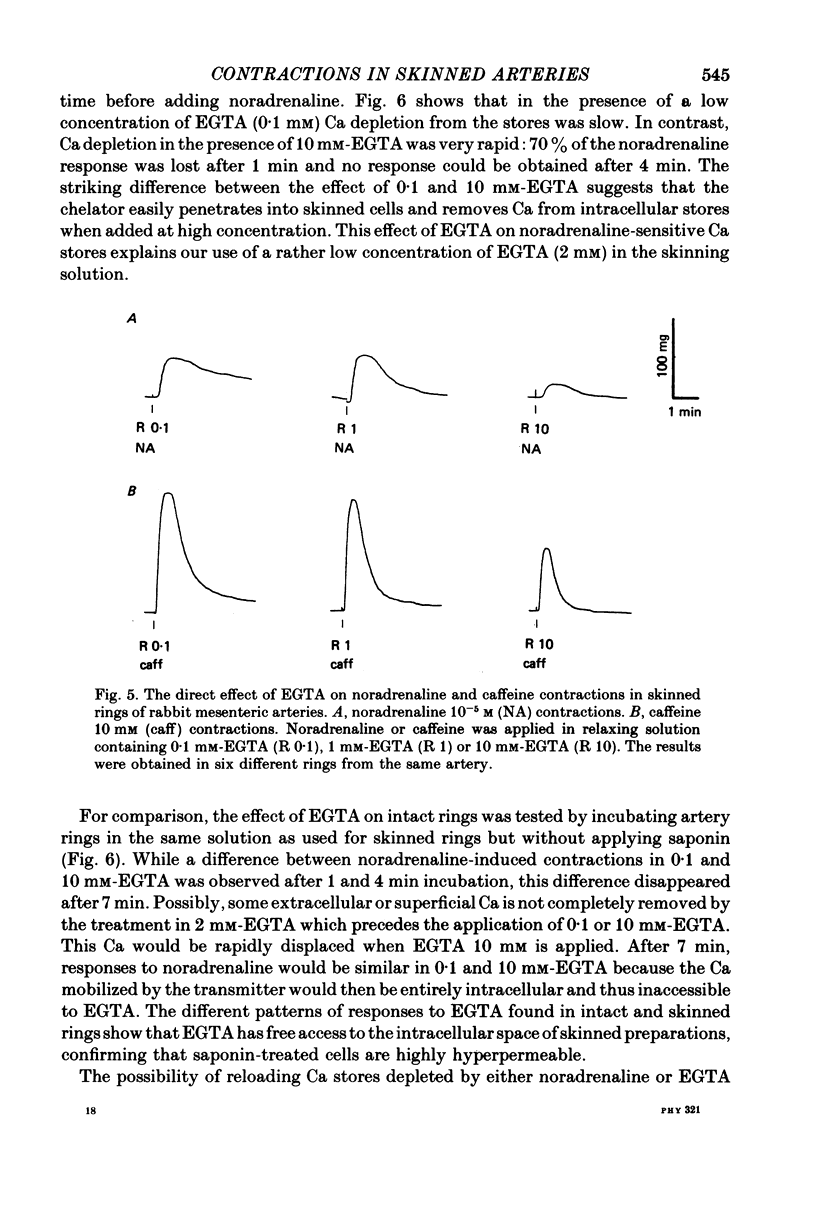
Abstract
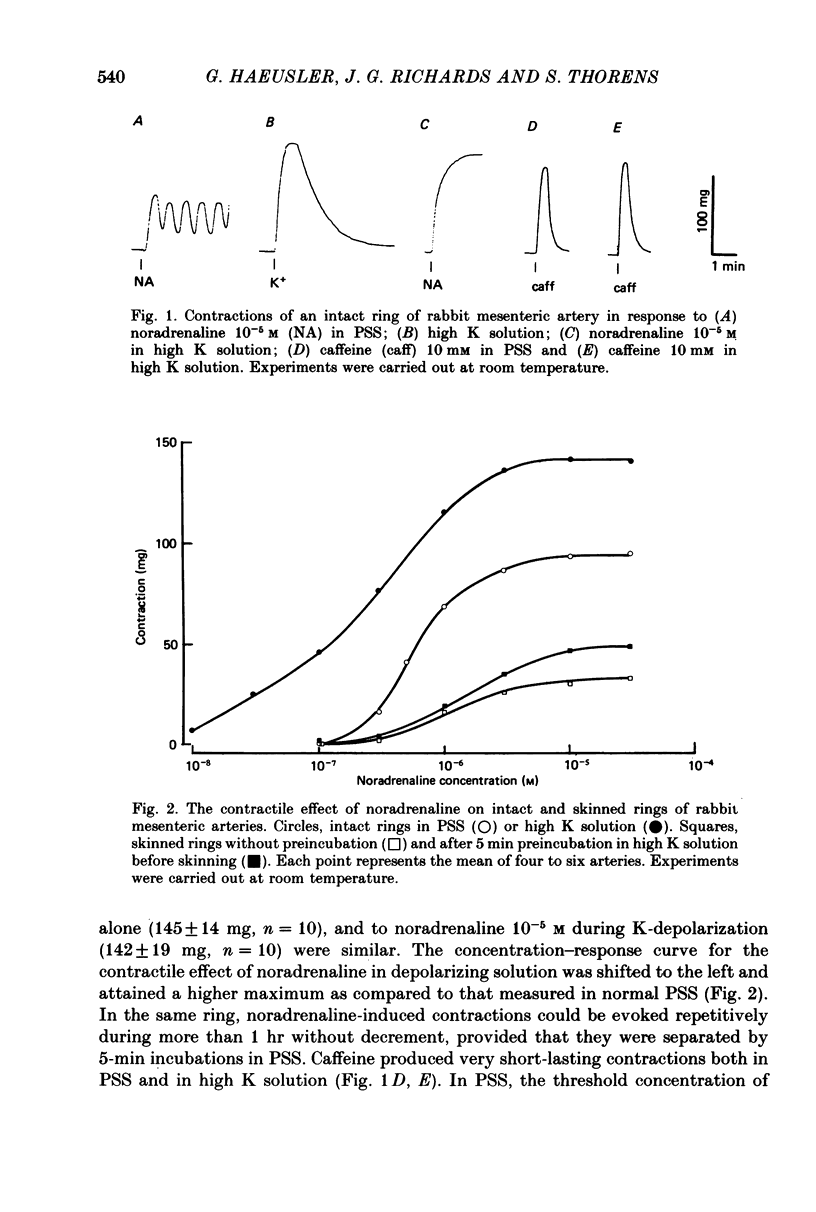
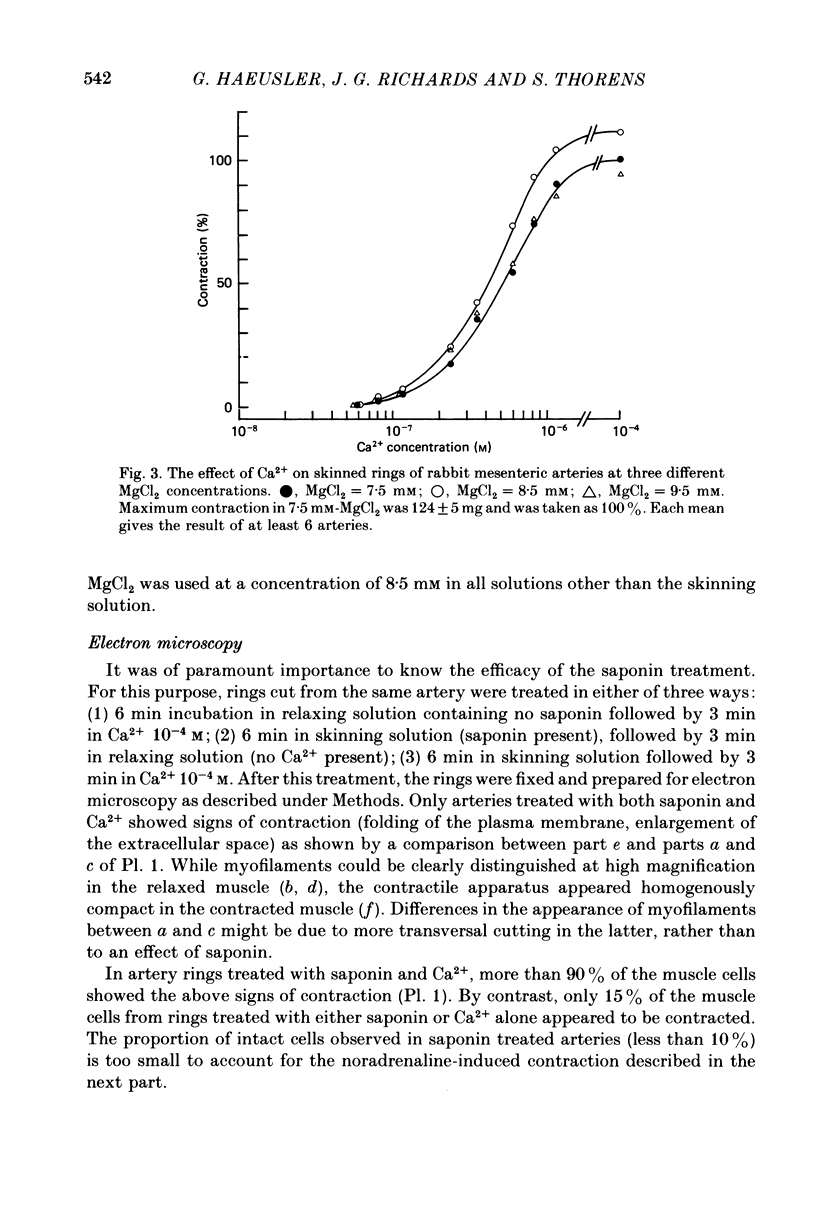
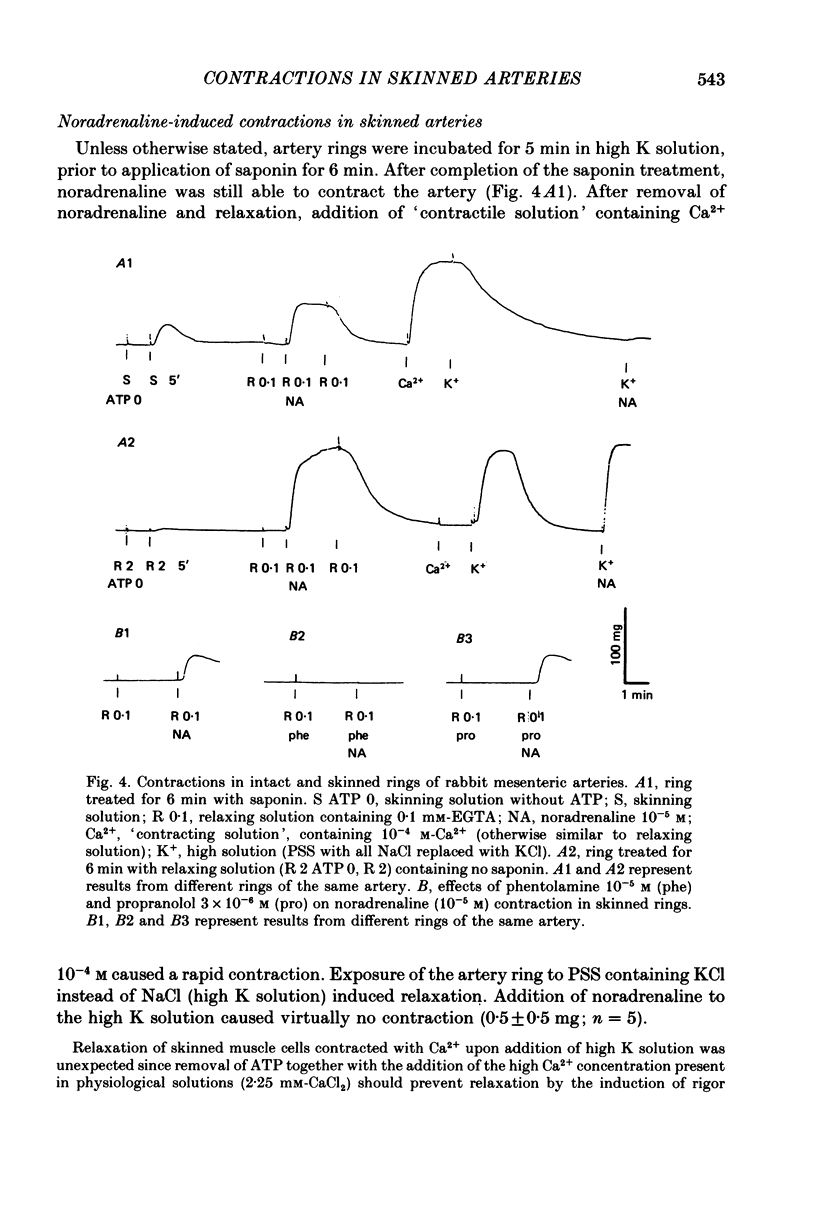
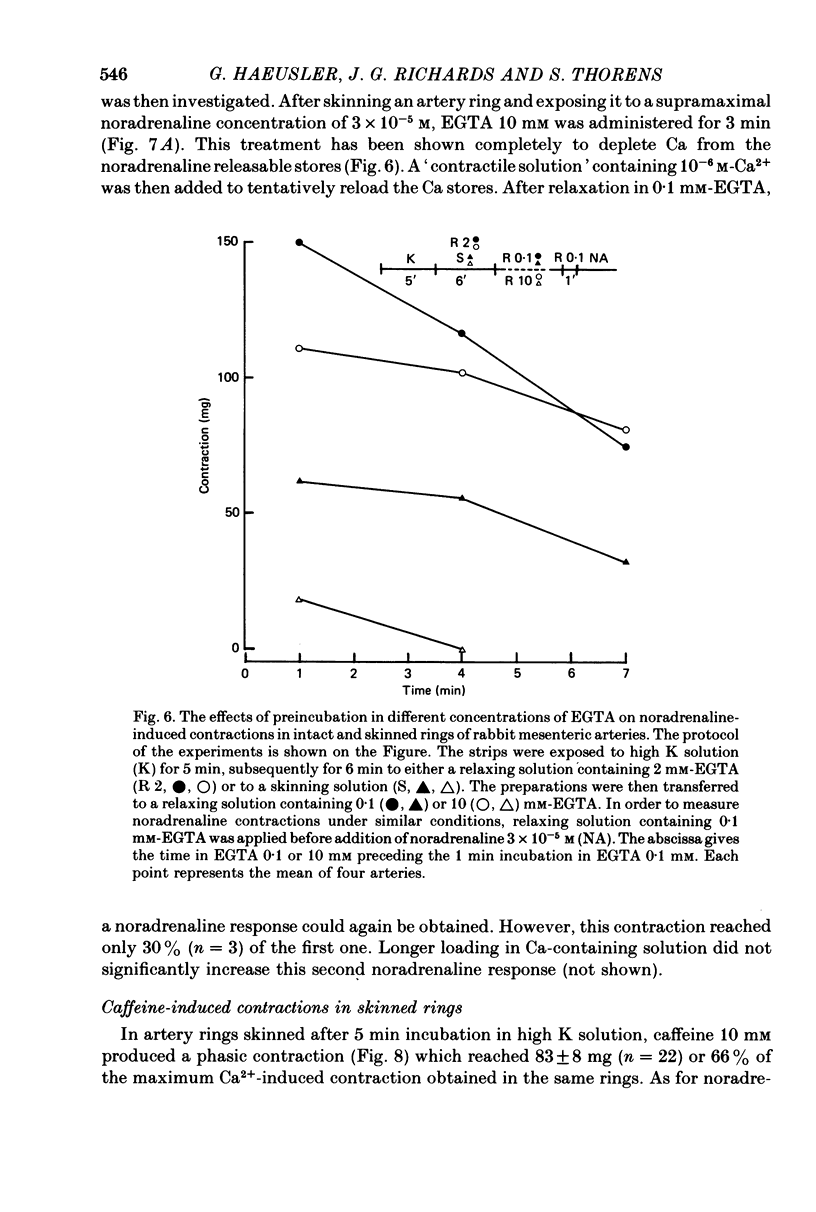
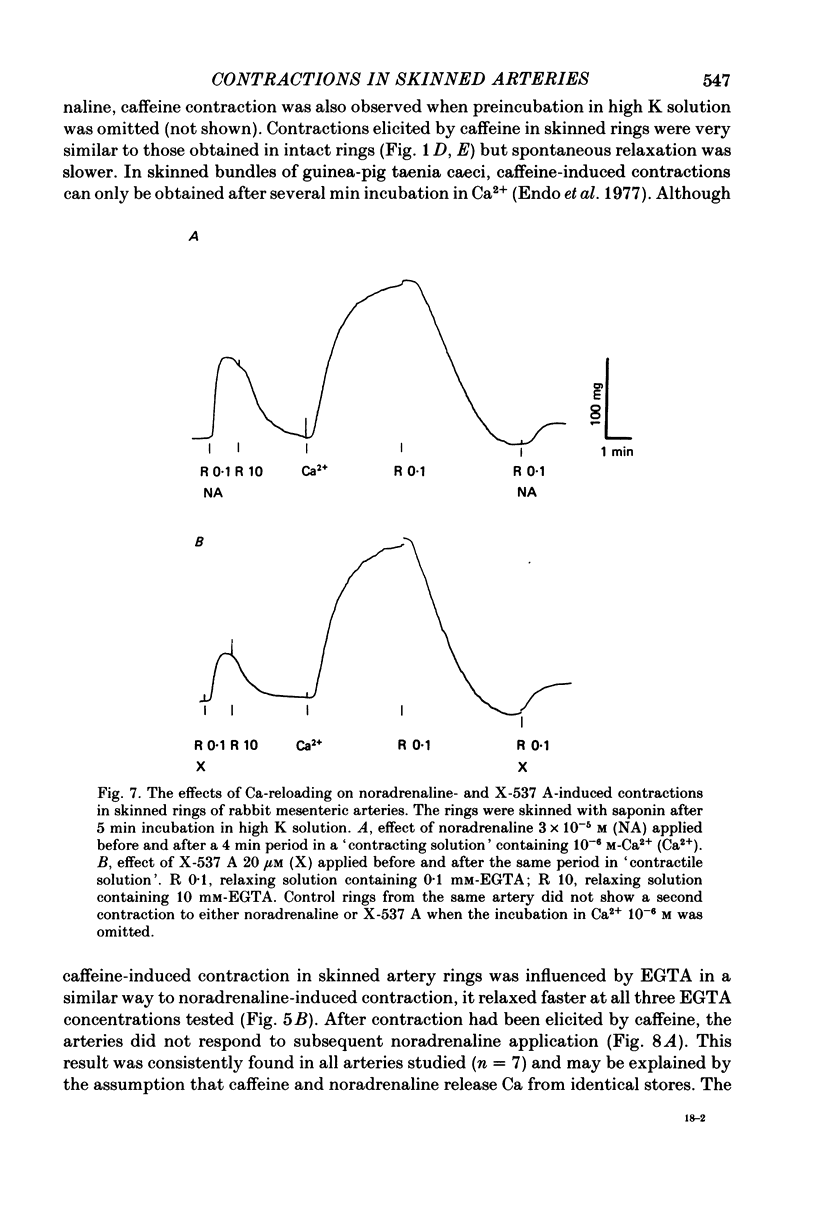
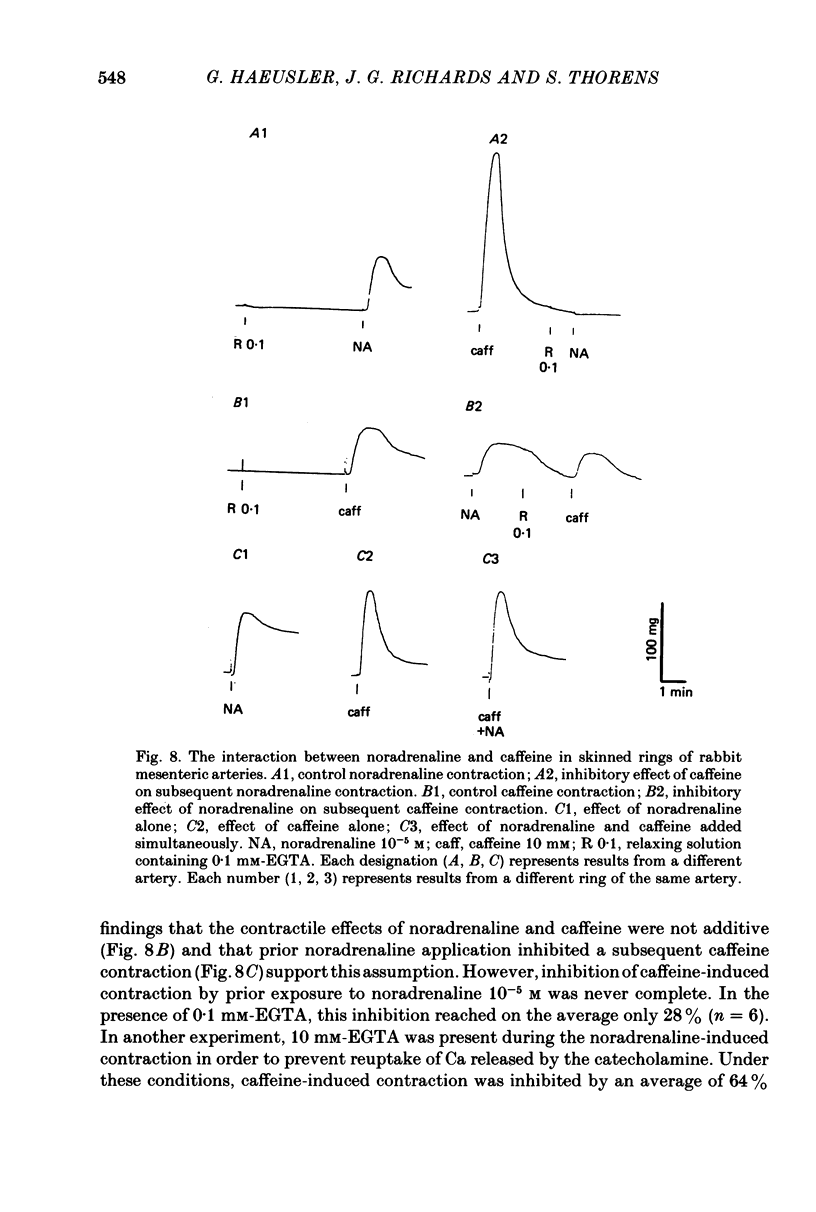
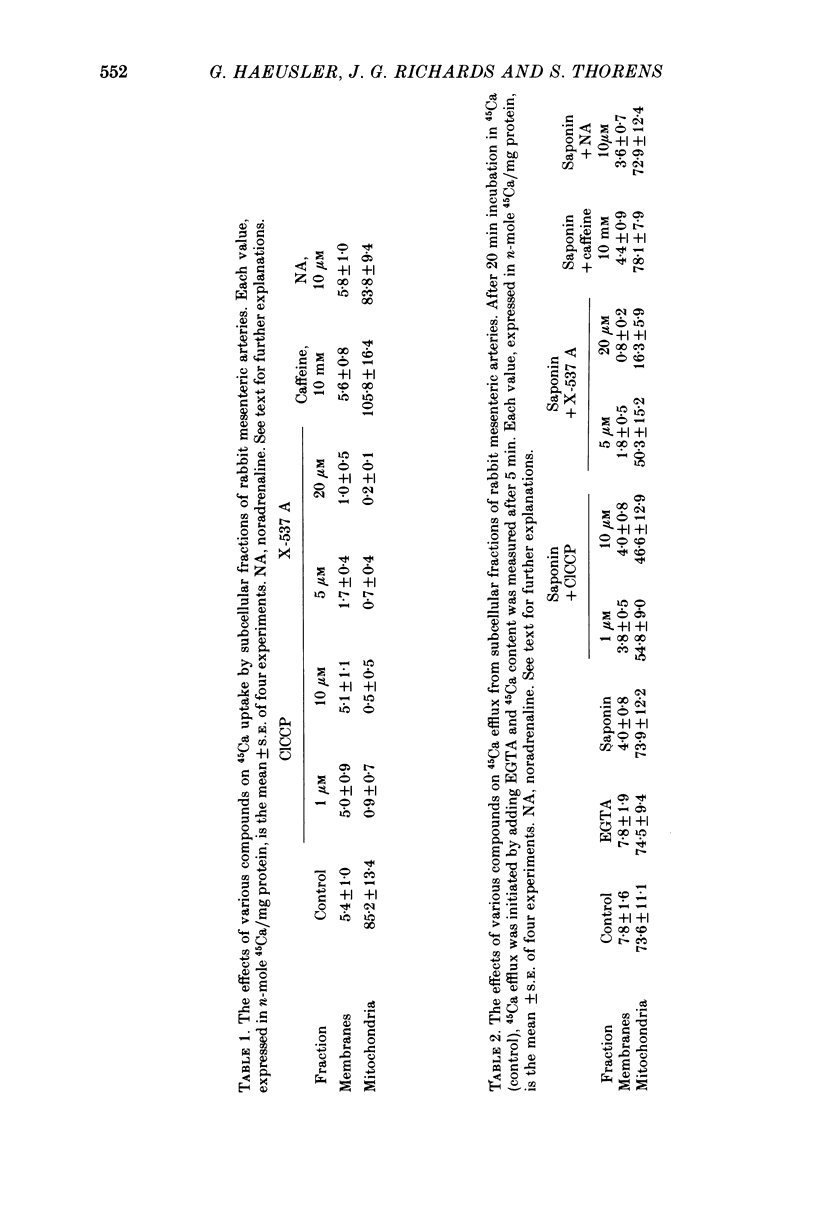
1. In rings of small rabbit mesenteric arteries, noradrenaline induced oscillatory contractions. After depolarization with potassium, which produced in this preparation only a transient contraction, the arteries responded to noradrenaline with tonic contraction. 2. Artery rings, skinned for 6 min with saponin (0.5 mg/ml.), were highly sensitive to calcium (half-maximum contraction at 4 x 10(-7) M-Ca2+). In the skinned preparations, a contraction was still elicited by noradrenaline. 3. Treatment with saponin renders virtually all smooth muscle cells of the mesenteric artery preparation hyperpermeable as indicated by both physiological and morphological criteria. 