Abstract
1. Recordings have been made from seventy-three neurones responding to electrical stimulation of pelvic, hypogastric or lumbar colonic nerves, in decerebrate or anaesthetized cats. Fifty-two of the units had long projections that ascended to the first cervical segment, and no units with visceral inputs were found to belong to the spino-cervical tract. Twenty-one units had long descending projections.
2. Twenty percent (i.e. 11/46) responded to parasympathetic (pelvic) nerve stimulation (group 1) whilst 80% (35/46) responded to stimulation of hypogastric and/or lumbar colonic nerves (group 2). Ninety percent of group 2 neurones also responded to pelvic nerve stimulation.
3. The electrical thresholds for activation of the units indicated that the largest peripheral nerve fibres responsible for the response were of the Aδ size.
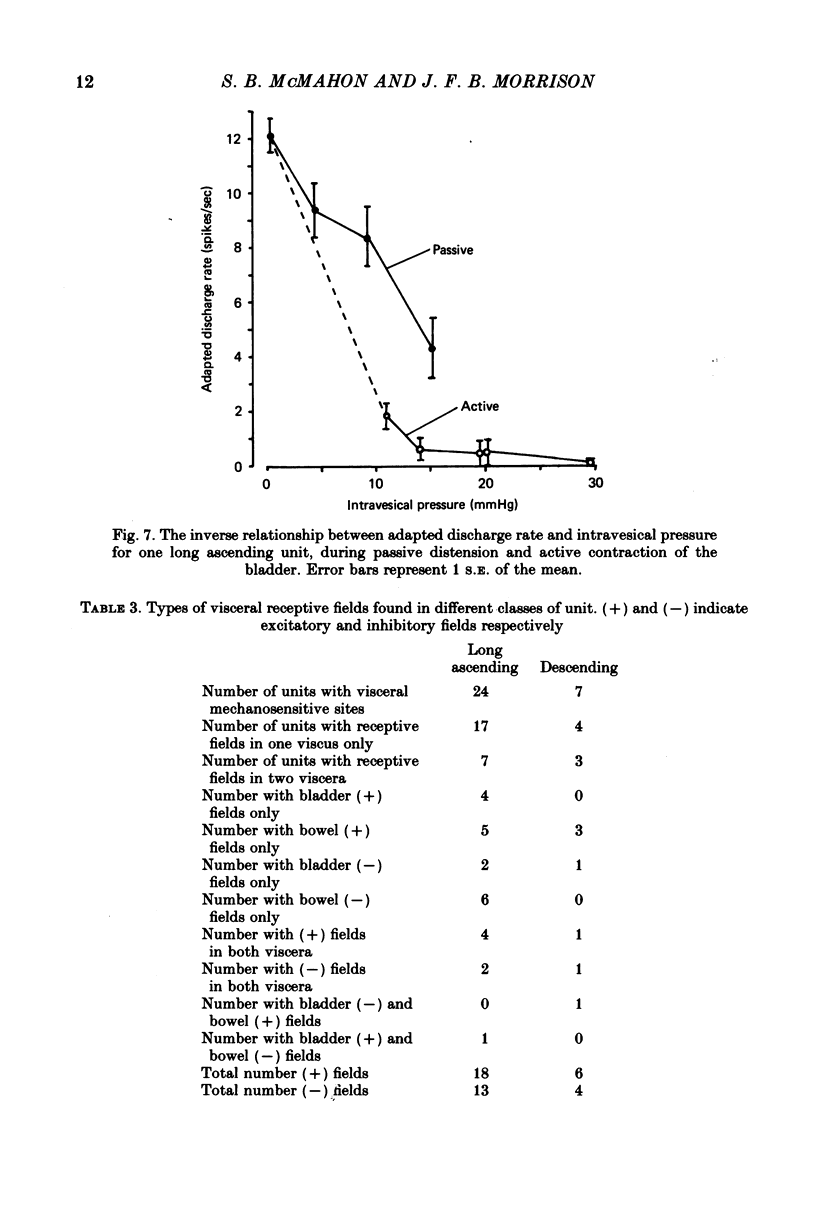
4. Thirty-one of the neurones had visceral mechanosensitive receptive fields; twenty-one had simple receptive fields in the bladder (seven) or in the colon (fourteen), ten units had compound receptive fields. The response of units with simple receptive fields to mechanical stimulation were either inhibitory or excitatory, and slowly adapting or rapidly adapting. Forty-two units appeared to have no visceral mechano-sensitive receptive fields in spite of showing responses to visceral nerve stimulation.
