Abstract
To establish the timing of lineage restriction among endodermal derivatives, we developed a method to label permanently subsets of lung precursor cells at defined times during development by using Cre recombinase to activate floxed alkaline phosphatase or green fluorescent protein genes under control of doxycycline-dependent surfactant protein C promoter. Extensive or complete labeling of peripheral lung, thyroid, and thymic epithelia, but not trachea, bronchi, or gastrointestinal tract occurred when mice were exposed to doxycycline from embryonic day (E) 4.5 to E6.5. Nonoverlapping cell lineages of conducting airways (trachea and bronchi), as distinct from those of peripheral airways (bronchioles, acini, and alveoli), were established well before formation of the definitive lung buds at E9–9.5. At E11.5, the labeled precursors of peripheral lung were restricted to relatively few cells along the bronchial tubes and clusters in bronchial tips and lateral buds. Thereafter, these cells underwent marked expansion to form the entire gas-exchange region in the lung. This study demonstrates early restriction of endodermal progenitor cells forming peripheral as compared with proximal airways, identifies distinct cell lineages in conducting airways, and distinguishes neuroepithelial and tracheal–bronchial gland cell lineages from those lining peripheral regions of the lung. This system for conditional gene addition or deletion is useful for the study of lung morphogenesis and gene function in vivo, and identifies progenitor cells that may serve as useful targets for cell or gene replacement for pulmonary disorders.
Keywords: Cre recombinase, pulmonary morphogenesis, transgenic mice
Epithelial cells forming thyroid, parathyroid, esophagus, and thymus are derived from adjacent foregut tissues at approximately embryonic day (E) 9–9.5 in the mouse (1, 2). The lung buds, also derived from the foregut, invade the splanchnic mesenchyme, the trachea elongates, and peripheral tubules undergo stereotypic branching to form the bronchi and bronchioles. As development proceeds, formation of acini and alveoli (peripheral lung) creates the gas-exchange region critical for postnatal survival. The timing, identity, and mechanisms by which endodermal cells produce distinct cell types in various organs derived from the foregut, and in particular, the lung, have not been well defined. Cell-fate mapping studies demonstrated that, as early as E7.5, discrete regions of the presomitic embryo contributed to ventral foregut-derived organs, including lung, pancreas, and liver (3). Later in development, temporal–spatial appearance of various morphological markers has been used to identify subsets of respiratory epithelial cells derived from putative lung-precursor cells (4, 5). Although the genetic programs directing lung formation are not well understood, experiments in which critical genes were added or deleted during development demonstrate the importance of paracrine signaling between splanchnic mesenchyme and the epithelium for morphogenesis of the lung. Sonic hedgehog and fibroblast growth factor-dependent pathways, as well as various classes of transcription factors, e.g., Nkx-2, forkhead (Fox), and GATA-family members, are involved in the differentiation of the lung from foregut endoderm (6–8).
The lung buds appear on the lateral aspects of the foregut before formation of the trachea. Distinct patterns of gene expression distinguish the extra pulmonary trachea and bronchi in the proximal lung from the more peripheral regions of the developing lung, and from thymus, thyroid, parathyroid, esophagus, and other foregut derivatives. It is generally assumed, but not formally proven, that proximal and peripheral regions of the pulmonary epithelium arise by proliferation and differentiation of common precursors. However, whether the diverse epithelial cells lining the mature respiratory tract share common or distinct lineage relationships with each other and other endodermally derived progenitor cells is unclear. Understanding the processes by which endodermal cells are committed and proliferate to form the lung may provide strategies to replace or genetically modify progenitor cells, which could be introduced for treatment of pulmonary diseases, such as hereditary surfactant protein B (SP-B) deficiency, cystic fibrosis, and other genetic disorders affecting the lung.
The present study was undertaken to identify the timing of cell fate restriction and lineage relationships among cells that serve as progenitors of the respiratory epithelium during lung morphogenesis. Endodermal precursors were permanently marked by the timed expression of Cre recombinase (Cre) in subsets of respiratory epithelial cells, which activated green fluorescent protein (GFP) or alkaline phosphatase (AP) in floxed genes. Cre expression was controlled by the doxycycline-inducible reverse tetracycline transactivator (rtTA), which is expressed under control of the surfactant protein C (SP-C) promoter (9–11). This study demonstrates: (i) the early divergence of cell lineages during organogenesis from the endoderm, (ii) the early spatial restriction of progenitor cells forming the proximal conducting airways as compared with the peripheral lung, (iii) the presence of two distinct cell lineages lining proximal conducting airways, and (iv) that tracheal-bronchial glands and neuroepithelial cells are not derived from precursors that form the peripheral subset of respiratory epithelial cells.
Materials and Methods
Transgenic Constructs.
