Abstract
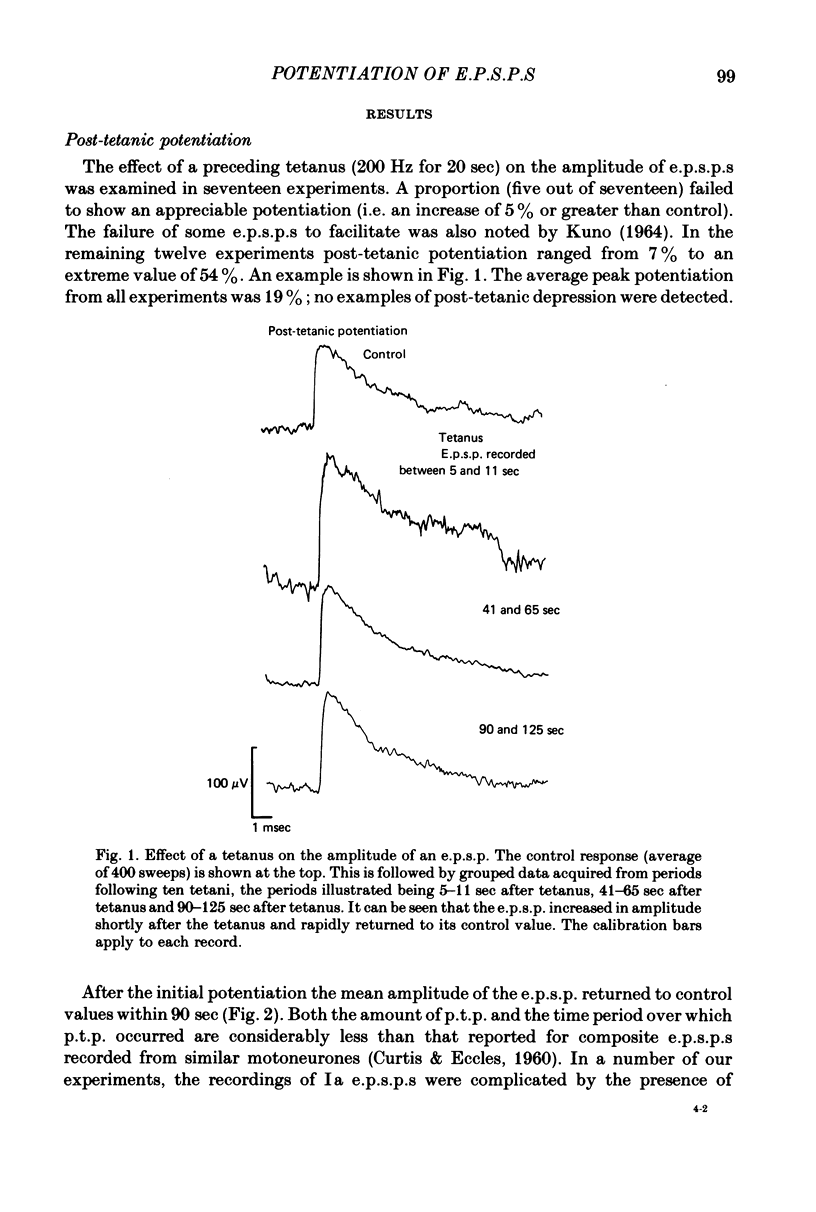
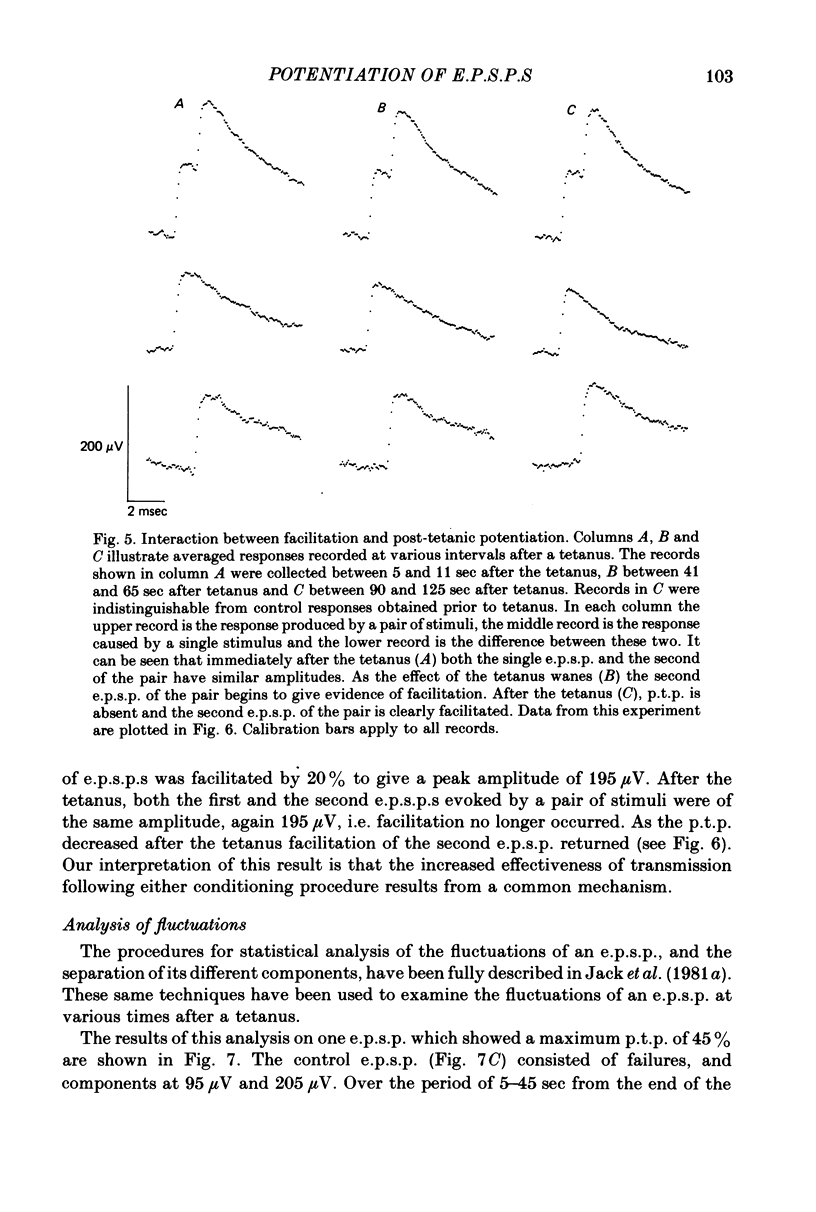
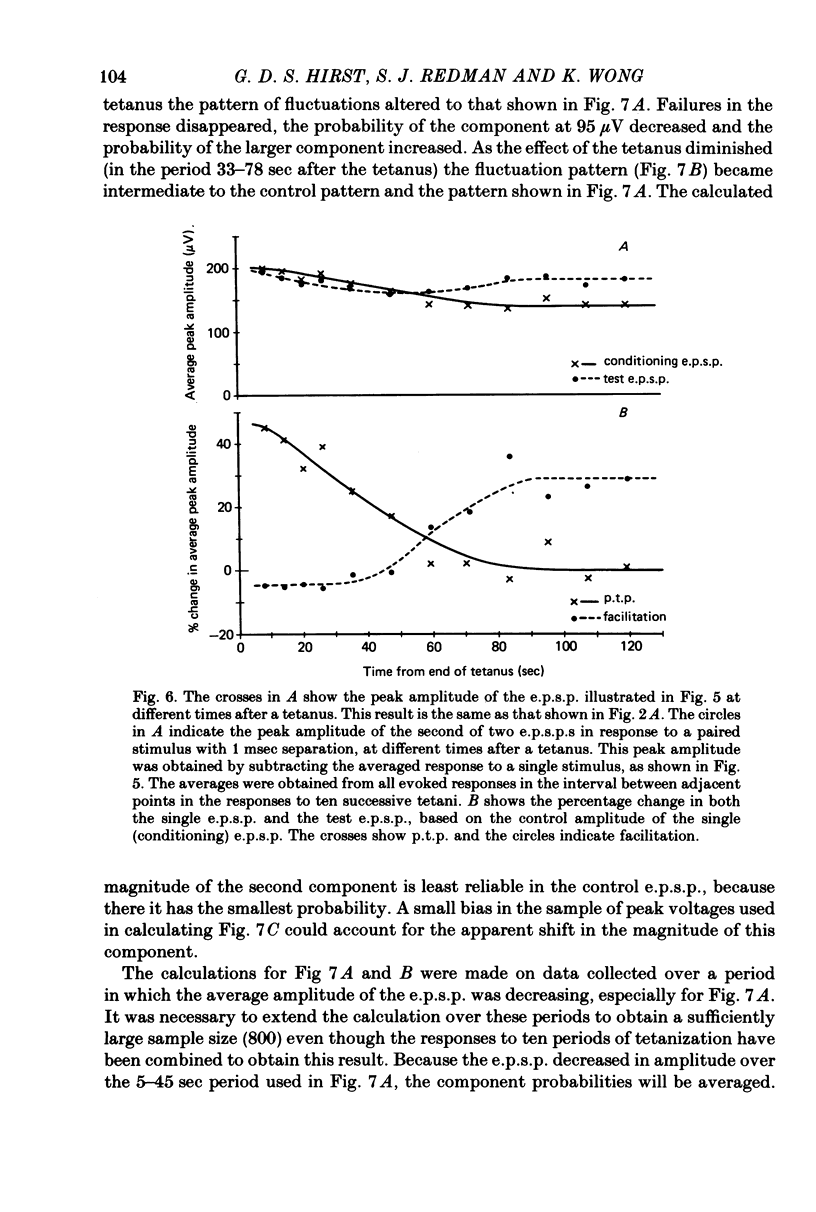
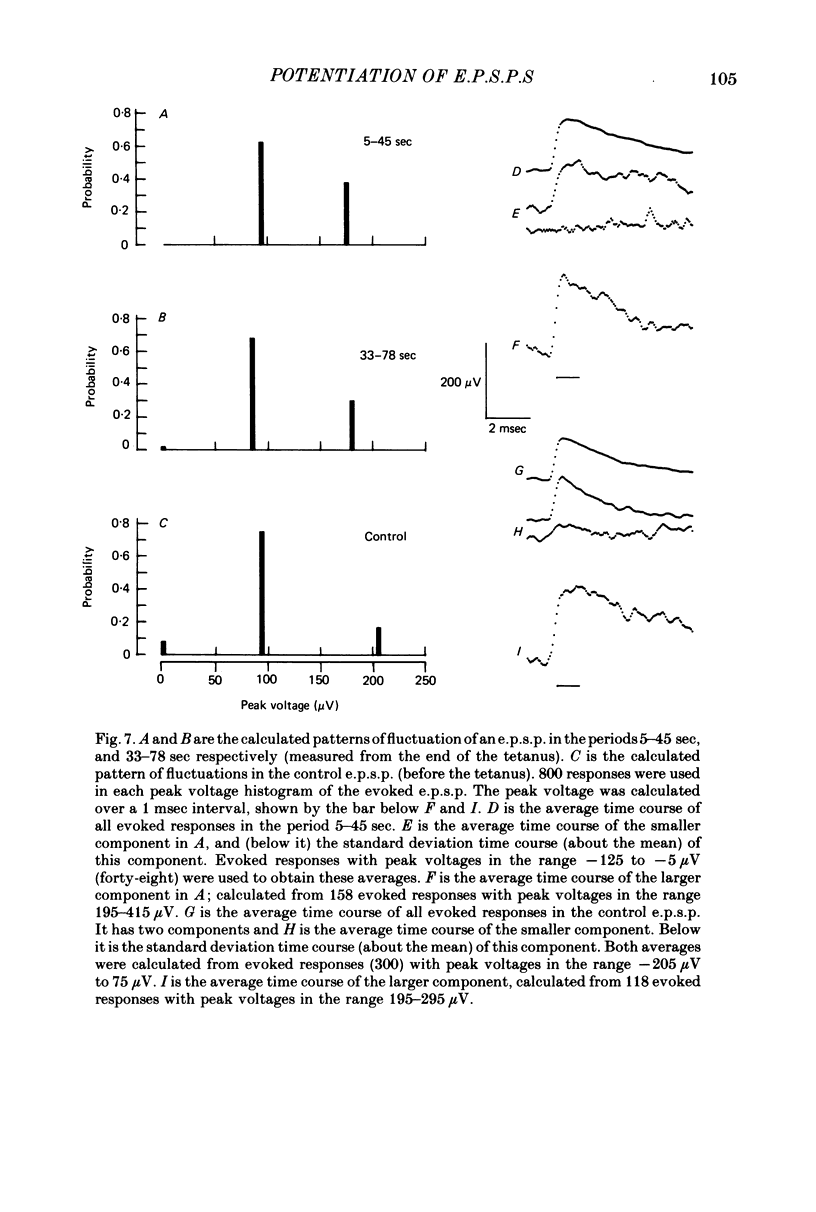
1. Excitatory post-synaptic potentials (e.p.s.p.s) were evoked in spinal alpha-motoneurones of the cat by impulses in single group Ia nerve fibres. 2. The average peak amplitude of some of these e.p.s.p.s was increased by a conditioning tetanus. The maximum increase observed was 54% of the control amplitude. 3. The average peak amplitude of some e.p.s.p.s was increased by a single conditioning stimulus which preceded the test stimulus by 1 or 2 msec. The maximum increase observed was 28% of the control amplitude. 4. The ability of e.p.s.p.s to potentiate following a tetanus was correlated with their ability to be facilitated by a single conditioning stimulus. 5. If an e.p.s.p. could be facilitated prior to a tetanus, the amount of facilitation was reduced after the tetanus, with all facilitation being abolished when post-tetanic potentiation was maximal. 6. The fluctuations of an e.p.s.p. were analysed before and after a tetanus. The peak amplitudes that an e.p.s.p. fluctuated between while potentiated did not gradually diminish as the effect of the tetanus disappeared. Post-tetanic potentiation, when it occurred, was accompanied by a decrease in the probability of occurrence of components with smaller peak amplitudes and an increase in the probability of occurrence of components with larger peak amplitudes. 7. These results are consistent with the suggestion that the magnitude of the synaptic potential generated at a single bouton does not vary from trial to trial (Jack, Redman & Wong, 1981a). Nor does the amplitude of this potential vary following a single conditioning stimulus or a tetanus. Post-tetanic potentiation and facilitation result from a decrease in the probability of failure to release transmitter following the conditioning stimuli.
