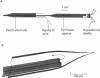
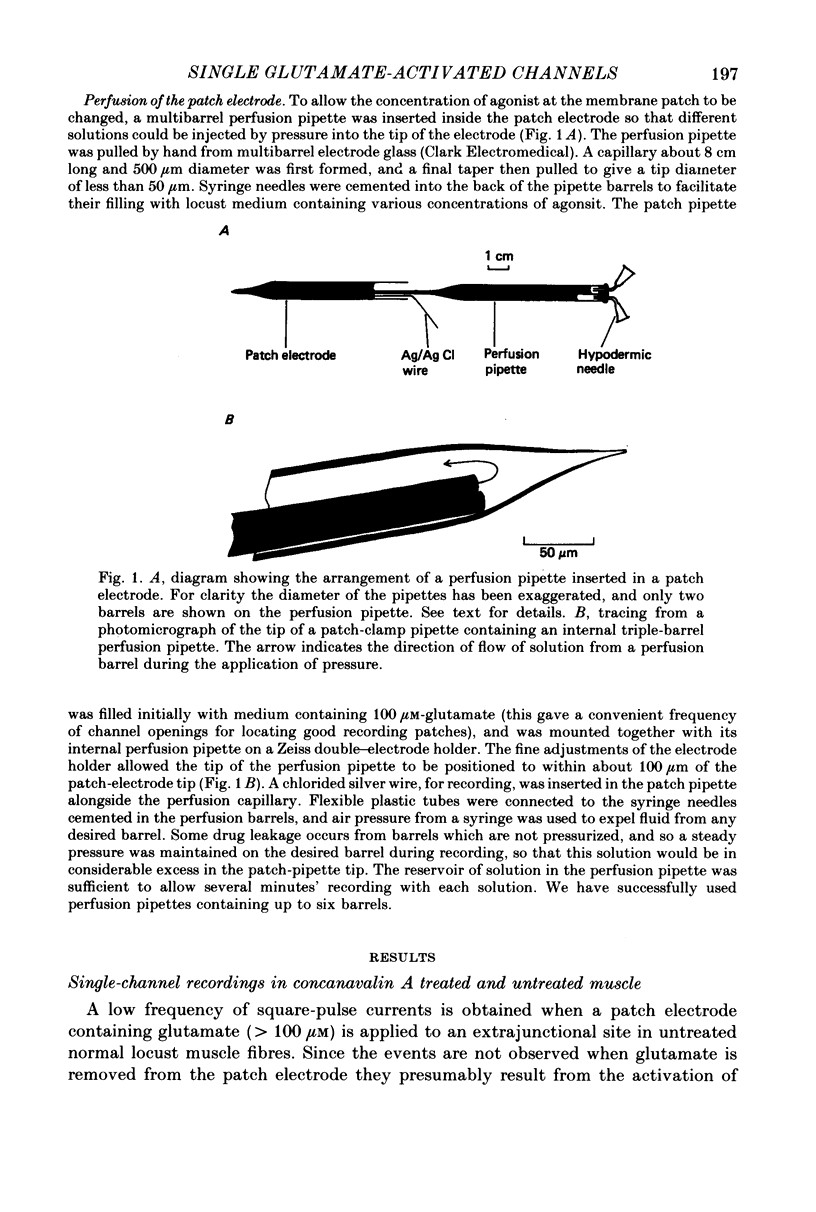
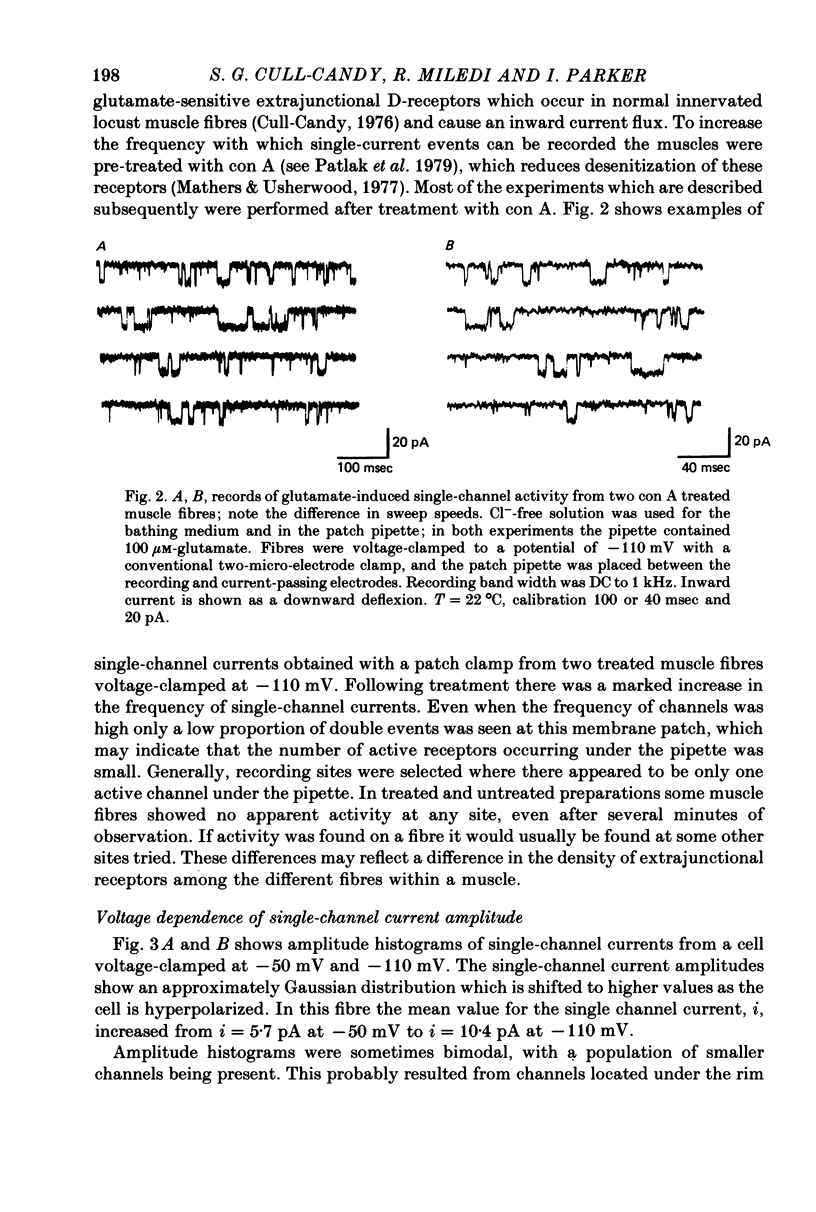
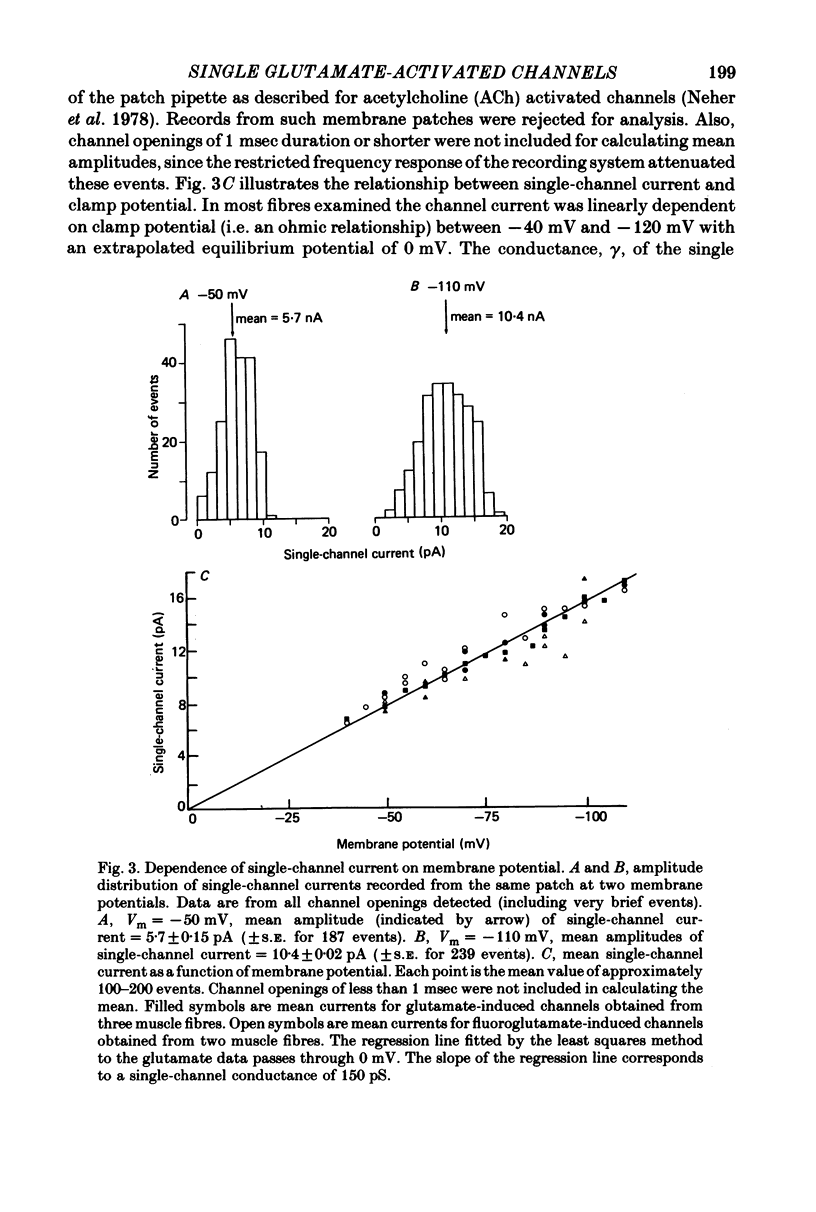
Abstract
1. Glutamate-activated single channels have been examined with