Two different floxed reporter transgenic mice used for this study were described previously. Excision of flanking loxP sites by Cre in ZEG (10) or in ZAP (11) transgenic mice, activates the expression of AP or GFP, respectively (Fig. 1). These mice were mated to SP-C/rtTA mice that express the rtTA activator under control of the human SP-C promoter (12–15). The 3.7-kb human SP-C promoter directs gene expression exclusively to the developing and mature pulmonary epithelium in epithelial cells of the primordial lung buds, with maintenance of expression in alveolar and bronchiolar epithelial cells after birth (13, 16). For regulated expression of Cre, the (tetO)7-Cre plasmid was cloned with the tet promoter from 13–3 and the Cre region and MT-1 polyadenylation signals from pBS185 (9). Transgenic (tetO)7-Cre mice were generated by pronuclear injection and crossed to 3.7 SP-C-rtTA, ZEG, or ZAP mice to produce triple transgenic mice. Triple transgenic mice resulted from crosses of SP-C-rtTA/(tetO)7-Cre and (tetO)7-Cre/ZAP or (tetO)7-Cre/ZEG double-transgenic parents.
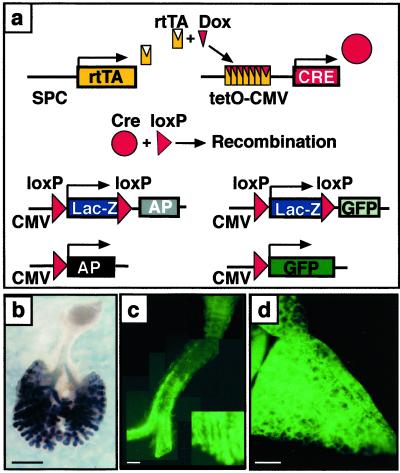
Fig 1.
Permanent cell labeling by doxycycline-inducible recombination in triple-transgenic mice. (a) Triple-transgenic mice were produced in which doxycycline activates rtTA expressed under control of the SP-C promoter. The rtTA then activates Cre that excises and religates the target gene, inactivating β-galactosidase (lacZ), and permanently labeling the cell by activating AP or GFP in the ZAP or ZEG mice, respectively. Dams were treated with doxycycline from E6.5 to day of killing. (b) Whole mount AP staining on an E13.5 lung from an SP-C-rtTA/tetO-Cre/ZAP embryo. Most intrapulmonary epithelial cells and some extrapulmonary airway cells stained for AP. (c) GFP fluorescence in trachea from an SP-C-rtTA/tetO-Cre/ZEG transgenic mouse on PN7. GFP-positive cells are organized in a linear fashion along the trachea and numbers of labeled cells increased from proximal to peripheral extrapulmonary airways (lower right corner, magnification, ×5). (d) Widespread GFP fluorescence was seen in lung parenchyma from an SP-C-rtTA/tetO-Cre/ZEG mouse on PN7. (Bars = 1 mm.)
Animal Use and Doxycycline Administration.
Animals were housed in pathogen-free conditions in accordance with institutional guidelines. Gestation was dated by detection of the vaginal plug and correlated with length and weight of each pup at the time of killing. Dams bearing double- and triple-transgenic pups were maintained on doxycycline in food (25 mg/g; Harlan Teklad, Madison, WI) or drinking water (1 mg/ml; Sigma) for various time spans. The mice were killed by either placing them in a CO2 chamber or by injecting them with 0.2–0.3 ml of anesthetic (ketamine, xylazine, acepromazine).
Histochemistry.
All experiments shown are representative of findings from at least three independent dams, generating at least five triple transgenic offspring, that were compared with littermates for each observation. Whole-mount AP staining was performed on all samples E14.5 and younger, before embedding and sectioning (11). Colocalization of GFP with other markers was assessed in samples fixed in 4% paraformaldehyde in PBS. Immunohistochemistry for Cre (Novagen, 1:20,000) and GFP (Chemicon, 1:10) was performed by using rabbit polyclonal antibodies and an immunoperoxidase method. Colocalization of calcitonin gene-related peptide (CGRP) and GFP was performed with rabbit anti-rat CGRP (Sigma, 1:500), and Texas red conjugated goat anti-rabbit (Jackson ImmunoResearch, 1:100) polyclonal antibodies. In situ hybridization was performed with 35S-UTP-labeled riboprobes to assess sites of mRNA expression as described (13). Antisense riboprobes for Cre were generated from a Bluescript II SK plasmid (gift from J. Hébert, Stanford University) containing the full-length Cre cDNA.
Results
Conditional Control of Gene Targeting in the Lung.
