Abstract
Duchenne muscular dystrophy (DMD) is the most frequent, progressive disease caused by a genetic defect that leads to the production of a nonfunctional form of dystrophin, thereby causing premature death. Ways to improve, adapt, and facilitate the care of people with DMD are still being explored. This viewpoint, developed by an accredited Duchenne center, aims to present current telemedicine options specifically tailored for patients with DMD and to discuss the advantages and limitations of these approaches across various health care domains. As one of the first centers in Poland to implement such an approach, the accredited Duchenne center provides targeted home-based care by using digital platforms and telemedicine tools. Additionally, we explore the potential of telemedicine to support different types of remote communication, including provider-to-provider, between patient/caregiver and provider, and between patient/caregiver and patient/caregiver interactions. This model has the potential to significantly enhance access to specialized care and improve the continuity and quality of life for those living with DMD.
Keywords: neuromuscular disorders, Duchenne muscular dystrophy, telemedicine, eHealth, digital medicine
Introduction
A patient with Duchenne muscular dystrophy (DMD) has unique specific needs since the disease is not only chronic but also progressive and leads to premature death in the second or third decade of life. The essence of DMD is progressive muscle weakness caused by a genetic defect that leads to the production of a nonfunctional form of dystrophin. As a result, muscle cells are irreversibly damaged [1,2].
To date, there is no effective causal treatment for patients with DMD. Gene therapies such as exon skipping, stop codon read-through, or adeno-associated virus gene therapy are promising but limited by many factors, for example, the type of mutation [3]. Therefore, the basic treatment still remains—apart from corticosteroid therapy, multidisciplinary specialist care. Due to the interplay of muscles and organs, an interdisciplinary care team (according to the guidelines) should include a neurologist, pulmonologist, physiotherapist, cardiologist, dietitian, psychologist, endocrinologist, and other health workers [4,5]. The frequency of the follow-up visits depends on the patient’s clinical condition (at least once a year), but the increase in the survival rate has resulted in an increase in the severity of the disease and the need for more frequent contact with health care professionals [1,2,4]. This may be inconvenient or impossible, particularly for people with reduced mobility and/or who live a long way from the research center. Ways to improve, adapt, and facilitate the care of people with DMD are still being explored.
One way may be the use of eHealth, which is a rapidly growing field and can support many health care processes. As of 2012, only 31% of the hospitals and 15% of the outpatient clinics were using telemedicine services in the European Union [6,7]. Currently, digital health interventions are available for most people in the United States, Europe, Canada, and Australia [8,9]. Notably, the pandemic was a catalyst for the development of new forms of virtual medical care, and it significantly accelerated the introduction of telemedicine in patients with DMD.
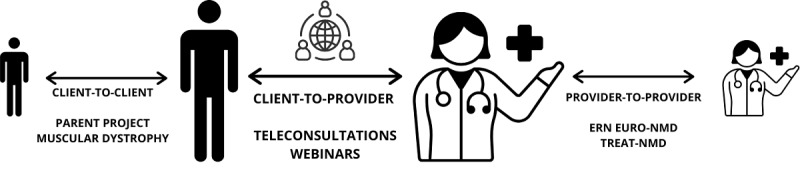
This up-to-date viewpoint based on the experience of our accredited center and collaboration with other centers for children with DMD aims to provide current telemedicine options dedicated to patients with DMD and discusses the pros and cons in different health care areas. We also explore the possible use of telemedicine services at a distance for communication conducted between 2 or more providers (provider-to-provider telemedicine), between patients/caregivers and providers (client-to-provider telemedicine), and between 2 or more patients/caregivers (client-to-client telemedicine).
In this paper, we have focused only on telemedicine due to its rapid development and increasing widespread use, even though it is only one of the components of the broadly understood eHealth according to the World Health Organization (WHO) [10].
Types of Telemedicine
Client-To-Provider Telemedicine
The rapid increase in the use of telemedicine during the COVID-19 pandemic was evident, and many countries implemented individualized DMD telecare approaches [11-16]. For example, in Italy, remote clinical evaluation and administration of functional scales were lacking for neuromuscular diseases before the pandemic [17], but these increased rapidly during the pandemic. Similarly, in France, teleconsultation guidelines underwent rapid changes, allowing various forms of teleconsultation, including telephone, whereas videoconferencing was the primary method used before the pandemic [18]. In Norway, at the same time, 14% of the medical professionals used telemedicine to contact a patient with a rare disease and monitor its development during the pandemic [19].
Many well-organized telemedicine programs have been related to other neuromuscular diseases. In 2014, in Massachusetts, TelePALS (Telemedicine for People with Amyotrophic Lateral Sclerosis) was performed with follow-up structured care via video televisits [20]. In another study in 2019, a trained nurse traveled to patients’ homes and performed and recorded video assessments for later review by members of the multidisciplinary team [21]. Yet another project in 2018 and 2020 included a mobile app for weekly surveys about symptoms, complications, noninvasive ventilation use, and gastrostomy use to communicate with clinic staff [22,23]. These projects showed that telemedicine is feasible and acceptable to both health care professionals and patients with amyotrophic lateral sclerosis. A telemedicine program in 2018 for another neuromuscular disease, facio-scapulo-humeral muscular dystrophy, has also shown improvement in the quality of life and the possibility of reducing hospitalizations [24].
Provider-To-Provider Telemedicine
Telemedicine has opened up opportunities for remote connections between health care professionals. The e-community of health care providers has become a common tool not only for scientific purposes but also for everyday clinical practice involving patients with DMD [25]. In Italy, during the COVID-19 pandemic, efforts were made to create a network for professionals and less-experienced doctors to enhance their knowledge of DMD [26]. This network also facilitated education through webinars and collaboration among distant researchers, projects, and clinical trials. Collaborative efforts between different centers to create large databases have helped in developing new medicines and policies for rare diseases. As of May 14, 2023, over 300 clinical trials on DMD have been registered on the clinical trial platforms (clinicaltrials.gov and clinicaltrialsregister.eu).
