Abstract
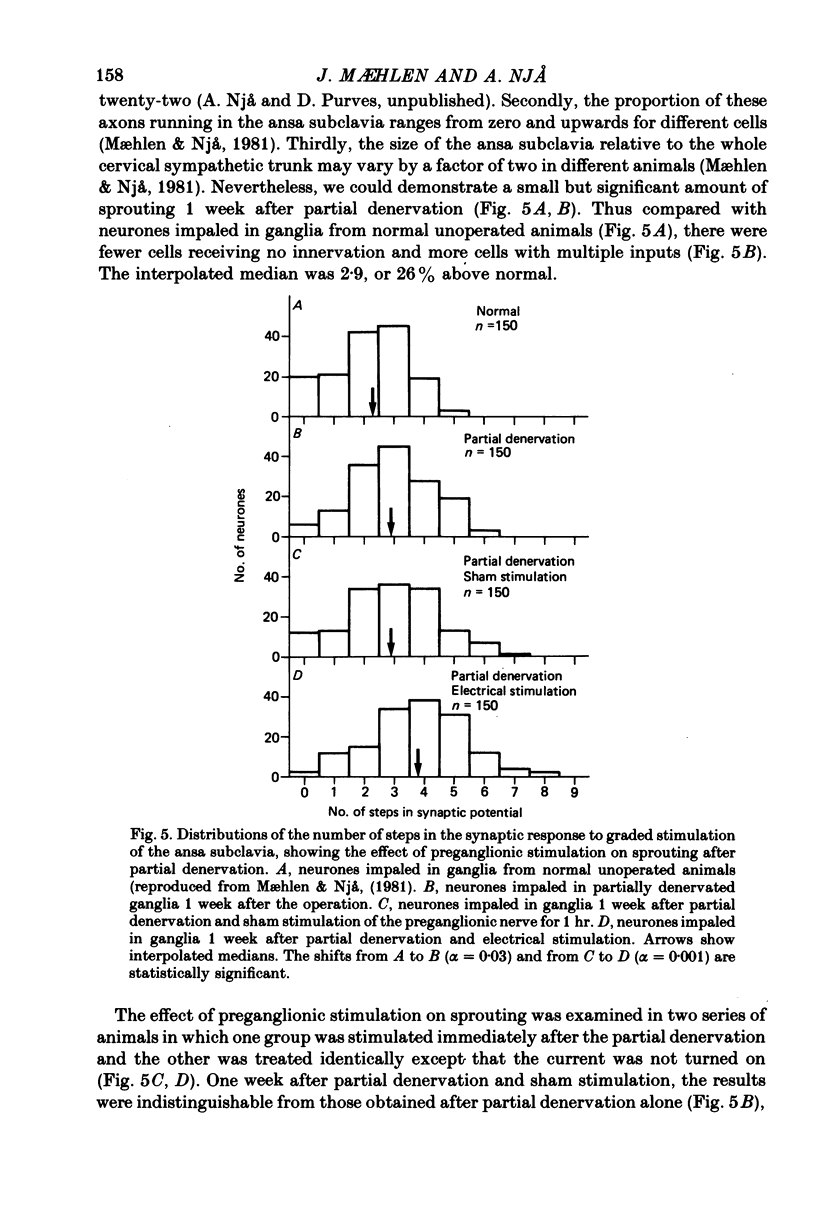
1. The effects of electrical stimulation of presynaptic and post-synaptic cells on sprouting after partial denervation were examined in the guinea-pig superior cervical ganglion with intracellular recording. The partial denervation reduced the mean number of preganglionic axons innervating each ganglion cell from about eleven to about two (Maehlen & Njå, 1981). 2. One week after partial denervation alone, the number of residual preganglionic axons innervating each superior cervical ganglion cell was increased by about 30%. This means that the number of ganglion cells contacted by each residual preganglionic axon was increased by the same amount, which represents an early stage of sprouting. 3. Preganglionic stimulation for 1 hr immediately after the partial denervation (with 100 pulses at 20 Hz every 25 sec) increased the rate of sprouting. Thus 1 week after partial denervation and preganglionic stimulation the number of residual preganglionic axons innervating each ganglion cell was increased by about 70%. Preganglionic stimulation had no similar effect on the innervation of normal ganglia. 4. The acceleration of sprouting caused by preganglionic stimulation was abolished by blocking ganglionic transmission with hexamethonium (30--60 mg kg-1 hr-1 I.V.) during the stimulation. 5. Furthermore, the rate of sprouting of residual preganglionic axons was increased by electrical stimulation of the ganglion cells alone. 6. These results show that after partial denervation of the superior cervical ganglion, a period of impulse activity in ganglion cells enhances their subsequent ability to receive innervation from sprouts arising from residual preganglionic axons.