In triple transgenic animals, the rtTA is expressed in epithelial cells under control of the SP-C promoter. Doxycycline, in combination with rtTA, activates expression of Cre, which causes recombination of the floxed reporter genes (Fig. 1a). Thus, the sites and timing of rtTA and Cre expression, and the pharmacodynamics of doxycycline, may influence the timing and extent of recombination in these experiments. Once progenitor cells are labeled, their daughter cells will be labeled, even if Cre is no longer expressed. Doxycycline induces target gene expression within 6–12 h, activity decreasing 12–24 h after withdrawal in this system (12, 15). Cre-dependent recombination permanently inactivates β-galactosidase, activating either AP or GFP (Fig. 1a).
Cre-mediated activation of the floxed AP or GFP genes was induced by treatment of the dam with doxycycline during pregnancy. After continuous treatment of the dam with doxycycline from E6.5 to E13.5 and E6.5 to postnatal day (PN) 7, AP staining or GFP fluorescence was observed in virtually all intrapulmonary respiratory epithelial cells in peripheral regions of the lung, as well as in a restricted subset of cells in the extrapulmonary conducting airways (distal trachea and bronchi) (Fig. 1 b–d).
Cre Recombinase Expression.
To understand the pharmacodynamics of recombination, the expression of Cre was assessed over time. After exposure to doxycycline for 48 h (E16.5–18.5), Cre mRNA was readily detected in epithelial cells throughout the lung at E18.5. Cre mRNA decreased markedly 48 h after withdrawal from doxycycline and was not detectable after 96 h (Fig. 2 a–c). Consistent with these findings, immunostaining of Cre was observed after 48 h of exposure to doxycycline in virtually all epithelial cells of the fetal lung (at E18) in a distribution consistent with that of Cre mRNA (Fig. 2d). Intensity of Cre staining decreased markedly at 48 h and was undetectable 96 h after removed from doxycycline, demonstrating that Cre expression was entirely doxycycline-dependent in the fetal lung (Fig. 2 e and f). No Cre staining could be detected in the absence of the rtTA transgene (Fig. 2 g–i).
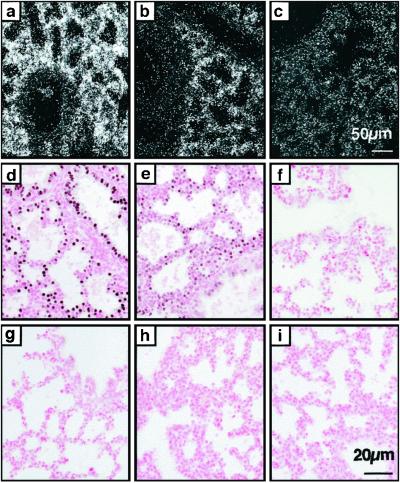
Fig 2.
Decreased Cre mRNA and protein after removal of doxycycline. Dams were treated for 48 h with doxycycline in the food. In situ hybridization for Cre mRNA (a–c) and immunohistochemistry for Cre protein (d–i) was performed on lung sections from embryos on E18.5, whereas on doxycycline (a, d, and g), 48 h (b, e, and h) and 96 h (c, f, and i) after removal from doxycycline, triple transgenic embryos (a–f), and double-transgenic embryos (g–i). (a–c) Cre-mRNA was detected throughout the respiratory epithelium, whereas on doxycycline, decreased after 48 h and was not different from nontransgenic controls (data not shown) 96 h after removal from doxycycline. [Bar = 50 μm (a–c).] (d–f) Whereas on doxycycline, Cre protein was readily detected in nuclei of most pulmonary epithelial cells, Cre protein was reduced 48 h after cessation of doxycycline and absent after 96 h. (g–i) In embryos lacking the SP-C-rtTA transgene (double-transgenic Cre/ZAP), Cre protein was not detected on or off doxycycline. [Bar = 20 μm (d–i).]
Recombination was doxycycline-sensitive and detected in epithelial cells of diverse cell types, including ciliated and nonciliated columnar cells in bronchi and bronchioles, and type II and type I cells in the alveolar region (Figs. 3 e and f and 4). Because the SP-C promoter is not active in ciliated or type I epithelial cells, these findings are consistent with recombination occurring in progenitor cells whose daughters were permanently labeled earlier by expression of Cre. Before E15.5, recombination did not occur in the fetal lung unless the dam was treated with doxycycline. Rare, nondoxycycline-dependent recombination occurred at E16.5 and thereafter (Fig. 4 a–c and g). Continuous treatment with doxycycline from E6.5 to E14.5, E6.5 to E15.5, and E6.5 to E16.5 resulted in labeling of virtually all peripheral respiratory epithelial cells (Fig. 4 d–f). Labeling of extrapulmonary conducting airways was also observed, the numbers of labeled cells increasing from proximal to distal regions of the trachea and bronchi (Fig. 1 b and c). When mice were treated postnatally with doxycycline, recombination was observed but rare (Fig. 4 h and i). Exposure of mature adult mice to doxycycline for 3 weeks resulted in rare labeling of peripheral lung epithelial cells (data not shown).