Another example of collaborative cooperation is the European Reference Networks established in 2017. These networks connect medical specialists across different disciplines through a dedicated information technology platform and telemedicine tools, effectively forming a virtual advisory board. EURO-NMD (European Reference Network for the thematic grouping of rare neuromuscular diseases) is a European Reference Network dedicated to rare neuromuscular diseases, aiming to facilitate diagnostic processes, standardize therapeutic approaches, and improve the efficiency and accessibility of health care systems [27] (Figure 1).
Figure 1.
EURO-NMD (European Reference Network for the thematic grouping of rare Neuromuscular Diseases). ERN: European Reference Network; NMD: neuromuscular disease.
Client-To-Client Telemedicine
Nearly 2 decades ago, the first teleproject was proposed in England to provide computer equipment and connectivity for families with DMD to reduce social isolation and to create a window into the world [28]. However, not all families were willing to participate in the program at that time. Since then, significant progress has been made in the use of electronic devices, equipment, social media, and applications. Organizations like the Parent Project of Muscular Dystrophy have developed their websites [29]. Technological barriers are diminishing, as mobile computing equipment becomes smaller and more affordable and wireless connectivity becomes more reliable and widespread. Currently, smartphones offer many possibilities to improve communication, access, and participation, and give a unique opportunity to directly deliver functionality to people with disabilities and their caregivers.
Applications of Telemedicine in Different Areas of DMD Health Care
Neurology
Neurological examinations for DMD require personal contact between doctors and patients, making virtual examinations during teleconsultations challenging [30]. Available data comparing virtual examinations to traditional physical examinations are limited, and standardized protocols for televisits are lacking in the literature [31-34].
To address these challenges, researchers have developed their televisit systems and protocols. Structured surveys such as the Brook and Vignos scales or Egen Scale Classification used for clinical evaluation and functionality can be useful for collecting standardized data [35-38]. Additionally, other scales commonly used in clinical trials (the 6-Minute Walk Test, Quantitative Muscle Testing, the DMD Functional Ability Self-Assessment Tool, or the Performance of the Upper Limb Scale) can be utilized in telemedicine for assessing muscle function and progression of disease [39-41]. For children or adult patients with advanced-stage DMD, the presence of a telepresenter during the virtual examination can be helpful [17,28].
Although telemedicine became prevalent during the pandemic, hybrid programs combining telemedicine with in-person care emerged after the pandemic. Nowadays, it is recommended that the initial diagnostic visit for patients with DMD takes place in-person at the center, with possible follow-up teleconsultations [42,43].
Pulmonology
Respiratory function is crucial for the quality and length of life of patients with DMD; therefore, annual evaluation of respiratory function is recommended for patients with gait preservation and every 6 months for those without or even more frequent if clinical symptoms dictate [44]. This includes collecting information on the type, number, and duration of respiratory infections, presence of cough reflex, daytime hypoventilation symptoms and sleep quality, as well as pulmonary function tests, including spirometry, peak cough flow, respiratory muscle strength (sniff nasal inspiratory pressure, maximal static inspiratory and expiratory pressures), and sleep study (polysomnography) with overnight capnography. Some of these procedures can be supported by telemedicine, for example, during virtual visits, health care professionals can provide information on respiratory care and collect data on chest infections and symptoms of hypoventilation or ineffective sleep (preferably using validated questionnaires, eg, Sleep-Disordered Breathing Questionnaire In Neuromuscular Patients [SiNQ‑5]) [45].
In a case series involving 3 patients with neuromuscular disorders—including one with DMD—video consultations, supported by baseline physiological monitoring, facilitated early identification of respiratory complications and helped avoid hospitalization [46]. Further, telemonitoring of pulmonary function by using portable devices at home (e-spirometry with assessment of forced vital capacity), in addition to traditional spirometry in reference centers, can be employed in patients with DMD [47,48].
Web-based care services could potentially be beneficial for adolescent and young adults in the later stages of the disease who require ventilatory support, aiding in decision-making and providing timely interventions for respiratory issues. A study conducted during the pandemic found that weekly video consultations (averaged 23 minutes) and ventilator telemonitoring successfully addressed most problems related to general clinical status and ventilation in a group of 21 children (including 5 with neuromuscular diseases), who relied on long-term ventilatory support (noninvasive and invasive) and living at home [49]. That study also identified challenges with telemedicine, particularly the absence of sufficient technology at home, such as low internet signal or lack of internet connectivity, which affected 17% of the participants [49]. Studies presenting comprehensive virtual respiratory care dedicated exclusively to patients with DMD are generally lacking. However, data from pediatric patients with other conditions requiring long-term respiratory support at home indicate that although clinical complications are common and respiratory decompensation is the most common cause, telemedicine can facilitate early diagnosis and treatment of life-threatening events [50]. In addition, it has been shown to reduce the frequency of hospitalizations and improve caregivers’ sense of security and confidence in home care among ventilated patients [51].
Cardiology
DMD can lead to significant cardiac complications, including cardiomyopathy, arrhythmias, and heart failure, which are all potentially lethal [52,53]. Early diagnosis and treatment of cardiomyopathy leads to favorable ventricular remodeling [54]. Because adverse myocardial changes can occur at a younger age than previously thought and due to the importance of diagnosis before overt cardiac dysfunction, yearly cardiac screening is recommended [54]. Cardiac diagnostics with electrocardiography, echocardiography, Holter monitoring, and magnetic resonance imaging require in-person visits. However, telemedicine can provide access to specialized care for patients who may not have local access to cardiologists [55]. Telemedicine enables remote monitoring of cardiac function and arrhythmias by using wearable sensors and implantable devices, allowing for timely interventions to prevent or treat cardiac complications [56].
