Abstract
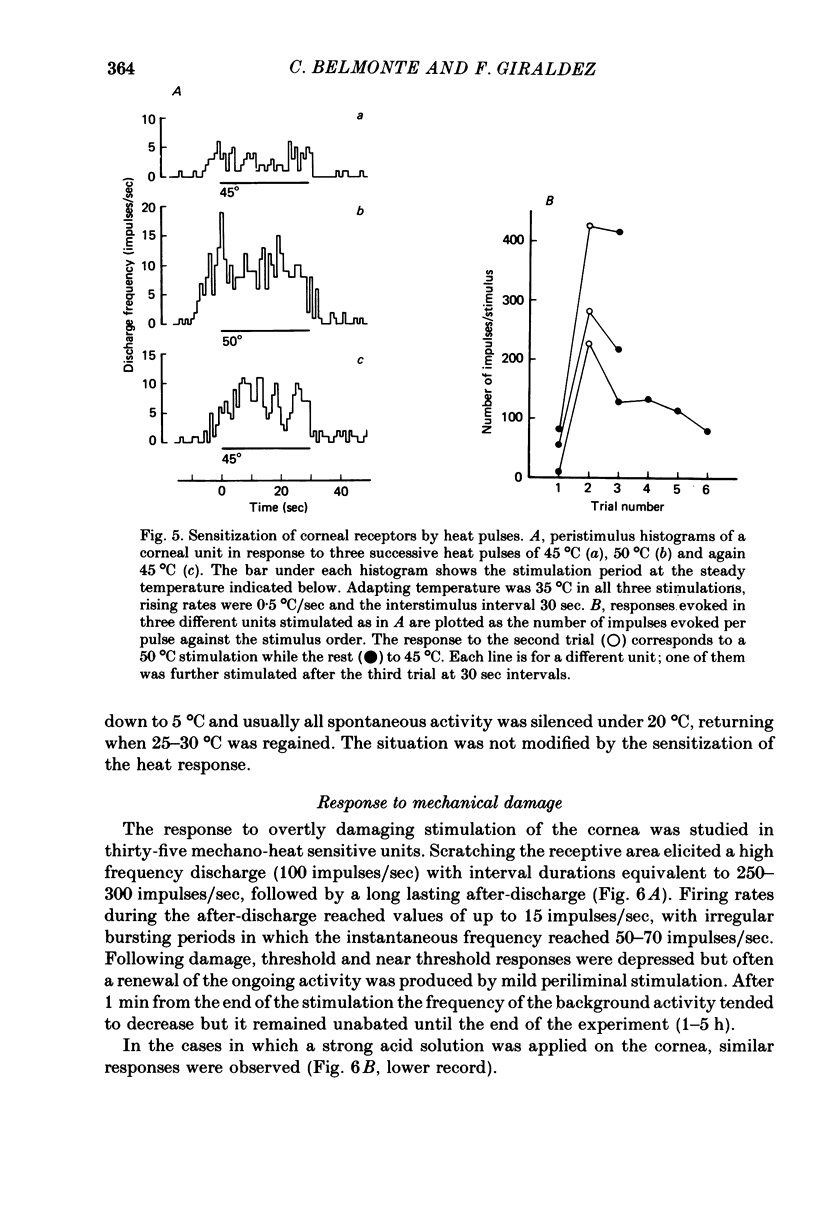
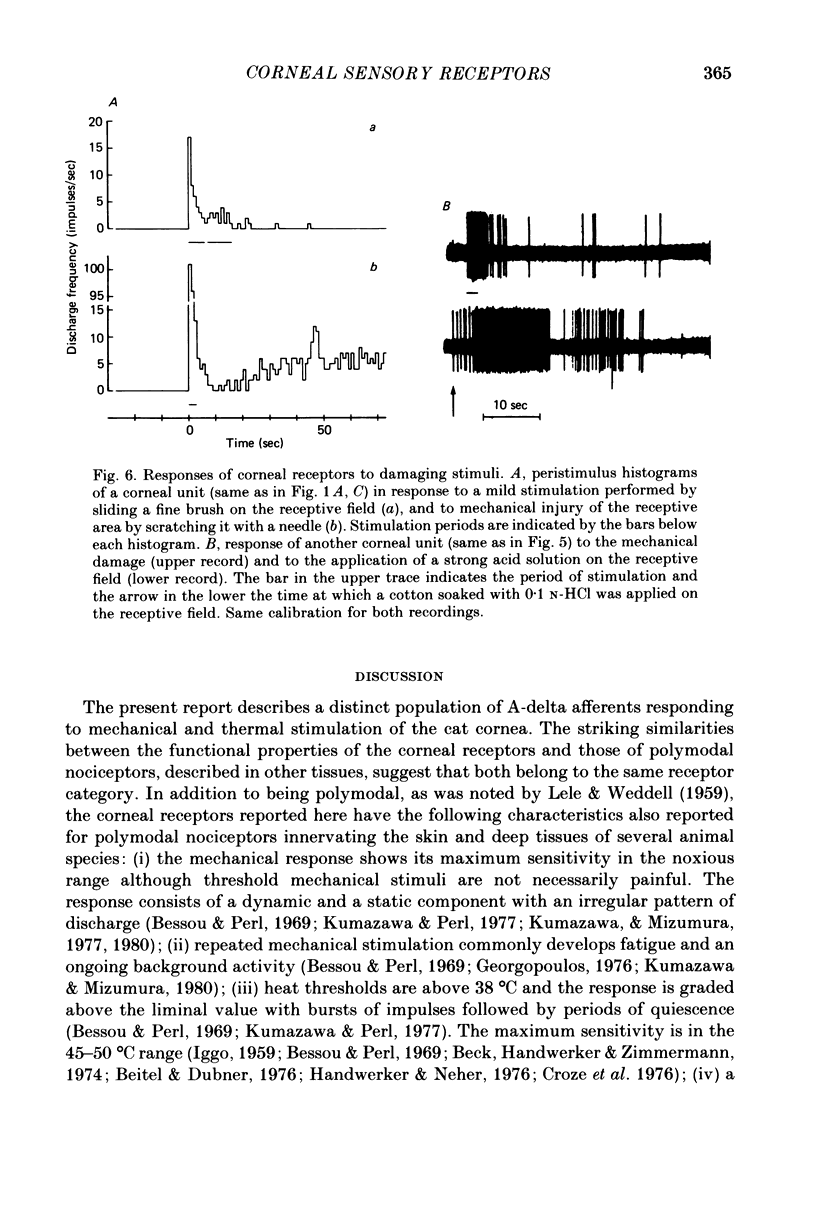
1. The afferent responses evoked by mechanical and thermal stimulation of the cat cornea were recorded extracellularly from strands of long and mixed ciliary nerves under deep anaesthesia. 94% of the units studied (n = 53) responded consistently to both stimuli. 2. Conduction velocities, measured by electrical stimulation of the receptive field, corresponded to the lower range of the A-delta fibre group (average = 5.4 m/sec). Receptive fields covered approximately a quadrant of the corneal surface and showed continuous sensitivity and overlapping. Units were silent in the absence of stimulation but an ongoing activity was commonly present after repeated mechanical and thermal stimulation. 3. Mechanical responses were evoked at low thresholds and consisted of a dynamic and static response that paralleled the amplitude of the stimulus. The pattern of the discharge was irregular and fatigue was easily developed by repeated stimulation. 4. Thresholds to heating were above 38 degrees C and the response increased monotonically with the temperature over the range from threshold to 50 degrees C. The heat response could be sensitized by repeated long suprathreshold stimulation while variable changes in the response were induced by briefer stimuli. Also depression was observed in some circumstances. A weak response to cooling was present in 50% of the units tested. 5. Damaging mechanical stimulation or the application of a strong acid solution evoked a vigorous response followed by an earlier discharge that persisted for hours. 6. The relation of these receptors to other polymodal nociceptors and corneal sensation is considered.