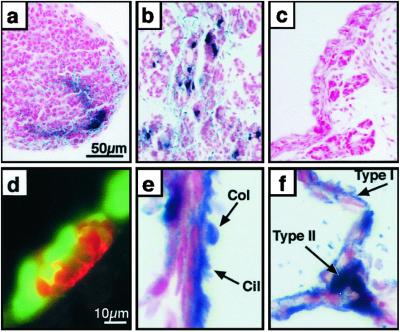
Fig 3.
Cre-mediated recombination in diverse epithelial cell types: thymus, thyroid, and peripheral lung. Pregnant dams were treated with doxycycline from E0.5 to E14.5 (a) or E0.5 to PN7 (b–f). AP staining (a–c, e, and f) was performed on paraffin sections counterstained with nuclear fast red. (a) AP staining of cells in the apical region of the thymus. (b) Subsets of thyroid cells expressed AP. (c) Tracheal-bronchial glands do not stain for AP. (d) Immunohistochemistry for CGRP (red) stained neuroendocrine bodies (NEBs) that did not coexpress GFP (green). (e) Most ciliated (cil) and nonciliated Clara (col) cells in the bronchioles stain for AP. (f) Staining in alveolar type I (type I) and type II (type II) cells was detected. [Bar = 50 μm (a–c) and 10 μm (d–f).]
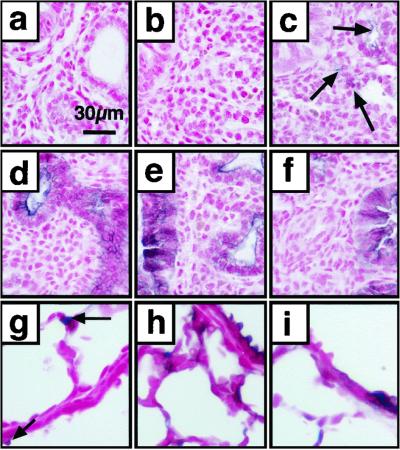
Fig 4.
Cre-mediated recombination in the embryonic and postnatal lung. Lung sections were obtained from mice killed at E14.5 (a and d), E15.5 (b and e), E16.5 (c and f), and PN 21 (g–i), and stained for AP with nuclear fast red counterstain. Recombination in the absence of doxycycline is rare (arrows) and can be seen only after E15.5 (a–c and g). Cell labeling was widespread after treatment of the dam with doxycycline from E6.5 to E14.5, E15.5, and E16.5 (d–f). Rare labeling was observed when exposed to doxycycline from E18.5 to PN9 (h) or PN2 to PN9 (i). [Bar = 30 μm (a–i).]
Early Recombination Distinguishes the Lung from Other Foregut Endodermally Derived Organs.
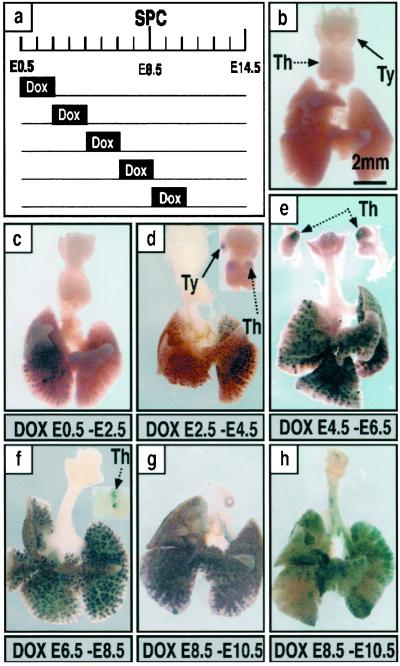
To assess the timing of restriction of progenitor cells contributing to distal and proximal regions of the lungs, the dams were exposed to doxycycline for 48-h periods to induce recombination; labeling was then assessed at E14.5 by whole-mount AP staining (Fig. 5). Widespread, but not complete, labeling was observed when the dam was exposed to doxycycline from E0 to E2.5 or E2.5 to E4.5 (Fig. 5 c and d). During these early periods, labeling was seen only in peripheral lung tubules and not in proximal conducting airways. After exposure to doxycycline from E0 to E2.5, labeling was distributed in a lobar pattern, one lobe or discrete regions of a lobe being labeled, the other being completely unlabeled. This pattern was occasionally seen after exposure from E2.5 to E4.5. Labeling of peripheral lung, thymus, and thyroid but not trachea, main bronchi, or esophagus, was detected after labeling between E2.5–4.5, E4.5–6.5, and E6.5–8.5, but not thereafter, suggesting that common progenitors of thymus, thyroid, and peripheral lung transiently entered the labeling field defined by activation of the SP-C promoter (Figs. 3 a and b and 5 d–f). Possible labeling in other organs or foregut derivatives was also carefully assessed in the GFP-expressing embryos and was never observed.