Nutrition
Proper nutrition is vital for patients with DMD. Eating disorders at the early stage of the disease due to steroid therapy and immobilization cause concern for excessive body weight. At later stages of the disease, there is a risk of malnutrition due to the weakening of the muscles of the gastrointestinal tract [4]. Teleconsultations can involve assessing the nutritional status, monitoring diet and fluid intake, and providing nutritional counseling. Web-based surveys such as the Food Frequency Questionnaire-6 item questionnaire [57] are useful in collecting dietary data. Software applications can assist patients in monitoring their dietary goals and measurements [58]. These interventions can address both excessive body weight in the early stages of the disease and the risk of malnutrition in later stages.
Psychology
Psychological support is needed at every stage of chronic disease. Studies [59] have shown that psychological and peer support via telehealth programs such as video sessions are useful and helpful for people with DMD and their caregivers. Telehealth programs can include health promotion causing improvement in the health-related quality of life. One study demonstrated the benefits of virtually delivered health coaching sessions (the first session was face-to-face, followed by 4 virtual sessions) related to physical activity and diet for males with DMD [60].
Telerehabilitation
According to the World Physiotherapy and the International Network of Physical Therapy Regulatory Authorities, the purpose of digital physical therapy practice is to facilitate the effective delivery of physical therapy services by improving access to care and information and managing health care resources [61]. Telerehabilitation consists of consulting, diagnostics, and therapy conducted with the use of interactive digital technologies in real-time connection (synchronous) or through prerecorded videos (asynchronous). Caregiver involvement is crucial for individuals with DMD to ensure the proper execution of exercises during telerehabilitation [62]. Web-based video instructions are more acceptable than live workshops [63]. Another study showed that 8-week telerehabilitation sessions enhanced muscle strength in ambulatory patients with DMD and that regular web-based meetings with physiotherapists were more effective than prerecorded or streaming video exercises [64]. Telerehabilitation can be supported by telemonitoring in patients with DMD (eg, cardiovascular parameters with electrocardiogram [65], overall physical activity level, step activity with accelerometers [66], effects of respiratory rehabilitation with home spirometers [47]). Virtual reality, including video games, has proven to be a valuable assistive technology for home-based rehabilitation [47].
Terminal Phase
Some studies [67-69] have shown that telemedicine can be used at every stage of chronic disease, including patients in the terminal phase of the disease, both in adults and children. This is important because patients with DMD can present hospice phase and have a premature death at a young age. Hospice programs utilizing telemedicine have mostly focused on children with oncological diseases, but the benefits extend to patients with neuromuscular diseases even in the form of simple teleconsultations [67-73]. Devices with touch screens, large letters, and easy-to-understand pictograms have proven useful in telemedicine for patients in the terminal phase [74]. Despite this, patients with end-stage DMD (requiring always multidimensional palliative support) remain underrepresented in telehealth research. Formative studies are needed to develop and evaluate integrated telemedicine approaches that address the specific needs of these patients.
Home health and nursing services may complement telemedicine in end-stage DMD by enabling in-home assessments, medication administration, and laboratory sample collection. This type of care can serve as an effective bridge between virtual supervision and hands-on clinical support, ensuring continuity for patients with complex needs. Evidence from other chronic conditions shows that nurse-led telehomecare can reduce hospitalizations and improve the quality of life [75,76]. However, this hybrid approach remains underexplored in neuromuscular disorders. Future studies should evaluate its feasibility by using indicators such as hospital admissions, clinical stability, and caregiver satisfaction.
Summary
Telemedicine provides numerous benefits for individuals with DMD, their caregivers, and health care professionals. It facilitates seamless communication among medical professionals from different centers, resulting in faster information exchange and the sharing of valuable experiences. By creating communities on social media platforms, individuals with DMD and their caregivers can find mutual support and gain a broader perspective of the world. Telemedicine has found applications in many areas of DMD health care, including neurology, pulmonology, cardiology, dietetics, psychology, and even terminally ill patients. Telerehabilitation with virtual reality can be especially attractive to patients with DMD.
A limitation of telemedicine is the absence of standardized televisit protocols and a comprehensive virtual physical examination, including neurological assessments. As a result, the initial diagnostic visit necessitates in-person presence. Telerehabilitation is also not free of limitations. For example, it requires the caregiver’s assistance, and for optimal outcomes, real-life physiotherapy cannot be fully replaced by web-based treatment. Technical barriers and privacy concerns can also limit the use of telemedicine. Although there is no substitute for personal contact with a doctor, it seems that patients with DMD can benefit greatly from digital health. Furthermore, patients with DMD and their family caregivers are relatively young and therefore can more easily adapt to the daily use of technology.
Clinical Consequences
Telemedicine cannot replace face-to-face visits—especially the first diagnostic visit must take place at the hospital. Telemedicine can support DMD health care when hospital visits are not possible. Telemedicine can be helpful as a window to the world for patients and their families and a quick way to exchange information and consultations between medical centers.
Abbreviations
- DMD
Duchenne muscular dystrophy
- EURO-NMD
European Reference Network for the thematic grouping of rare Neuromuscular Diseases
- SiNQ‑5
Sleep-Disordered Breathing Questionnaire In Neuromuscular Patients
- TelePALS
Telemedicine for People with Amyotrophic Lateral Sclerosis
- WHO
World Health Organization
Footnotes
Authors' Contributions: Conceptualization: EW
Writing – original draft preparation: EW, AW, JW, DS, JMS
Writing – reviewing: AO, MN, SM
Editing: EW, AW
Selection and preparation of literature: ASR, KS, JW, MN
All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest: None declared.
