Abstract
Objective. The Newborn Individualized Developmental Care and Assessment Program (NIDCAP) is widely used in neonatal intensive care units and comprises 85 discrete infant behaviors, some of which may communicate infant distress. The objective of this study was to identify developmentally relevant movements indicative of pain in preterm infants.
Methods. Forty-four preterm infants were assessed at 32 weeks' gestational age (GA) during 3 phases (baseline, lance/squeeze, and recovery) of routine blood collection in the neonatal intensive care unit. The NIDCAP and Neonatal Facial Coding System (NFCS) were coded from separate continuous bedside video recordings; mean heart rate (mHR) was derived from digitally sampled continuous electrographic recordings. Analysis of variance (phase × gender) with Bonferroni corrections was used to compare differences in NIDCAP, NFCS, and mHR. Pearson correlations were used to examine relationships between the NIDCAP and infant background characteristics.
Results. NFCS and mHR increased significantly to lance/squeeze. Eight NIDCAP behaviors also increased significantly to lance/squeeze. Another 5 NIDCAP behaviors decreased significantly to lance/squeeze. Infants who had lower GA at birth, had been sicker, had experienced more painful procedures, or had greater morphine exposure showed increased hand movements indicative of increased distress.
Conclusions. Of the 85 NIDCAP behaviors, a subset of 8 NIDCAP movements were associated with pain. Particularly for infants who are born at early GAs, addition of these movements to commonly used measures may improve the accuracy of pain assessment.
Major advances in neonatal care now enable a high proportion of preterm infants to survive. During a time when medical concerns focused primarily on stabilizing the physiologic needs of preterm infants, Als, a developmental psychologist, developed a theory and systematic method of assessing the developmental needs of preterm newborns, the Newborn Individualized Developmental Care and Assessment Program (NIDCAP).1 Als hypothesized that early exposure to stress, or the mismatch of the preterm infant brain with the environment, could be linked to the long-term developmental impairments reported in these children at school age.2 Since the early 1980s, the NIDCAP observation system has become widely used in neonatal intensive care units (NICUs) throughout North America and Europe.3,4 With this system, infant responses, which include motor behaviors, state organizational behaviors, and autonomically related indicators (eg, respiratory pattern), are recorded continuously in 2-minute time blocks before, during, and after a procedure.5
Although the NIDCAP is not designed specifically to assess pain, determining whether specific NIDCAP behaviors occur during acute pain might provide more accurate recognition of valid cues that may be useful for clinical pain assessment and pain management. For clinical reasons, accurate interpretation of preterm infant pain behaviors is crucial. For example, infants who are in pain or are “stressed” may be given sedatives or analgesics. Analgesics may act differently in the brain according to whether pain is present.6 Therefore, appropriate administration of analgesics only when pain is present may be critical for preventing unwanted long-term side effects of opioid use.7,8 Thus, identifying and treating pain in preterm infants is a high priority for caregivers in the NICU because early pain exposure may alter nociceptive pathways9,10 and may also contribute to changes in other areas of development.11,12
Although improved pain assessment and management is a clinical priority, accurate identification of pain responses is complex for a number of reasons. First, preterm infants respond with facial, motor, and physiologic changes to acute pain, but they differ from term-born infants in that their responses are of smaller magnitude, particularly at younger gestational ages (GAs).13,14 Second, preterm infants at earlier GAs may display different pain behaviors than infants at later GAs as a result of neurologic immaturity. Such behaviors may not be captured in the current pain scales because the behaviors chosen have been based on behaviors seen in term infants. Third, no physiologic or behavioral threshold specifically marks the presence of pain. Finally, although using a single pain index is easier for clinicians, the physiologic and behavioral responses of preterm infants to painful stimuli are often dissociated15; therefore, reliance on current pain indices may not capture the range of responses in this population.16 Currently, changes in facial activity, shifts in infant sleep/waking state, and physiologic indices of heart rate are the most promising biobehavioral pain indicators in preterm infants.17-20
In contrast to the research using facial activity and heart rate as pain cues, body movements have not been evaluated thoroughly. Some researchers include knee or leg flexion in their pain index,21-23 and others use a measure of total body movements.13 These measures are problematic because, first, flexion and extension are not pain specific; and, second, many movements are combined, thus making them more difficult to interpret. Alternatively, using specific behaviors from the NIDCAP to assess pain is appealing because it is an established tool that is developmentally appropriate for preterm infants, and the coding descriptors of each of the facial and body movements are very specific.
Several researchers have used the NIDCAP to assess body movements of infants in the NICU, but these studies either did not describe the procedures observed or did not include painful procedures.24-26 Recently, 1 study did examine NIDCAP behaviors in response to events that varied in degree of intrusiveness (endotracheal suctioning, chest physical therapy, diaper change, and nasogastric feed); however, this study used brief observation periods and did not include a pain procedure (eg, skin breaking).27 Only 2 studies have used the full NIDCAP to study pain in premature infants. The first of these investigations did not evaluate the system along with other reliable, valid behavioral and physiologic pain measures or take into account GA at assessment, baseline behavioral state, or handling before the invasive procedure.28 Although the second study measured pain responses over longer periods of time and compared the NIDCAP with other reliable infant biobehavioral pain measures, it included only 10 infants, and the time of each handling phase was not controlled.29 The purpose of the present study was to determine whether a subset of NIDCAP movements could be used as valid acute pain cues in preterm infants.