Full text
PDF

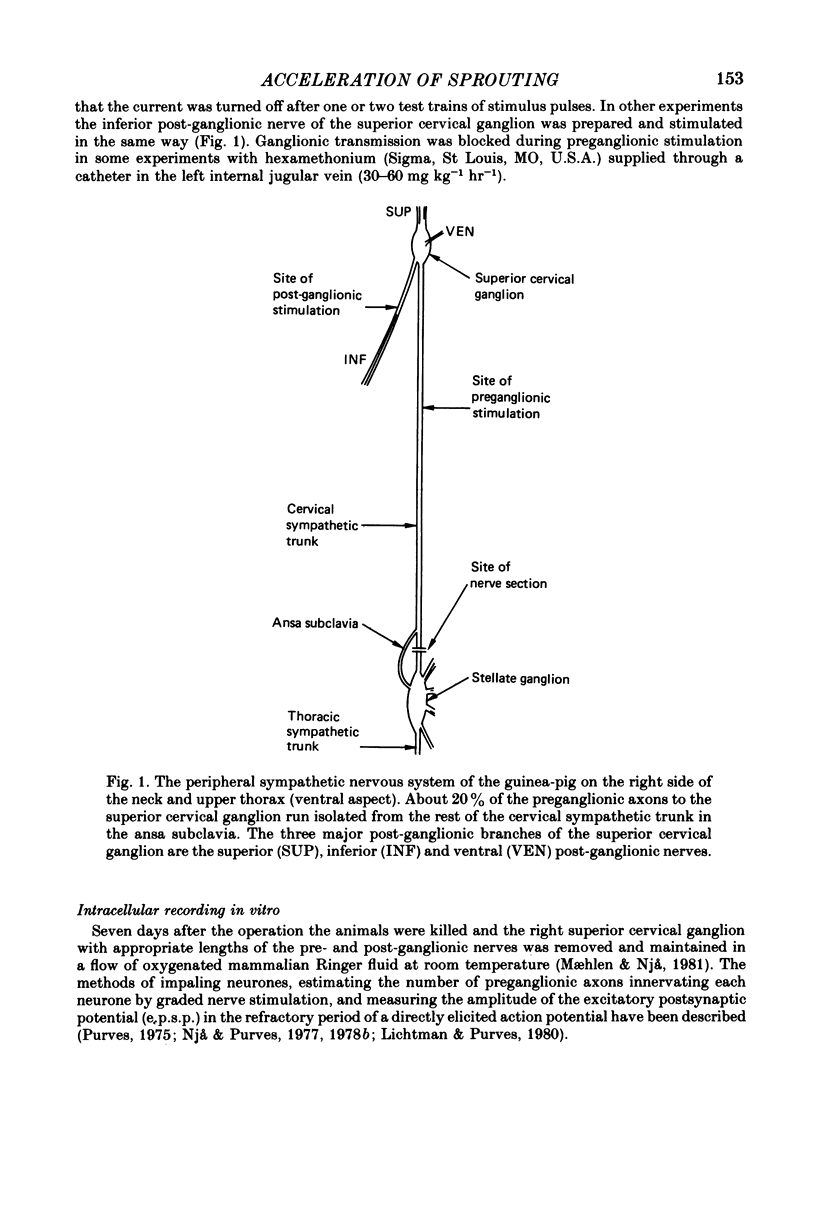

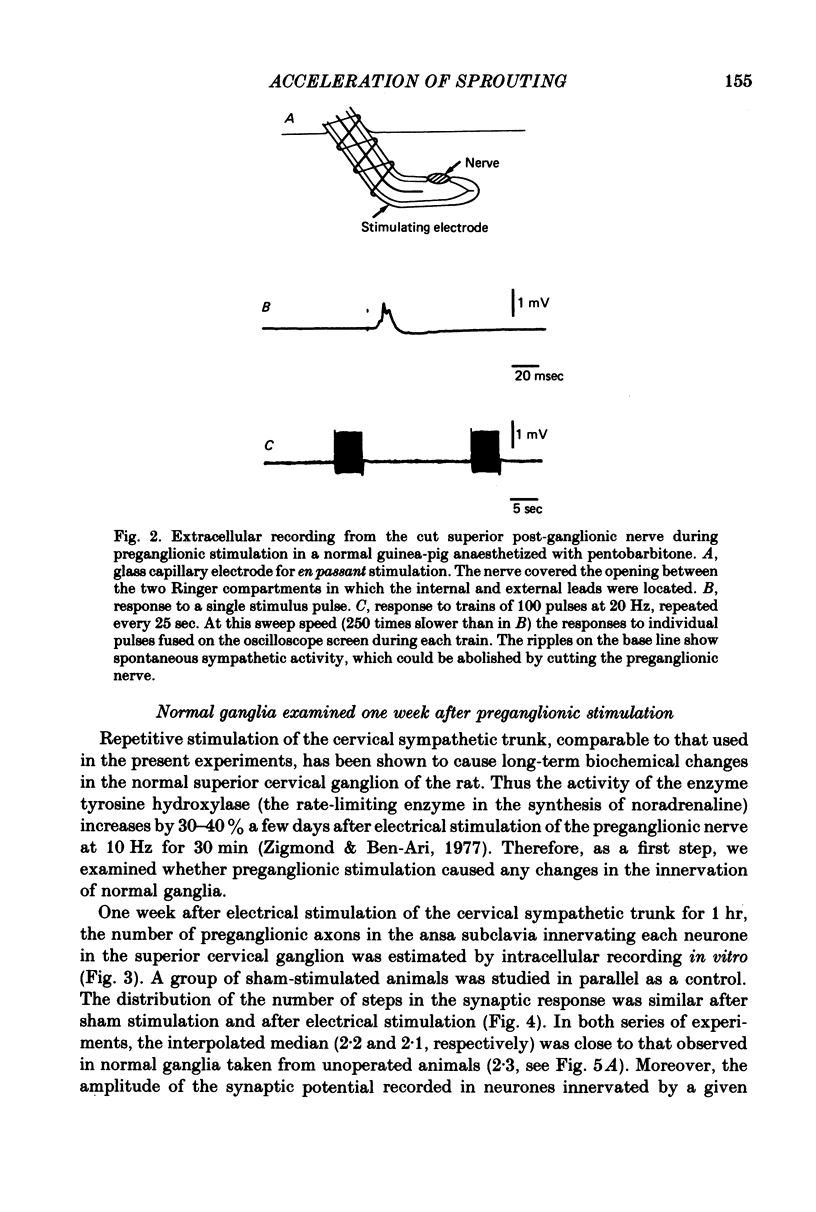
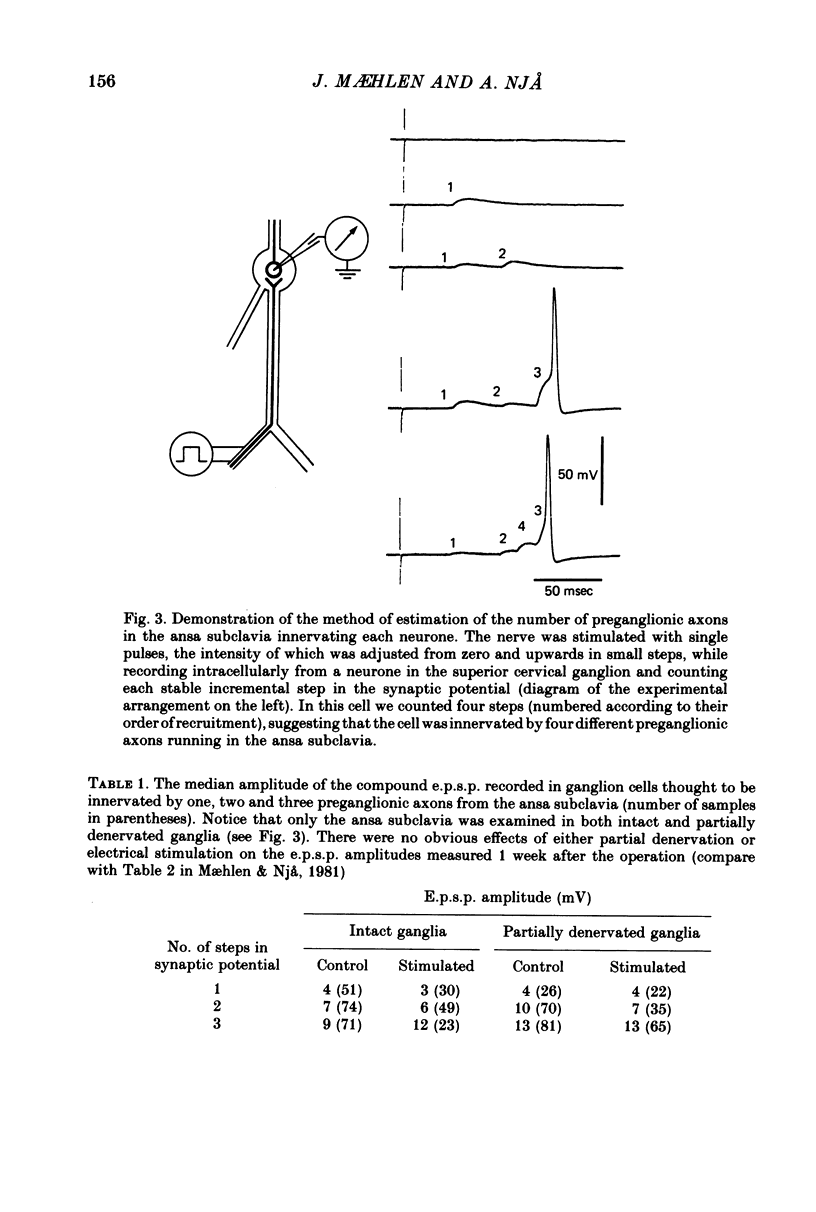
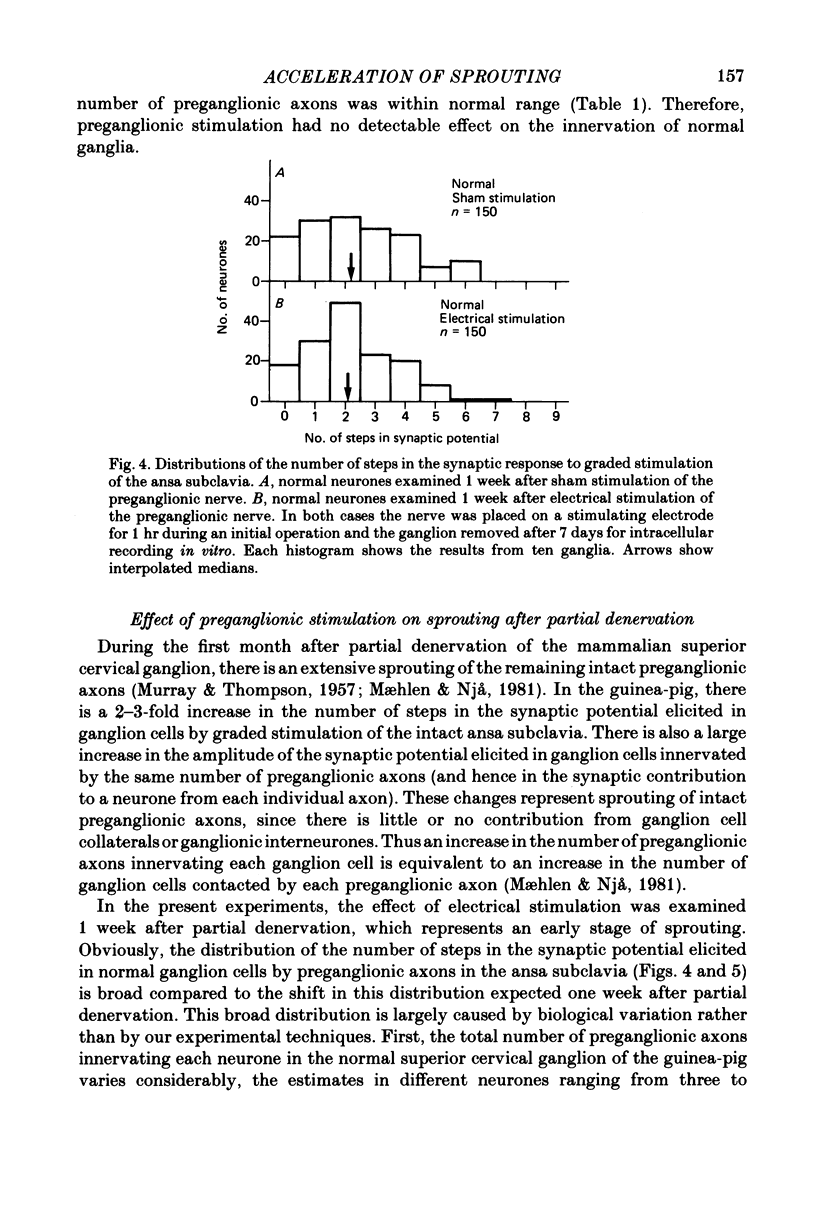

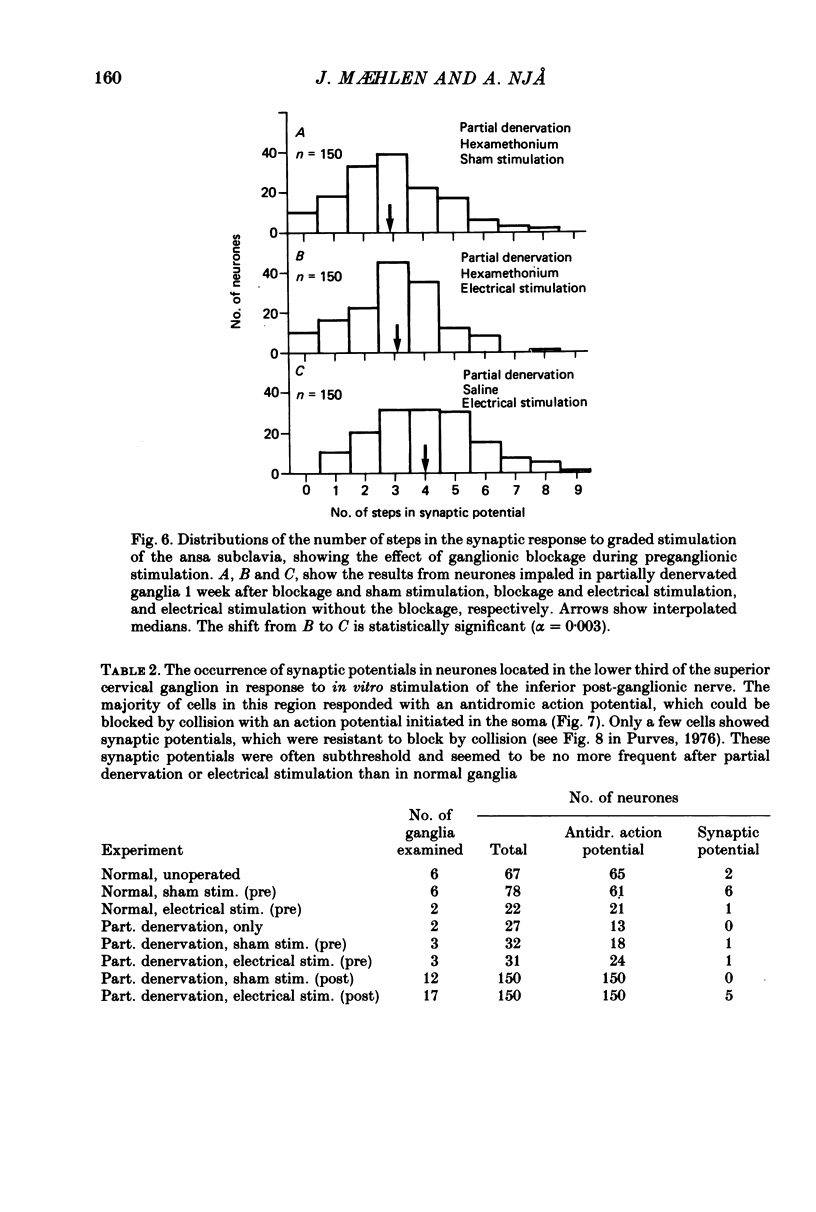




Selected References
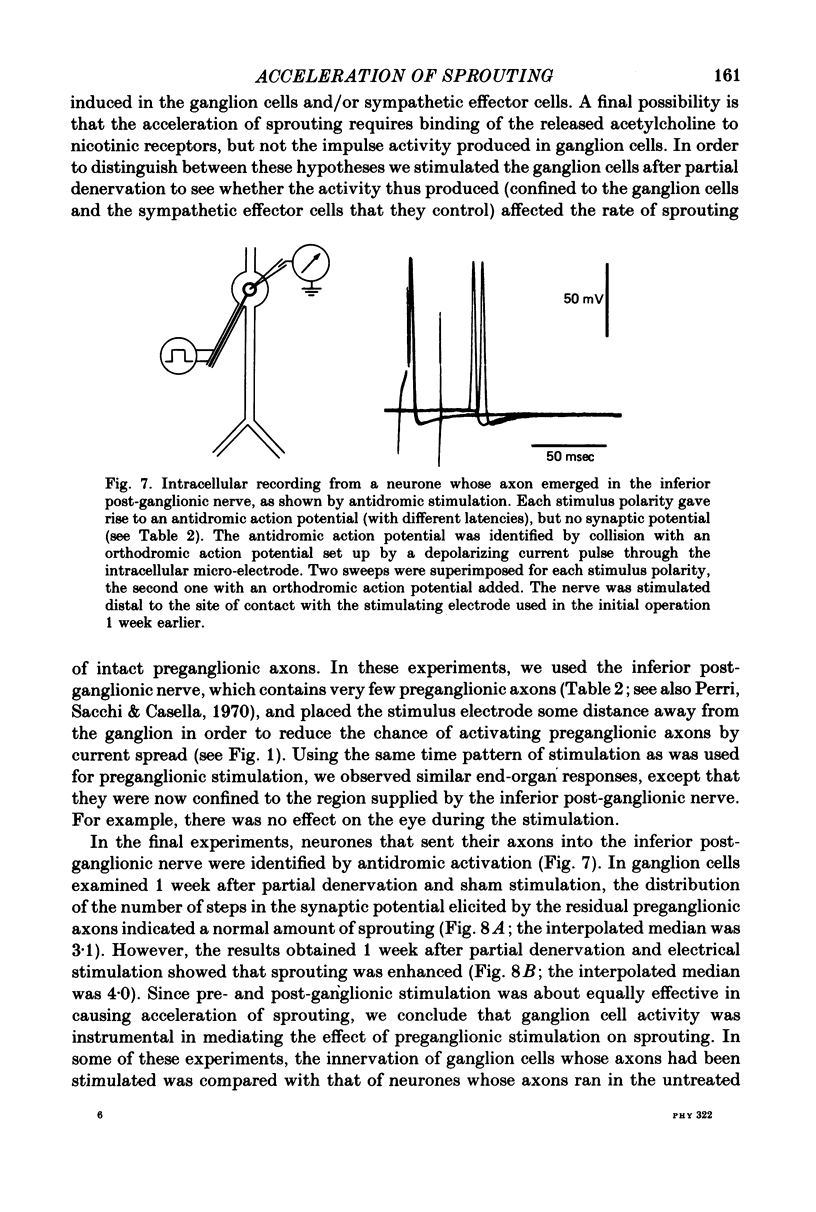
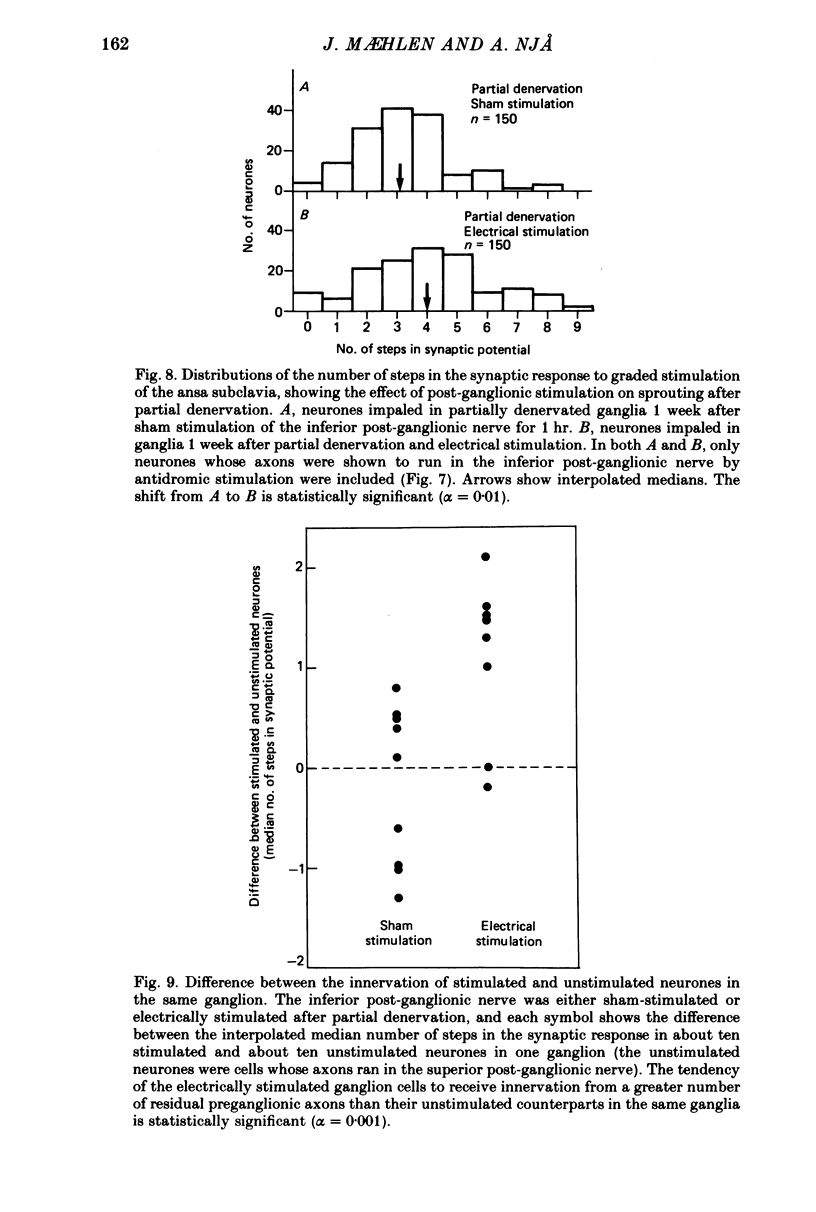
These references are in PubMed. This may not be the complete list of references from this article.
- Aguilar C. E., Bisby M. A., Cooper E., Diamond J. Evidence that axoplasmic transport of trophic factors is involved in the regulation of peripheral nerve fields in salamanders. J Physiol. 1973 Oct;234(2):449–464. doi: 10.1113/jphysiol.1973.sp010354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz W. J., Caldwell J. H., Ribchester R. R. Sprouting of active nerve terminals in partially inactive muscles of the rat. J Physiol. 1980 Jun;303:281–297. doi: 10.1113/jphysiol.1980.sp013285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black I. B. Regulation of autonomic development. Annu Rev Neurosci. 