4. While the Ca stores responsible for the noradrenaline-induced contraction of skinned arteries were depleted at a slow rate by 0.1 mM-EGTA, they were completely emptied by a 4 min exposure to 10 mM-EGTA. After release of intracellular Ca by noradrenaline, the Ca stores could be partially replenished by incubating the preparation in 10(-6) M-Ca2+ for 4 min. 5. Noradrenaline failed to contract skinned arteries after part of the intracellular Ca had been released by caffeine but not after Ca release by the ionophore X-537 A. 6. The mitochondrial uncoupler, carbonyl cyanide m-chlorophenylhydrazone, inhibited noradrenaline-induced contractions of skinned arteries. 7. Noradrenaline had no effect on 45Ca translocation in either membrane vesicles or mitochondria isolated from mesenteric arteries. 8. The present results show that in vascular smooth muscle a certain degree of structural integrity of the cell membrane, but not its selective permeability, is required for the coupling between alpha-adrenoceptors and Ca release from intracellular stores; the data also suggest that alpha-adrenoceptor stimulation results in release of Ca bound to the plasma membrane rather than indirect release of Ca accumulated in intracellular organelles.
Full text
PDF




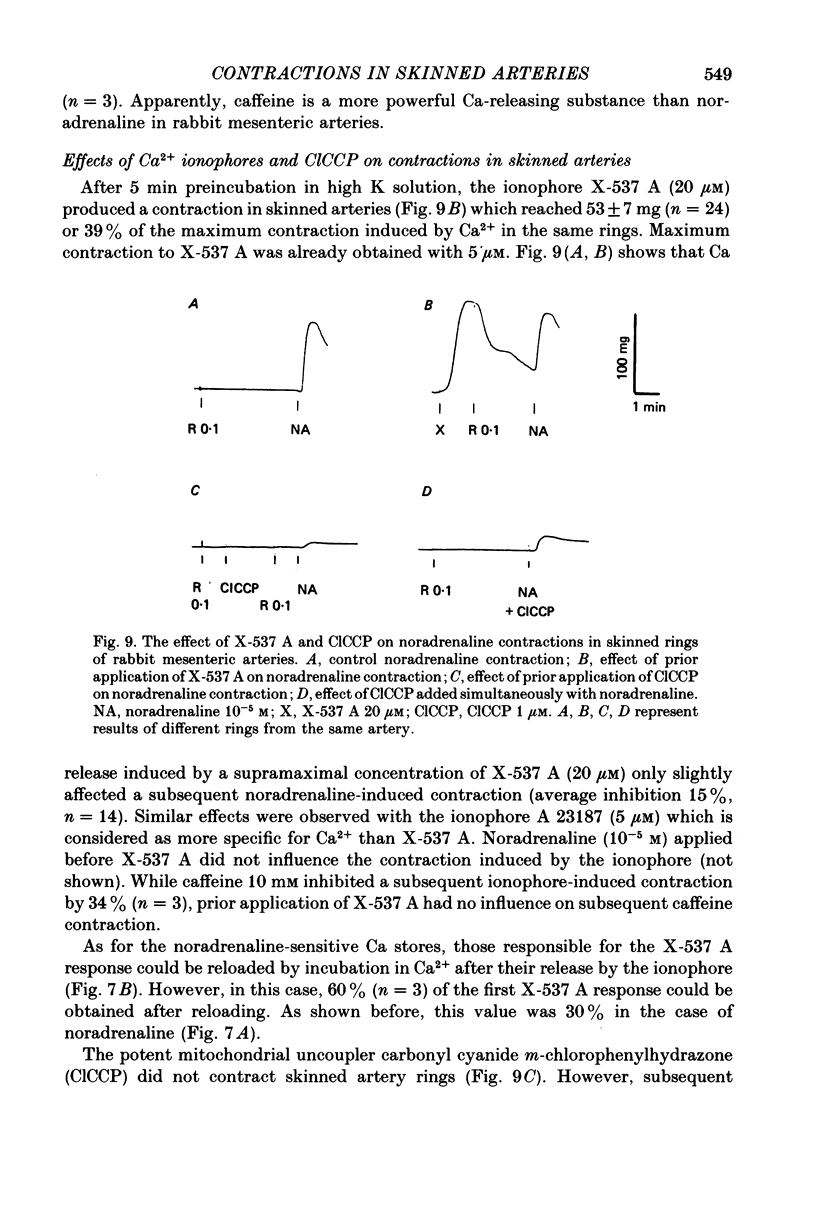






