Abstract
Previous studies using rabbits and ferrets found that electrical stimulation of the pontine nuclei or middle cerebellar peduncle could serve as a conditioned stimulus (CS) in eyeblink conditioning (Bao, Chen, & Thompson, 2000; Hesslow, Svensson, & Ivarsson, 1999; Steinmetz, 1990; Steinmetz, Lavond, & Thompson, 1985; 1989; Steinmetz et al., 1986; Tracy, Thompson, Krupa, & Thompson, 1998). The current study used electrical stimulation of the pontine nuclei as a CS to establish eyeblink conditioning in rats. The goals of this study were to develop a method for directly activating the CS pathway in rodents and to compare the neural circuitry underlying eyeblink conditioning in different mammalian species. Rats were given electrical stimulation through a bipolar electrode implanted in the pontine nuclei paired with a periorbital shock unconditioned stimulus (US). Paired training was followed by extinction training. A subset of rats was given a test session of paired training after receiving an infusion of muscimol into the anterior interpositus nucleus. Rats given paired presentations of the stimulation CS and US developed CRs rapidly and showed extinction. Muscimol infusion prior to the test session resulted in a reversible loss of the eyeblink CR. The results demonstrate that electrical stimulation of the pontine nuclei can be used as a CS in rodents and that the CS pathway is similar in rats, rabbits, and ferrets. In addition, the loss of CRs following muscimol inactivation shows that the conditioning produced with pontine stimulation depends on cerebellar mechanisms.
Keywords: learning, memory, cerebellum, pontine nucleus, pontine stimulation
Introduction
The central neural pathway for a tone conditioned stimulus (CS) in eyeblink conditioning originates in the cochlear nuclei (Steinmetz, Logan, Rosen, Thompson, Lavond, & Thompson, 1987). The cochlear nuclei then project to the pontine nuclei, which transmit auditory stimulation to the cerebellar nuclei and the granule cells of the cerebellar cortex via mossy fibers (Mihailoff, 1993; McCrea, Bishop, & Kitai, 1977; Steinmetz et al., 1987; Steinmetz & Sengelaub, 1992). The axons of granule cells, the parallel fibers, synapse with Purkinje cells. Electrical stimulation of the pontine nuclei or mossy fibers serves as a sufficient CS for conditioning with a peripheral US in rabbits and ferrets (Bao et al., 2000; Hesslow et al., 1999; Steinmetz, 1990; Steinmetz et al. 1985; 1986; 1989; Tracy et al. 1998). Mossy fiber stimulation has also been used as an effective CS when paired with stimulation of the climbing fiber pathway as the US in rabbits (Steinmetz et al. 1989). The induction of eyeblink conditioning in rabbits with direct stimulation of the mossy and climbing fiber projections demonstrates that these cerebellar afferents are the most likely pathways used during conditioning with peripheral stimuli.
Associative learning that is established using pontine or mossy fiber stimulation as a CS is abolished by lesions of the cerebellar interpositus nucleus in rabbits (Steinmetz et al. 1986). The loss of CRs following interpositus damage indicates that stimulation of the mossy fiber pathway most likely produced learning through its projection to the cerebellum rather than through antidromic stimulation of pontine afferents or through stimulation of neighboring nuclei. Additional evidence in support of the view that mossy fiber stimulation produces cerebellar learning comes from a study that demonstrated learning-specific changes in interpositus nucleus neuronal activity when pontine stimulation was used as a CS in rabbits (Steinmetz, 1990). The learning-specific neuronal activity in the interpositus nucleus was similar during trials with the stimulation CS and trials in which a tone was substituted for the stimulation CS. Stimulation of mossy fibers, therefore, produces cerebellum-dependent learning through afferent stimulation that is functionally equivalent to the afferent stimulation produced by presentation of a tone CS.
The goals of the current study were to develop a method for obtaining eyeblink conditioning with direct stimulation of the CS pathway in rats and to compare the neural circuitry underlying eyeblink conditioning in different mammalian species. The application of the pontine stimulation paradigm to rats could be useful for quantitative analyses of the CS pathway in studies that typically use rodent models such as developmental studies of eyeblink conditioning (e.g., Freeman & Nicholson, 2004), genetic/molecular biological studies in mice (e.g., Chen et al., 1996; Chen, Bao, & Thompson, 1999), and studies of the behavioral effects of early ethanol exposure (e.g., Green, Johnson, Goodlett, & Steinmetz, 2002; Green, Rogers, Goodlett, & Steinmetz, 2000). In the current experiment, rats were given electrical stimulation through a bipolar electrode implanted in the pontine nuclei (300 ms, 0.1 ms pulses, at 200 Hz) paired with a periorbital shock US (25 ms, 2.5 mA). Paired training was followed by two 100-trial extinction sessions. A subset of rats was given an infusion of muscimol (1.0 μl, 2.0 mM) into the anterior interpositus nucleus followed by a test session of paired training. Muscimol infusions were used to determine whether CRs established using pontine stimulation as a CS would be abolished by cerebellar inactivation.