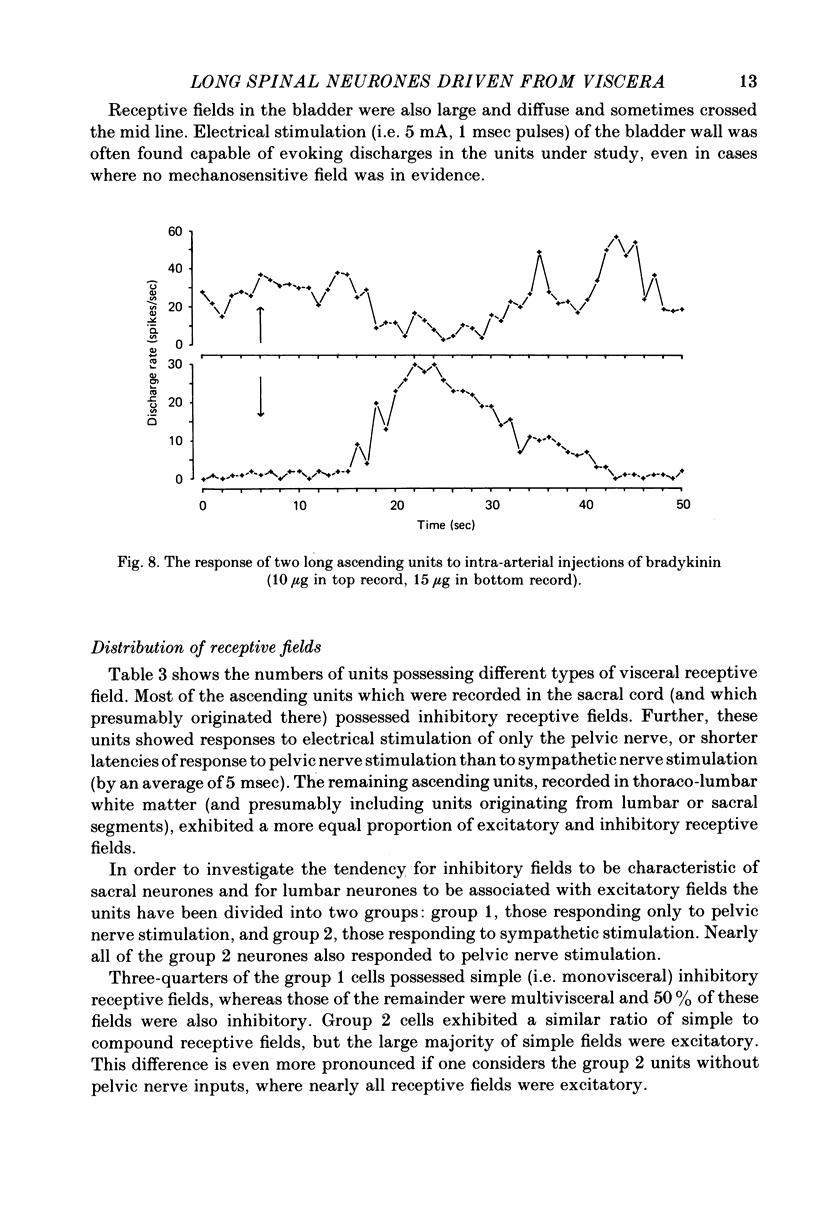
5. Fifty percent of the units tested responded to innocuous somatic stimuli, mostly derived from muscle or joint receptors. Some of the units were found to respond to injections of bradykinin (10-15 μg) into a hindlimb artery.