Fig 5.
Temporal specificity of recombination. Dams were treated with doxycycline for 48 h and labeling assessed at E14.5 by whole-mount AP staining. A diagram of the treatment protocols used during embryonic development is shown (a). E0.5 is 12 h after fertilization as determined by detection of a vaginal plug. Whole-mount AP staining of lungs from triple-transgenic mice was assessed on E14.5 to determine the extent of recombination (b–h). Single-transgenic control littermate (b). Partial lobar labeling in the peripheral lung after doxycycline from E0.5 to E2.5 (c). Extensive labeling of intrapulmonary tissue with variable staining in the thymus and thyroid seen among littermates (d). Labeling of most intrapulmonary epithelial cells. Staining was observed in the thymus of all mice but not in the thyroid (e). Labeling in the peripheral lung was found in all mice; recombination in the thymus was found frequently but not in all mice (f). Labeling of extrapulmonary airways was never observed from E0.5to E8.5 (c–f). From E8.5 to E10.5, labeling of intrapulmonary epithelial cells was widespread or focal with variation seen among littermates; subsets of cells in extrapulmonary airways were labeled, the numbers of labeled cells being more abundant in the periphery, and labeling was not seen in thymus or thyroid (g and h). Extensive, but not complete labeling of intrapulmonary epithelial cells was observed in association with labeling of some cells in the extrapulmonary airways (h). (Bar = 2 mm.) Th, thymus; Ty, thyroid.
Labeling of most or all peripheral respiratory epithelial cells was usually observed after exposure to doxycycline from E2.5 to E4.5, E4.5 to E6.5, or E6.5 to E8.5. Labeling was completely excluded from extrapulmonary conducting airways during these periods of doxycycline exposure (Fig. 5 c–f). The homogeneity of labeling in the lung and lack of labeling in trachea and bronchi, occurring before formation of the lung buds, demonstrates that distinct progenitor cell(s) give rise to the conducting airways as compared with the peripheral lung parenchyma during early embryogenesis.
Identification of Progenitors of the Lung Periphery.
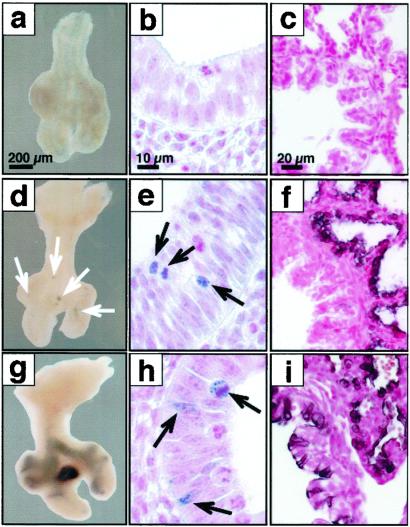
To identify the subset of precursor cells that were labeled during the early period of doxycycline exposure, the dams were placed on doxycycline from E6.5to E8.5, and the pups killed on E11.5 before extensive branching morphogenesis occurred, or on E18.5 after formation of the gas exchange area. At E11.5, labeled precursor cells were visualized with both AP (Fig. 6 d and g) and GFP (Fig. 6 e and h) in a highly restricted subset of cells at lateral buds along the bronchial tubes and in clusters at the tips of the lung buds (Fig. 6 d and e). Thus relatively few, highly localized, epithelial cells that were labeled before lung bud formation serve as progenitors of the uniformly labeled cells seen in the lung periphery at E14.5 (Fig. 5f) and E18.5 (Fig. 6f). After labeling from E4.5to E6.5 or E6.5 to E8.5 and killing at either E14.5 or E18.5, no labeled cells were observed in the extrapulmonary or larger conducting airways (Figs. 6f and 5 c–f). The daughter cells derived from this population of peripheral progenitor cells expand markedly after E11.5 with resultant labeling of nearly all of the intrapulmonary epithelial cells. After exposure to doxycycline from E8.5 to E10.5 and analysis at E11.5, recombination was observed in a patchy but more widely distributed pattern with labeling being observed in some cells in the extrapulmonary airways (Fig. 6 g and h). These findings are consistent with the more extensive labeling of subsets of tracheal-bronchial cells seen on E14.5 or E18.5 after exposure to doxycycline from E8.5 to E10.5 (Figs. 5 g and h and 6i). The nonoverlapping labeling characteristics (or patterns) induced by doxycycline exposure from E6.5 to E8.5 and E8.5 to E10.5 demonstrate that active labeling does not extend beyond 48 h after removal from doxycycline.
Fig 6.