Method
Subjects
The subjects were 13 male Long-Evans rats (250–400 g). The rats were housed in the animal colony in Spence Laboratories of Psychology at the University of Iowa. All rats were maintained on a 12-hr light/dark cycle with light onset at 7:00 a.m. and given ad libitum access to food and water. All procedures used in this study were approved by the Institutional Animal Care and Use Committee at the University of Iowa.
Surgery
One week before training, rats were removed from their home cages and anesthetized with an i.p. injection of sodium pentobarbital (60 mg/kg). In order to reduce respiratory tract secretions and excess salivation during anesthesia, the rats were also given injections of atropine sulfate (0.45 mg/kg). After the onset of anesthesia, the rats were fitted with differential electromyograph (EMG) electrodes that were implanted in the left orbicularis oculi muscle in the upper eyelid. The reference electrode was attached to a stainless steel skull screw. The EMG electrode leads terminated in gold pins in a plastic connector. A bipolar stimulating electrode (PlasticsOne, Roanoke, VA) for delivering the shock US was implanted subdermally, caudal to the left eye. A second bipolar stimulating electrode was implanted in the right pontine nucleus. The stereotaxic coordinates for the pontine nuclei taken from bregma were 6.9 mm posterior, 1.5 mm lateral, and 10.2 mm ventral to the skull surface. In three rats, a 23-gauge guide cannula was implanted .5 mm dorsal to the left anterior interpositus nucleus. A 30-gauge stylet was inserted into the guide cannula. The stereotaxic coordinates for the anterior interpositus nucleus taken from bregma were 11.5 mm posterior, 2.3 mm lateral, and 5.2 mm ventral to the skull surface. The plastic connector housing the EMG electrode leads, the bipolar stimulating electrodes, the guide cannula, and three screws were secured to the skull with dental acrylic (Dentsply International, Inc., York, PA). The rats were given 0.006% Sulfatrim® (Alpharma, Inc., Baltimore, MD) in water for four days following surgery.
Stimulation Procedure
Electrical stimulation of the pontine nucleus functioned as the CS, which was administered in a 200 Hz train of 0.1 ms monophasic pulses for 300 ms. The stimulation threshold for the CS was found by setting the stimulating current at 50 μA, and either increasing or decreasing the current in ten mA increments, until a slight movement was detected (Tracy et al., 1998). Observable movements included, but were not limited to, eye blinks and head movements. The level of stimulation during training was set at half the movement threshold intensity (range 60–120 μA).
Muscimol Infusion Procedure
Prior to the muscimol infusions, the stylet was removed from the guide cannula and replaced with a 30-gauge infusion cannula. The infusion cannula was connected to polyethylene tubing (PE 10, 110 to 120 cm), which was connected to a 10 ml gas tight syringe (Hamilton, Reno, NV). The syringe was placed in an infusion pump (Harvard Apparatus, Holliston, MA) and 1.0 ml muscimol (2.0 nmol) was infused at a rate of 30 ml/hr. The tubing connected to the infusion cannula was cut and sealed with candle wax. The infusion cannula remained in place for the duration of the experimental session.
Apparatus
The conditioning apparatus consisted of a small animal sound-attenuating chamber (BRS/LVE, Laurel, MD). Within the sound-attenuating chamber was a small animal operant chamber (BRS/LVE) in which the rats were kept during conditioning. One wall of the operant chamber was fitted with two speakers that independently produce tones of up to 120 dB (SPL), with a frequency range of approximately 1000–9000 Hz. The back wall of the sound-attenuating chamber was equipped with a small light (6 W). An exhaust fan on one of the walls provided a 65 dB masking noise.
The electrode leads from the rat’s head stage were connected to peripheral equipment by lightweight cables that allowed the rat to move freely during conditioning. A desktop computer was connected to the peripheral equipment. Computer software controlled the delivery of stimuli and recorded eyelid EMG activity (JSA Designs, Raleigh, NC). An output hardware circuit permitted the delivery of a shock stimulus through a stimulus isolator (Model number 365A, World Precision Instruments, Sarasota, FL). An input hardware circuit amplified differentially (gain = 2000; sampling rate = 250 Hz), filtered (500–5000Hz), and integrated (time constant = 20 ms) the EMG activity.