References
- 1.Birnkrant DJ, Bushby K, Bann CM, Apkon SD, Blackwell A, Brumbaugh D, Case LE, Clemens PR, Hadjiyannakis S, Pandya S, Street N, Tomezsko J, Wagner KR, Ward LM, Weber DR. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and neuromuscular, rehabilitation, endocrine, and gastrointestinal and nutritional management. The Lancet Neurology. 2018 Mar;17(3):251–267. doi: 10.1016/s1474-4422(18)30024-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Birnkrant DJ, Bushby K, Bann CM, Alman BA, Apkon SD, Blackwell A, Case LE, Cripe L, Hadjiyannakis S, Olson AK, Sheehan DW, Bolen J, Weber DR, Ward LM. Diagnosis and management of Duchenne muscular dystrophy, part 2: respiratory, cardiac, bone health, and orthopaedic management. The Lancet Neurology. 2018 Apr;17(4):347–361. doi: 10.1016/s1474-4422(18)30025-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elangkovan N, Dickson G. Gene therapy for Duchenne muscular dystrophy. JND. 2021 Nov 30;8(s2):S303–S316. doi: 10.3233/jnd-210678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Birnkrant DJ, Bushby K, Bann CM, Apkon SD, Blackwell A, Colvin MK, Cripe L, Herron AR, Kennedy A, Kinnett K, Naprawa J, Noritz G, Poysky J, Street N, Trout CJ, Weber DR, Ward LM, DMD Care Considerations Working Group Diagnosis and management of Duchenne muscular dystrophy, part 3: primary care, emergency management, psychosocial care, and transitions of care across the lifespan. Lancet Neurol. 2018 May;17(5):445–455. doi: 10.1016/S1474-4422(18)30026-7. https://europepmc.org/abstract/MED/29398641 .S1474-4422(18)30026-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wasilewska E, Małgorzewicz S, Sobierajska-Rek A, Jabłońska-Brudło J, Górska Lucyna, Śledzińska K, Bautembach-Minkowska J, Wierzba J. Transition from childhood to adulthood in patients with Duchenne muscular dystrophy. Medicina (Kaunas) 2020 Aug 24;56(9):426. doi: 10.3390/medicina56090426. https://www.mdpi.com/resolver?pii=medicina56090426 .medicina56090426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oh J, Park Y, Jo EC, Kim S. Current status and progress of telemedicine in Korea and other countries. Healthc Inform Res. 2015 Oct;21(4):239–243. doi: 10.4258/hir.2015.21.4.239. https://europepmc.org/abstract/MED/26618029 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scott Kruse C, Karem P, Shifflett K, Vegi L, Ravi K, Brooks M. Evaluating barriers to adopting telemedicine worldwide: a systematic review. J Telemed Telecare. 2016 Oct 16;24(1):4–12. doi: 10.1177/1357633x16674087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Craig J, Patterson V. Introduction to the practice of telemedicine. J Telemed Telecare. 2005 Jan 01;11(1):3–9. doi: 10.1258/1357633053430494. [DOI] [PubMed] [Google Scholar]
- 9.Singhal Arvind, Cowie Martin. What is e-Health? e-Journal of Cardiology Practice Vol 18. [2024-10-28]. https://www.escardio.org/Journals/E-Journal-of-Cardiology-Practice/Volume-18/what-is-e-health .
- 10.Global observatory for eHealth. World Health Organization. [2024-10-29]. https://www.who.int/observatories/global-observatory-for-ehealth#_blank .
- 11.Mauri E, Abati E, Musumeci O, Rodolico C, D'Angelo Maria Grazia, Mirabella M, Lucchini Matteo, Bello Luca, Pegoraro Elena, Maggi Lorenzo, Manneschi Letizia, Gemelli Chiara, Grandis Marina, Zuppa Angela, Massucco Sara, Benedetti Luana, Caponnetto Claudia, Schenone Angelo, Prelle Alessandro, Previtali Stefano C, Scarlato Marina, D'Amico Adele, Bertini Enrico, Pennisi Elena M, De Giglio Laura, Pane Marika, Mercuri Eugenio, Mongini Tiziana, Ricci Federica, Berardinelli Angela, Astrea Guja, Lenzi Sara, Battini Roberta, Ricci Giulia, Torri Francesca, Siciliano Gabriele, Santorelli Filippo M, Ariatti Alessandra, Filosto Massimiliano, Passamano Luigia, Politano Luisa, Scutifero Marianna, Tonin Paola, Fossati Barbara, Panicucci Chiara, Bruno Claudio, Ravaglia Sabrina, Monforte Mauro, Tasca Giorgio, Ricci Enzo, Petrucci Antonio, Santoro Lucio, Ruggiero Lucia, Barp Andrea, Albamonte Emilio, Sansone Valeria, Gagliardi Delia, Costamagna Gianluca, Govoni Alessandra, Magri Francesca, Brusa Roberta, Velardo Daniele, Meneri Megi, Sciacco Monica, Corti Stefania, Bresolin Nereo, Moroni Isabella, Messina Sonia, Di Muzio Antonio, Nigro Vincenzo, Liguori Rocco, Antonini Giovanni, Toscano Antonio, Minetti Carlo, Comi Giacomo Pietro, Italian Association of Myology Estimating the impact of COVID-19 pandemic on services provided by Italian Neuromuscular Centers: an Italian Association of Myology survey of the acute phase. Acta Myol. 2020 Jun;39(2):57–66. doi: 10.36185/2532-1900-008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bamaga AK, Alghamdi F, Alshaikh N, Altwaijri W, Bashiri FA, Hundallah K, Abukhaled M, Muthaffar OY, Al-Mehmadi S, Jamaly TA, Al-Muhaizea MA, Al-Saman A. Consensus statement on the management of Duchenne muscular dystrophy in Saudi Arabia during the coronavirus disease 2019 pandemic. Front Pediatr. 2021;9:629549. doi: 10.3389/fped.2021.629549. https://europepmc.org/abstract/MED/33681102 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levine H, Prais D, Aharoni S, Nevo Y, Katz J, Rahmani E, Goldberg L, Scheuerman O. COVID-19 in advanced Duchenne/Becker muscular dystrophy patients. Neuromuscul Disord. 