Abstract
1. High-energy phosphate metabolism and energy liberated as heat and work were measured in 3 sec tetani of frog sartorius muscles at 0 °C.
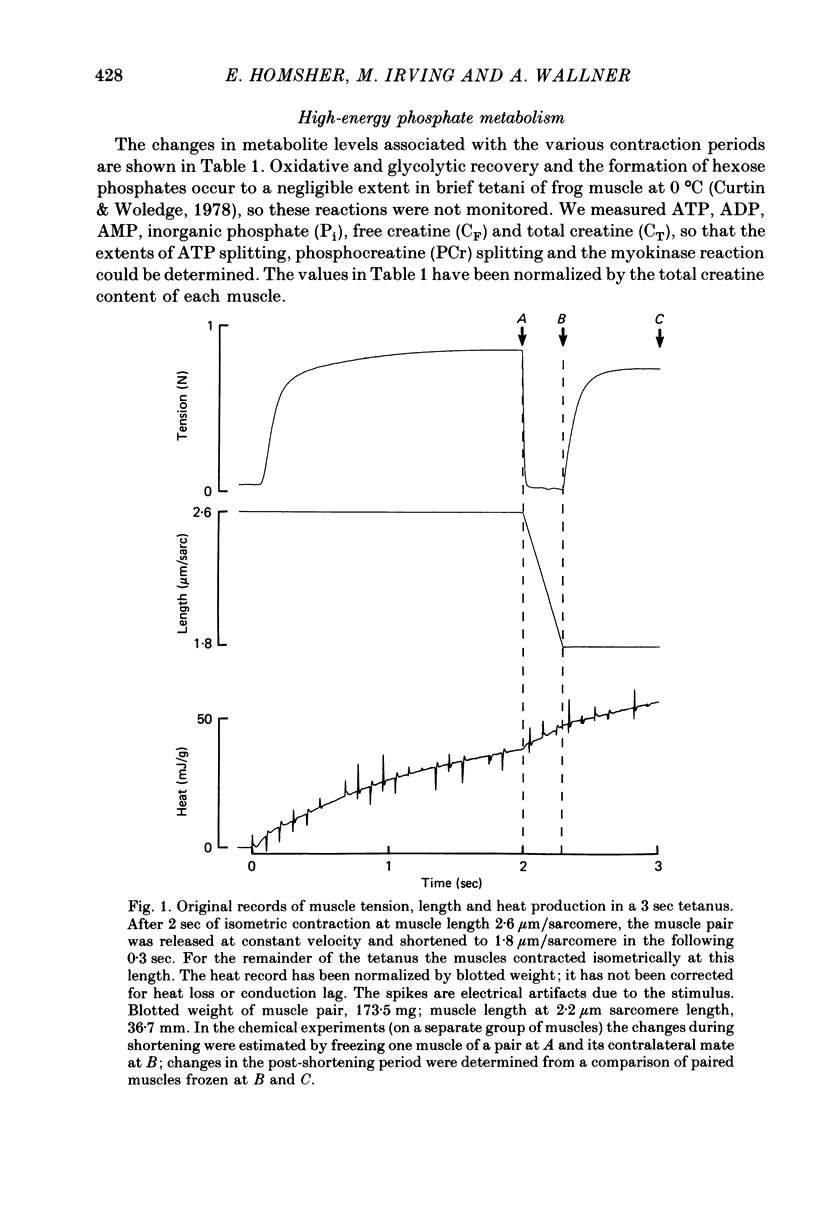
2. Three contraction periods were studied: (a) shortening at near-maximum velocity for 0.3 sec from sarcomere length 2.6 to 1.8 μm, beginning after 2 sec of isometric stimulation, (b) the 0.7 sec isometric period immediately following such rapid shortening, (c) the period from 2 to 3 sec in an isometric tetanus at sarcomere length 1.8 μm.
3. There were no significant changes in levels of ATP, ADP or AMP in any contraction period. The observed changes in inorganic phosphate and creatine levels indicated that the only significant reaction occurring was phosphocreatine splitting.
4. The mean rate of high-energy phosphate splitting during rapid shortening, 0.48 ± 0.24 μmole/g.sec (mean ± s.e. of mean, n = 29; `g' refers to blotted muscle weight), was not significantly different from that in the 1 sec period in the isometric tetanus, 0.32 ± 0.11 μmole/g.sec (n = 17). The mean rate in the post-shortening period, 0.71 ± 0.10 μmole/g.sec (n = 22), was greater than that in the 1 sec period in the isometric tetanus, and this difference is significant (P < 0.02, t test).