Conditioning Procedure
The rats were given adaptation to the apparatus for 10 min before each training session. Daily training sessions included 100 trials of classical delay eyeblink conditioning with an intertrial interval of 30 s (range 18–42 s). The training sessions consisted of ten blocks of ten trials. Of the ten trials in each block, there were nine trials consisting of paired presentations of the CS and US and one CS-alone trial. During paired trials, a 300 ms stimulation train delivered to the pontine nuclei served as the CS and coterminated with a 25 ms shock US, yielding an interstimulus interval of 275 ms. The CS-alone trials were included to assess behavioral responses (integrated EMG activity) uncontaminated by the unconditioned response (UR; Gormezano, Kehoe, & Marshall, 1983). The three rats given muscimol received infusions after the third training session. The infusion session was followed by a recovery training session with no infusion. The last two training sessions for all rats included 100 CS-alone trials (extinction). The digitized response data were the voltage values of integrated EMG activity sampled every 2.5 ms with a time constant of 20 ms. The CR threshold was set at 0.4 V above the amplified and integrated EMG activity at baseline. The EMG baseline was usually zero (except for the DC offset) because the orbicularis oculi muscle does not exhibit spontaneous or tonal activity. Integrated EMG responses exceeding the threshold value during the first 80 ms of the CS period were considered startle responses to the tone CS; responses that exceeded the threshold value during the last 220 ms of the CS were considered CRs; and responses that crossed the threshold after US-onset were defined as URs.
Data Analysis
The behavioral data were examined for each training session. Repeated measures analysis of variance (ANOVA) was performed for the CR percentage, CR amplitude, CR onset latency, and CR peak latency. The amplitude and latency data were taken from CS-alone trials in which a response occurred. Significant differences were evaluated by Tukey’s honestly significant difference (HSD) test (all p’s < .05).
Histology
On the day after training, the rats were euthanized with a lethal injection of sodium pentobarbital (120 mg/kg) and transcardially perfused with approximately 100 ml of physiological saline followed by approximately 300 ml of 3% formalin. After perfusion, the brains were post-fixed in the same fixative for a minimum of 48 hrs, and subsequently sectioned at 50 μm with a sliding microtome. Sections were then stained with cresyl violet. The locations of the stimulating electrodes were confirmed by examining serial sections.
Results
Rats given pontine stimulation as the CS acquired eyeblink conditioning rapidly, reaching asymptotic performance by the second training session (Figure 1). The increase in CR percentage was paralleled by a decrease in CR onset latency and CR peak latency (Figure 2). The peak of the eyelid EMG response occurred within 50 ms of the onset time of the US during the second and third training sessions. Extinction was rapid and responding continued to decrement during the second day of extinction training (Figures 1 and 2).
Fig. 1.
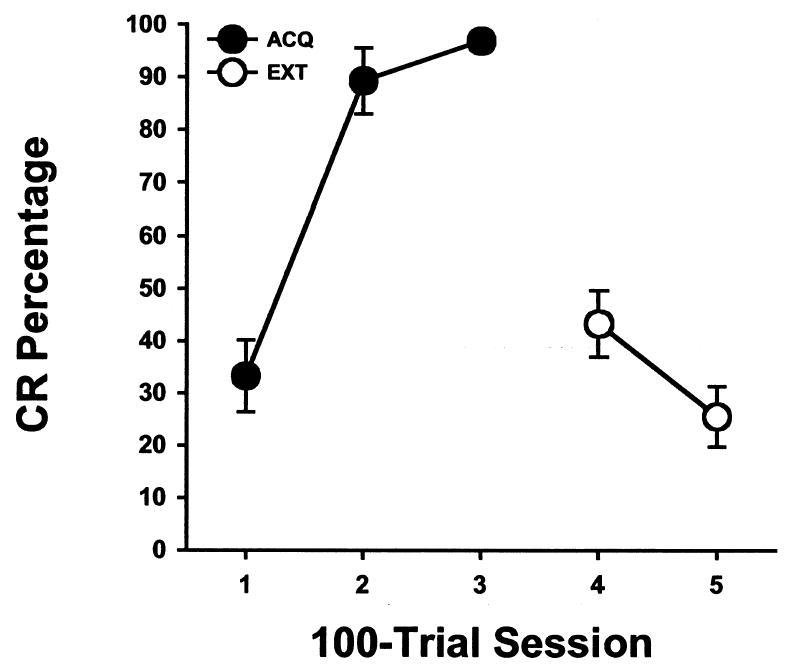
Mean (± SE) eyeblink conditioned response (CR) percentage for rats given pontine stimulation as the conditioned stimulus during three acquisition training sessions (ACQ) and two CS-alone extinction sessions (EXT).
Fig. 2.