2021 Jul;31(7):607–611. doi: 10.1016/j.nmd.2021.03.011. https://europepmc.org/abstract/MED/34053847 .S0960-8966(21)00076-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Veerapandiyan A, Wagner KR, Apkon S, McDonald CM, Mathews KD, Parsons JA, Wong BL, Eichinger K, Shieh PB, Butterfield RJ, Rao VK, Smith EC, Proud CM, Connolly AM, Ciafaloni E. The care of patients with Duchenne, Becker, and other muscular dystrophies in the COVID-19 pandemic. Muscle Nerve. 2020 Jul;62(1):41–45. doi: 10.1002/mus.26902. https://europepmc.org/abstract/MED/32329920 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Costamagna G, Abati E, Bresolin N, Comi GP, Corti S. Management of patients with neuromuscular disorders at the time of the SARS-CoV-2 pandemic. J Neurol. 2021 May;268(5):1580–1591. doi: 10.1007/s00415-020-10149-2. https://air.unimi.it/handle/2434/760993 .10.1007/s00415-020-10149-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spina E, Trojsi F, Tozza S, Iovino A, Iodice R, Passaniti C, Abbadessa G, Bonavita S, Leocani L, Tedeschi G, Manganelli F, Lavorgna L, Digital Technologies‚ WebSocial Media Study Group of the Italian Society of Neurology (SIN) How to manage with telemedicine people with neuromuscular diseases? Neurol Sci. 2021 Sep;42(9):3553–3559. doi: 10.1007/s10072-021-05396-8. https://europepmc.org/abstract/MED/34173087 .10.1007/s10072-021-05396-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giannotta M, Petrelli C, Pini A. Telemedicine applied to neuromuscular disorders: focus on the COVID-19 pandemic era. Acta Myol. 2022;41(1):6. doi: 10.36185/2532-1900-066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Solé G, Salort-Campana E, Pereon Y, Stojkovic T, Wahbi K, Cintas P, Adams D, Laforet P, Tiffreau V, Desguerre I, Pisella L, Molon A, Attarian S, FILNEMUS COVID-19 study group Guidance for the care of neuromuscular patients during the COVID-19 pandemic outbreak from the French Rare Health Care for Neuromuscular Diseases Network. Rev Neurol (Paris) 2020 Jun;176(6):507–515. doi: 10.1016/j.neurol.2020.04.004. https://hal.archives-ouvertes.fr/hal-03141170 .S0035-3787(20)30523-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hagen K, Hummelvoll G. Practical videoconference training: experience from a Norwegian resource centre for rare disorders. Commun Med. 2015;12(2-3):199–209. doi: 10.1558/cam.16690. [DOI] [PubMed] [Google Scholar]
- 20.Van De Rijn M, Paganoni S, Levine-Weinberg M, Campbell K, Swartz Ellrodt A, Estrada J, Cohen AB, Schwamm LH, Berry JD. Experience with telemedicine in a multi-disciplinary ALS clinic. Amyotroph Lateral Scler Frontotemporal Degener. 2018 Feb;19(1-2):143–148. doi: 10.1080/21678421.2017.1392577. [DOI] [PubMed] [Google Scholar]
- 21.Pulley MT, Brittain R, Hodges W, Frazier C, Miller L, Matyjasik-Liggett Maria, Maurer S, Peters M, Solomon K, Berger AR. Multidisciplinary amyotrophic lateral sclerosis telemedicine care: the store and forward method. Muscle Nerve. 2019 Jan;59(1):34–39. doi: 10.1002/mus.26170. [DOI] [PubMed] [Google Scholar]
- 22.Hobson EV, Baird WO, Partridge R, Cooper CL, Mawson S, Quinn A, Shaw PJ, Walsh T, Wolstenholme D, Mcdermott CJ. The TiM system: developing a novel telehealth service to improve access to specialist care in motor neurone disease using user-centered design. Amyotroph Lateral Scler Frontotemporal Degener. 2018 Aug;19(5-6):351–361. doi: 10.1080/21678421.2018.1440408. https://www.tandfonline.com/doi/10.1080/21678421.2018.1440408?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub0pubmed . [DOI] [PubMed] [Google Scholar]
- 23.Helleman J, Van Eenennaam R, Kruitwagen ET, Kruithof WJ, Slappendel MJ, Van Den Berg LH, Visser-Meily JM, Beelen A. Telehealth as part of specialized ALS care: feasibility and user experiences with "ALS home-monitoring and coaching". Amyotroph Lateral Scler Frontotemporal Degener. 2020 May;21(3-4):183–192. doi: 10.1080/21678421.2020.1718712. https://www.tandfonline.com/doi/10.1080/21678421.2020.1718712?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub0pubmed . [DOI] [PubMed] [Google Scholar]
- 24.Portaro S, Calabrò Rocco Salvatore, Bramanti P, Silvestri G, Torrisi M, Conti-Nibali V, Caliri S, Lunetta C, Alagna B, Naro A, Bramanti A. Telemedicine for facio-scapulo-humeral muscular dystrophy: a multidisciplinary approach to improve quality of life and reduce hospitalization rate? Disabil Health J. 2018 Apr;11(2):306–309. doi: 10.1016/j.dhjo.2017.09.003.S1936-6574(17)30168-1 [DOI] [PubMed] [Google Scholar]
- 25.How telehealth can help patients with muscular dystrophy. Rare Disease Advisor. [2024-10-29]. https://www.rarediseaseadvisor.com/conferences/mda-2022/how-telehealth-can-help-patients-with-muscular-dystrophy;
- 26.Astrea G, Marinella G, Agosto C, Gagliardi D, Grandis M, Giuliano M, Politano L. How to define and enhance diagnostic and assistance pathways in neuromuscular diseases during the COVID-19 pandemic: the concept of network. Acta Myol. 2021 Dec;40(4):172–176. doi: 10.36185/2532-1900-060. https://air.unimi.it/handle/2434/919510 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.EURO-NMD. European Reference Network. [2025-01-10]. https://ern-euro-nmd.eu/educational-resources/
- 28.Soutter J, Hamilton N, Russell P, Russell C, Bushby K, Sloper P, Bartlett K. The Golden Freeway: a preliminary evaluation of a pilot study advancing information technology as a social intervention for boys with Duchenne muscular dystrophy and their families. Health Soc Care Community. 2004 Jan;12(1):25–33. doi: 10.1111/j.1365-2524.2004.00465.x. [DOI] [PubMed] [Google Scholar]
- 29.Parent Project Muscular Dystrophy. [2024-10-29]. https://www.parentprojectmd.org/