conventional and internally perfused patch-clamp electrodes applied to the extrajunctional membrane of locust muscle fibres which were usually treated with concanavalin A to reduce desensitization. Channels opened by glutamate and other agonists have been compared.
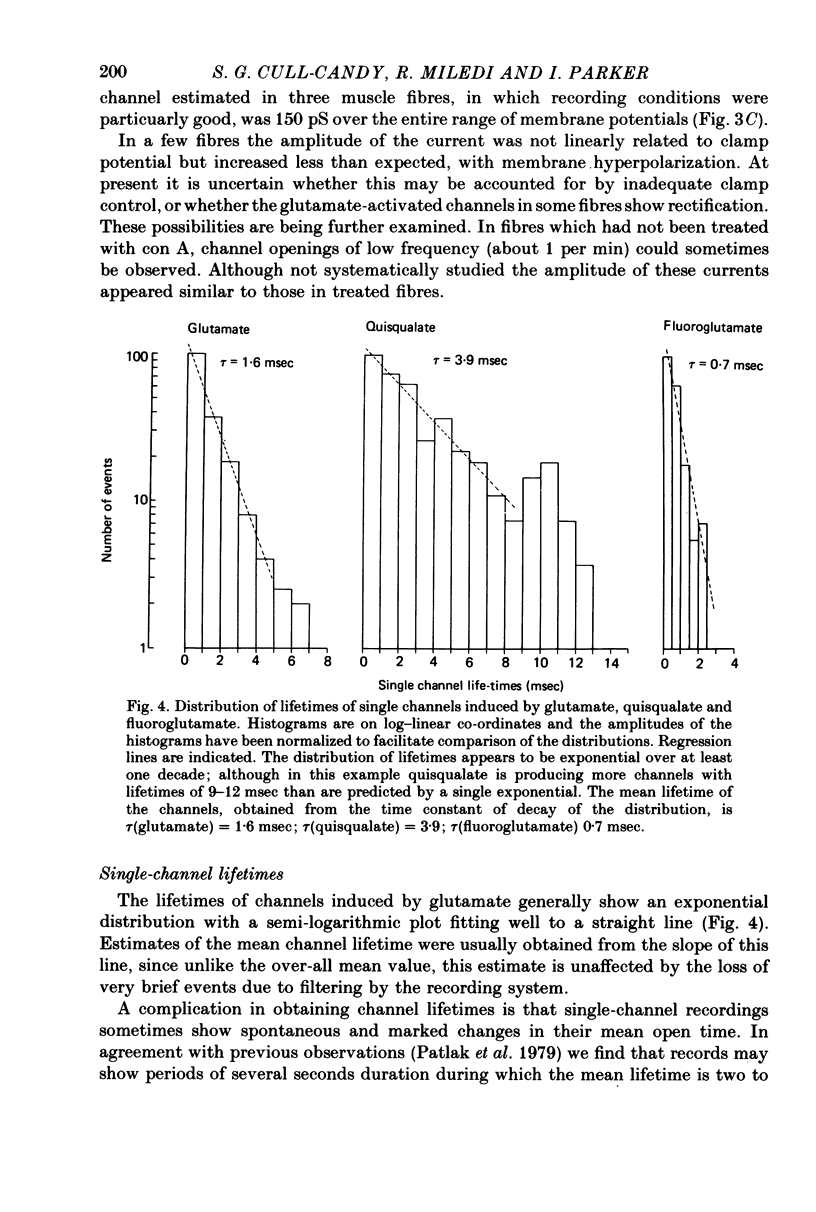
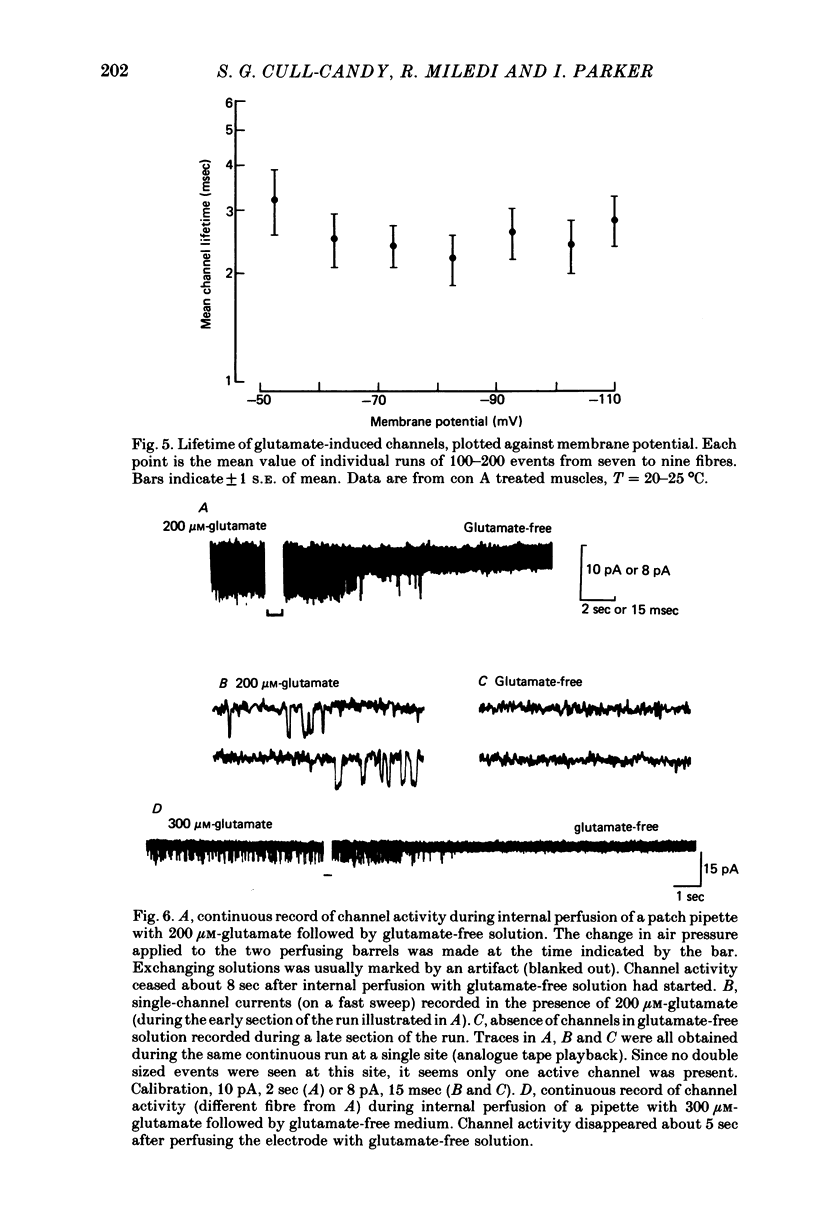
2. Recording patches were selected where there appeared to be only one active channel under the pipette. The conductance for single glutamate-activated channels was 150 pS and was not markedly dependent on clamp potential. The lifetimes of the channels were usually exponentially distributed with a mean of τ(glutamate) = 2.3 ± 0.12 msec, T = 23 °C, Vm = -60 mV.