1978;1:183–214. doi: 10.1146/annurev.ne.01.030178.001151. [DOI] [PubMed] [Google Scholar]
- Brown M. C., Holland R. L., Ironton R. Degenerating nerve products affect innervated muscle fibres. Nature. 1978 Oct 19;275(5681):652–654. doi: 10.1038/275652a0. [DOI] [PubMed] [Google Scholar]
- Brown M. C., Holland R. L., Ironton R. Nodal and terminal sprouting from motor nerves in fast and slow muscles of the mouse. J Physiol. 1980 Sep;306:493–510. doi: 10.1113/jphysiol.1980.sp013410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. C., Ironton R. Motor neurone sprouting induced by prolonged tetrodotoxin block of nerve action potentials. Nature. 1977 Feb 3;265(5593):459–461. doi: 10.1038/265459a0. [DOI] [PubMed] [Google Scholar]
- Brown M. C., Ironton R. Suppression of motor nerve terminal sprouting in partially denervated mouse muscles [proceedings]. J Physiol. 1977 Oct;272(1):70P–71P. [PubMed] [Google Scholar]
- EDDS M. V., Jr Collateral nerve regeneration. Q Rev Biol. 1953 Sep;28(3):260–276. doi: 10.1086/399699. [DOI] [PubMed] [Google Scholar]
- Ebendal T., Olson L., Seiger A., Hedlund K. O. Nerve growth factors in the rat iris. Nature. 1980 Jul 3;286(5768):25–28. doi: 10.1038/286025a0. [DOI] [PubMed] [Google Scholar]
- HOFFMAN H. Acceleration and retardation of the process of axon-sprouting in partially devervated muscles. Aust J Exp Biol Med Sci. 1952 Dec;30(6):541–566. doi: 10.1038/icb.1952.52. [DOI] [PubMed] [Google Scholar]
- HOFFMAN H. Local re-innervation in partially denervated muscle; a histophysiological study. Aust J Exp Biol Med Sci. 1950 Jul;28(4):383–397. doi: 10.1038/icb.1950.39. [DOI] [PubMed] [Google Scholar]
- LILEY A. W. An investigation of spontaneous activity at the neuromuscular junction of the rat. J Physiol. 