- 30.Wechsler LR. Advantages and limitations of teleneurology. JAMA Neurol. 2015 Mar;72(3):349–354. doi: 10.1001/jamaneurol.2014.3844.2089220 [DOI] [PubMed] [Google Scholar]
- 31.Contesse MG, Hodges J, Staunton H, Lowes LP, Elmankabadi B, Elder SJ, Ormazabal MG, Dalle Pazze L, Leffler MG. Home-based video assessment of ease of movement for patients with Duchenne: interviews with physical therapists to select movement tasks. Physiother Res Int. 2023 Jan 30;28(3):e1993. doi: 10.1002/pri.1993. [DOI] [PubMed] [Google Scholar]
- 32.Contesse MG, Lowes LP, White MK, Dalle Pazze L, McSherry C, Alfano LN, Iammarino M, Reash N, Bonarrigo K, Kiefer M, Laubscher K, McIntyre M, Mockler S, Nelson L, Vogel L, Leffler MG. Development of Duchenne Video Assessment scorecards to evaluate ease of movement among those with Duchenne muscular dystrophy. PLoS One. 2022;17(4):e0266845. doi: 10.1371/journal.pone.0266845. https://dx.plos.org/10.1371/journal.pone.0266845 .PONE-D-21-36797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Contesse MG, Sapp ATL, Apkon SD, Lowes LP, Dalle Pazze L, Leffler MG. Reliability and construct validity of the Duchenne Video Assessment. Muscle Nerve. 2021 Aug;64(2):180–189. doi: 10.1002/mus.27335. https://europepmc.org/abstract/MED/34050939 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.White MK, Leffler M, Rychlec K, Jones C, McSherry C, Walker L, Kosinski M. Adapting traditional content validation methods to fit purpose: an example with a novel video assessment and training materials in Duchenne muscular dystrophy (DMD) Qual Life Res. 2019 Nov;28(11):2979–2988. doi: 10.1007/s11136-019-02245-2. https://europepmc.org/abstract/MED/31302840 .10.1007/s11136-019-02245-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brooke MH, Griggs RC, Mendell JR, Fenichel GM, Shumate JB, Pellegrino RJ. Clinical trial in Duchenne dystrophy. I. The design of the protocol. Muscle Nerve. 1981;4(3):186–197. doi: 10.1002/mus.880040304. [DOI] [PubMed] [Google Scholar]
- 36.Vignos PJ. Management of progressive muscular dystrophy in childhood. JAMA. 1963 Apr 13;184(2):89–96. doi: 10.1001/jama.1963.03700150043007. [DOI] [PubMed] [Google Scholar]
- 37.Miliani Capelini C, da Silva TD, Tonks J, Watson S, Boscolo Alvarez MP, Del Ciello de Menezes L, Favero FM, Caromano F, Massetti T, Bandeira de Mello Monteiro C. Improvements in motor tasks through the use of smartphone technology for individuals with Duchenne muscular dystrophy. NDT. 2017 Aug;13:2209–2217. doi: 10.2147/ndt.s125466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ansary AM, Martinez JN, Scott JD. The virtual physical exam in the 21st century. J Telemed Telecare. 2019 Nov 06;27(6):382–392. doi: 10.1177/1357633x19878330. [DOI] [PubMed] [Google Scholar]
- 39.Bushby K, Connor E. Clinical outcome measures for trials in Duchenne muscular dystrophy: report from International Working Group meetings. Clin Investig (Lond) 2011 Sep;1(9):1217–1235. doi: 10.4155/cli.11.113. https://europepmc.org/abstract/MED/22639722 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Herrington WG, Goldsack JC, Landray MJ. Increasing the use of mobile technology-derived endpoints in clinical trials. Clin Trials. 2018 Jun;15(3):313–315. doi: 10.1177/1740774518755393. https://journals.sagepub.com/doi/10.1177/1740774518755393?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub0pubmed . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fujii T, Takeshita E, Iwata Y, Yajima H, Nozaki F, Mori M, Kumada T. Cumulative jerk as an outcome measure in nonambulatory Duchenne muscular dystrophy. Brain Dev. 2019 Oct;41(9):796–802. doi: 10.1016/j.braindev.2019.06.002.S0387-7604(19)30101-9 [DOI] [PubMed] [Google Scholar]
- 42.Saporta MA, Granit V, Lewis R, Benatar M. Yes, we can: neuromuscular examination by telemedicine. Muscle Nerve. 2020 Dec;62(6):E83–E85. doi: 10.1002/mus.27056. [DOI] [PubMed] [Google Scholar]
- 43.Grogan J, Simmons Z. Telemedicine for the care of neuromuscular disorders. Curr Treat Options Neurol. 2020 May 11;22(6):1. doi: 10.1007/s11940-020-00625-5. https://www.scopus.com/pages/publications/85084478505#_blank . [DOI] [Google Scholar]
- 44.Childs A, Turner C, Astin R, Bianchi S, Bourke J, Cunningham V, Edel L, Edwards C, Farrant P, Heraghty J, James M, Massey C, Messer B, Michel Sodhi J, Murphy PB, Schiava M, Thomas A, Trucco F, Guglieri M. Development of respiratory care guidelines for Duchenne muscular dystrophy in the UK: key recommendations for clinical practice. Thorax. 2024 Apr 15;79(5):476–485. doi: 10.1136/thorax-2023-220811. http://thorax.bmj.com/lookup/pmidlookup?view=long&pmid=38123347 .thorax-2023-220811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Steier J, Jolley C, Seymour J, Teschler H, Luo Y, Polkey M, Moxham J. Screening for sleep-disordered breathing in neuromuscular disease using a questionnaire for symptoms associated with diaphragm paralysis. Eur Respir J. 2011 Feb;37(2):400–405. doi: 10.1183/09031936.00036210. https://publications.ersnet.org/lookup/pmid/20595146 .09031936.00036210 [DOI] [PubMed] [Google Scholar]