Progenitors of the peripheral lung are highly restricted at E11.5. Lungs were obtained at E11.5 (a, b, d, e, g, and h), at E18.5 (c, f, and i), from control mice (a–c) or triple-transgenic mice SP-C-rtTA/(tetO)Cre/Zap (d, f, g, and i) and SP-C-rtTA/(tetO)Cre/ZEG (e and h) after doxycycline treatment from E6.5 to E8.5 (d–f) or E8.5 to E10.5 (g–i). No AP or GFP staining was seen in controls. At E11.5, AP-labeled cells seen after treatment with doxycycline from E6.5 to E8.5 were highly restricted to small clusters along the bronchial tubes and to the tips of the lobar bronchi (d). At higher magnification, small clusters of GFP-immunostaining cells were noted at these locations (e). This same period of doxycycline exposure resulted in nearly complete labeling of peripheral lung with exclusion of large conducting and extrapulmonary airways at E18.5 (f). After doxycycline exposure, from E8.5 to E10.5, more extensive AP (g) or GFP (h) was seen in proximal and peripheral lung tubules at E11.5. Consistent with the more widespread labeling of extrapulmonary conducting airways seen at E18.5, patchy labeling of the peripheral lung parenchyma was also noted at E18.5 (i). [Bars = 200 μm, (a, d, g), 10 μm, (b, e, h), and 20 μm (c, f, i).]
Cre-Mediated Labeling of the Trachea and Bronchi Later in Lung Morphogenesis.
A subset of cells in the trachea and bronchi were consistently labeled when the mice were exposed to doxycycline from, but not before, E8.5 to E10.5 and E10.5–12.5. Labeling was less extensive in the proximal trachea. Labeled cells were located in discrete longitudinal stripes, the number of labeled cells increasing in a proximal–distal gradient along the airway (Figs. 1c and 5 g and h). These findings support the concept that a distinct subset of conducting airway cells entered the SP-C-labeling field later in development (E8.5–10.5) (Fig. 6 g–i). The lack of labeled cells in the conducting airway after earlier exposures to doxycycline defines a temporal limit on doxycycline-induced Cre activity. Because these conducting airway cells were not labeled during doxycycline exposure between E6.5 and E8.5, they are not derived from migration of peripheral cells labeled earlier in development. Estimates of an approximately 2-day lag in extinguishing labeling after removal of doxycycline is consistent with the timing of decreased Cre mRNA and staining (Fig. 2).
Tracheal-Bronchial Glands and Neuroepithelial Bodies.
Labeling of tracheal glands was never observed (Fig. 3c). Clusters of neuroendocrine cells (neuroepithelial bodies) in peripheral conducting airways stained for CGRP (17), but did not express GFP (Fig. 3c). Thus, neither glands nor neuroepithelial cells share a common lineage with other peripheral epithelial cells that were extensively labeled by expression of Cre under control of the SP-C-rtTA transgene (Fig. 3 e and f).
Discussion
Cre was directed to lung precursor cells to label progenitor cells of the respiratory epithelium during endoderm differentiation and lung morphogenesis. Cell lineages lining bronchioles, acini, and alveoli were distinguished from conducting airway cell lineages lining trachea and bronchi early in development, before formation of lung buds. Remarkably, expansion of a small, spatially restricted, subset of progenitor cells located along the bronchial tubes at sites of lateral budding and at the tips of the lung buds produced nearly the entire repertoire of respiratory epithelial cells of the peripheral lung. Cells lining the peripheral lung shared labeling relationships with thymus and thyroid but not with other foregut derivatives including trachea, bronchi, esophagus, and other gastrointestinal organs. These nonoverlapping proximal and distal lung cell lineages were identified after exposure to doxycycline before E6.5, likely before completion of gastrulation. Later in development, a second subset of respiratory epithelial cells in the extrapulmonary conducting airways was labeled (E8.5–12.5), identifying two distinct lineages of cells in the conducting airways. Tracheal-bronchial glands and neuroepithelial cells were never labeled, distinguishing their lineage from the peripheral subset of lung epithelial cells. This model provides a system for addition or deletion of genes of interest in the lung epithelium, and provides methodology useful for cell lineage analysis and study of gene function during lung morphogenesis, oncogenesis, and repair.