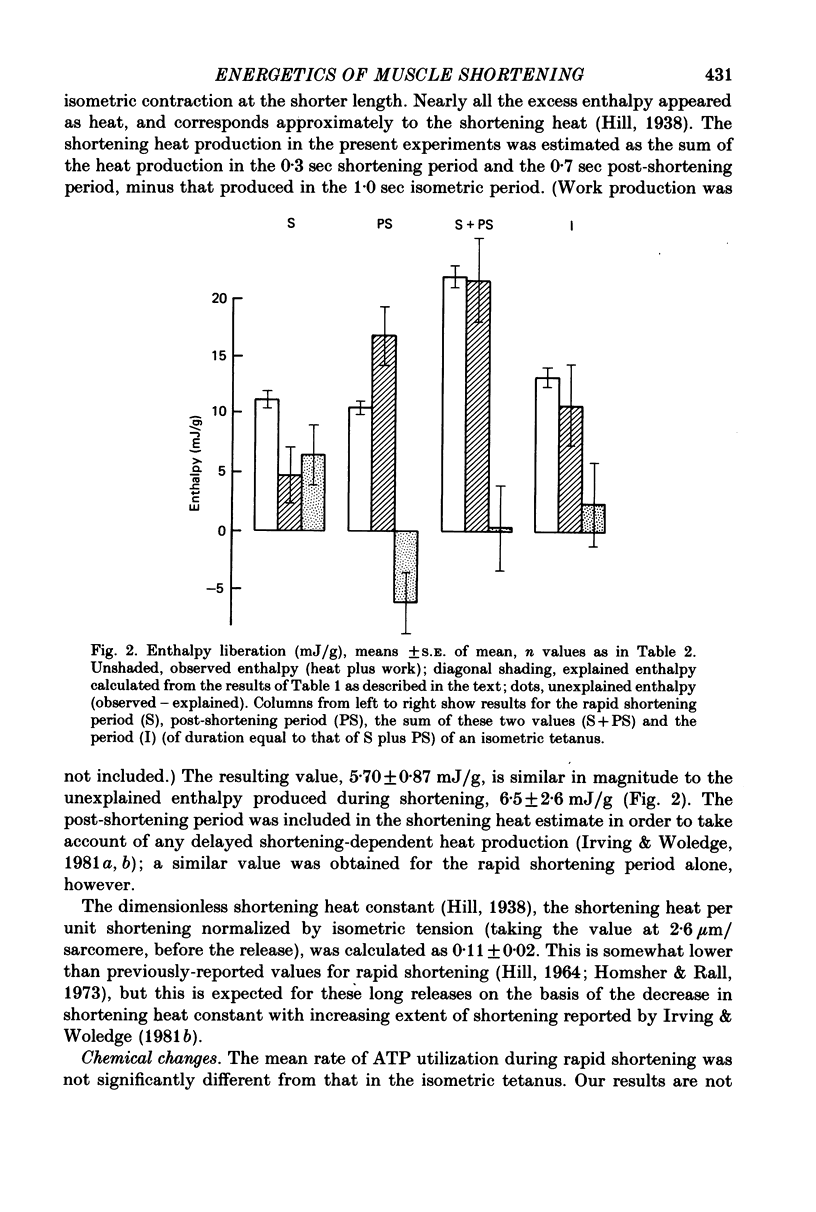
5. A large quantity of heat plus work was produced during the rapid shortening period, but less than half of this could be accounted for by simultaneous chemical reactions. The unexplained enthalpy production was 6.5 ± 2.6 mJ/g (mean ± s.e. of mean). No significant unexplained enthalpy was produced in the 1 sec period in the isometric tetanus.
6. In the post-shortening period the observed enthalpy was less, by 6.2 ± 2.6 mJ/g, than that expected from the simultaneous chemical reactions.