METHODS
Study Subjects
The infants were recruited by a NICU-trained research nurse, and written informed consent was obtained from the mother or other legal guardian according to a protocol approved by the Clinical Research Ethics Board of the University of British Columbia. The study sample included 44 preterm infants in the level III NICU in the Children's & Women's Health Centre of British Columbia (Vancouver, BC, Canada). The infants were ≤32 completed weeks' GA at birth, had no major congenital anomalies, and had no reported illicit maternal drug use during pregnancy. Infants who had received analgesics or sedatives within 72 hours of the assessment or who had significant intraventricular hemorrhage (grade III or IV) and/or parenchymal brain injury (periventricular leukomalacia) were excluded. All infants were 32 weeks' postconceptional age (PCA; ±7 days) at the time of testing. Infant characteristics are presented in Tables 1 to 3. Sample size estimates were calculated as though we were using a between-groups design; this provides a conservative estimate given that we used a repeated measures design. GPOWER30 was used to calculate the estimate, and effect sizes entered into the program were based on changes in Neonatal Facial Coding System (NFCS) scores during blood collection at 31 to 33 weeks.13 Using this method, 15 infants were needed to detect differences between each phase for a power of .90 with the statistical significance set at .05. For examining gender and associations of clinical variables, the sample size was increased to 44.
TABLE 1.
Demographic Characteristics (n = 44)
| Mean (SD) | Range | n (%) | |
|---|---|---|---|
| Birth weight, g | 1289 (388) | 590–2345 | |
| GA at birth, wk | 29.6 (2.0) | 25–32 | |
| Male gender | 23 (52) | ||
| Small for gestational age | 8 (18) | ||
| SNAP-II day 1 | 12 (9) | 0–34 | |
| SNAP-II day 3 | 3 (4) | 0–14 | |
| Ventilation, d | 5.34 (9) | 0–38 | |
| Other respiratory support, d | 8.5 (9) | 1–32 | |
| Dexamethazone, d | 0.05 (0.3) | 0–2 | |
| Pain exposure* | 60.36 (41) | 2–157 | |
| Morphine exposure† | 0.29 (0.67) | 0–3.99 | |
| Ethnicity (white) | 34 (77) | ||
| Maternal age, y | 32.1 (5.7) | 19–47 |
Number of invasive (skin-breaking) procedures from birth to the study day.
Morphine exposure = (daily average/kg per os dose/3 + daily average intravenous mg/kg) × days.
TABLE 3.
Characteristics of Earlier and Later Born Infants
| Characteristic | Early Born (<30 Weeks; n = 25; Mean [SD]) | Later Born (30–32 Weeks; n = 19; Mean [SD]) | P Value |
|---|---|---|---|
| NS indicates not significant. | |||
| Postnatal age on study day, d | 26.7 (11) | 7.1 (3) | <.0001 |
| Pain exposure* | 83 (41) | 30 (15) | <.001 |
| Ventilation, d | 8.9 (0.6) | 0.63 (1) | <.0001 |
| Other respiratory support, d | 12.8 (9) | 2.7 (3) | <.001 |
| No. of invasive procedures during 24 h before study day | 1.2 (1) | 1.9 (3) | NS |
| Time since last handling | 86.6 (55) | 96.4 (47) | NS |
Number of invasive (skin-breaking) procedures from birth to study day.
Measures
All infants were observed during blood collection that was required for clinical management. The 3 phases of the procedure included in this study were a baseline period of 6 minutes of no handling immediately before the first contact by the laboratory technician (baseline); a blood collection period of 6 minutes from insertion of the lancet into the heel, which included the heel lance and squeezing (lance/squeeze); and an undisturbed recovery period of 6 minutes from the last contact of the laboratory technician (recovery).
Infant State
Infant sleep/wake state was coded according to the NIDCAP protocol5: 1 = deep sleep; 2 = light sleep; 3 = drowsy; 4 = quiet awake; 5 = active awake; 6 = highly aroused/crying; and 7 = AA (prolonged respiratory pause >8 seconds). The predominant state during each 2-minute period was coded for each phase.
Facial Activity (NFCS)
The NFCS is a reliable, well-validated behavioral pain measure that is used widely in studies of term-born19,31 and preterm infants.13,14,32-34 Traditionally, the full NFCS has been applied for brief periods (eg, 20 seconds per phase) to capture the acute pain response. However, for this study, the frequency of NFCS brow bulge was coded continuously for 18 minutes using the Noldus Observer system35 (throughout 6 minutes of baseline, 6 minutes of lance/squeeze, and 6 minutes of recovery) to match the NIDCAP coding. Brow bulge was selected as a proxy for upper facial actions because it has been shown to correlate highly with the other upper facial actions of the NFCS.36 Lower facial actions were not used in that they are sometimes obscured in preterm infants. Videotapes were edited for coding in random order of events, and coders were blind to all clinical information about the infants and to events. To establish reliability, both the primary NFCS coder (L.H.) and the reliability coder were trained on the entire tool to achieve a reliability coefficient of .87.19 In addition, reliability coding was conducted on 20% of the sample. For data analysis, the frequency of NFCS brow bulge was summed across all infants for each 6-minute phase.
Full Body (NIDCAP)
The NIDCAP behaviors were coded continuously, from video recordings of each infant, for the 3 phases (baseline, lance/squeeze, and recovery), and coding was conducted blind to all clinical information. Following published NIDCAP procedures, the frequency of each infant's movements was recorded systematically in 2-minute time blocks.5 The primary coder (L.H.) was an occupational therapist, and the reliability coder was a physiotherapist, both of whom were NIDCAP certified. Reliability for the NIDCAP was initially established during the certification process.37 In addition, a randomly selected sample of 5% of NIDCAP video segments from the study (baseline segment, lance/squeeze segment, or recovery segment) was coded to evaluate reliability. NIDCAP reliability was calculated by determining the percentage of agreement of occurrence (both coders indicating the presence or absence of a behavior) within every 2-minute time segment during each 6-minute phase for each infant. Interrater agreement was 87%. Physiologic measures (heart rate) were recorded by custom computer software and so were not scored using the NIDCAP observation record.
Heart Rate
Continuous electrocardiographic activity was recorded from a single lead of surface electrocardiogram (lead II) and was digitally sampled at 360 Hz off-line using a specially adapted computer acquisition system. Custom physiologic signal processing software38 was used to acquire, process, and analyze heart rate. R waves were detected from the sampled electrocardiogram and were used to form a smoothed instantaneous 4-Hz time series as described previously.39 Mean heart rate was calculated for each 2-minute segment of each study period to correspond to the 2-minute NIDCAP time blocks and averaged over 6 minutes of each of the 3 phases (baseline, lance/squeeze, and recovery). Before statistical analysis, 25 (6%) of the 2-minute HR segments were dropped because of poor signal and were replaced by the group mHR for that phase.