In the present experiments, labeling of precursor cells depends on the pharmacodynamics of doxycycline, the timing of activity of the fragment of the SP-C promoter used to express the reverse tetracycline transactivator protein, and activity of the (tetO)7CMV construct used to express Cre. SP-C mRNA and protein are expressed in a highly lung-specific manner in various species including mouse and human (17–19). In earlier studies, neither endogenous SP-C mRNA nor SP-C promoter-driven transgene expression were detected in trachea, extrapulmonary conducting airways, thyroid, thymus, or in other foregut derivatives when assessed from E10 and thereafter (13). Thus, the early Cre-mediated labeling of epithelial cells represents recombination in progenitors during early embryogenesis. The human SP-C element used for the present experiments contains cis-acting elements active earlier than the endogenous SP-C gene (13), perhaps lacking inhibitory elements present in the normal SP-C locus. Although the present work does not provide formal proof, the early timing and restriction of labeling in thymus, thyroid, and peripheral lung is most consistent with recombination in shared precursors well before formation of lung buds. Thus, for the present experiments, the human SP-C element does not recapitulate endogenous SP-C expression, but fortuitously contains elements useful in identifying early lineage relationships and the timing of cell-type restriction.
Recombination in the Postnatal Lung.
Continuous exposure of adult mice to doxycycline resulted in detectable but relatively rare labeling of respiratory epithelial cells despite the fact that the SP-C promoter is highly active in adult bronchiolar and alveolar type II cells, and that rtTA and Cre recombinase expression by the SP-C element was observed in the postnatal lung (refs. 13, 17, and 18; data not shown). Postnatally, Cre-dependent labeling was generally observed in isolated clusters of cells that included type I and ciliated cells, both terminally differentiated cells that do not express SP-C. It is unclear whether the lower number of labeled cells observed reflects a requirement for proliferation for Cre-mediated recombination, an intrinsic resistance to recombination, or slow cell turnover with lack of expansion of late-labeled precursors in the mature lung. In the present experiments, the extent of recombination differed developmentally and was not directly correlated with activity of the human SP-C promoter or cell division. In the alveolar regions, some spontaneous recombination was observed in the postnatal period without exposure to doxycycline. The lesser extent of labeled cells observed after treatment with doxycycline in the postnatal period suggests that individually labeled cells do not amplify greatly thereafter.
Estimation of Timing of Recombination.
We chose 48-h-dosing windows to provide time for adequate transfer of doxycycline and activation of Cre in the fetal lung. However, the precise timing of recombination is complicated by the pharmacodynamics of doxycycline, the stability of induced rtTA mRNA, activity of Cre, and, perhaps, replication rates and fates of the targeted cells. Previous studies from our laboratory demonstrated activation of luciferase by SP-C-rtTA in the fetal lung occurs within 6–12 h after exposure to doxycycline, whereas activity of target genes was decreased or absent 24 h after removal of the drug (12, 14, 15). In the present experiments, widespread expression of Cre mRNA and protein were detected in the fetal lung, and both were decreased or absent 48–96 h after removal from doxycycline. Although the precise temporal window for doxycycline labeling is unclear, it most likely extends at least 48 h after withdrawal of doxycycline but not substantially beyond. These labeling periods or windows are functionally defined and clearly demarcated, because distinct patterns of labeling were exhibited between each of the 2-day periods. For example, patchy, lobar-restricted labeling was seen from E0 to E2.5, more extensive labeling from E2.5–4.5, and virtually complete labeling from E4.5 to E6.5 or E6.5 to E8.5, indicating distinct periods in which all or only some progenitor cells were marked. Thus, if labeling after a short exposure from E0 to E2.5 extended through to E6.5–8.5, more widespread or homogenous labeling would have been observed after early exposure to doxycycline. Likewise, tracheal-bronchial labeling was not observed after labeling from E6.5 to E8.5, but was reproducibly seen during all 2-day periods of doxycycline exposure after E8.5. Thus, the labeling period cannot extend beyond 2–4 days after removal of doxycycline. The differential labeling observed after each 2-day period of doxycycline exposure provides a reasonable temporal estimate for the time of labeling of precursor cells and for marking peripheral vs. proximal lung progenitors before or during gastrulation. Thus, lineage restriction of proximal and peripheral lung structures may occur remarkably early in development, well before formation of the lung primordia.
Implications for Cell and Gene Replacement.
Knowledge of the lineage relationships and timing of restriction among respiratory epithelial cells has been limited by the lack of clearly defined markers or precursors that give rise to the numerous distinct cell types found in the mature respiratory epithelium. The present study identifies a few highly, spatially restricted precursors that ultimately produce virtually all cells lining the periphery of the lung. Because of their capacity to form the gas-exchange region of the lung, peripheral progenitor cells may serve as therapeutic targets for the introduction of genetic material for correction of a genetic or acquired lung disease in the future. Identification of lung progenitor cells and determination of the timing and extent of their distribution provides a conceptual framework that may be useful for optimizing strategies for stem cell or gene therapy for genetic and acquired pulmonary diseases. The early commitment of proximal–distal epithelial cell lineages and paucity of progenitor cells in the postnatal lung, however, complicates strategies for gene or stem cell therapy in the lung. The highly restricted nature of the peripheral precursor cells detected at E11.5 and their remarkable ability to expand to form the majority of cells lining the peripheral lung makes them an attractive target for gene transfer only during early lung morphogenesis. The rarity of recombination later in the perinatal and postnatal period suggests a lack of active progenitors with significant capacity for contribution to the respiratory surface during late development or in the adult. Finally, this system for conditional regulation of Cre-mediated gene expression will be highly useful for study of gene function in general.