1956 Jun 28;132(3):650–666. doi: 10.1113/jphysiol.1956.sp005555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman J. W., Purves D. The elimination of redundant preganglionic innervation to hamster sympathetic ganglion cells in early post-natal life. J Physiol. 1980 Apr;301:213–228. doi: 10.1113/jphysiol.1980.sp013200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomo T., Westgaard R. H. Control of ACh sensitivity in rat muscle fibers. Cold Spring Harb Symp Quant Biol. 1976;40:263–274. doi: 10.1101/sqb.1976.040.01.027. [DOI] [PubMed] [Google Scholar]
- MURRAY J. G., THOMPSON J. W. The occurrence and function of collateral sprouting in the sympathetic nervous system of the cat. J Physiol. 1957 Jan 23;135(1):133–162. doi: 10.1113/jphysiol.1957.sp005700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maehlen J., Njå A. Selective synapse formation during sprouting after partial denervation of the guinea-pig superior cervical ganglion. J Physiol. 1981;319:555–567. doi: 10.1113/jphysiol.1981.sp013926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njå A., Purves D. Specific innervation of guinea-pig superior cervical ganglion cells by preganglionic fibres arising from different levels of the spinal cord. J Physiol. 1977 Jan;264(2):565–583. doi: 10.1113/jphysiol.1977.sp011683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njå A., Purves D. Specificity of initial synaptic contacts made on guinea-pig superior cervical ganglion cells during regeneration of the cervical sympathetic trunk. J Physiol. 1978 Aug;281:45–62. doi: 10.1113/jphysiol.1978.sp012408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njå A., Purves D. The effects of nerve growth factor and its antiserum on synapses in the superior cervical ganglion of the guinea-pig. J Physiol. 1978 Apr;277:53–75. [PMC free article] [PubMed] [Google Scholar]
- Passatore M., Pettorossi V. E. Efferent fibers in the cervical sympathetic nerve influenced by light. Exp Neurol. 1976 Jul;52(1):66–82. doi: 10.1016/0014-4886(76)90201-6. [DOI] [PubMed] [Google Scholar]