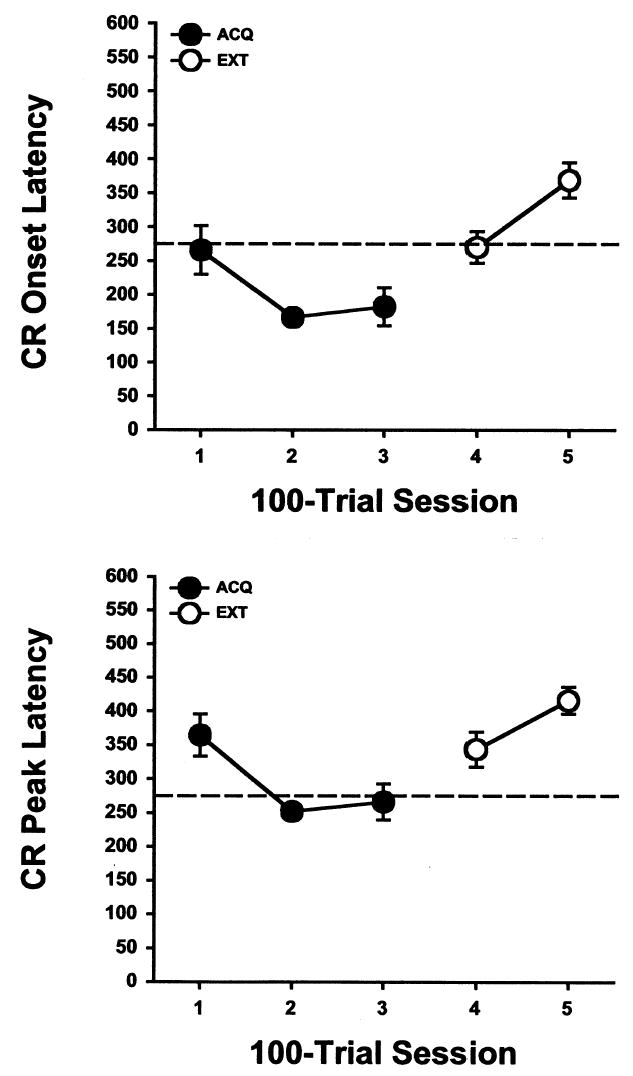
Mean (± SE) eyeblink conditioned response (CR) onset latency (upper) and peak latency (lower) for rats given pontine stimulation as the conditioned stimulus during three acquisition training sessions (ACQ) and two CS-alone extinction sessions (EXT). The dashed line indicates the onset time of the unconditioned stimulus.
The behavioral data were examined statistically with repeated measures analysis of variance (ANOVA). Analysis of the CR percentage data during training and extinction revealed an effect of the Training Session factor, F(4, 40) = 38.228, p < .0001. The Training Session effect was due to a significant increase in CR percentage from Session 1 to Session 2 and a significant decrease in CR percentage from Session 3 to the first extinction session (Session 4) and from Session 4 to Session 5 (all comparisons, p < .05).
Analyses of CR onset and peak latencies also revealed an effect of the Training Session factor, F(4, 40) = 10.164, p < .0001 and F(4, 40) = 8.631, p < .0001, respectively. The onset and peak latencies of the CR decreased from Session 1 to Session 2 and then increased from Session 3 to Session 4 (all comparisons, p < .05). A further increase in CR onset and peak latencies was observed from Session 4 to Session 5 (both comparisons, p < .05).
The rats given muscimol infusions into the cerebellar deep nuclei during the fourth training session showed nearly complete loss of CRs followed by full recovery of CR percentage on the following day (Figure 3). This group then exhibited a reduction in responding during the extinction sessions. An ANOVA of the CR percentage data revealed an effect of the Training Session factor, F(4, 8) = 18.449, p < .0001. The Training Sessions effect was due to an increase in CR percentage from Session 1 to Session 2, a decrease in CRs from Session 3 to the muscimol test session (Session 4) and a subsequent increase from the muscimol test session to the recovery session (Session 5, all comparisons, p < .05).
Fig. 3.

Mean (± SE) eyeblink conditioned response (CR) percentage for rats given pontine stimulation as the conditioned stimulus during three acquisition training sessions (ACQ), a session following muscimol infusion into the anterior interpositus nucleus (MUS), a recovery session with no drug infusion (REC), and two CS-alone extinction sessions (EXT).
The bipolar electrode was successfully implanted in the pontine nuclei in 11 of the 13 rats (Figure 4). Most of the electrodes were placed in the lateral pontine nucleus and supported rapid conditioning. The four electrodes that were in the ventral nucleus also supported robust conditioning. Two of the placements were ventral to the pontine nuclei and did not support conditioning.