6. Group 1 had predominantly inhibitory visceral receptive fields, and somatic receptive fields in structures innervated from sacral segments of the spinal cord. Group 2 units all received inputs from visceral nerves entering the spinal cord over lumbar segments; many also received projections from sacral segmental inputs. These inputs showed an equal mixture of excitatory and inhibitory visceral receptive fields and convergence from somatic inputs arising from lumbar as well as sacral dermatomes. It seems likely that this group represents units originating in lumbar as well as sacral segments of the cord.
7. The possible role of these neurones as mediators of visceral sensations and visceral reflexes is discussed.
Full text
PDF





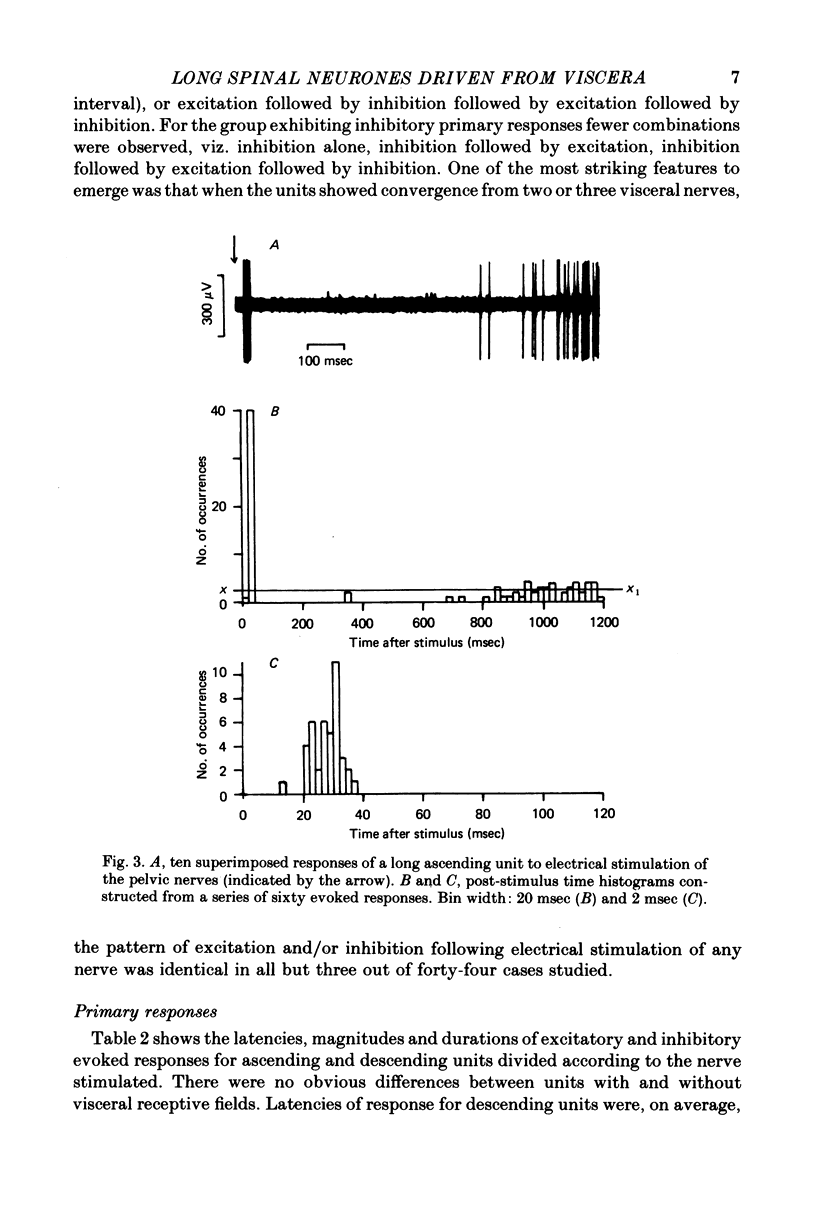
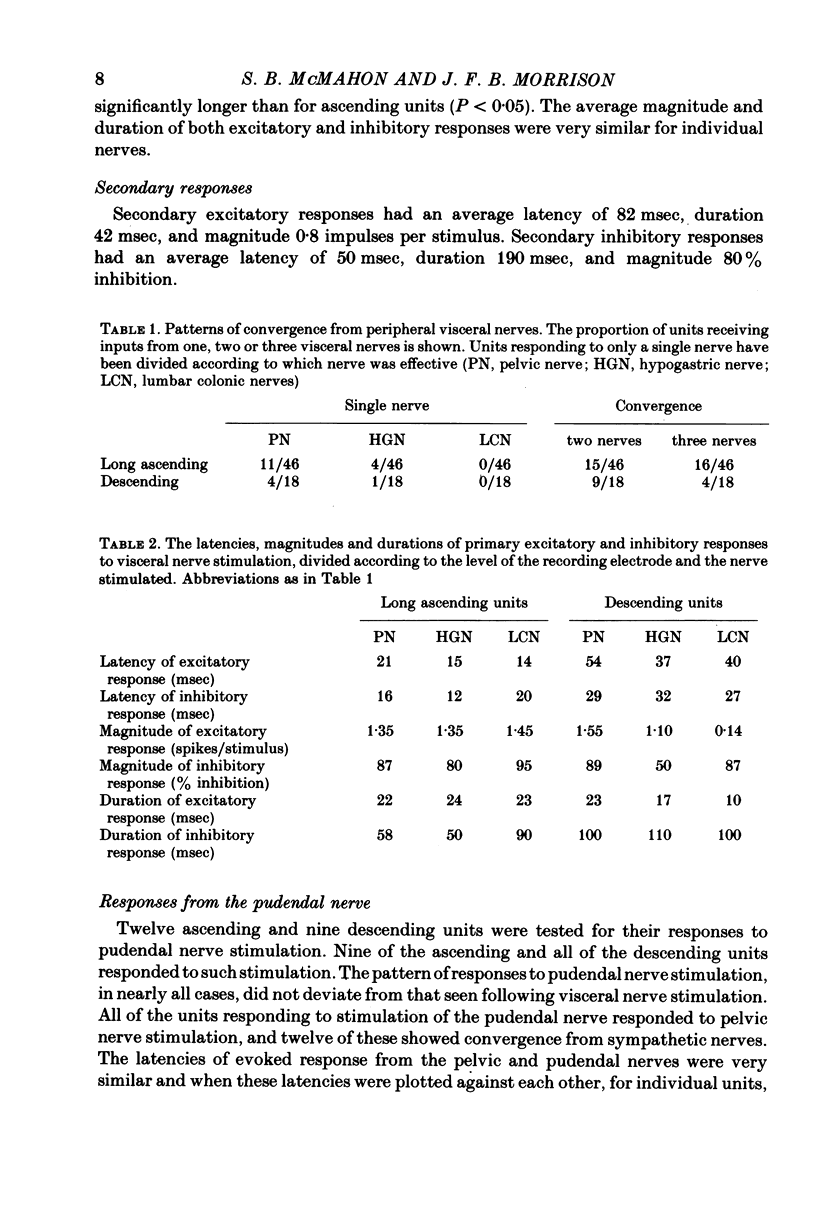
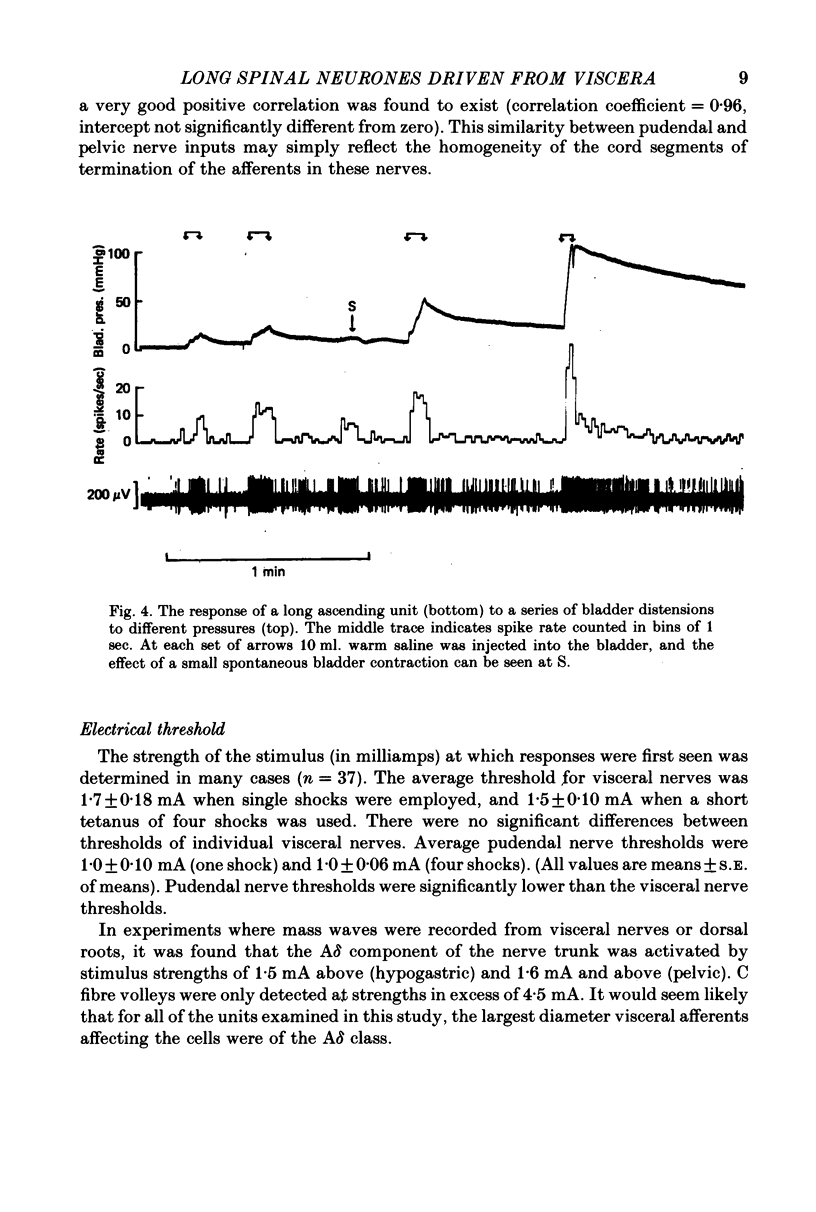
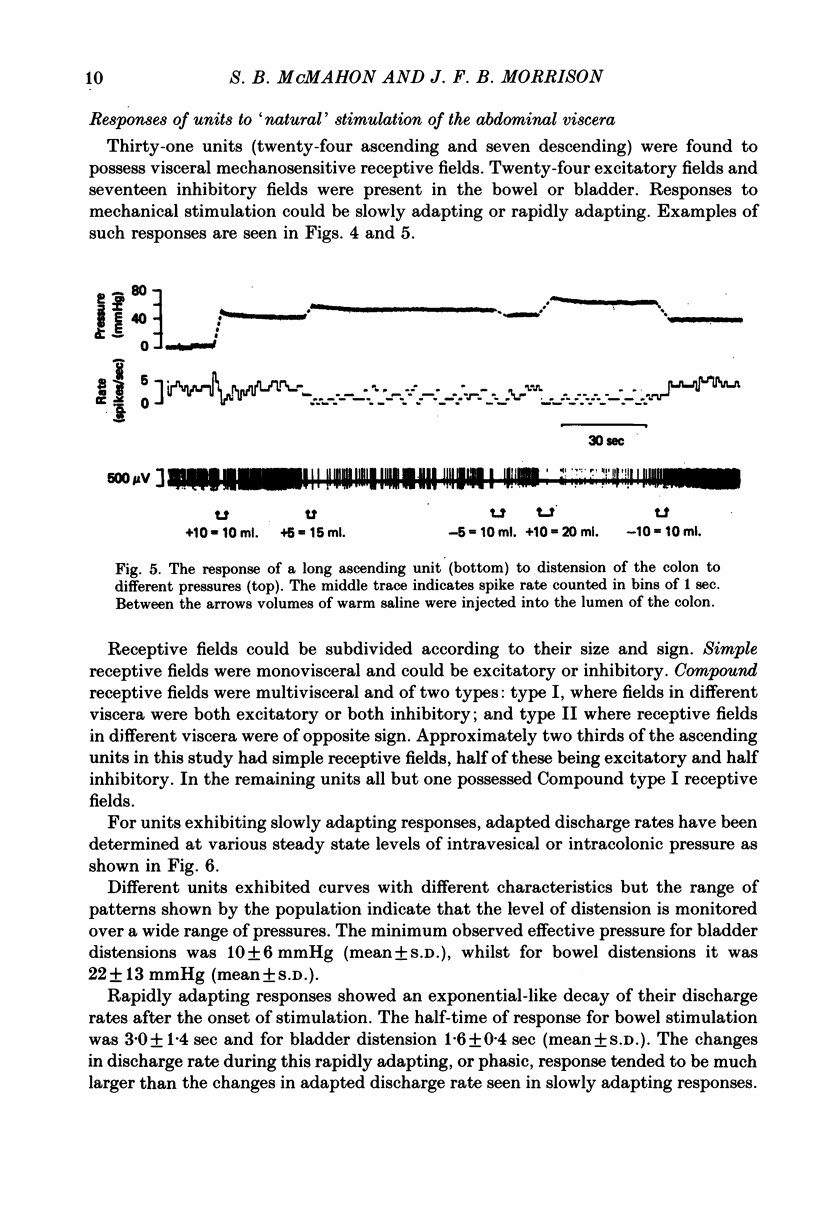








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURNS B. D. The central control of respiratory movements. Br Med Bull. 1963 Jan;19:7–9. doi: 10.1093/oxfordjournals.bmb.a070010. [DOI] [PubMed] [Google Scholar]
- Brown A. G., Franz D. N. Responses of spinocervical tract neurones to natural stimulation of identified cutaneous receptors. Exp Brain Res. 1969;7(3):231–249. doi: 10.1007/BF00239031. [DOI] [PubMed] [Google Scholar]
- Cervero F., Iggo A. Natural stimulation of urinary bladder afferents does not affect transmission through lumbosacral spinocervical tract neurones in the cat. Brain Res. 1978 Nov 10;156(2):375–379. doi: 10.1016/0006-8993(78)90522-x. [DOI] [PubMed] [Google Scholar]