3. Channels opened by fluoroglutamate had a mean lifetime of τ(fluoroglutamate) = 1.4 ± 0.1 msec; channels opened by quisqualate had a mean lifetime of τ(quisqualate) = 6.4 ± 1.0 msec. The conductances of channels opened by fluoroglutamate, quisqualate and glutamate were not significantly different.
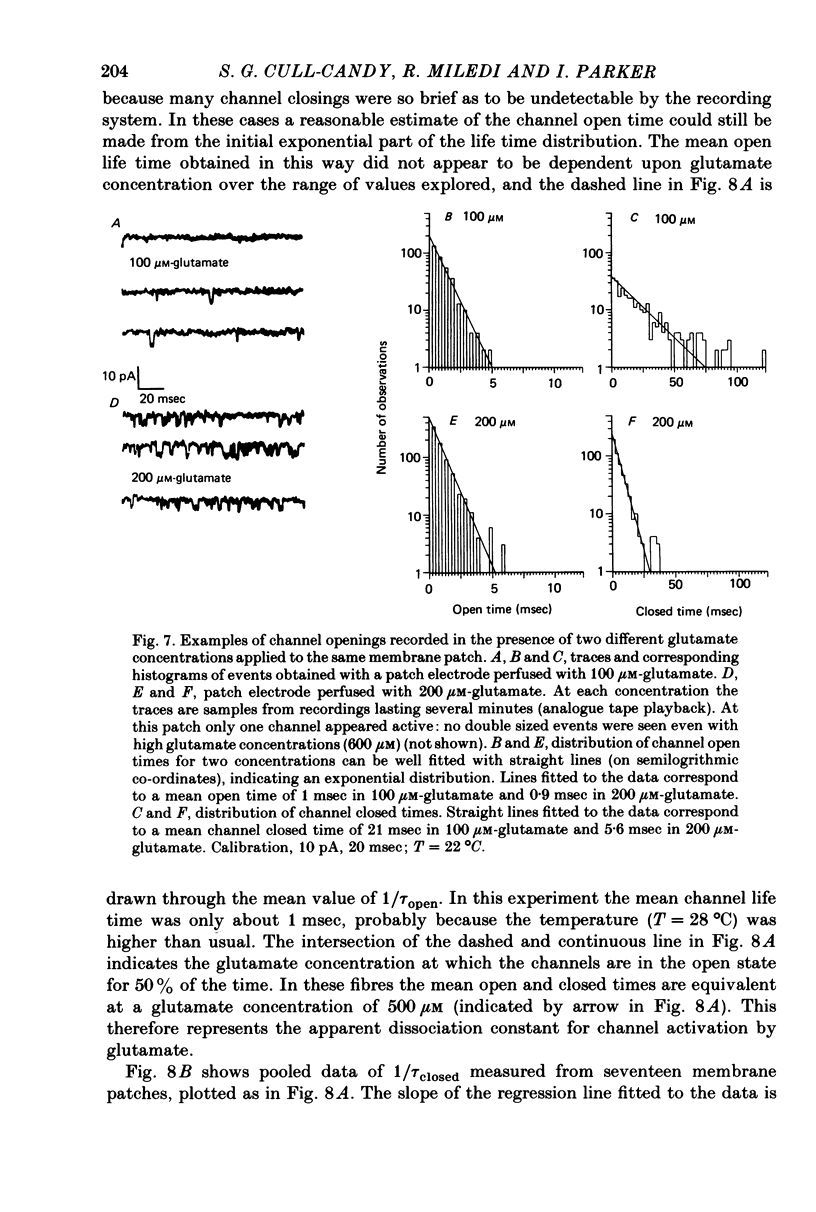
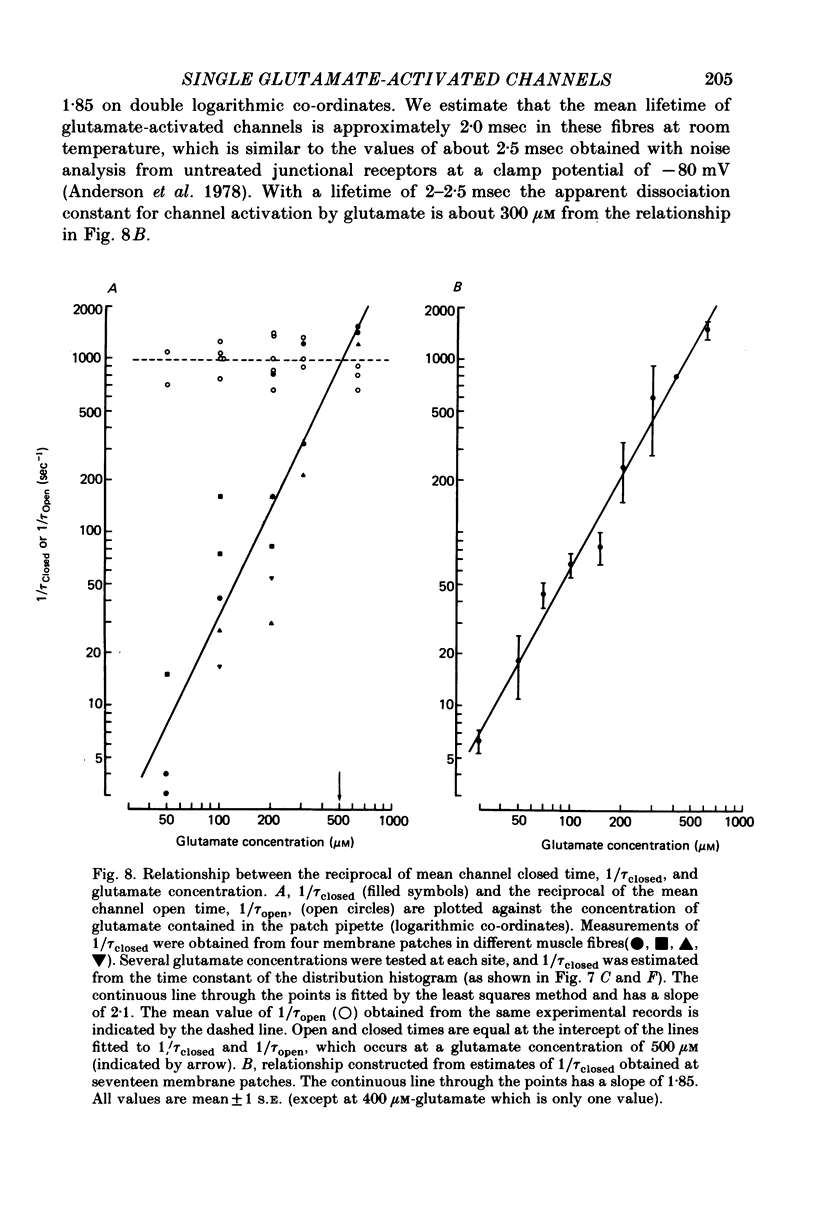
4. The behaviour of individual receptor—channel complexes has been examined at various concentrations of glutamate. Drug solutions were applied through an internal perfusion pipette which allowed exchange of the solution in the patch-electrode tip within 10 sec. The distribution of channel closed times could be fitted with a single exponential. Channel lifetime was not markedly dependent on glutamate concentration (30-600 μm) whereas the channel closed time decreased with increasing glutamate concentration.