Background Data
A NICU-trained research nurse completed the prospective clinical chart review and obtained information from birth to day of testing, including but not limited to the following: birth weight, GA at birth, Apgar score at 1 minute, illness severity using the Score for Neonatal Acute Physiology II (SNAP-II),40 amount of opioid and other analgesic and sedative exposure, numbers and types of invasive skin-breaking procedures, respiratory support, and type and time of last handling just before blood collection. Invasive procedures were defined as those involving skin breaking, such as heel lance, venipuncture, insertion of arterial and venous lines, lumbar puncture, and chest-tube insertion. In addition, number of endotracheal intubations was collected (Table 1). Study day characteristics of the infants are presented in Table 2.
TABLE 2.
Infant Characteristics on the Study Day (n = 44)
| Mean (SD) | Range | n (%) | |
|---|---|---|---|
| PCA on study day, wk | 32.3 (0.7) | 31–33 | |
| Postnatal age on study day, d | 18.2 (13) | 3–51 | |
| Mechanical ventilation on study day | 4 (9) | ||
| Time since last feed, min* | 58.8 (4) | 0–116 | |
| No. of painful procedures in 24 h before study day | 1.5 (1) | 0–11 | |
| Time since last invasive procedure, min | 1242.2 (1187) | 40–6690 |
Four infants were not on oral feeds.
Procedures
Each infant was lying in the incubator undisturbed for a period of at least 30 minutes before recording. Heart rate data were collected by attaching the leads from the bedside monitor to a custom-designed computer data acquisition system. Two cameras (one positioned for close-up on the face, the other on the full body) were attached to a custom-made recording set-up on a moveable cart, including two 9-in video monitors. The signals were fed directly to 2 VCRs, and a time code was imprinted automatically. Each study phase was marked with an inaudible event cue signal recorded simultaneously on the videotape and physiologic acquisition systems. During recording, the incubator was partially covered with a blanket, and the infant's position was supported (nested) using a continuous roll around both sides and feet. At the time the infants were studied, 10 were supine, 27 were prone, and 7 were side lying with the face and full body in view for video coding. The infant's position was not altered before or during the procedure, because handling to alter the position may affect the infant pain response.41 For the blood-collection procedure, the research nurse applied a foot-warming pack 5 minutes before the laboratory technician drew the blood. The research nurse determined which foot would be used for the blood collection according to which foot would be most easily accessed by the laboratory technician to minimize extreme stretching of the leg and foot during the procedure. Fifteen different laboratory technicians conducted the blood collection on the 44 infants. The laboratory technician's standard protocol involved checking the infant's identification band on the incubator, removing the warming pack from the foot, swabbing the heel with a small gauze pad with disinfectant, lancing the heel, and then gently squeezing the heel intermittently until the amount of blood that was required for clinical care was collected. A research technician set up the video camera and the VCR machines, operated the cardiac data acquisition computer, and marked each phase during the procedure.
Data Analysis
The frequencies of each NIDCAP behavior were reviewed, and the 31 movements that occurred in <25% of the infants were excluded from statistical analysis (Fig 1).27 Total frequencies of each NIDCAP movement were summed for each 6-minute phase to reveal in a clinically meaningful way the amount of infant movement exhibited throughout the procedure. Repeated measures analysis of variance was conducted across the 3 phases of blood collection, with gender examined as a between-subjects variable. Bonferroni corrections were used to correct for overall error. Statistically significant analysis of variance was followed by planned t tests for paired comparisons to identify differences between specific phases. Pearson product-moment correlations were used to examine associations between perinatal variables and to describe relationships between the NIDCAP and infant background characteristics during lance/squeeze.
Fig 1.
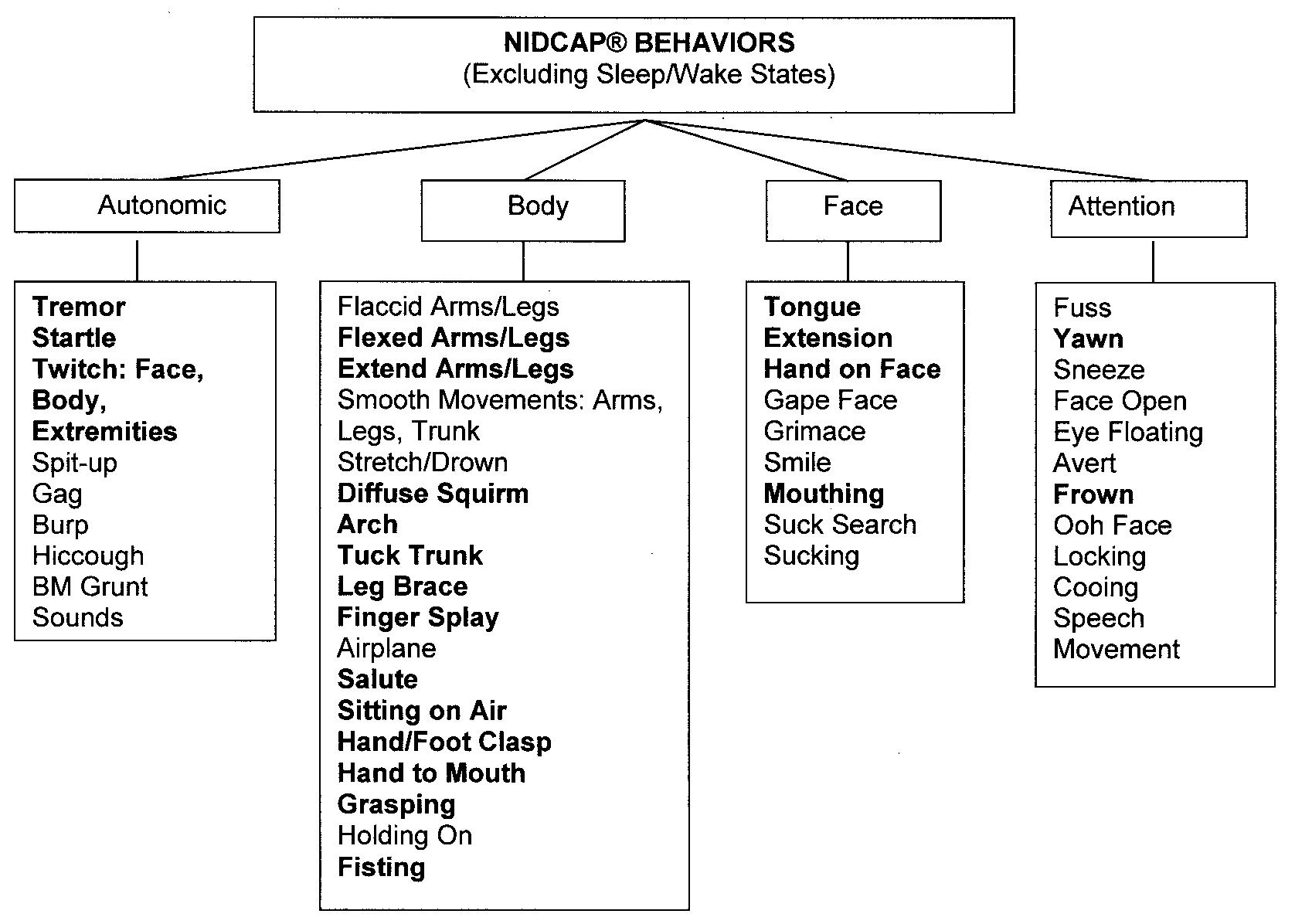
NIDCAP behaviors. Behaviors in bold were included in analyses.