Fig. 4.
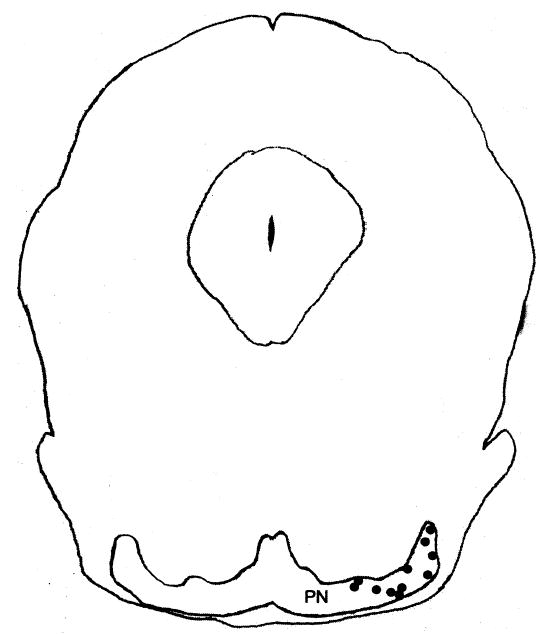
Coronal section of the rat brainstem depicting the electrode placements (black dots) in the pontine region. PN, pontine nuclei.
Discussion
Rats trained with electrical stimulation of the pontine nuclei as a CS acquired eyeblink CRs rapidly. All of the rats reached asymptotic levels of conditioning by the second day of training. As seen with tone and light CSs, the onset and peak response latencies decreased as the CR was acquired and the peak of the CR coincided with the onset time of the US (Gormezano et al., 1983; Kehoe & Napier, 1991). Extinction was evident during the two 100-trial sessions.
The rate of conditioning with the stimulation CS was more rapid than the rate of conditioning seen when a tone CS is used (e.g., Nicholson, Sweet, & Freeman, 2003), a finding that was also observed in pontine stimulation experiments that used rabbits (Steinmetz et al., 1986). However, the timing of the CRs from conditioning with the stimulation CS is very similar to that seen during conditioning with a tone CS (Nicholson et al., 2003). The CR onset latency was 185.4 ms for a tone CS and 182.3 ms for the stimulation CS. The latency of the peak amplitude of the CR was 276.0 ms for a tone CS and 266.1 ms for the stimulation CS. The latency data indicate that the stimulation CS produces CRs that are very similar to the CRs produced by conditioning with a tone CS, as seen in the rabbit studies.
Inactivation of the cerebellum with muscimol reversibly abolished CRs established through conditioning with the stimulation CS. The reversible loss of CRs following muscimol inactivation suggests that the conditioning established during eyeblink conditioning with a pontine stimulation CS requires cerebellar activity for performance of the CR. The demonstration of cerebellar dependence for conditioning with a stimulation CS helps rule out the possibility that stimulation of the pontine nuclei induced conditioning by antidromic stimulation of pontine afferents or through stimulation of non-cerebellar efferent projections. However, cerebellar inactivation alone does not conclusively establish that the memory underlying conditioning with the stimulation CS is stored in the cerebellum. Further studies that reversibly inactivate the red nucleus are needed to establish that the memory for conditioning with a pontine stimulation CS is stored in the cerebellum in rats.
The demonstration of eyeblink conditioning with pontine stimulation as the CS in rats and rabbits suggests that CS pathways are similar in these species. However, the degree of similarity in the functional organization of the pons in rats and rabbits is not clear. A developmental study of unit activity in the pontine nuclei during eyeblink conditioning in infant rats found pontine neurons that respond robustly to a tone CS in the lateral, dorsal lateral, and ventral pontine nuclei (Freeman & Muckler, 2003). In contrast, robust auditory responses in the pons are primarily restricted to the lateral and dorsal lateral nuclei in rabbits (Bao et al., 2000; Clark, Gohl, & Lavond, 1997; Steinmetz et al., 1987). It is possible that the auditory projection from the cochlear nuclei to the pontine nuclei in rats is organized differently than in rabbits. However, a comprehensive analysis of the auditory CS pathway using anatomical tract tracing, auditory evoked potentials, and discrete lesions has only been conducted in rabbits (Steinmetz et al., 1987). The paucity of evidence concerning the specific auditory CS pathway in rats makes it difficult to definitively determine whether the stimulation sites used in the current study activated auditory inputs to the cerebellum or some other stimulus modality.