7. The results are interpreted in terms of an exothermic shift in the population of cross-bridge states during rapid shortening. It is suggested that a relatively slow subsequent step prevents many of these cross-bridges from completing the cycle and splitting ATP until after the end of shortening.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bárány K., Bárány M., Gillis J. M., Kushmerick M. J. Phosphorylation-dephosphorylation of the 18,000-dalton light chain of myosin during the contraction-relaxation cycle of frog muscle. J Biol Chem. 1979 May 10;254(9):3617–3623. [PubMed] [Google Scholar]
- CAIN D. F., INFANTE A. A., DAVIES R. E. Chemistry of muscle contraction. Adenosine triphosphate and phosphorylcreatine as energy supplies for single contractions of working muscle. Nature. 1962 Oct 20;196:214–217. doi: 10.1038/196214a0. [DOI] [PubMed] [Google Scholar]
- CARLSON F. D., HARDY D. J., WILKIE D. R. Total energy production and phosphocreatine hydrolysis in the isotonic twitch. J Gen Physiol. 1963 May;46:851–882. doi: 10.1085/jgp.46.5.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARLSON F. D., SIGER A. The creatine phosphoryltransfer reaction in iodoacetate-poisoned muscle. J Gen Physiol. 1959 Nov;43:301–313. doi: 10.1085/jgp.43.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplain R. A., Frommelt B. The energetics of muscular contraction. I. Total energy output and phosphoryl creatine splitting in isovelocity and isotonic tetani of frog sartorius. Pflugers Arch. 1972;334(2):167–180. doi: 10.1007/BF00586789. [DOI] [PubMed] [Google Scholar]
- Curtin N. A., Woledge R. C. Chemical change and energy production during contraction of frog muscle: how are their time courses related? J Physiol. 1979 Mar;288:353–366. [PMC free article] [PubMed] [Google Scholar]
- Curtin N. A., Woledge R. C. Energy balance in DNFB-treated and untreated frog muscle. J Physiol. 1975 Apr;246(3):737–752. doi: 10.1113/jphysiol.1975.sp010913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin N. A., Woledge R. C. Energy changes and muscular contraction. Physiol Rev. 1978 Jul;58(3):690–761. doi: 10.1152/physrev.1978.58.3.690. [DOI] [PubMed] [Google Scholar]
- DAVIES R. E. A MOLECULAR THEORY OF MUSCLE CONTRACTION: CALCIUM-DEPENDENT CONTRACTIONS WITH HYDROGEN BOND FORMATION PLUS ATP-DEPENDENT EXTENSIONS OF PART OF THE MYOSIN-ACTIN CROSS-BRIDGES. Nature. 1963 Sep 14;199:1068–1074. doi: 10.1038/1991068a0. [DOI] [PubMed] [Google Scholar]
- Ebashi S., Endo M., Otsuki I. Control of muscle contraction. Q Rev Biophys. 1969 Nov;2(4):351–384. doi: 10.1017/s0033583500001190. [DOI] [PubMed] [Google Scholar]
- Edman K. A. Mechanical deactivation induced by active shortening in isolated muscle fibres of the frog. J Physiol. 1975 Mar;246(1):255–275. doi: 10.1113/jphysiol.1975.sp010889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg E., Hill T. L., Chen Y. Cross-bridge model of muscle contraction. Quantitative analysis. Biophys J. 1980 Feb;29(2):195–227. doi: 10.1016/S0006-3495(80)85126-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferenczi M. A., Homsher E., Simmons R. M., Trentham D. R. Reaction mechanism of the magnesium ion-dependent adenosine triphosphatase of frog muscle myosin and subfragment 1. Biochem J. 1978 Apr 1;171(1):165–175. doi: 10.1042/bj1710165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford L. E., Huxley A. F., Simmons R. M. Tension responses to sudden length change in stimulated frog muscle fibres near slack length. J Physiol. 1977 Jul;269(2):441–515. doi: 10.1113/jphysiol.1977.sp011911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert C., Kretzschmar K. M., Wilkie D. R., Woledge R. C. Chemical change and energy output during muscular contraction. J Physiol. 1971 Oct;218(1):163–193. doi: 10.1113/jphysiol.1971.sp009609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILL A. V. THE EFFECT OF LOAD ON THE HEAT OF SHORTENING OF MUSCLE. Proc R Soc Lond B Biol Sci. 1964 Jan 14;159:297–318. doi: 10.1098/rspb.1964.0004. [DOI] [PubMed] [Google Scholar]