Acknowledgments
We thank Paula Blair, Jean Carter, and Ann Maher for assistance and Glenn Fishmann for construction of the (tetO)7Cre plasmid. This work was supported by F. W. F. Schroedinger (A.-K.T.P.), National Institutes of Health (J.A.W. and A.-K.T.P.), the Cystic Fibrosis Foundation (J.A.W. and S.E.W.), and National Institutes of Health Grant HL56387 (to J.A.W. and S.E.W.).
Abbreviations
Cre, Cre recombinase
GFP, green fluorescent protein
AP, alkaline phosphatase
rtTA, reverse tetracycline transactivator
E, embryonic day
PN, postnatal day
CGRP, calcitonin gene-related peptide
SP, surfactant protein
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Wells J. M. & Melton, D. A. (1999) Annu. Rev. Cell. Dev. Biol. 15, 393-410. [DOI] [PubMed] [Google Scholar]
- 2.Wells J. M. & Melton, D. A. (2000) Development (Cambridge, U.K.) 127, 1563-1572. [DOI] [PubMed] [Google Scholar]
- 3.Lawson K. A., Meneses, J. J. & Pedersen, R. A. (1986) Dev. Biol. 115, 325-339. [DOI] [PubMed] [Google Scholar]
- 4.Perl A.-K. T. & Whitsett, J. A. (1999) Clin. Genet. 56, 14-27. [DOI] [PubMed] [Google Scholar]
- 5.Warburton D., Schwarz, M., Tefft, D., Flores-Delgado, G., Anderson, K. D. & Cardoso, W. V. (2000) Mech. Dev. 92, 55-81. [DOI] [PubMed] [Google Scholar]
- 6.Hogan B. L. M. (1999) Cell 96, 225-233. [DOI] [PubMed] [Google Scholar]
- 7.Costa R. H., Kalinichenko, V. V. & Lim, L. (2001) Am. J. Physiol. 280, L823-L838. [DOI] [PubMed] [Google Scholar]
- 8.Whitsett J. (1998) Nat. Genet. 20, 7-8. [DOI] [PubMed] [Google Scholar]
- 9.Sauer B. (1998) Methods Companion Methods Enzymol. 14, 381-392. [Google Scholar]
- 10.Novak A., Guo, C., Yang, W., Nagy, A. & Lobe, C. G. (2000) Genesis 28, 147-155. [PubMed] [Google Scholar]
- 11.Lobe C. G., Koop, K. E., Kreppner, W., Lomeli, H., Gertsenstein, M. & Nagy, A. (1999) Dev. Biol. 208, 281-292. [DOI] [PubMed] [Google Scholar]
- 12.Perl A.-K. T., Tichelaar, J. W. & Whitsett, J. A. (2002) Transgenic Res. 11, 21-29. [DOI] [PubMed] [Google Scholar]
- 13.Wert S. E., Glasser, S. W., Korfhagen, T. R. & Whitsett, J. A. (1993) Dev. Biol. 156, 426-443. [DOI] [PubMed] [Google Scholar]
- 14.Clark J. C., Tichelaar, J. W., Wert, S. E., Itoh, N., Perl, A.-K. T., Stahlman, M. T. & Whitsett, J. A. (2001) Am. J. Physiol. 280, L705-L715. [DOI] [PubMed] [Google Scholar]
- 15.Tichelaar J. W., Lu, W. & Whitsett, J. A. (2000) J. Biol. Chem. 275, 11858-11864. [DOI] [PubMed] [Google Scholar]
- 16.Khoor A., Gray, M. E., Singh, G. & Stahlman, M. T. (1996) J. Histochem. Cytochem. 44, 1429-1438. [DOI] [PubMed] [Google Scholar]
- 17.Glasser S. W., Korfhagen, T. R., Wert, S. E., Bruno, M. D., McWilliams, K. M., Vorbroker, D. K. & Whitsett, J. A. (1991) Am. J. Physiol. 261, L349-L356. [DOI] [PubMed] [Google Scholar]
- 18.Khoor A., Stahlman, M. T., Gray, M. E. & Whitsett, J. A. (1994) J. Histochem. Cytochem. 42, 1187-1199. [DOI] [PubMed] [Google Scholar]
- 19.Glasser S. W., Burhans, M. S., Eszterhas, S. K., Bruno, M. D. & Korfhagen, T. R. (2000) Am. J. Physiol. 278, L933-L945. [DOI] [PubMed] [Google Scholar]