The results of the current study provide further evidence that the neural circuitry underlying eyeblink conditioning in different mammalian species is similar. It is well established that lesions of the rabbit cerebellar interpositus nucleus abolish CRs and prevent acquisition of CRs (for review see Christian & Thompson, 2003). Lesions of the mouse and rat cerebellar nuclei also prevent eyeblink conditioning (Chen et al., 1996; 1999; Freeman, Carter, & Stanton, 1995; Lee & Kim, 2004; Skelton, 1988) and cerebellar pathology produces conditioning deficits in humans (Daum et al., 1993). Unit recording studies demonstrated that neurons in the rat cerebellum show activity profiles during eyeblink conditioning that are very similar to the conditioning-specific activity profiles seen in rabbits (Berthier & Moore, 1986; 1990; Freeman & Nicholson, 1999; Gould & Steinmetz, 1996; McCormick, Clark, Lavond, & Thompson, 1982; McCormick & Thompson, 1984a,b; Nicholson & Freeman, 2002; 2003a; 2004; Rogers, Britton, & Steinmetz, 2001). Studies using adult and infant rats also showed that neurons in the pontine nuclei respond to tones and develop conditioning-specific patterns of activity during eyeblink conditioning, as seen in rabbits (Bao et al, 2000; Clark et al., 1997; Freeman & Muckler, 2003; Freeman & Nicholson, 1999; McCormick, Lavond, & Thompson, 1983). The rapid cerebellum-dependent conditioning seen in the current study with stimulation of the pontine nuclei as a CS suggests that the primary CS pathway for eyeblink conditioning in rats is the mossy fiber projection to the cerebellum, as observed in rabbits and ferrets (Hesslow et al., 1999; Steinmetz et al., 1987). The US pathway in rodents has been investigated primarily in developing rats (Freeman & Nicholson, 2004). These developmental studies provide evidence using unit recording and stimulation that the inferior olive and its climbing fiber projection to the cerebellum are the US pathway in rats, which is consistent with the previously identified US pathway in rabbits (Kim, Krupa, & Thompson, 1998; Mauk, Steinmetz, & Thompson, 1986; Nicholson & Freeman, 2003a,b; Sears & Steinmetz, 1991; Steinmetz et al., 1989). The studies cited above suggest a strong concordance between rabbit, rat, and ferret neural circuitry underlying eyeblink conditioning. However, some of the eyeblink neural circuitry including the red nucleus has not been examined thoroughly in rats.
Footnotes
This research was supported by National Institute for Neurological Disorders and Stroke grant NS38890 and National Institute for Mental Health grant MH065483 to JHF
References
- Bao S, Chen L, Thompson RF. Learning- and cerebellum-dependent neuronal activity in the lateral pontine nucleus. Behavioral Neuroscience. 2000;114:254–261. doi: 10.1037//0735-7044.114.2.254. [DOI] [PubMed] [Google Scholar]
- Berthier NE, Moore JW. Cerebellar Purkinje cell activity related to the classically conditioned nictitating membrane response. Experimental Brain Research. 1986;63:341–350. doi: 10.1007/BF00236851. [DOI] [PubMed] [Google Scholar]
- Berthier NE, Moore JW. Activity of deep cerebellar nuclear cells during classical conditioning of nictitating membrane extension in rabbits. Experimental Brain Research. 1990;83:44–54. doi: 10.1007/BF00232192. [DOI] [PubMed] [Google Scholar]
- Chen L, Bao S, Lockard JM, Kim JJ, Thompson RF. Impaired classical eyeblink conditioning in cerebellar lesioned and Purkinje cell degeneration (pcd) mutant mice. Journal of Neuroscience. 1996;16:2829–2838. doi: 10.1523/JNEUROSCI.16-08-02829.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Bao S, Thompson RF. Bilateral lesions of the interpositus nucleus completely prevent eyeblink conditioning in Purkinje cell-degeneration mutant mice. Behavioral Neuroscience. 1999;113:204–210. doi: 10.1037//0735-7044.113.1.204. [DOI] [PubMed] [Google Scholar]
- Christian KM, Thompson RF. Neural substrates of eyeblink conditioning: acquisition and retention. Learning & Memory. 2003;10:427–455. doi: 10.1101/lm.59603. [DOI] [PubMed] [Google Scholar]
- Clark RE, Gohl EB, Lavond DG. The learning-related activity that develops in the pontine nuclei during classical eye-blink conditioning is dependent on the interpositus nucleus. Learning and Memory. 1997;3:532–544. doi: 10.1101/lm.3.6.532. [DOI] [PubMed] [Google Scholar]