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baudouin-Legros M., Meyer P. Effects of angiotensin, catecholamines and cyclic AMP on calcium storage in aortic microsomes. Br J Pharmacol. 1973 Feb;47(2):377–385. doi: 10.1111/j.1476-5381.1973.tb08335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohr D. F. Vascular smooth muscle updated. Circ Res. 1973 Jun;32(6):665–672. doi: 10.1161/01.res.32.6.665. [DOI] [PubMed] [Google Scholar]
- Bolton T. B. Mechanisms of action of transmitters and other substances on smooth muscle. Physiol Rev. 1979 Jul;59(3):606–718. doi: 10.1152/physrev.1979.59.3.606. [DOI] [PubMed] [Google Scholar]
- Brading A. F., Sneddon P. Evidence for multiple sources of calcium for activation of the contractile mechanism of guinea-pig taenia coli on stimulation with carbachol. Br J Pharmacol. 1980 Oct;70(2):229–240. doi: 10.1111/j.1476-5381.1980.tb07928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caswell A. H., Pressman B. C. Kinetics of transport of divalent cations across sarcoplasmic reticulum vesicles induced by ionophores. Biochem Biophys Res Commun. 1972 Oct 6;49(1):292–298. doi: 10.1016/0006-291x(72)90043-5. [DOI] [PubMed] [Google Scholar]