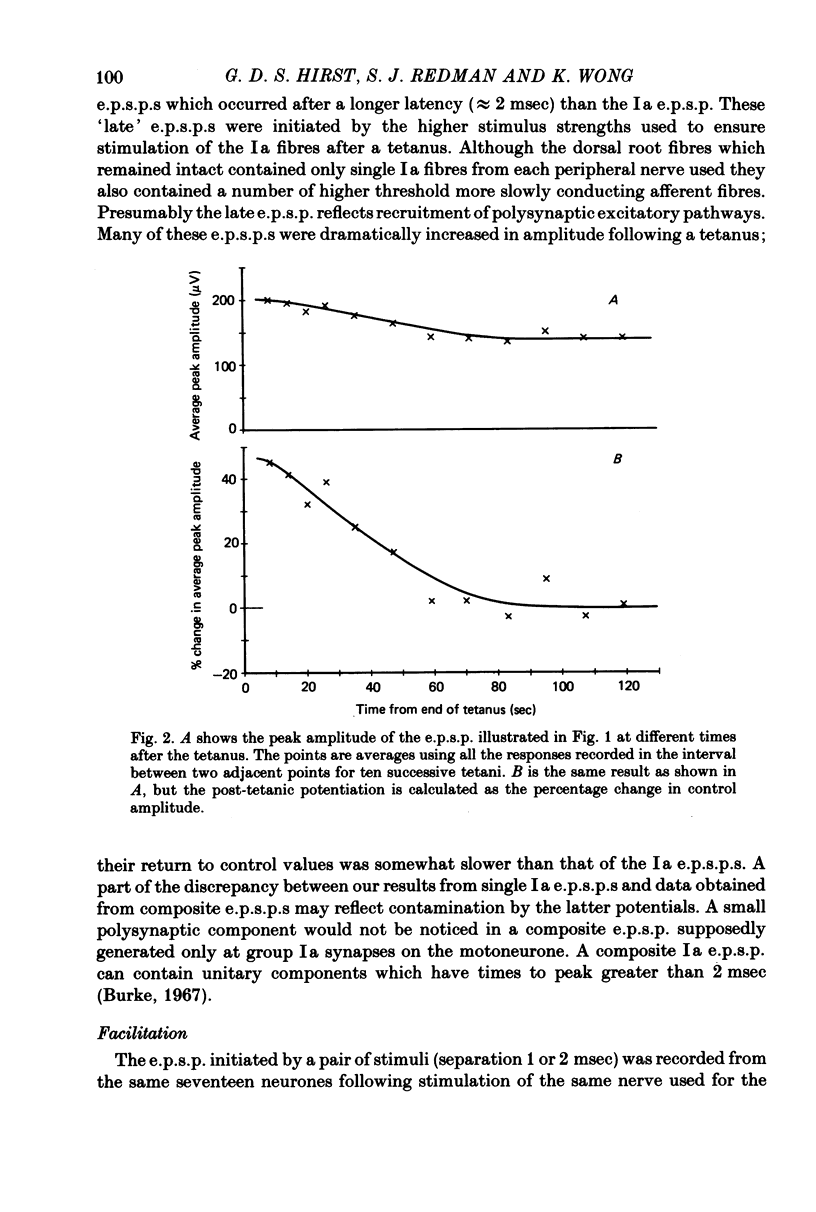
Full text
PDF

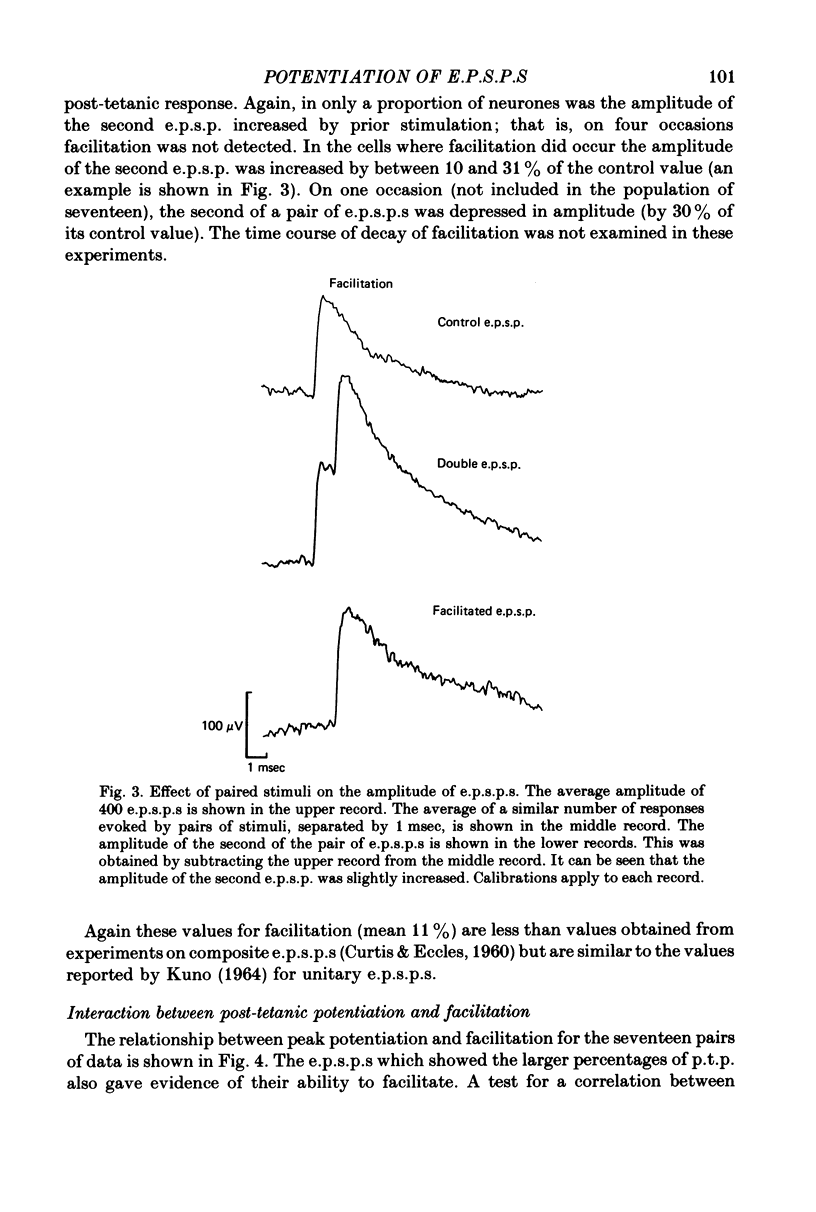
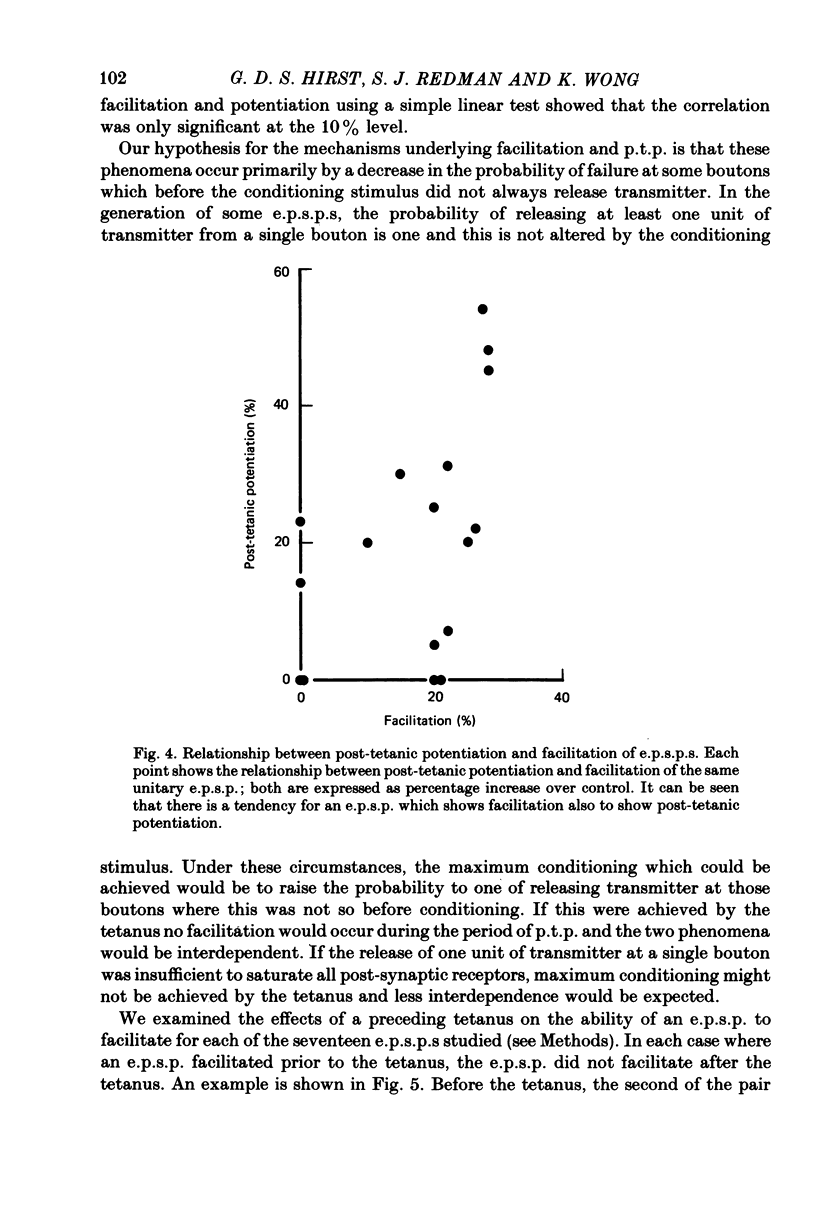




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burke R. E. Composite nature of the monosynaptic excitatory postsynaptic potential. J Neurophysiol. 1967 Sep;30(5):1114–1137. doi: 10.1152/jn.1967.30.5.1114. [DOI] [PubMed] [Google Scholar]
- CURTIS D. R., ECCLES J. C. Synaptic action during and after repetitive stimulation. J Physiol. 1960 Feb;150:374–398. doi: 10.1113/jphysiol.1960.sp006393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards F. R., Redman S. J., Walmsley B. Non-quantal fluctuations and transmission failures in charge transfer at Ia synapses on spinal motoneurones. J Physiol. 