- Coote J. H., Downman C. B. Central pathways of some autonomic reflex discharges. J Physiol. 1966 Apr;183(3):714–729. doi: 10.1113/jphysiol.1966.sp007894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groat W. C., Lalley P. M. Reflex firing in the lumbar sympathetic outflow to activation of vesical afferent fibres. J Physiol. 1972 Oct;226(2):289–309. doi: 10.1113/jphysiol.1972.sp009985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groat W. C. Nervous control of the urinary bladder of the cat. Brain Res. 1975 Apr 11;87(2-3):201–211. doi: 10.1016/0006-8993(75)90417-5. [DOI] [PubMed] [Google Scholar]
- Edvardsen P. Nervous control of urinary bladder in cats. I. The collecting phase. Acta Physiol Scand. 1968 Jan-Feb;72(1):157–171. doi: 10.1111/j.1748-1716.1968.tb03838.x. [DOI] [PubMed] [Google Scholar]
- Fields H. L., Meyer G. A., Partridge L. D., Jr Convergence of visceral and somatic input onto spinal neurons. Exp Neurol. 1970 Jan;26(1):36–52. doi: 10.1016/0014-4886(70)90086-5. [DOI] [PubMed] [Google Scholar]
- Fields H. L., Partridge L. D., Jr, Winter D. L. Somatic and visceral receptive field properties of fibers in ventral quadrant white matter of the cat spinal cord. J Neurophysiol. 1970 Nov;33(6):827–837. doi: 10.1152/jn.1970.33.6.827. [DOI] [PubMed] [Google Scholar]