- Colucci W. S., Gimbrone M. A., Jr, Alexander R. W. Characterization of postsynaptic alpha-adrenergic receptors by [3H]-dihydroergocryptine binding in muscular arteries from the rat mesentery. Hypertension. 1980 Mar-Apr;2(2):149–155. doi: 10.1161/01.hyp.2.2.149. [DOI] [PubMed] [Google Scholar]
- DRAHOTA Z., CARAFOLI E., ROSSI C. S., GAMBLE R. L., LEHNINGER A. L. THE STEADY STATE MAINTENANCE OF ACCUMULATED CA++ IN RAT LIVER MITOCHONDRIA. J Biol Chem. 1965 Jun;240:2712–2720. [PubMed] [Google Scholar]
- Deth R., van Breemen C. Agonist induced release of intracellular Ca2+ in the rabbit aorta. J Membr Biol. 1977 Jan 28;30(4):363–380. doi: 10.1007/BF01869677. [DOI] [PubMed] [Google Scholar]
- Droogmans G., Raeymaekers L., Casteels R. Electro- and pharmacomechanical coupling in the smooth muscle cells of the rabbit ear artery. J Gen Physiol. 1977 Aug;70(2):129–148. doi: 10.1085/jgp.70.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo M., Tanaka M., Ogawa Y. Calcium induced release of calcium from the sarcoplasmic reticulum of skinned skeletal muscle fibres. Nature. 1970 Oct 3;228(5266):34–36. doi: 10.1038/228034a0. [DOI] [PubMed] [Google Scholar]