1976 Aug;259(3):689–704. doi: 10.1113/jphysiol.1976.sp011489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards F. R., Redman S. J., Walmsley B. Statistical fluctuations in charge transfer at Ia synapses on spinal motoneurones. J Physiol. 1976 Aug;259(3):665–688. doi: 10.1113/jphysiol.1976.sp011488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack J. J., Redman S. J., Wong K. Modifications to synaptic transmission at group Ia synapses on cat spinal motoneurones by 4-aminopyridine. J Physiol. 1981 Dec;321:111–126. doi: 10.1113/jphysiol.1981.sp013974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack J. J., Redman S. J., Wong K. The components of synaptic potentials evoked in cat spinal motoneurones by impulses in single group Ia afferents. J Physiol. 1981 Dec;321:65–96. doi: 10.1113/jphysiol.1981.sp013972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUNO M. MECHANSIM OF FACILITATION AND DEPRESSION OF THE EXCITATORY SYNAPTIC POTENTIAL IN SPINAL MOTONEURONES. J Physiol. 1964 Dec;175:100–112. doi: 10.1113/jphysiol.1964.sp007505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau E. M., Smolinsky A., Lass Y. Post-tetanic potentiation and facilitation do not share a common calcium-dependent mechanism. Nat New Biol. 1973 Aug 1;244(135):155–157. doi: 10.1038/newbio244155a0. [DOI] [PubMed] [Google Scholar]
- Lüscher H. R., Ruenzel P., Henneman E. How the size of motoneurones determines their susceptibility to discharge. Nature. 1979 Dec 20;282(5741):859–861. doi: 10.1038/282859a0. [DOI] [PubMed] [Google Scholar]
- Redman S. Junctional mechanisms at group Ia synapses. Prog Neurobiol. 1979;12(1):33–83. doi: 10.1016/0301-0082(79)90010-8. [DOI] [PubMed] [Google Scholar]