RESULTS
Infant State
Most infants were in “sleep” (75%) or “drowsy” (25%) states during baseline, and then shifted significantly from baseline to lance/squeeze (t = -7.40, P < .0001). Seven percent of infants remained in “deep sleep” during lance/squeeze, no infants were in “active sleep,” 56% were “drowsy,” and 36% were crying. Sleep/wake state also shifted significantly from lance/squeeze to recovery (t = 6.43, P < .0001); 70% of infants returned to sleep, and only 2% remained highly aroused.
NFCS and NIDCAP
There were no statistically significant differences in gender with any of the behavioral measures. The frequency of NFCS brow bulge increased significantly across the 3 phases (F[1,43] = 49.43; P < .0001). Of the 25 NIDCAP behaviors included in the statistical analyses, the frequency of a subset of 8 NIDCAP behaviors—flex arms, flex legs, extend arms, extend legs, hand on face, finger splay, fisting, and frown—increased significantly across the 3 phases. The frequency of a second subset of 5 NIDCAP behaviors—twitch face, twitch body, twitch extremities, mouthing, and foot clasping—decreased significantly across the 3 phases. In addition, the frequency of 2 NIDCAP behaviors—diffuse squirms (F[1,43] = 5.22; P < .008) and arching (F[1,43] = 4.22; P < .03)—decreased significantly from lance/squeeze to recovery. Finally, during recovery, all but 1 of the NIDCAP behaviors returned to baseline frequencies; the frequency of fisting (t = -2.5, P < 0.02) remained increased. The total frequencies of the NIDCAP behaviors across the 3 phases of blood collection are shown in Table 4.
TABLE 4.
Changes in Frequency of NIDCAP Behaviors Across the Phases
| NIDCAP Behavior | Total Frequencies Across Phases |
|||
|---|---|---|---|---|
| Baseline | Lance/Squeeze | Recovery | P Value | |
| Flex arms | 38 | 88 | 49 | <.01 |
| Flex legs | 118 | 310 | 117 | <.0001 |
| Extend arms | 26 | 52 | 27 | <.02 |
| Extend legs | 103 | 216 | 79 | <.01 |
| Hand on face | 14 | 45 | 11 | <.001 |
| Finger splay | 42 | 86 | 51 | <.048 |
| Fisting | 0 | 12 | 7 | <.004 |
| Frown | 12 | 51 | 16 | <.0001 |
| Twitch face | 153 | 19 | 127 | <.0001 |
| Twitch body | 74 | 12 | 60 | <.001 |
| Twitch extremities | 371 | 39 | 287 | <.0001 |
| Mouthing | 22 | 8 | 36 | <.02 |
| Foot clasping | 18 | 1 | 16 | <.01 |
Heart Rate
Heart rate increased significantly across the 3 phases (F[1,43] = 40.67; P < .0001). Heart rate (mean ± standard deviation) increased from baseline (157.5 ± 12) to lance/squeeze (174.0 ± 20; t = - 6.74, P < .0001) and decreased during recovery (156.3 ± 16; t = 9.48, P < .0001).
Infant Background Characteristics
GA at birth and postnatal age (days) at testing were highly correlated (r = -0.91, P < .0001); GA at birth was used to examine the relationships between infant characteristics and the NIDCAP behaviors. Perinatal and study day characteristics of the earlier born and later born infants are shown in Table 3.
During blood collection at 32 weeks, infants who were born at lower GAs (<30 weeks) showed significantly higher frequencies of finger splay, fisting, and mouthing during lance/squeeze than infants who were born at later GAs. Infants who had been exposed to greater numbers of invasive procedures since birth showed significantly more finger splays and fisting. Moreover, infants who had greater opioid exposure displayed significantly more fisting and hand on face. Finally, infants who were sicker earlier in the neonatal course (SNAP-II day 1) displayed more facial twitches. Finally, infants who continued to be physiologically unstable on day 3 (SNAP-II day 3) continued to show more facial twitches, greater numbers of hand on face, and fisting than infants who became medically more stable (Table 5).
TABLE 5.