Full text
PDF



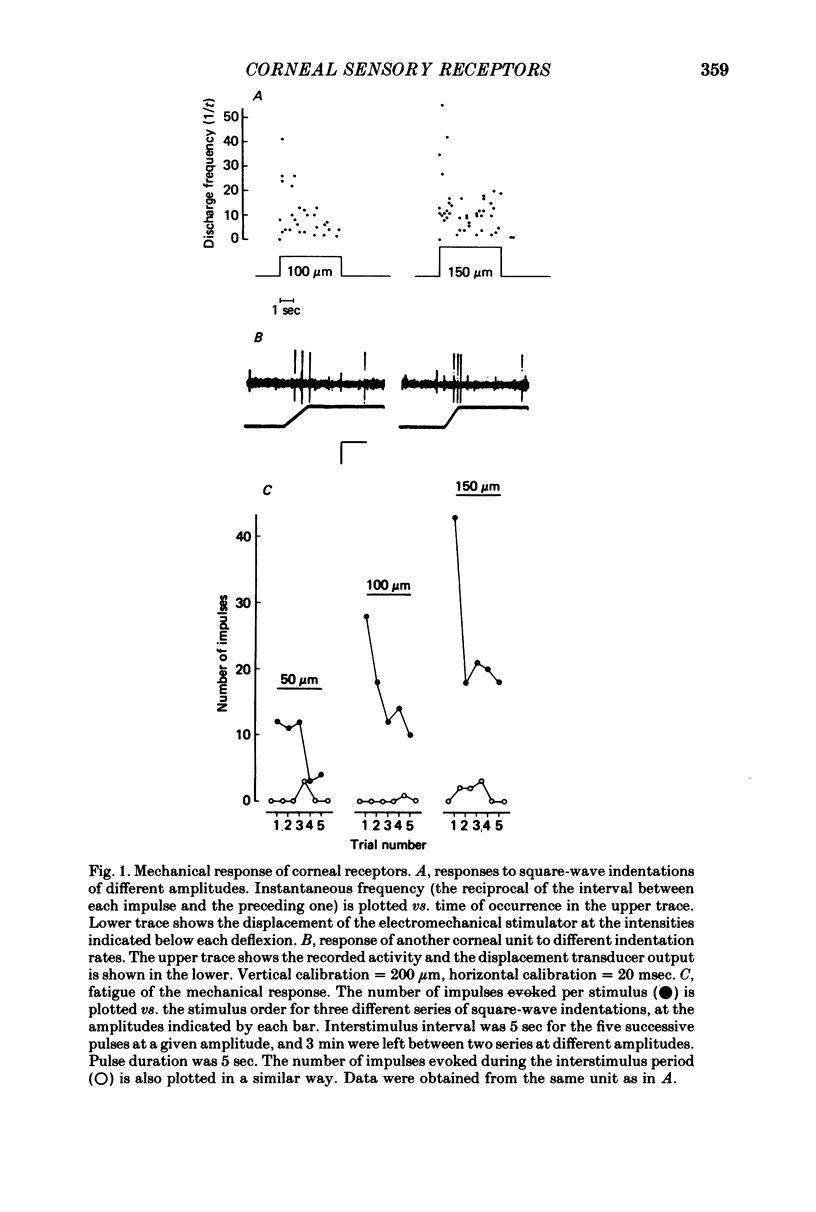
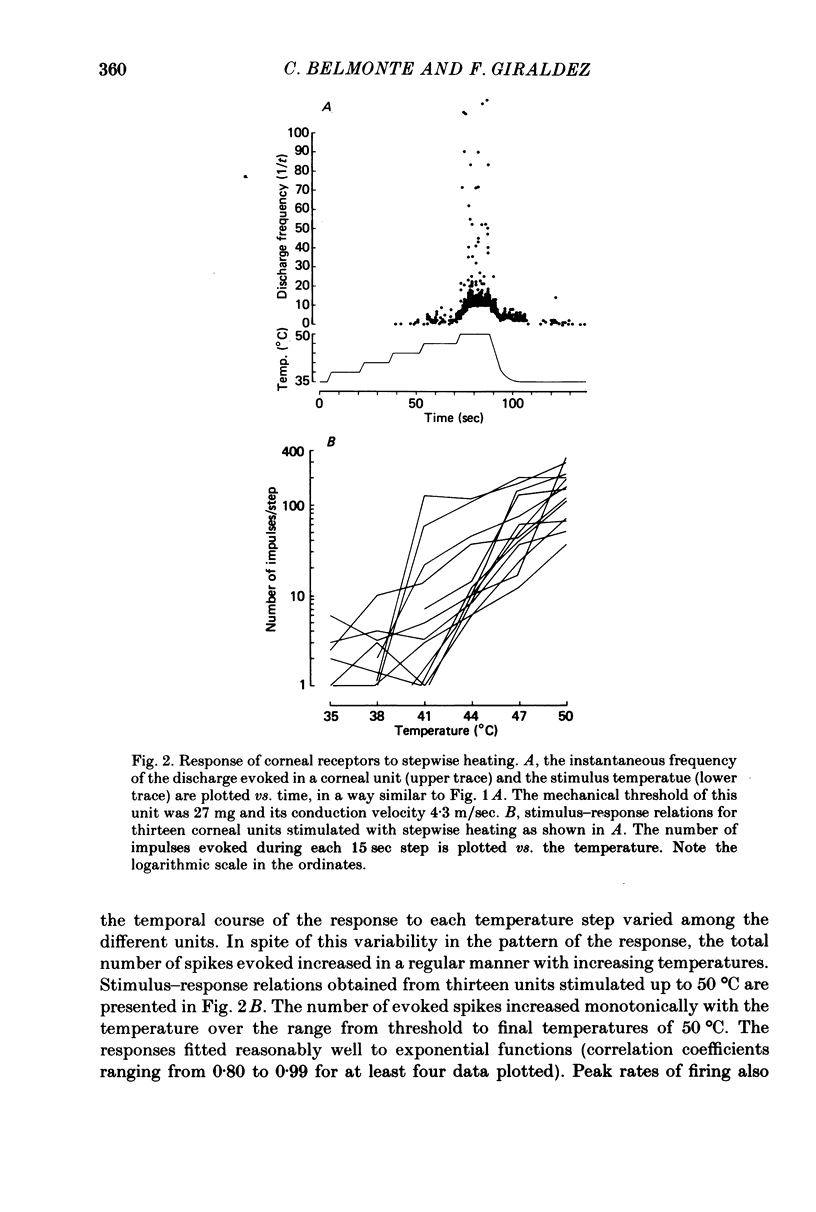

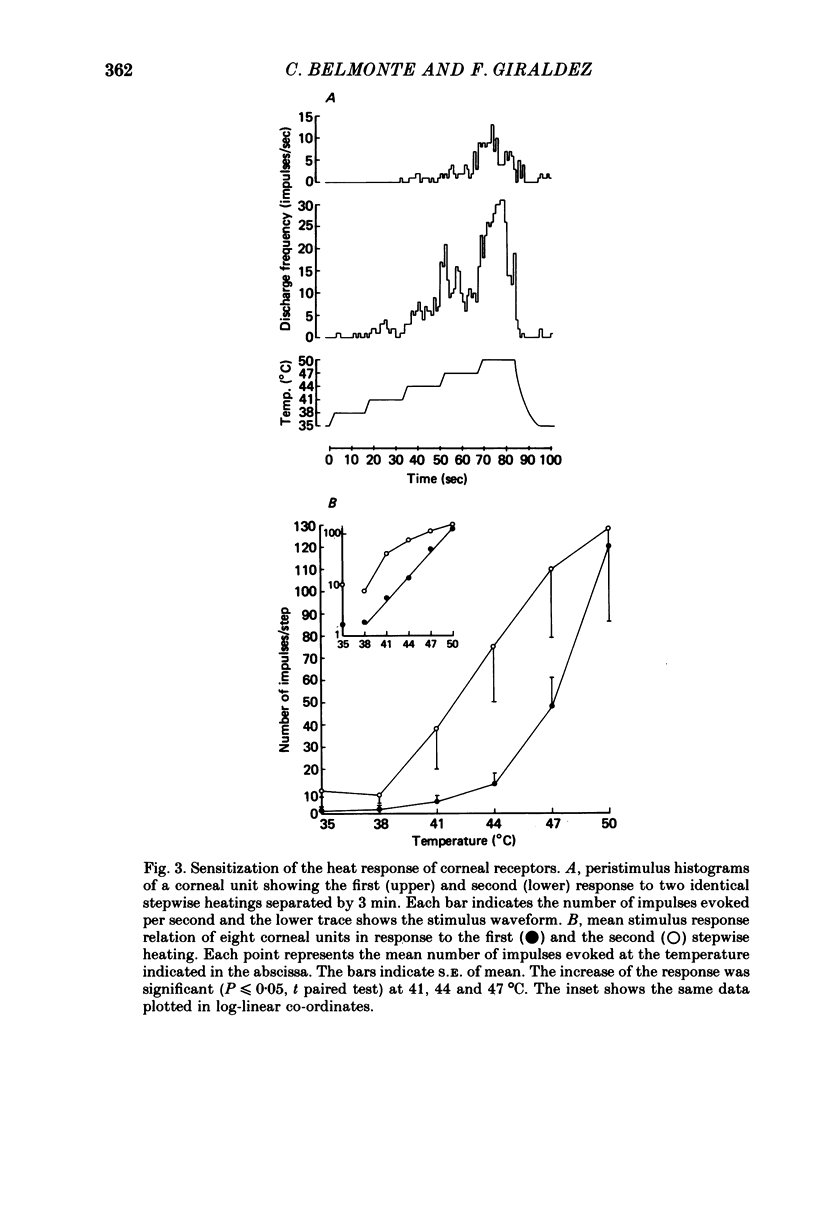
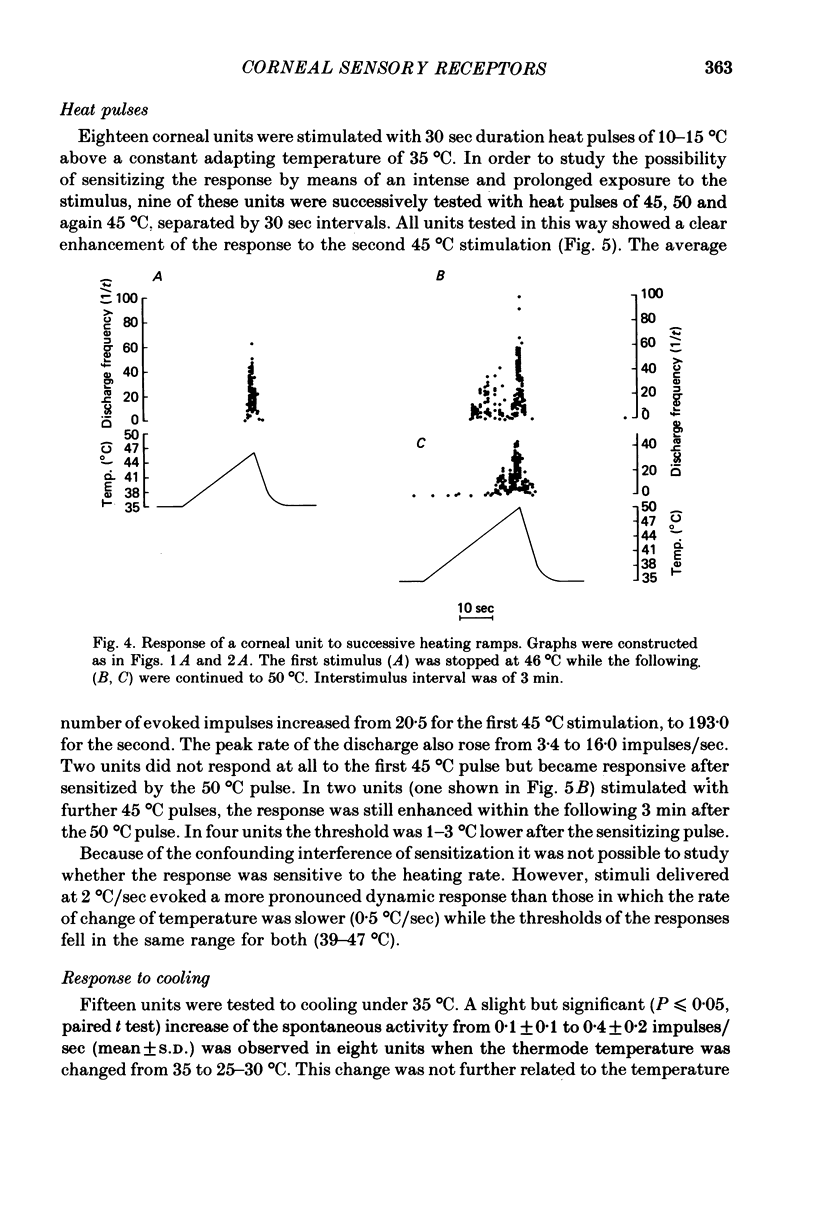



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck P. W., Handwerker H. O., Zimmermann M. Nervous outflow from the cat's foot during noxious radiant heat stimulation. Brain Res. 1974 Mar 8;67(3):373–386. doi: 10.1016/0006-8993(74)90488-0. [DOI] [PubMed] [Google Scholar]
- Beitel R. E., Dubner R. Response of unmyelinated (C) polymodal nociceptors to thermal stimuli applied to monkey's face. J Neurophysiol. 1976 Nov;39(6):1160–1175. doi: 10.1152/jn.1976.39.6.1160. [DOI] [PubMed] [Google Scholar]
- Bessou P., Perl E. R. Response of cutaneous sensory units with unmyelinated fibers to noxious stimuli. J Neurophysiol. 1969 Nov;32(6):1025–1043. doi: 10.1152/jn.1969.32.6.1025. [DOI] [PubMed] [Google Scholar]
- Beuerman R. W., Tanelian D. L. Corneal pain evoked by thermal stimulation. Pain. 1979 Aug;7(1):1–14. doi: 10.1016/0304-3959(79)90102-7. [DOI] [PubMed] [Google Scholar]