- Floyd K., Hick V. E., Morrison J. F. Mechanosensitive afferent units in the hypogastric nerve of the cat. J Physiol. 1976 Jul;259(2):457–471. doi: 10.1113/jphysiol.1976.sp011476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd K., Lawrenson G. Mechanosensitive afferents in the cat pelvic nerve [proceedings]. J Physiol. 1979 May;290(2):51P–52P. [PubMed] [Google Scholar]
- Floyd K., McMahon S. B., Morrison J. F. Inhibitory interactions between colonic and vesical afferents in the micturition reflex of the cat. J Physiol. 1982 Jan;322:45–52. doi: 10.1113/jphysiol.1982.sp014021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjone R. Peripheral autonomic influence on the motility of the urinary bladder in the cat. I. Rhythmic contractions. Acta Physiol Scand. 1965 Dec;65(4):370–377. doi: 10.1111/j.1748-1716.1965.tb04287.x. [DOI] [PubMed] [Google Scholar]
- HUNT C. C., KUNO M. Properties of spinal interneurones. J Physiol. 1959 Sep 2;147:346–363. doi: 10.1113/jphysiol.1959.sp006248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock M. B., Rigamonti D. D., Bryan R. N. Convergence in the lumbar spinal cord of pathways activated by splanchnic nerve and hind limb cutaneous nerve stimulation. Exp Neurol. 1973 Feb;38(2):337–348. doi: 10.1016/0014-4886(73)90157-x. [DOI] [PubMed] [Google Scholar]
- Harding R., Leek B. F. Differentiation between motoneurone and interneurone activity recorded from the medullary gastiic centres of sheep. J Physiol. 1970 Jul;209(1 Suppl):42P+–42P+. [PubMed] [Google Scholar]