Correlations Between Infant Background Characteristics and NIDCAP Behaviors
| Infant Characteristics | NIDCAP Behavior | r | P Value |
|---|---|---|---|
| GA at birth | Finger splay | –0.39 | <.009 |
| Fisting | –0.42 | <.004 | |
| Mouthing | –0.42 | <.03 | |
| Illness severity (SNAP-II day 1) | Facial twitches | 0.33 | <.03 |
| Illness severity (SNAP-II day 3) | Facial twitches | 0.40 | <.01 |
| Hand on face | 0.35 | <.02 | |
| Fisting | 0.58 | <.0001 | |
| Pain exposure* | Finger splay | 0.37 | <.013 |
| Fisting | 0.40 | <.007 | |
| Morphine exposure† | Fisting | 0.57 | <.0001 |
| Hand on face | 0.34 | <.02 |
Number of invasive (skin-breaking) procedures from birth to study day.
Morphine exposure = (daily average/kg per os dose/3 + daily average intravenous mg/kg) × days.
DISCUSSION
Although the NIDCAP has been used widely in NICUs throughout North America and Europe since the 1980s, this study is the first to examine whether the NIDCAP catalog behaviors can be used to identify acute pain in preterm infants under well-controlled conditions using a relatively large sample. The infants in this study showed facial, behavioral state, and heart rate responses similar to responses documented in other studies of responses of preterm infants to acute pain.13,42 Although we found that a significant proportion (37%) of the NIDCAP movements either did not occur or occurred in <25% of the infants, this finding is consistent with another study that recently reported that more than one third of the movements described by the NIDCAP were not observed in infants <30 weeks' GA in the NICU even when observed sequentially over a 7-week period. However, these investigators did not specify the procedures that they observed.26
The most important finding of this study was that we found a subset of 8 NIDCAP movements that seem to be associated with acute pain in preterm infants. Although some of the 8 NIDCAP movements that we identified are included in other pain measures, others have not been described as behavioral pain cues before this study. For example, the increased frequency of flexing and extending the extremities has been reported in other studies that described and assessed pain responses in both term-born23,43 and preterm infants.22,28,44 In particular, extending the legs seems to be a consistently observed distress cue.27,29 The combined flexing and extending of the legs observed in these infants may be reflexive in nature (ie, the flexor withdrawal response),45,46 but because the flexor withdrawal response is not pain specific, the use of these movements for pain assessment is unlikely to improve the identification of pain if facial activity, sleep/wake state, and change in heart rate are also used.
As in previous studies, we found that finger splay increased during the lance phase. Moreover, finger splay may be a developmentally specific distress cue, because the infants who were born before 30 weeks' GA had a higher frequency of finger splays to lance than those who were born ≥30 weeks. These earlier born infants show greater finger splays not only during lance/squeeze but also during the baseline phase. This suggests that these infants may be relatively more stressed. This finding is consistent with previous studies that examined procedural stress and pain responses in this population27,29 and may be indicative of “sensitization” that results from greater early pain exposure.47,48
Fisting has been described in 1 other study that examined preterm infants' responses to pain.49 Furthermore, a recent study showed that fisting is considered by a majority of nurses to be a pain indicator.50 Like finger splay, fisting seems to be a sensitive distress cue in infants who are born at earlier gestational ages (<30 weeks) and may be useful in identification of pain in infants who are sicker, who have been exposed to greater numbers of invasive procedures, and who require opioids during their care. This finding is important because preterm infants' behavioral pain expression is influenced by their previous experience in the NICU; that is, infants who are born at earlier gestational ages and who have experienced greater numbers of painful procedures may show diminished facial responses to acute pain.14,42 In addition to fisting and finger splaying, which have been previously associated with painful experiences in the NICU, the movement of hand on face (which involves a defensive-like action with the infant placing a hand on its face) may represent an additional pain cue in the preterm population.
The only NIDCAP facial movement that increased significantly during lance/squeeze was frowning (brow lowering). Frowning is a flexor motion that involves knitting of the eyebrows or darkening of the eyes, a motion that is consistent with brow lowering shown in facial responses to pain.13,19
Whereas we found a subset of 8 NIDCAP behaviors associated with pain, we also found 5 behaviors that decreased significantly during the lance/squeeze phase. As in our previous study of stress during endotracheal suctioning, twitches decreased and startles did not change significantly during a more invasive procedure. Although in some infant pain scales, jitteriness and startles have been included with other behavioral indicators of pain,44,51 we conclude that, in preterm infants, these are not specific pain cues.27,29 Indeed, many fetal ultrasound studies show that twitches and startles are behaviors associated with sleep states in the normal fetus.52-54 Rather than indicators of pain, twitches are necessary movements for normal infant development that influence neuron cell death, synapse elimination, muscle fiber differentiation, and formation of topographic maps.55 Although we concluded that twitches may not be specific pain cues, infants who were sicker on day 1 and who remained sicker on day 3 had more facial twitches associated with the lance/squeeze phase at 32 weeks' PCA, a finding that has not been reported in previous studies. We cannot attribute this finding to the infants' being in active sleep during the lance, because none of our infants remained in this sleep state during blood collection.
Another NIDCAP behavior that decreased to lance/squeeze was foot clasping. This finding was not unexpected, because infants who are rapidly flexing and extending their legs are less likely to clasp their feet together. Similarly, the frequency of mouthing (more than 1 opening and closing of the mouth) decreased during lance/squeeze. Indeed, fetal ultrasound studies have shown that mouthing is a regularly observed movement in utero (ie, under optimal, nonstressed conditions).56,57
Like finger splay, the frequency of 2 behaviors—diffuse squirms and arching— occurred at high frequencies during baseline. However, they differed from finger splay in that they did not increase to lance/squeeze and furthermore dropped significantly during recovery. The high frequency of these movements during baseline may indicate a higher basal arousal in earlier born preterm infants; however, even infants who were born at ≥30 weeks and had little previous pain exposure showed these behaviors. It is possible that their diminished frequency during recovery was an indication of fatigue caused by the length of the procedure.
