272:E506–16. doi: 10.1152/ajpendo.1997.272.3.E506. [DOI] [PubMed] [Google Scholar]
- 36.Eastman CI, Martin SK, Hebert M. Failure of extraocular light to facilitate circadian rhythm reentrainment in humans. Chronobiol Int. 2000;17:807–26. doi: 10.1081/cbi-100102116. [DOI] [PubMed] [Google Scholar]
- 37.Sharkey KM, Eastman CI. Melatonin phase shifts human circadian rhythms in a placebo-controlled simulated night-work study. Am J Physiol Regul Integr Comp Physiol. 2002;282:R454–63. doi: 10.1152/ajpregu.00135.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nelson DE, Takahashi JS. Sensitivity and integration in a visual pathway for circadian entrainment in the hamster. J Physiol. 1991;439:115–45. doi: 10.1113/jphysiol.1991.sp018660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rimmer DW, Boivin DB, Shanahan TL, Kronauer RE, Duffy JF, Czeisler CA. Dynamic resetting of the human circadian pacemaker by intermittent bright light. Am J Physiol. 2000;279:R1574–9. doi: 10.1152/ajpregu.2000.279.5.R1574. [DOI] [PubMed] [Google Scholar]
- 40.Thapan K, Arendt J, Skene DJ. An action spectrum for melatonin suppression: evidence for a novel non-rod, non-cone photoreceptor system in humans. J Physiol. 2001;535:261–7. doi: 10.1111/j.1469-7793.2001.t01-1-00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brainard GC, Hanifin JP, Greeson JM, et al. Action spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptor. J Neurosci. 2001;21:6405–12. doi: 10.1523/JNEUROSCI.21-16-06405.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Warman VL, Dijk DJ, Warman GR, Arendt J, Skene DJ. Phase advancing human circadian rhythms with short wavelength light. Neurosci Lett. 2003;342:37–40. doi: 10.1016/s0304-3940(03)00223-4. [DOI] [PubMed] [Google Scholar]
- 43.Wright HR, Lack LC, Kennaway DJ. Differential effects of light wavelength in phase advancing the melatonin rhythm. J Pineal Res. 2004;36:140–4. doi: 10.1046/j.1600-079x.2003.00108.x. [DOI] [PubMed] [Google Scholar]
- 44.Hebert M, Martin SK, Lee C, Eastman CI. The effects of prior light history on the suppression of melatonin by light in humans. J Pineal Res. 2002;33:198–203. doi: 10.1034/j.1600-079x.2002.01885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eastman CI. High intensity light for circadian adaptation to a 12-h shift of the sleep schedule. Am J Physiol. 1992;263:R428–436. doi: 10.1152/ajpregu.1992.263.2.R428. [DOI] [PubMed] [Google Scholar]
- 46.Honma K-I, Honma S, Nakamura K, Sasaki M, Endo T, Takahashi T. Differential effects of bright light and social cues on reentrainment of human circadian rhythms. Am J Physiol. 1995;268:R528–35. doi: 10.1152/ajpregu.1995.268.2.R528. [DOI] [PubMed] [Google Scholar]
- 47.Czeisler CA, Kronauer RE, Allan JS, et al. Bright light induction of strong (type 0) resetting of the human circadian pacemaker. Science. 1989;244:1328–33. doi: 10.1126/science.2734611. [DOI] [PubMed] [Google Scholar]
- 48.Khalsa SBS, Jewett ME, Cajochen C, Czeisler CA. A phase response curve to single bright light pulses in human subjects. J Physiol. 2003;549.3:945–52. doi: 10.1113/jphysiol.2003.040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boivin DB, James FO. Phase-dependent effect of room light exposure in a 5-h advance of the sleep-wake cycle: Implications for jet lag. J Biol Rhythms. 2002;17:266–76. doi: 10.1177/074873040201700310. [DOI] [PubMed] [Google Scholar]
- 50.Lynch HJ, Rivest RW, Ronsheim PM, Wurtman RJ. Light intensity and the control of melatonin secretion in rats. Neuroendocrinology. 1981;33:181–5. doi: 10.1159/000123226. [DOI] [PubMed] [Google Scholar]
- 51.Meyer WE, Millam JR. Plasma melatonin levels in Japanese quail exposed to dim light are determined by subjective interpretation of day and night, not light intensity. Gen Comp Endocrinol. 1991;82:377–85. doi: 10.1016/0016-6480(91)90313-u. [DOI] [PubMed] [Google Scholar]
- 52.Martin SK, Eastman CI. Sleep logs of young adults with self-selected sleep times predict the dim light melatonin onset. Chronobiol Int. 2002;19:695–707. doi: 10.1081/cbi-120006080. [DOI] [PubMed] [Google Scholar]
- 53.Burgess HJ, Savic N, Sletten T, Roach G, Gilbert SS, Dawson D. The relationship between the dim light melatonin onset and sleep on a regular schedule in young healthy adults. Behav Sleep Med. 2003;1:102–14. doi: 10.1207/S15402010BSM0102_3. [DOI] [PubMed] [Google Scholar]
- 54.Baehr EK, Revelle W, Eastman CI. Individual differences in the phase and amplitude of the human circadian temperature rhythm: with an emphasis on morningness-eveningness. J Sleep Res. 2000;9:117–27. doi: 10.1046/j.1365-2869.2000.00196.x. [DOI] [PubMed] [Google Scholar]
- 55.Campbell SS, Dijk D-J, Boulos Z, Eastman CI, Lewy AJ, Terman M. Light treatment for sleep disorders: consensus report. III. Alerting and activating effects. J Biol Rhythms. 1995;10:129–32. doi: 10.1177/074873049501000205. [DOI] [PubMed] [Google Scholar]
- 56.Lewy AJ, Bauer VK, Ahmed S, et al. The human phase response curve (PRC) to melatonin is about 12 hours out of phase with the PRC to light. Chronobiol Int. 1998;15:71–83. doi: 10.3109/07420529808998671. [DOI] [PubMed] [Google Scholar]
- 57.Burgess HJ, Sharkey KM, Eastman CI. Bright light, dark and melatonin can promote circadian adaptation in night shift workers. Sleep Med Rev. 2002;6:407–20. [PubMed] [Google Scholar]