- KAMIKAWA K., KURO M. Analysis of lateral column units related to vesical reflexes. Exp Neurol. 1962 Oct;6:271–284. doi: 10.1016/0014-4886(62)90043-2. [DOI] [PubMed] [Google Scholar]
- KERR F. W., ALEXANDER S. DESCENDING AUTONOMIC PATHWAYS IN THE SPINAL CORD. Arch Neurol. 1964 Mar;10:249–261. doi: 10.1001/archneur.1964.00460150019002. [DOI] [PubMed] [Google Scholar]
- KURU M., KURATI T., KOYAMA Y. The bulbar vesico-constrictor center and the bulbo-sacral connections arising from it: a study of the function of the lateral reticulo-spinal tract. J Comp Neurol. 1959 Dec;113:365–388. doi: 10.1002/cne.901130303. [DOI] [PubMed] [Google Scholar]
- KURU M. The spino-bulbar tracts and the pelvic sensory vagus; further contributions to the theory of the sensory dual innervation of the viscera. J Comp Neurol. 1956 Apr;104(2):207–231. doi: 10.1002/cne.901040203. [DOI] [PubMed] [Google Scholar]
- McMahon S. B., Morrison J. F. Factors that determine the excitability of parasympathetic reflexes to the cat bladder. J Physiol. 1982 Jan;322:35–43. doi: 10.1113/jphysiol.1982.sp014020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon S. B., Morrison J. F. Two group of spinal interneurones that respond to stimulation of the abdominal viscera of the cat. J Physiol. 1982 Jan;322:21–34. doi: 10.1113/jphysiol.1982.sp014019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson A. The effects of somatic stimuli on the bladder in the cat. J Physiol. 