- Campbell J. N., Meyer R. A., LaMotte R. H. Sensitization of myelinated nociceptive afferents that innervate monkey hand. J Neurophysiol. 1979 Nov;42(6):1669–1679. doi: 10.1152/jn.1979.42.6.1669. [DOI] [PubMed] [Google Scholar]
- Croze S., Duclaux R., Kenshalo D. R. The thermal sensitivity of the polymodal nociceptors in the monkey. J Physiol. 1976 Dec;263(3):539–562. doi: 10.1113/jphysiol.1976.sp011644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubner R., Sumino R., Wood W. I. A peripheral "cold" fiber population responsive to innocuous and noxious thermal stimuli applied to monkey's face. J Neurophysiol. 1975 Nov;38(6):1373–1389. doi: 10.1152/jn.1975.38.6.1373. [DOI] [PubMed] [Google Scholar]
- Fidone S. J., Sato A. A study of chemoreceptor and baroreceptor A and C-fibres in the cat carotid nerve. J Physiol. 1969 Dec;205(3):527–548. doi: 10.1113/jphysiol.1969.sp008981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald M., Lynn B. The sensitization of high threshold mechanoreceptors with myelinated axons by repeated heating. J Physiol. 1977 Feb;265(2):549–563. doi: 10.1113/jphysiol.1977.sp011730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulos A. P. Functional properties of primary afferent units probably related to pain mechanisms in primate glabrous skin. J Neurophysiol. 1976 Jan;39(1):71–83. doi: 10.1152/jn.1976.39.1.71. [DOI] [PubMed] [Google Scholar]
- Giraldez F., Geijo E., Belmonte C. Response characteristics of corneal sensory fibers to mechanical and thermal stimulation. Brain Res. 1979 Nov 30;177(3):571–576. doi: 10.1016/0006-8993(79)90475-x. [DOI] [PubMed] [Google Scholar]
- Handwerker H. O., Neher K. D. Characteristics of C-fibre receptors in the cat's foot responding to stepwise increase of skin temperature ot noxious levels. Pflugers Arch. 1976 Sep 30;365(2-3):221–229. doi: 10.1007/BF01067022. [DOI] [PubMed] [Google Scholar]
- Iggo A., Ogawa H. Primate cutaneous thermal nociceptors. J Physiol. 1971 Jul;216(2):77P–78P. [PubMed] [Google Scholar]
- KENSHALO D. R. Comparison of thermal sensitivity of the forehead, lip, conjunctiva and cornea. J Appl Physiol. 1960 Nov;15:987–991. doi: 10.1152/jappl.1960.15.6.987. [DOI] [PubMed] [Google Scholar]