- Daum I, Schugens MM, Ackermann H, Lutzenberger W, Dichgans J, Birbaumer N. Classical conditioning after cerebellar lesions in humans. Behavioral Neuroscience. 1993;107:748–756. doi: 10.1037//0735-7044.107.5.748. [DOI] [PubMed] [Google Scholar]
- Freeman JH, Jr, Carter CS, Stanton ME. Early cerebellar lesions impair eyeblink conditioning in developing rats: differential effects of unilateral lesions on postnatal day 10 or 20. Behavioral Neuroscience. 1995;109:893–902. doi: 10.1037//0735-7044.109.5.893. [DOI] [PubMed] [Google Scholar]
- Freeman JH, Jr, Muckler AS. Developmental changes in eyeblink conditioning and neuronal activity in the pontine nuclei. Learning & Memory. 2003;10:337–345. doi: 10.1101/lm.63703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman JH, Jr, Nicholson DA. Neuronal activity in the cerebellar interpositus and lateral pontine nuclei during inhibitory classical conditioning of the eyeblink response. Brain Research. 1999;833:225–233. doi: 10.1016/s0006-8993(99)01547-4. [DOI] [PubMed] [Google Scholar]
- Freeman JH, Jr, Nicholson DA. Developmental changes in the neural mechanisms of eyeblink conditioning. Behavioral and Cognitive Neuroscience Reviews. 2004;3:3–13. doi: 10.1177/1534582304265865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gormezano I, Kehoe EJ, Marshall BS. Twenty years of classical conditioning research with the rabbit. Progress in Psychobiology and Physiological Psychology. 1983;10:197–275. [Google Scholar]
- Gould TJ, Steinmetz JE. Changes in rabbit cerebellar cortical and interpositus nucleus activity during acquisition, extinction, and backward classical eyelid conditioning. Neurobiology of Learning and Memory. 1996;65:17–34. doi: 10.1006/nlme.1996.0003. [DOI] [PubMed] [Google Scholar]
- Green JT, Rogers RF, Goodlett CR, Steinmetz JE. Impairment in eyeblink classical conditioning in adult rats exposed to ethanol as neonates. Alcoholism: Clinical & Experimental Research. 2000;24:438–447. [PubMed] [Google Scholar]
- Green JT, Johnson TB, Goodlett CR, Steinmetz JE. Eyeblink classical conditioning and interpositus nucleus activity are disrupted in adult rats exposed to ethanol as neonates. Learning & Memory. 2002;9:304–320. doi: 10.1101/lm.47602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesslow G, Svensson P, Ivarsson M. Learned movements elicited by direct stimulation of cerebellar mossy fiber afferents. Neuron. 1999;24:179–185. doi: 10.1016/s0896-6273(00)80831-4. [DOI] [PubMed] [Google Scholar]
- Kehoe EJ, Napier RM. Temporal specificity in cross-modal transfer of the rabbit nictitating membrane response. Journal of Experimental Psychology: Animal Behavior Processes. 1991;17:26–35. [PubMed] [Google Scholar]
- Kim JJ, Krupa DJ, Thompson RF. Inhibitory cerebello-olivary projectionsand blocking effect in classical conditioning. Science. 1998;279:570–573. doi: 10.1126/science.279.5350.570. [DOI] [PubMed] [Google Scholar]
- Lee T, Kim JJ. Differential effects of cerebellar, amygdalar, and hippocampal lesions on classical eyeblink conditioning in rats. Journal of Neuroscience. 2004;24:3242–3250. doi: 10.1523/JNEUROSCI.5382-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauk MD, Steinmetz JE, Thompson RF. Classical conditioning using stimulation of the inferior olive as the unconditioned stimulus. Proceedings of the National Academy of Sciences. 1986;83:5349–5353. doi: 10.1073/pnas.83.14.5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Clark GA, Lavond DG, Thompson RF. Initial localization of the memory trace for a basic form of learning. Proceedings of the National Academy of Sciences. 1982;79:2731–2735. doi: 10.1073/pnas.79.8.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Lavond DG, Thompson RF. Neuronal responses of the rabbit brainstem during performance of the classically conditioned nictitating membrane (NM)/eyelid response. Brain Research. 1983;271:73–88. doi: 10.1016/0006-8993(83)91366-5. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Thompson RF. Cerebellum: essential involvement in the classically conditioned eyelid response. Science. 1984a;223:296–299. doi: 10.1126/science.6701513. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Thompson RF. Neuronal responses of the rabbit cerebellum during acquisition and performance of a classically conditioned nictitating membrane-eyelid response. Journal of Neuroscience. 1984b;11:2811–2822. doi: 10.1523/JNEUROSCI.04-11-02811.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrea RA, Bishop GA, Kitai ST. Electrophysiological and horseradish peroxidase studies of precerebellar afferents to the nucleus interpositus anterior. II. mossy fiber system. Brain Research. 1977;122:215–228. doi: 10.1016/0006-8993(77)90290-6. [DOI] [PubMed] [Google Scholar]