1966 Jul;185(1):185–196. doi: 10.1113/jphysiol.1966.sp007980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSwiney B. A., Suffolk S. F. Segmental distribution of certain visceral afferent neurones of the pupillo-dilator reflex in the cat. J Physiol. 1938 Jul 14;93(2):104–116. doi: 10.1113/jphysiol.1938.sp003629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NATHAN P. W., SMITH M. C. The centrifugal pathway for micturition within the spinal cord. J Neurol Neurosurg Psychiatry. 1958 Aug;21(3):177–189. doi: 10.1136/jnnp.21.3.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NATHAN P. W. Sensations associated with micturition. Br J Urol. 1956 Jun;28(2):126–131. doi: 10.1111/j.1464-410x.1956.tb04744.x. [DOI] [PubMed] [Google Scholar]
- NATHAN P. W. Thermal sensation in the bladder. J Neurol Neurosurg Psychiatry. 1952 Aug;15(3):150–151. doi: 10.1136/jnnp.15.3.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomeranz B., Wall P. D., Weber W. V. Cord cells responding to fine myelinated afferents from viscera, muscle and skin. J Physiol. 1968 Dec;199(3):511–532. doi: 10.1113/jphysiol.1968.sp008666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds D. V. Surgery in the rat during electrical analgesia induced by focal brain stimulation. Science. 1969 Apr 25;164(3878):444–445. doi: 10.1126/science.164.3878.444. [DOI] [PubMed] [Google Scholar]
- Sato A., Sato Y., Schmidt P. F. Effects on reflex bladder activity of chemical stimulation of small diameter afferents from skeletal muscle in the cat. Neurosci Lett. 1979 Jan;11(1):13–17. doi: 10.1016/0304-3940(79)90048-x. [DOI] [PubMed] [Google Scholar]