Before this study, most of the literature describing the responses of preterm infants to acute pain used very short periods of observation. Our findings demonstrated that many preterm infants remain in a higher state of physiologic and behavioral arousal not only during the tissue-damaging portion but also during the entire blood collection. It may be that this higher and sustained level of stress contributes to altered reactivity and self-regulation observed later in these children.11,58
Another important finding of this study is that we found increased body movements associated with acute pain in infants who were born at earlier gestational ages. This finding is contrary to the dampened facial activity that has been associated with infants of lower GAs36 and those who experience greater numbers of painful procedures.14,42 Increased body movements during pain may be indicative of increased pain sensitivity as a result of sensitization, which is then followed by the “wind-up” phenomenon, both of which are spinal cord-mediated effects.48
Although our study carefully controlled for age at assessment, length of assessment time, and procedure order, there remain some limitations. First, it was not possible to be blinded to events when coding body movements. However, with the use of a second video camera, facial coding was conducted blinded to events. Second, the infants' position during the lance was not controlled; more than half of the infants were positioned in prone during the assessment. It is standard practice in our nursery to promote prone positioning to support physiologic stability, particularly for infants with respiratory difficulties.59 We deliberately did not alter the position of the infant for the study because change of position would likely alter biobehavioral reactions. Although prone position does not seem to affect the facial responses during blood work,41 it may have altered the frequency of 1 NIDCAP body movement, sitting on air, an action whereby the legs are flexed at the hips and extended at the knees. This movement would be very unlikely to occur in prone.
CONCLUSION
The NIDCAP is a developmentally sensitive model of care that provides a detailed behavioral catalog of infant behaviors. We found a subset of 8 NIDCAP movements that were associated with acute pain in preterm infants at 32 weeks' PCA. By adding a few discrete body movements to the assessment of pain, particularly hand movements such as finger splay, fisting, and hand on face, we can use some elements of the NIDCAP assessment to provide additional behavioral cues that may make identifying pain in preterm infants more accurate, particularly for infants who are born at early GAs.
ACKNOWLEDGMENTS
This study was supported by National Institutes of Health Grant HD39783, Canadian Institutes of Health Research Grant MOP42469, Canadian Institutes of Health Research/Canadian Occupational Therapy Foundation Post-Doctoral Fellowship (Dr Holsti), and a Senior Scholar Award from the Michael Smith Foundation for Health Research (Dr Grunau).
We thank the staff and families of the Special Care Nursery at B.C.'s Children's Hospital for participation in this study; Colleen Fitzgerald, Study Co-ordinator; Gisella Gosse and Adi Amir for data collection; and Colleen Jantzen for carrying out reliability NFCS coding, all of whom are staff of the Biobehavioral Research Unit of Centre for Community Health and Health Evaluation Research, B.C. Research Institute for Children's & Women's Health. We also thank Linda Williams, Clinical Supervisor, Physiotherapy Department, B.C.'s Children's Hospital, for carrying out NIDCAP reliability coding.
ABBREVIATIONS
- NIDCAP
Newborn Individualized Developmental Care and Assessment Program
- NICU
neonatal intensive care unit
- GA
gestational age
- PCA
postconceptional age
- NFCS
Neonatal Facial Coding System
- SNAP-II
Score for Neonatal Acute Physiology II
REFERENCES
- 1.Als H. Toward a synactive theory of development: promise for the assessment and support of infant individuality. Infant Ment Health J. 1982;3:229–243. [Google Scholar]
- 2.Als H, Gilkerson L. The role of relationship-based developmentally supportive newborn intensive care in strengthening outcome of preterm infants. Semin Perinatol. 1997;21:178–189. doi: 10.1016/s0146-0005(97)80062-6. [DOI] [PubMed] [Google Scholar]
- 3.Symington A, Pinelli J. Developmental care for promoting development and preventing morbidity in preterm infants. Cochrane Database Syst Rev. 2003;(4):CD001814. doi: 10.1002/14651858.CD001814. [DOI] [PubMed] [Google Scholar]
- 4.Jacobs SE, Sokol J, Ohlsson A. The Newborn Individualized Developmental Care and Assessment Program is not supported by metaanalysis of the data. J Pediatr. 2002;140:699–706. doi: 10.1067/mpd.2002.123667. [DOI] [PubMed] [Google Scholar]
- 5.Als H. Manual for the Naturalistic Observation of Newborn Behavior (Preterm and Fullterm) The Children's Hospital; Boston, MA: 1984. [Google Scholar]
- 6.Rahman W, Fitzgerald M, Aynsley-Green A, Dickenson A. The effects of neonatal exposure to inflammation and/or morphine on neuronal responses and morphine analgesia in adult rats. In: Jensen TS, Turner JA, Weisenfeld-Halling Z, editors. Progress in Pain Research and Management. Vol. 8. IASP Press; Seattle, WA: 1997. pp. 738–794. [Google Scholar]
- 7.Ng E, Taddio A, Ohlsson A. Intravenous midazolam infusion for sedation of infants in the neonatal intensive care unit. Cochrane Database Syst Rev. 2003;(1):CD002052. doi: 10.1002/14651858.CD002052. [DOI] [PubMed] [Google Scholar]