- Entman M. L., Gillette P. C., Wallick E. T., Pressman B. C., Schwartz A. A study of calcium binding and uptake by isolated cardiac sarcoplasmic reticulum: the use of a new ionophore (X537A). Biochem Biophys Res Commun. 1972 Aug 21;48(4):847–853. doi: 10.1016/0006-291x(72)90685-7. [DOI] [PubMed] [Google Scholar]
- Exton J. H. Mechanisms involved in alpha-adrenergic phenomena: role of calcium ions in actions of catecholamines in liver and other tissues. Am J Physiol. 1980 Jan;238(1):E3–12. doi: 10.1152/ajpendo.1980.238.1.E3. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calcium release from the sarcoplasmic reticulum. Circ Res. 1977 Feb;40(2):119–129. doi: 10.1161/01.res.40.2.119. [DOI] [PubMed] [Google Scholar]
- Godfraind T., Kaba A. Actions phasique et tonique de l'adrénaline sur un muscle lisse vasculaire et leur inhibition par des agents pharmacologiques. Arch Int Pharmacodyn Ther. 1969 Apr;178(2):488–491. [PubMed] [Google Scholar]
- Godt R. E. Calcium-activated tension of skinned muscle fibers of the frog. Dependence on magnesium adenosine triphosphate concentration. J Gen Physiol. 1974 Jun;63(6):722–739. doi: 10.1085/jgp.63.6.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harafuji H., Ogawa Y. Re-examination of the apparent binding constant of ethylene glycol bis(beta-aminoethyl ether)-N,N,N',N'-tetraacetic acid with calcium around neutral pH. J Biochem. 1980 May;87(5):1305–1312. doi: 10.1093/oxfordjournals.jbchem.a132868. [DOI] [PubMed] [Google Scholar]
- Janis R. A., Crankshaw D. J., Daniel E. E. Control of intracellular Ca2+ activity in rat myometrium. Am J Physiol. 1977 Jan;232(1):C50–C58. doi: 10.1152/ajpcell.1977.232.1.C50. [DOI] [PubMed] [Google Scholar]
- Karaki H., Kubota H., Urakawa N. Mobilization of stored calcium for phasic contraction induced by norepinephrine in rabbit aorta. Eur J Pharmacol. 1979 Jun 15;56(3):237–245. doi: 10.1016/0014-2999(79)90176-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mekata F. Electrophysiological studies of the smooth muscle cell membrane of the rabbit common carotid artery. J Gen Physiol. 1971 Jun;57(6):738–751. doi: 10.1085/jgp.57.6.