- Sato A., Sato Y., Shimada F., Torigata Y. Changes in vesical function produced by cutaneous stimulation in rats. Brain Res. 1975 Sep 5;94(3):465–474. doi: 10.1016/0006-8993(75)90229-2. [DOI] [PubMed] [Google Scholar]
- Selzer M., Spencer W. A. Convergence of visceral and cutaneous afferent pathways in the lumbar spinal cord. Brain Res. 1969 Jul;14(2):331–348. doi: 10.1016/0006-8993(69)90114-0. [DOI] [PubMed] [Google Scholar]
- Selzer M., Spencer W. A. Interactions between visceral and cutaneous afferents in the spinal cord: reciprocal primary afferent fiber depolarization. Brain Res. 1969 Jul;14(2):349–366. doi: 10.1016/0006-8993(69)90115-2. [DOI] [PubMed] [Google Scholar]
- TAUB A. LOCAL, SEGMENTAL AND SUPRASPINAL INTERACTION WITH A DORSOLATERAL SPINAL CUTANEOUS AFFERENT SYSTEM. Exp Neurol. 1964 Oct;10:357–374. doi: 10.1016/0014-4886(64)90006-8. [DOI] [PubMed] [Google Scholar]
- White J. C., Verlot M. G., Ehrentheil O. NEUROGENIC DISTURBANCES OF THE COLON AND THEIR INVESTIGATION BY THE COLONMETROGRAM: A PRELIMINARY REPORT. Ann Surg. 1940 Dec;112(6):1042–1057. doi: 10.1097/00000658-194012000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter D. L. Receptor characteristics and conduction velocites in bladder afferents. J Psychiatr Res. 1971 Aug;8(3):225–235. doi: 10.1016/0022-3956(71)90021-5. [DOI] [PubMed] [Google Scholar]