- 46.Zamarrón Carlos, Morete E, González Francisco. Telemedicine system for the care of patients with neuromuscular disease and chronic respiratory failure. Arch Med Sci. 2014 Oct 27;10(5):1047–1051. doi: 10.5114/aoms.2014.46223. https://doi.org/10.5114/aoms.2014.46223 .23768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wasilewska E, Sobierajska-Rek A, Małgorzewicz S, Soliński M, Jassem E. Benefits of telemonitoring of pulmonary function-3-month follow-up of home electronic spirometry in patients with Duchenne muscular dystrophy. J Clin Med. 2022 Feb 06;11(3):856. doi: 10.3390/jcm11030856. https://www.mdpi.com/resolver?pii=jcm11030856 .jcm11030856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wasilewska E, Sobierajska-Rek A, Małgorzewicz S, Soliński M, Szalewska D, Jassem E. Is it possible to have home e-monitoring of pulmonary function in our patients with Duchenne muscular dystrophy in the COVID-19 pandemic?-a one center pilot study. Int J Environ Res Public Health. 2021 Aug 26;18(17):8967. doi: 10.3390/ijerph18178967. https://www.mdpi.com/resolver?pii=ijerph18178967 .ijerph18178967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Onofri A, Pavone M, De Santis S, Verrillo E, Caggiano S, Ullmann N, Cutrera R. Telemedicine in children with medical complexity on home ventilation during the COVID-19 pandemic. Pediatr Pulmonol. 2021 Jun;56(6):1395–1400. doi: 10.1002/ppul.25289. https://europepmc.org/abstract/MED/33524228 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Muñoz-Bonet Juan I, López-Prats José L, Flor-Macián Eva M, Cantavella T, Domínguez Amparo, Vidal Y, Brines J. Medical complications in a telemedicine home care programme for paediatric ventilated patients. J Telemed Telecare. 2020;26(7-8):462–473. doi: 10.1177/1357633X19843761. [DOI] [PubMed] [Google Scholar]
- 51.Muñoz-Bonet JI, López-Prats JL, Flor-Macián EM, Cantavella T, Bonet L, Domínguez A, Brines J. Usefulness of telemedicine for home ventilator-dependent children. J Telemed Telecare. 2018 Dec 11;26(4):207–215. doi: 10.1177/1357633x18811751. https://doi.org/10.1177/1357633X18811751 . [DOI] [PubMed] [Google Scholar]
- 52.Birnkrant DJ, Ararat E, Mhanna MJ. Cardiac phenotype determines survival in Duchenne muscular dystrophy. Pediatr Pulmonol. 2015 Jun 10;51(1):70–76. doi: 10.1002/ppul.23215. [DOI] [PubMed] [Google Scholar]
- 53.Paramsothy P, Wang Y, Cai B, Conway KM, Johnson NE, Pandya S, Ciafaloni E, Mathews KD, Romitti PA, Howard JF, Riley C. Selected clinical and demographic factors and all-cause mortality among individuals with Duchenne muscular dystrophy in the Muscular Dystrophy Surveillance, Tracking, and Research Network. Neuromuscul Disord. 2022 Jun;32(6):468–476. doi: 10.1016/j.nmd.2022.04.008. https://europepmc.org/abstract/MED/35597713 .S0960-8966(22)00132-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spurney C, Shimizu R, Morgenroth LP, Kolski H, Gordish-Dressman Heather, Clemens PR, CINRG Investigators Cooperative International Neuromuscular Research Group Duchenne Natural History Study demonstrates insufficient diagnosis and treatment of cardiomyopathy in Duchenne muscular dystrophy. Muscle Nerve. 2014 Aug;50(2):250–256. doi: 10.1002/mus.24163. https://europepmc.org/abstract/MED/24395289 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grant B, Wallace JG, Hobson RA, Craig BG, Mulholland HC, Casey FA. Telemedicine applications for the regional paediatric cardiology service in Northern Ireland. J Telemed Telecare. 2002 Jan 01;8(2_suppl):31–33. doi: 10.1177/1357633x020080s214. [DOI] [PubMed] [Google Scholar]
- 56.Imberti JF, Tosetti A, Mei DA, Maisano A, Boriani G. Remote monitoring and telemedicine in heart failure: implementation and benefits. Curr Cardiol Rep. 2021 May 07;23(6):55. doi: 10.1007/s11886-021-01487-2. https://europepmc.org/abstract/MED/33959819 .10.1007/s11886-021-01487-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Freedman LS, Commins JM, Moler JE, Willett W, Tinker LF, Subar AF, Spiegelman D, Rhodes D, Potischman N, Neuhouser ML, Moshfegh AJ, Kipnis V, Arab L, Prentice RL. Pooled results from 5 validation studies of dietary self-report instruments using recovery biomarkers for potassium and sodium intake. Am J Epidemiol. 2015 Apr 01;181(7):473–487. doi: 10.1093/aje/kwu325. https://europepmc.org/abstract/MED/25787264 .kwu325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wills AM, Garry J, Hubbard J, Mezoian T, Breen CT, Ortiz-Miller C, Nalipinski P, Sullivan S, Berry JD, Cudkowicz M, Paganoni S, Chan J, Macklin EA. Nutritional counseling with or without mobile health technology: a randomized open-label standard-of-care-controlled trial in ALS. BMC Neurol. 2019 May 29;19(1):104. doi: 10.1186/s12883-019-1330-6. https://bmcneurol.biomedcentral.com/articles/10.1186/s12883-019-1330-6 .10.1186/s12883-019-1330-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sadasivan A, Warrier Mg, Vengalil S, Thomas Pt, Nalini A. Telehealth services for an adolescent with Duchenne muscular dystrophy (DMD) during the COVID-19 pandemic. Australian Social Work. 2024 May 06;78(1):108–114. doi: 10.1080/0312407X.2024.2340469. [DOI] [Google Scholar]
- 60.McPherson AC, McAdam L, Keenan S, Schwellnus H, Biddiss E, DeFinney A, English K. A feasibility study using solution-focused coaching for health promotion in children and young people with Duchenne muscular dystrophy. Dev Neurorehabil. 2018 Feb;21(2):121–130. doi: 10.1080/17518423.2017.1289271. [DOI] [PubMed] [Google Scholar]
- 61.Lee A, Finnin K, Holdsworth L, Milette D, Peterson C. Report of the WCPT/INPTRA digital physical therapy practice task force. World Confederation for Physical Therapy and International Network of Physiotherapy Regulatory Authorities. [2025-01-10]. https://world.physio/sites/default/files/2020-06/WCPT-INPTRA-Digital-Physical-Therapy-Practice-Task-force-March2020.pdf .