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekata F., Niu H. Biophysical effects of adrenaline on the smooth muscle of the rabbit common carotid artery. J Gen Physiol. 1972 Jan;59(1):92–102. doi: 10.1085/jgp.59.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobley B. A. Calcium ionophores and tension production in skinned frog muscle fibers. Eur J Pharmacol. 1977 Sep 15;45(2):101–104. doi: 10.1016/0014-2999(77)90079-6. [DOI] [PubMed] [Google Scholar]
- Saida K., Nonomura Y. Characteristics of Ca2+- and Mg2+-induced tension development in chemically skinned smooth muscle fibers. J Gen Physiol. 1978 Jul;72(1):1–14. doi: 10.1085/jgp.72.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somlyo A. P., Somlyo A. V., Shuman H. Electron probe analysis of vascular smooth muscle. Composition of mitochondria, nuclei, and cytoplasm. J Cell Biol. 1979 May;81(2):316–335. doi: 10.1083/jcb.81.2.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somlyo A. V., Somlyo A. P. Electromechanical and pharmacomechanical coupling in vascular smooth muscle. J Pharmacol Exp Ther. 1968 Jan;159(1):129–145. [PubMed] [Google Scholar]
- Thorens S. Ca2+-ATPase and Ca uptake without requirement for Mg2+ in membrane fractions of vascular smooth muscle. FEBS Lett. 1979 Feb 1;98(1):177–180. doi: 10.1016/0014-5793(79)80178-7. [DOI] [PubMed] [Google Scholar]
- Tsai B. S., Lefkowitz R. J. [3H]Dihydroergocryptine binding to alpha adrenergic receptors in canine aortic membranes. J Pharmacol Exp Ther. 1978 Mar;204(3):606–614. [PubMed] [Google Scholar]
- Vallières J., Scarpa A., Somlyo A. P. Subcellular fractions of smooth muscle. Isolation, substrate utilization and Ca++ transport by main pulmonary artery and mesenteric vein mitochondria. Arch Biochem Biophys. 1975 Oct;170(2):659–669. doi: 10.1016/0003-9861(75)90162-9. [DOI] [PubMed] [Google Scholar]
- Van Breemen C., Farinas B. R., Gerba P., McNaughton E. D. Excitation-contraction coupling in rabbit aorta studied by the lanthanum method for measuring cellular calcium influx. Circ Res. 1972 Jan;30(1):44–54. doi: 10.1161/01.res.30.1.44. [DOI] [PubMed] [Google Scholar]
- Weber A., Herz R. The relationship between caffeine contracture of intact muscle and the effect of caffeine on reticulum. J Gen Physiol. 1968 Nov;52(5):750–759. doi: 10.1085/jgp.52.5.750. [DOI] [PMC free article] [PubMed] [Google Scholar]