5. The reciprocal of channel closed time vs. glutamate concentration had a slope value of 1.85 on logarithmic co-ordinates. The approximately second power dependence of net forward reaction rate on glutamate concentration suggests that at least two glutamate molecules activate a single receptor—channel complex.
6. The apparent dissociation constant for the glutamate—receptor complex is large, being about 300-500 μM. If the receptors have an equally low affinity for neurally released transmitter, then only a small amount of the transmitter packet is expected to bind to receptors. Quisqualate and glutamate have similar receptor affinities whereas receptor affinity for fluoroglutamate is smaller.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. R., Cull-Candy S. G., Miledi R. Glutamate and quisqualate noise in voltage-clamped locust muscle fibres. Nature. 1976 May 13;261(5556):151–153. doi: 10.1038/261151a0. [DOI] [PubMed] [Google Scholar]
- Anderson C. R., Cull-Candy S. G., Miledi R. Glutamate current noise: post-synaptic channel kinetics investigated under voltage clamp. J Physiol. 1978 Sep;282:219–242. doi: 10.1113/jphysiol.1978.sp012459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson C. R., Stevens C. F. Voltage clamp analysis of acetylcholine produced end-plate current fluctuations at frog neuromuscular junction. J Physiol. 1973 Dec;235(3):655–691. doi: 10.1113/jphysiol.1973.sp010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biscoe T. J., Evans R. H., Headley P. M., Martin M., Watkins J. C. Domoic and quisqualic acids as potent amino acid excitants of frog and rat spinal neurones. Nature. 1975 May 8;255(5504):166–167. doi: 10.1038/255166a0. [DOI] [PubMed] [Google Scholar]
- Clark R. B., Gration K. A., Usherwood P. N. Influence of glutamate and aspartate on time course of decay of excitatory synaptic currents at locust neuromuscular junctions. Brain Res. 1980 Jun 16;192(1):205–216. doi: 10.1016/0006-8993(80)91020-3. [DOI] [PubMed] [Google Scholar]
- Clements A. N., May T. E. Pharmacological studies on a locust neuromuscular preparation. J Exp Biol. 1974 Oct;61(2):421–442. doi: 10.1242/jeb.61.2.421. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Dionne V. E., Steinbach J. H., Stevens C. F. Conductance of channels opened by acetylcholine-like drugs in muscle end-plate. Nature. 1975 Jan 17;253(5488):204–206. doi: 10.1038/253204a0. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. On the stochastic properties of single ion channels. Proc R Soc Lond B Biol Sci. 1981 Mar 6;211(1183):205–235. doi: 10.1098/rspb.1981.0003. [DOI] [PubMed] [Google Scholar]
- Crawford A. C., McBurney R. N. On the elementary conductance event produced by L-glutamate and quanta of the natural transmitter at the neuromuscular junctions of Maia squinado. J Physiol. 1976 Jun;258(1):205–225. doi: 10.1113/jphysiol.1976.sp011415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford A. C., McBurney R. N. The post-synaptic action of some putative excitatory transmitter substances. Proc R Soc Lond B Biol Sci. 1976 Mar 16;192(1109):481–489. doi: 10.1098/rspb.1976.0026. [DOI] [PubMed] [Google Scholar]
- Crawford A. C., McBurney R. N. The termination of transmitter action at the crustacean excitatory neuromuscular junction. J Physiol. 1977 Jul;268(3):711–729. doi: 10.1113/jphysiol.1977.sp011878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S. G. Glutamate sensitivity and distribution of receptors along normal and denervated locust muscle fibres. J Physiol. 1978 Mar;276:165–181. doi: 10.1113/jphysiol.1978.sp012226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S. G. Two types of extrajunctional L-glutamate receptors in locust muscle fibres. J Physiol. 1976 Feb;255(2):449–464. doi: 10.1113/jphysiol.1976.sp011289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudel J. Nonlinear voltage dependence of excitatory synaptic current in crayfish muscle. Pflugers Arch. 1974;352(3):227–241. doi: 10.1007/BF00590488. [DOI] [PubMed] [Google Scholar]