- HILL A. V., WOLEDGE R. C. An examination of absolute values in myothermic measurements. J Physiol. 1962 Jul;162:311–333. doi: 10.1113/jphysiol.1962.sp006935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUXLEY A. F. Muscle structure and theories of contraction. Prog Biophys Biophys Chem. 1957;7:255–318. [PubMed] [Google Scholar]
- Homsher E., Kean C. J., Wallner A., Garibian-Sarian V. The time-course of energy balance in an isometric tetanus. J Gen Physiol. 1979 May;73(5):553–567. doi: 10.1085/jgp.73.5.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homsher E., Mommaerts W. F., Ricchiuti N. V., Wallner A. Activation heat, activation metabolism and tension-related heat in frog semitendinosus muscles. J Physiol. 1972 Feb;220(3):601–625. doi: 10.1113/jphysiol.1972.sp009725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homsher E., Rall J. A. Energetics of shortening muscles in twitches and tetanic contractions. I. A reinvestigation of Hill's concept of the shortening heat. J Gen Physiol. 1973 Dec;62(6):663–676. doi: 10.1085/jgp.62.6.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homsher E., Rall J. A., Wallner A., Ricchiuti N. V. Energy liberation and chemical change in frog skeletal muscle during single isometric tetanic contractions. J Gen Physiol. 1975 Jan;65(1):1–21. doi: 10.1085/jgp.65.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley A. F. A note suggesting that the cross-bridge attachment during muscle contraction may take place in two stages. Proc R Soc Lond B Biol Sci. 1973 Feb 27;183(1070):83–86. doi: 10.1098/rspb.1973.0006. [DOI] [PubMed] [Google Scholar]
- Irving M., Woledge R. C. The dependence on extent of shortening of the extra energy liberated by rapidly shortening frog skeletal muscle. J Physiol. 1981 Dec;321:411–422. doi: 10.1113/jphysiol.1981.sp013993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving M., Woledge R. C. The energy liberation of frog skeletal muscle in tetanic contractions containing two periods of shortening. J Physiol. 1981 Dec;321:401–410. doi: 10.1113/jphysiol.1981.sp013992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving M., Woledge R. C., Yamada K. The heat produced by frog muscle in a series of contractions with shortening. J Physiol. 1979 Aug;293:103–118. doi: 10.1113/jphysiol.1979.sp012880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian F. J., Sollins K. R., Sollins M. R. A model for the transient and steady-state mechanical behavior of contracting muscle. Biophys J. 1974 Jul;14(7):546–562. doi: 10.1016/S0006-3495(74)85934-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretzschmar K. M., Wilkie D. R. A new method for absolute heat measurement, utilizing the Peltier effect. J Physiol. 1972 Jul;224(1):18P–21P. [PubMed] [Google Scholar]
- Kushmerick M. J., Larson R. E., Davies R. E. The chemical energetics of muscle contraction. I. Activation heat, heat of shortening and ATP utilization for activation-relaxation processes. Proc R Soc Lond B Biol Sci. 1969 Dec 23;174(1036):293–313. doi: 10.1098/rspb.1969.0095. [DOI] [PubMed] [Google Scholar]
- Lymn R. W., Taylor E. W. Mechanism of adenosine triphosphate hydrolysis by actomyosin. Biochemistry. 1971 Dec 7;10(25):4617–4624. doi: 10.1021/bi00801a004. [DOI] [PubMed] [Google Scholar]
- MOMMAERTS W. F., SERAYDARIAN K., MARECHAL G. Work and chemical change in isotonic muscular contractions. Biochim Biophys Acta. 1962 Feb 12;57:1–12. doi: 10.1016/0006-3002(62)91071-5. [DOI] [PubMed] [Google Scholar]
- Mommaerts W. F., Wallner A. The break-down of adenosine triphosphate in the contraction cycle of the frog sartorius muscle. J Physiol. 1967 Nov;193(2):343–357. doi: 10.1113/jphysiol.1967.sp008361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rall J. A., Homsher E., Wallner A., Mommaerts W. F. A temporal dissociation of energy liberation and high energy phosphate splitting during shortening in frog skeletal muscles. J Gen Physiol. 1976 Jul;68(1):13–27. doi: 10.1085/jgp.68.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartorelli L., Fromm H. J., Benson R. W., Boyer P. D. Direct and 18-O-exchange measurements relevant to possible activated or phosphorylated states of myosin. Biochemistry. 1966 Sep;5(9):2877–2884. doi: 10.1021/bi00873a015. [DOI] [PubMed] [Google Scholar]