- Passatore M. Physiological characterization of efferent cervical sympathetic fibers influenced by changes of illumination. Exp Neurol. 1976 Oct;53(1):71–81. doi: 10.1016/0014-4886(76)90282-x. [DOI] [PubMed] [Google Scholar]
- Perri V., Sacchi O., Casella C. Synaptically mediated potentials elicited by the stimulation of post-ganglionic trunks in the guinea-pig superior cervical ganglion. Pflugers Arch. 1970;314(1):55–67. doi: 10.1007/BF00587046. [DOI] [PubMed] [Google Scholar]
- Purves D. Functional and structural changes in mammalian sympathetic neurones following colchicine application to post-ganglionic nerves. J Physiol. 1976 Jul;259(1):159–175. doi: 10.1113/jphysiol.1976.sp011459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves D. Functional and structural changes in mammalian sympathetic neurones following interruption of their axons. J Physiol. 1975 Nov;252(2):429–463. doi: 10.1113/jphysiol.1975.sp011151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves D., Lichtman J. W. Formation and maintenance of synaptic connections in autonomic ganglia. Physiol Rev. 1978 Oct;58(4):821–862. doi: 10.1152/physrev.1978.58.4.821. [DOI] [PubMed] [Google Scholar]
- Rauschecker J. P., Singer W. The effects of early visual experience on the cat's visual cortex and their possible explanation by Hebb synapses. J Physiol. 1981 Jan;310:215–239. doi: 10.1113/jphysiol.1981.sp013545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoenen H., Barde Y. A. Physiology of nerve growth factor. Physiol Rev. 1980 Oct;60(4):1284–1335. doi: 10.1152/physrev.1980.60.4.1284. [DOI] [PubMed] [Google Scholar]
- Zigmond R. E., Ben-Ari Y. Electrical stimulation of preganglionic nerve increases tyrosine hydroxylase activity in sympathetic ganglia. Proc Natl Acad Sci U S A. 1977 Jul;74(7):3078–3080. doi: 10.1073/pnas.74.7.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]