- 8.Mao J. Opioid-induced abnormal pain sensitivity: implications in clinical opioid therapy. Pain. 2002;100:213–217. doi: 10.1016/S0304-3959(02)00422-0. [DOI] [PubMed] [Google Scholar]
- 9.Anand KJ, Coskun V, Thrivikraman KV, Nemeroff CB, Plotsky PM. Long-term behavioral effects of repetitive pain in neonatal rat pups. Physiol Behav. 1999;66:627–637. doi: 10.1016/s0031-9384(98)00338-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anand KJS. Effects of perinatal pain and stress. Prog Brain Res. 2000;122:117–129. doi: 10.1016/s0079-6123(08)62134-2. [DOI] [PubMed] [Google Scholar]
- 11.Grunau RE. Long-term consequences of pain in human neonates. In: Anand KJS, Stevens BJ, McGrath PJ, editors. Pain in Neonates. 2nd Revised and Enlarged Edition, Pain Research and Clinical Management. Vol. 10. Elsevier Science; New York, NY: 2000. pp. 55–76. [Google Scholar]
- 12.Grunau R. Early pain in preterm infants. A model of long-term effects. Clin Perinatol. 2002;29:373–394. doi: 10.1016/s0095-5108(02)00012-x. [DOI] [PubMed] [Google Scholar]
- 13.Craig KD, Whitfield MF, Grunau RV, Linton RV, Hadjistavropoulos HD. Pain in the preterm neonate: behavioral and physiological indices. Pain. 1993;52:287–299. doi: 10.1016/0304-3959(93)90162-I. [DOI] [PubMed] [Google Scholar]
- 14.Johnston CC, Stevens BJ. Experience in a neonatal intensive care unit affects pain response. Pediatrics. 1996;98:925–903. [PubMed] [Google Scholar]
- 15.Morison SJ, Grunau RE, Oberlander TF, Whitfield MF. Relations between behavioral and cardiac autonomic reactivity to acute pain in preterm neonates. Clin J Pain. 2001;17:350–358. doi: 10.1097/00002508-200112000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Porter FL, Wolf CM, Miller JP.Procedural pain in newborn infants: the influence of intensity and development Pediatrics 19991041Available at: www.pediatrics/org/cgi/content/full/104/1/e13 [DOI] [PubMed]
- 17.Stevens BJ, Johnston CC, Grunau RV. Issues of assessment of pain and discomfort in neonates. J Obstet Gynecol Neonatal Nurs. 1995;24:849–855. doi: 10.1111/j.1552-6909.1995.tb02570.x. [DOI] [PubMed] [Google Scholar]
- 18.Stevens B, Johnston CC, Petryshen P, Taddio A. The premature infant pain profile. Clin J Pain. 1996;12:13–22. doi: 10.1097/00002508-199603000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Grunau RE, Craig KD. Pain expression in neonates: facial action and cry. Pain. 1987;28:395–410. doi: 10.1016/0304-3959(87)90073-X. [DOI] [PubMed] [Google Scholar]
- 20.Franck LS, Greenburg CS, Stevens B. Pain assessment in infants and children. Acute pain in children. Pediatr Clin North Am. 2000;47:487–512. doi: 10.1016/s0031-3955(05)70222-4. [DOI] [PubMed] [Google Scholar]
- 21.Evans JC, Vogelpohl DG, Bourguignon CM, Morcott CS. Pain behaviors in LBW infants accompany some “non-painful” caregiving procedures. Neonatal Netw. 1997;16:33–40. [PubMed] [Google Scholar]
- 22.Lawrence J, Alcock D, McGrath PJ, Kay, MacMurray SB, Dulberg C. The development of a tool to assess neonatal pain. Neonatal Netw. 1993;12:59–66. [PubMed] [Google Scholar]
- 23.Franck LS. A new method to quantitatively describe pain behavior in infants. Nurs Res. 1986;35:28–31. [PubMed] [Google Scholar]
- 24.Cheng CM, Chapman JS. Assessment of reliability and validity for the behavioral observation record for developmental care. Nurs Res. 1997;46:40–45. doi: 10.1097/00006199-199701000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Peters KL. Bathing premature infants: physiological and behavioral consequences. Am J Crit Care. 1998;7:90–100. [PubMed] [Google Scholar]
- 26.Pressler JL, Helm JM, Hepworth JT, Wells NL. Behaviors of very preterm neonates as documented using NIDCAP observations. Neonatal Netw. 2001;20:15–24. doi: 10.1891/0730-0832.20.8.15. [DOI] [PubMed] [Google Scholar]
- 27.Grunau RE, Holsti L, Whitfield MF, Ling E. Are twitches, startles and body movements pain indicators in extremely low birth weight infants? Clin J Pain. 2000;16:37–45. doi: 10.1097/00002508-200003000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Van Cleve L, Andrews S. Pain responses of hospitalized neonates to venipuncture. MCN Am J Matern Child Nurs. 1995;20:148–152. doi: 10.1097/00005721-199505000-00013. [DOI] [PubMed] [Google Scholar]
- 29.Morison SJ, Holsti L, Grunau RE, et al. Are there developmentally distinct motor indicators of pain in preterm infants? Early Hum Dev. 2003;72:131–146. doi: 10.1016/s0378-3782(03)00044-6. [DOI] [PubMed] [Google Scholar]
- 30.Faul F, Erdfelder E. GPOWER: A priori-, post hoc-, and compromise power analyses for MS-DOS (Computer program) Bonn University; Bonn, Germany: 1998. [Google Scholar]
- 31.Grunau RE, Johnston CC, Craig KD. Neonatal facial and cry responses to invasive and non-invasive procedures. Pain. 1990;42:295–305. doi: 10.1016/0304-3959(90)91142-6. [DOI] [PubMed] [Google Scholar]
- 32.Lindh V, Wiklund U, Sandman P-O, Hakansson S. Assessment of acute pain in preterm infants by evaluation of facial expression and frequency domain analysis of heart rate variability. Early Hum Dev. 1997;48:131–142. doi: 10.1016/s0378-3782(96)01851-8. [DOI] [PubMed] [Google Scholar]
- 33.Stevens BJ, Johnston CC, Horton L. Factors that influence the behavioral pain responses of premature infants. Pain. 1994;59:101–109. doi: 10.1016/0304-3959(94)90053-1. [DOI] [PubMed] [Google Scholar]