- Kumazawa T., Mizumura K. Mechanical and thermal responses of polymodal receptors recorded from the superior spermatic nerve of dogs. J Physiol. 1980 Feb;299:233–245. doi: 10.1113/jphysiol.1980.sp013122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumazawa T., Mizumura K. Thin-fibre receptors responding to mechanical, chemical, and thermal stimulation in the skeletal muscle of the dog. J Physiol. 1977 Dec;273(1):179–194. doi: 10.1113/jphysiol.1977.sp012088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumazawa T., Perl E. R. Primate cutaneous sensory units with unmyelinated (C) afferent fibers. J Neurophysiol. 1977 Nov;40(6):1325–1338. doi: 10.1152/jn.1977.40.6.1325. [DOI] [PubMed] [Google Scholar]
- LELE P. P., WEDDELL G. Sensory nerves of the cornea and cutaneous sensibility. Exp Neurol. 1959 Oct;1:334–359. doi: 10.1016/0014-4886(59)90025-1. [DOI] [PubMed] [Google Scholar]
- LELE P. P., WEDDELL G. The relationship between neurohistology and corneal sensibility. Brain. 1956 Mar;79(1):119–154. doi: 10.1093/brain/79.1.119. [DOI] [PubMed] [Google Scholar]
- Lynn B. The heat sensitization of polymodal nociceptors in the rabbit and its independence of the local blood flow. J Physiol. 1979 Feb;287:493–507. doi: 10.1113/jphysiol.1979.sp012672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark D., Maurice D. Sensory recording from the isolated cornea. Invest Ophthalmol Vis Sci. 1977 Jun;16(6):541–545. [PubMed] [Google Scholar]
- Millodot M. Objective measurement of corneal sensitivity. Acta Ophthalmol (Copenh) 1973;51(3):325–334. doi: 10.1111/j.1755-3768.1973.tb06010.x. [DOI] [PubMed] [Google Scholar]
- Mosso J. A., Kruger L. Receptor categories represented in spinal trigeminal nucleus caudalis. J Neurophysiol. 1973 May;36(3):472–488. doi: 10.1152/jn.1973.36.3.472. [DOI] [PubMed] [Google Scholar]
- SCHIRMER K. E. ASSESSMENT OF CORNEAL SENSITIVITY. Br J Ophthalmol. 1963 Aug;47:488–492. doi: 10.1136/bjo.47.8.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITEAR M. An electron microscope study of the cornea in mice, with special reference to the innervation. J Anat. 1960 Jul;94:387–409. [PMC free article] [PubMed] [Google Scholar]
- ZANDER E., WEDDELL G. Observations on the innervation of the cornea. J Anat. 1951 Jan;85(1):68–99. [PMC free article] [PubMed] [Google Scholar]