- Dudel J. The voltage dependence of the decay of the excitatory postsynaptic current and the effect of concanavalin A at the crayfish neuromuscular junction. J Physiol (Paris) 1979;75(6):601–604. [PubMed] [Google Scholar]
- Faeder I. R., Salpeter M. M. Glutamate uptake by a stimulated insect nerve muscle preparation. J Cell Biol. 1970 Aug;46(2):300–307. doi: 10.1083/jcb.46.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOYLE G. The anatomy and innervation of locust skeletal muscle. Proc R Soc Lond B Biol Sci. 1955 Jan 27;143(911):281–292. doi: 10.1098/rspb.1955.0011. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The binding of acetylcholine to receptors and its removal from the synaptic cleft. J Physiol. 1973 Jun;231(3):549–574. doi: 10.1113/jphysiol.1973.sp010248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The characteristics of 'end-plate noise' produced by different depolarizing drugs. J Physiol. 1973 May;230(3):707–717. doi: 10.1113/jphysiol.1973.sp010213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The statistical nature of the acetycholine potential and its molecular components. J Physiol. 1972 Aug;224(3):665–699. doi: 10.1113/jphysiol.1972.sp009918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathers D. A. The influence of concanavalin A on glutamate-induced current fluctuations in locust muscle fibres. J Physiol. 1981 Mar;312:1–8. doi: 10.1113/jphysiol.1981.sp013611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathers D. A., Usherwood P. N. Concanavalin A blocks desensitisation of glutamate receptors on insect muscle fibres. Nature. 1976 Feb 5;259(5542):409–411. doi: 10.1038/259409a0. [DOI] [PubMed] [Google Scholar]
- Neher E., Sakmann B. Single-channel currents recorded from membrane of denervated frog muscle fibres. Nature. 1976 Apr 29;260(5554):799–802. doi: 10.1038/260799a0. [DOI] [PubMed] [Google Scholar]
- Neher E., Sakmann B., Steinbach J. H. The extracellular patch clamp: a method for resolving currents through individual open channels in biological membranes. Pflugers Arch. 1978 Jul 18;375(2):219–228. doi: 10.1007/BF00584247. [DOI] [PubMed] [Google Scholar]
- Onodera K., Takeuchi A. Effects of membrane potential and temperature on the excitatory post-synaptic current in the crayfish muscle. J Physiol. 1978 Mar;276:183–192. doi: 10.1113/jphysiol.1978.sp012227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patlak J. B., Gration K. A., Usherwood P. N. Single glutamate-activated channels in locust muscle. Nature. 1979 Apr 12;278(5705):643–645. doi: 10.1038/278643a0. [DOI] [PubMed] [Google Scholar]
- Sakmann B., Patlak J., Neher E. Single acetylcholine-activated channels show burst-kinetics in presence of desensitizing concentrations of agonist. Nature. 1980 Jul 3;286(5768):71–73. doi: 10.1038/286071a0. [DOI] [PubMed] [Google Scholar]
- Shinozaki H., Shibuya I. A new potent excitant, quisqualic acid: effects on crayfish neuromuscular junction. Neuropharmacology. 1974 Jul;13(7):665–672. doi: 10.1016/0028-3908(74)90056-2. [DOI] [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. THE EFFECT ON CRAYFISH MUSCLE OF IONTOPHORETICALLY APPLIED GLUTAMATE. J Physiol. 1964 Mar;170:296–317. doi: 10.1113/jphysiol.1964.sp007332. [DOI] [PMC free article] [PubMed] [Google Scholar]