- 62.Sobierajska-Rek A, Mański. Jabłońska-Brudło J, Śledzińska K, Wasilewska E, Szalewska D. Respiratory telerehabilitation of boys and young men with Duchenne muscular dystrophy in the COVID-19 pandemic. Int J Environ Res Public Health. 2021 Jun 08;18(12):6179. doi: 10.3390/ijerph18126179. https://www.mdpi.com/resolver?pii=ijerph18126179 .ijerph18126179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sobierajska-Rek A, Mański. Jabłońska-Brudło J, Śledzińska K, Ucińska A, Wierzba J. Establishing a telerehabilitation program for patients with Duchenne muscular dystrophy in the COVID-19 pandemic. Wien Klin Wochenschr. 2021 Apr;133(7-8):344–350. doi: 10.1007/s00508-020-01786-8. https://europepmc.org/abstract/MED/33346889 .10.1007/s00508-020-01786-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kenis-Coskun O, Imamoglu S, Karamancioglu B, Kurt K, Ozturk G, Karadag-Saygi E. Comparison of telerehabilitation versus home-based video exercise in patients with Duchenne muscular dystrophy: a single-blind randomized study. Acta Neurol Belg. 2022 Oct;122(5):1269–1280. doi: 10.1007/s13760-022-01975-4. https://europepmc.org/abstract/MED/35616780 .10.1007/s13760-022-01975-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Peretti A, Amenta F, Tayebati SK, Nittari G, Mahdi SS. Telerehabilitation: review of the state-of-the-art and areas of application. JMIR Rehabil Assist Technol. 2017 Jul 21;4(2):e7. doi: 10.2196/rehab.7511. https://rehab.jmir.org/2017/2/e7/ v4i2e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Arteaga D, Donnelly T, Crum K, Markham L, Killian M, Burnette WB, Soslow J, Buchowski MS. Assessing physical activity using accelerometers in youth with Duchenne muscular dystrophy. JND. 2020 Jun 02;7(3):331–342. doi: 10.3233/jnd-200478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bradford N, Herbert A, Walker R, Pedersen L, Hallahan A, Irving H, Bensink Mark E, Armfield Nigel R, Smith Anthony C. Home telemedicine for paediatric palliative care. Stud Health Technol Inform. 2010;161:10–19. [PubMed] [Google Scholar]
- 68.Monterosso L, Kristjanson LJ, Aoun S, Phillips MB. Supportive and palliative care needs of families of children with life-threatening illnesses in Western Australia: evidence to guide the development of a palliative care service. Palliat Med. 2007 Dec;21(8):689–696. doi: 10.1177/0269216307083032.21/8/689 [DOI] [PubMed] [Google Scholar]
- 69.Bradford NK, Armfield NR, Young J, Smith AC. Paediatric palliative care by video consultation at home: a cost minimisation analysis. BMC Health Serv Res. 2014 Jul 28;14(1):328. doi: 10.1186/1472-6963-14-328. https://bmchealthservres.biomedcentral.com/articles/10.1186/1472-6963-14-328 .1472-6963-14-328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Holmen H, Riiser K, Winger A. Home-based pediatric palliative care and electronic health: systematic mixed methods review. J Med Internet Res. 2020 Feb 28;22(2):e16248. doi: 10.2196/16248. https://www.jmir.org/2020/2/e16248/ v22i2e16248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bradford NK, Young J, Armfield NR, Herbert A, Smith AC. Home telehealth and paediatric palliative care: clinician perceptions of what is stopping us? BMC Palliat Care. 2014 Jun 16;13(1):29. doi: 10.1186/1472-684x-13-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bird M, Li L, Ouellette C, Hopkins K, McGillion MH, Carter N. Use of synchronous digital health technologies for the care of children with special health care needs and their families: scoping review. JMIR Pediatr Parent. 2019 Nov 21;2(2):e15106. doi: 10.2196/15106. https://pediatrics.jmir.org/2019/2/e15106/ v2i2e15106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Weaver MS, Neumann ML, Navaneethan H, Robinson JE, Hinds PS. Human touch via touchscreen: rural nurses' experiential perspectives on telehealth use in pediatric hospice care. J Pain Symptom Manage. 2020 Nov;60(5):1027–1033. doi: 10.1016/j.jpainsymman.2020.06.003. https://linkinghub.elsevier.com/retrieve/pii/S0885-3924(20)30435-8 .S0885-3924(20)30435-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Duursma F, Schers HJ, Vissers KC, Hasselaar J. Study protocol: optimization of complex palliative care at home via telemedicine. A cluster randomized controlled trial. BMC Palliat Care. 2011 Aug 9;10(1):13. doi: 10.1186/1472-684x-10-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liang HY, Hann Lin L, Yu Chang C, Mei Wu F, Yu S. Effectiveness of a nurse-led tele-homecare program for patients with multiple chronic illnesses and a high risk for readmission: a randomized controlled trial. J Nurs Scholarsh. 2021 Mar;53(2):161–170. doi: 10.1111/jnu.12622. [DOI] [PubMed] [Google Scholar]
- 76.Bulto LN. The role of nurse‐led telehealth interventions in bridging healthcare gaps and expanding access. Nursing Open. 2024 Jan 11;11(1):e2092–e2092. doi: 10.1002/nop2.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]