- Mihailoff GA. Cerebellar nuclear projections from the basilar pontine nuclei and nucleus reticularis tegmenti pontis as demonstrated with PHA-L tracing in the rat. Journal of Comparative Neurology. 1993;330:130–146. doi: 10.1002/cne.903300111. [DOI] [PubMed] [Google Scholar]
- Nicholson DA, Freeman JH Jr. Neuronal correlates of conditioned inhibition of the eyeblink response in the anterior interpositus nucleus. Behavioral Neuroscience. 2002;116:22–36. [PubMed] [Google Scholar]
- Nicholson DA, Freeman JH., Jr Addition of inhibition in the olivocerebellar system and the ontogeny of a motor memory. Nature Neuroscience. 2003a;6:532–537. doi: 10.1038/nn1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson DA, Freeman JH., Jr Developmental changes in evoked Purkinje cell complex spike responses. Journal of Neurophysiology. 2003b;90:2349–2357. doi: 10.1152/jn.00481.2003. [DOI] [PubMed] [Google Scholar]
- Nicholson DA, Freeman JH., Jr Developmental changes in eyeblink conditioning and simple spike activity in the cerebellar cortex. Developmental Psychobiology. 2004;44:45–57. doi: 10.1002/dev.10149. [DOI] [PubMed] [Google Scholar]
- Nicholson DA, Sweet JA, Freeman JH., Jr Long-term retention of the classically conditioned eyeblink response in rats. Behavioral Neuroscience. 2003;117:871–875. doi: 10.1037/0735-7044.117.4.871. [DOI] [PubMed] [Google Scholar]
- Rogers RF, Britton GB, Steinmetz JE. Learning-related interpositus activity is conserved across species as studied during eyeblink conditioning in the rat. Brain Research. 2001;905:171–177. doi: 10.1016/s0006-8993(01)02532-x. [DOI] [PubMed] [Google Scholar]
- Sears LL, Steinmetz JE. Dorsal accessory inferior olive activity diminishes during acquisition of the rabbit classically conditioned eyelid response. Brain Research. 1991;545:114–122. doi: 10.1016/0006-8993(91)91276-7. [DOI] [PubMed] [Google Scholar]
- Skelton RW. Bilateral cerebellar lesions disrupt conditioned eyelid responses in unrestrained rats. Behavioral Neuroscience. 1988;102:586–590. doi: 10.1037//0735-7044.102.4.586. [DOI] [PubMed] [Google Scholar]
- Steinmetz JE. Neuronal activity in the rabbit interpositus nucleus during classical NM-conditioning with a pontine-nucleus-stimulation CS. Psychological Science. 1990;1:378–382. [Google Scholar]
- Steinmetz JE, Lavond DG, Thompson RF. Classical conditioning of the rabbit eyelid response with mossy fiber stimulation as the conditioned stimulus. Bulletin of the Psychonomic Society. 1985;23:245–248. [Google Scholar]
- Steinmetz JE, Lavond DG, Thompson RF. Classical conditioning in rabbits using pontine nucleus stimulation as a conditioned stimulus and inferior olive stimulation as an unconditioned stimulus. Synapse. 1989;3:225–233. doi: 10.1002/syn.890030308. [DOI] [PubMed] [Google Scholar]
- Steinmetz JE, Logan CG, Rosen DJ, Thompson JK, Lavond DG, Thompson RF. Initial localization of the acoustic conditioned stimulus projection system to the cerebellum essential for classical eyelid conditioning. Proceedings of the National Academy of Sciences. 1987;84:3531–3535. doi: 10.1073/pnas.84.10.3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz JE, Rosen DJ, Chapman PF, Lavond DG, Thompson RF. Classical conditioning of the rabbit eyelid response with a mossy fiber stimulation CS. I. Pontine nuclei and middle cerebellar peduncle stimulation. Behavioral Neuroscience. 1986;100:878–887. doi: 10.1037//0735-7044.100.6.878. [DOI] [PubMed] [Google Scholar]
- Steinmetz JE, Sengelaub DR. Possible conditioned stimulus pathway for classical eyelid conditioning in rabbits. Behavioral and Neural Biology. 1992;57:103–115. doi: 10.1016/0163-1047(92)90593-s. [DOI] [PubMed] [Google Scholar]
- Tracy JA, Thompson JK, Krupa DJ, Thompson RF. Evidence of plasticity in the pontocerebellar conditioned stimulus pathway during classical conditioning of the eyeblink response in the rabbit. Behavioral Neuroscience. 1998;112:267–285. doi: 10.1037//0735-7044.112.2.267. [DOI] [PubMed] [Google Scholar]