- 34.Johnston CC, Stevens B, Craig KD, Grunau RE. Developmental changes in pain expression in premature, full-term, two-and four-month-old infants. Pain. 1993;52:201–208. doi: 10.1016/0304-3959(93)90132-9. [DOI] [PubMed] [Google Scholar]
- 35.The Observer, Base Package for Windows Reference Manual. Noldus Information Technology; Wageningen, The Netherlands: 1995. [Google Scholar]
- 36.Johnston CC, Stevens BJ, Yang F, Horton L. Differential response to pain by very premature neonates. Pain. 1995;61:471–479. doi: 10.1016/0304-3959(94)00213-X. [DOI] [PubMed] [Google Scholar]
- 37.Pressler JL, Hepworth JT. A quantitative use of the NIDCAP tool. Clin Nurs Res. 2002;11:89–102. doi: 10.1177/105477380201100107. [DOI] [PubMed] [Google Scholar]
- 38.HR View Software. Boston Medical Technologies; Brighton, MA: 1996. [Google Scholar]
- 39.Berger RD, Saul P, Cohen RJ. Transfer function analysis of autonomic regulation: canine atrial rate response. Am J Physiol. 1989;256:H142–H152. doi: 10.1152/ajpheart.1989.256.1.H142. [DOI] [PubMed] [Google Scholar]
- 40.Lee SK, Ohlsson A, Synnes AR, et al. Mortality variations and illness severity (SNAP II) in Canadian NICUs. Pediatr Res. 1999;45:248A. [Google Scholar]
- 41.Grunau RE, Linhares MBM, Holsti L, Oberlander TF, Whitfield MF. Does prone or supine position influence pain responses in preterm infants at 32 weeks gestational age? Clin J Pain. 2004;20:76–82. doi: 10.1097/00002508-200403000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grunau RE, Oberlander TF, Whitfield MF, Fitzgerald C, Lee SK. Demographic and therapeutic determinants of pain reactivity in very low birth weight neonates at 32 weeks' postconceptional age. Pediatrics. 2001;107:105–112. doi: 10.1542/peds.107.1.105. [DOI] [PubMed] [Google Scholar]
- 43.Rich EC, Marshal RE, Volpe JJ. The normal neonatal response to pinprick. Dev Med Child Neurol. 1974;16:432–434. doi: 10.1111/j.1469-8749.1974.tb03367.x. [DOI] [PubMed] [Google Scholar]
- 44.Sparshott MM. The development of a clinical distress scale for ventilated newborn infants: identification of pain and distress based on validated behavioural scores. J Neonat Nurs. 1996:5–10. [Google Scholar]
- 45.Fitzgerald M, Shaw A, McIntosh N. Postnatal development of the cutaneous flexor reflex: comparative study of preterm infants and new-born rat pups. Dev Med Child Neurol. 1988;30:520–526. doi: 10.1111/j.1469-8749.1988.tb04779.x. [DOI] [PubMed] [Google Scholar]
- 46.Andrews K, Fitzgerald M. Cutaneous flexion reflex in human neonates: a quantitative study of threshold and stimulus-response characteristics after single and repeated stimuli. Dev Med Child Neurol. 1999;41:696–703. doi: 10.1017/s0012162299001425. [DOI] [PubMed] [Google Scholar]
- 47.Andrews K, Fitzgerald M. The cutaneous withdrawal reflex in human neonates: sensitization, receptive fields, and the effects of contralateral stimulation. Pain. 1994;56:95–101. doi: 10.1016/0304-3959(94)90154-6. [DOI] [PubMed] [Google Scholar]
- 48.Fitzgerald M, Millard C, McIntosh N. Cutaneous hypersensitivity following peripheral tissue damage in newborn infants and its reversal with topical anaesthesia. Pain. 1989;39:36. doi: 10.1016/0304-3959(89)90172-3. [DOI] [PubMed] [Google Scholar]
- 49.Bozzette M. Observation of pain behavior in the NICU: an exploratory study. J Perinat Neonat Nurs. 1993;7:76–87. doi: 10.1097/00005237-199306000-00010. [DOI] [PubMed] [Google Scholar]
- 50.Howard VA, Thurber FW. The interpretation of infant pain: physiological and behavioral indicators used by NICU nurses. J Pediatr Nurs. 1998;13:164–174. doi: 10.1016/s0882-5963(98)80075-4. [DOI] [PubMed] [Google Scholar]
- 51.Halimaa SL, Vehviläinen-Julkunen K, Heinonen K. Knowledge, assessment and management of pain related to nursing procedures used with premature babies: questionnaire study for caregivers. Int J Nurs Pract. 2001;7:422–430. doi: 10.1046/j.1440-172x.2001.00322.x. [DOI] [PubMed] [Google Scholar]
- 52.Kisilevsky BS, Low JA. Human fetal behavior: 100 years of study. Dev Rev. 1998;18:1–29. [Google Scholar]
- 53.DiPietro JA, Hodgson DM, Costigan KA, Hilton SC. Fetal neurobehavioral development. Child Dev. 1996;67:2553–2567. [PubMed] [Google Scholar]
- 54.Visser GHA. The second trimester. In: Nijhuis JG, editor. Fetal Behavior. Developmental and Perinatal Aspects. Oxford Medical Publications, Oxford University Press; Oxford, UK: 1992. pp. 17–25. [Google Scholar]
- 55.Blumberg MS, Lucas DE. A developmental and component analysis of active sleep. Dev Psychobiol. 1996;29:1–22. doi: 10.1002/(SICI)1098-2302(199601)29:1<1::AID-DEV1>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 56.Roodenburg PJ, Wladimiroff JW, Prechtl HFR. Classification and quantitative aspects of fetal movements during the second half of normal pregnancy. Early Hum Dev. 1991;25:19–35. doi: 10.1016/0378-3782(91)90203-f. [DOI] [PubMed] [Google Scholar]
- 57.D'Elia A, Pighetti M, Moccia G, Santangelo N. Spontaneous motor activity in normal fetuses. Early Hum Dev. 2001;65:139–147. doi: 10.1016/s0378-3782(01)00224-9. [DOI] [PubMed] [Google Scholar]
- 58.Grunau RE, Oberlander TF, Whitfield MF, Fitzgerald C, Morison SJ, Saul JP. Pain reactivity in former extremely low birth weight infants at corrected age 8 months compared with term born controls. Infant Behav Dev. 2001;24:41–55. [Google Scholar]
- 59.Hutchinson AA, Ross KR, Russel G. The effect of posture on ventilation and lung mechanisms in preterm and light-for-date infants. Pediatrics. 1979;64:429–432. [PubMed] [Google Scholar]


