Abstract
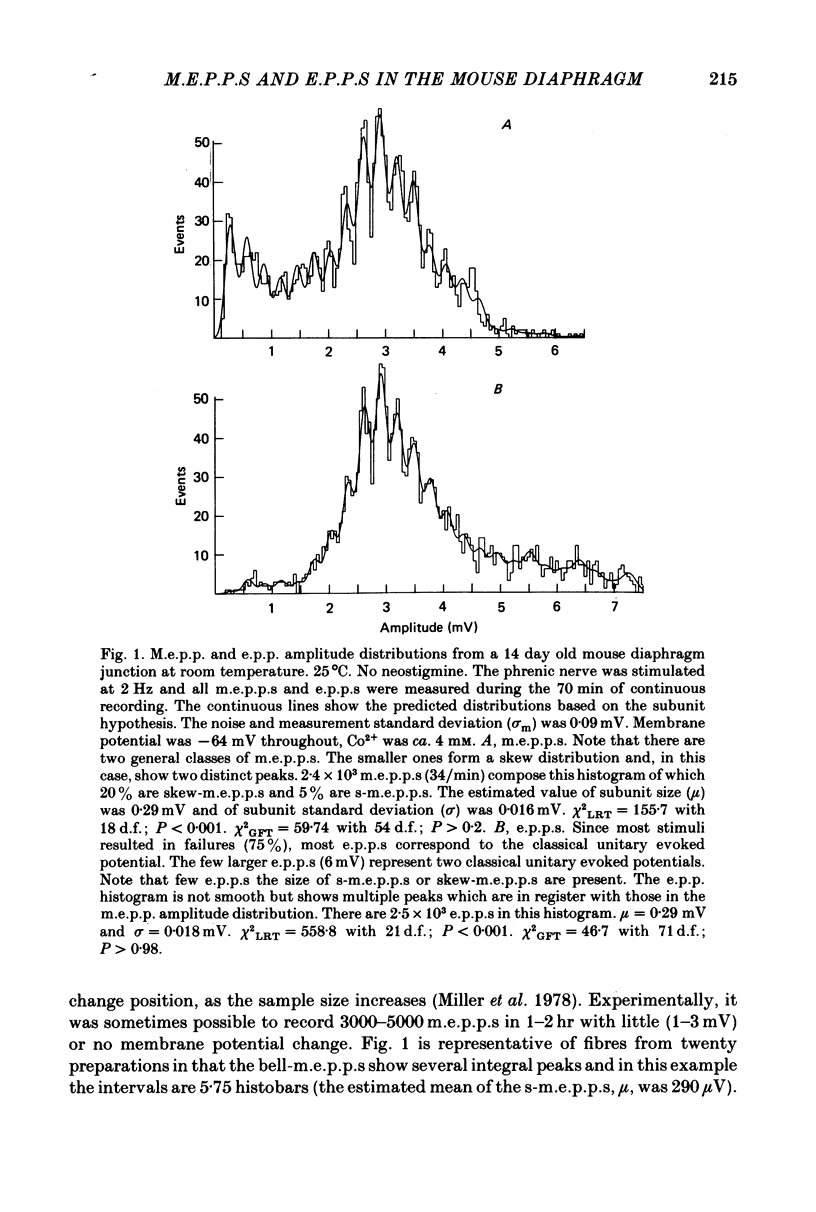
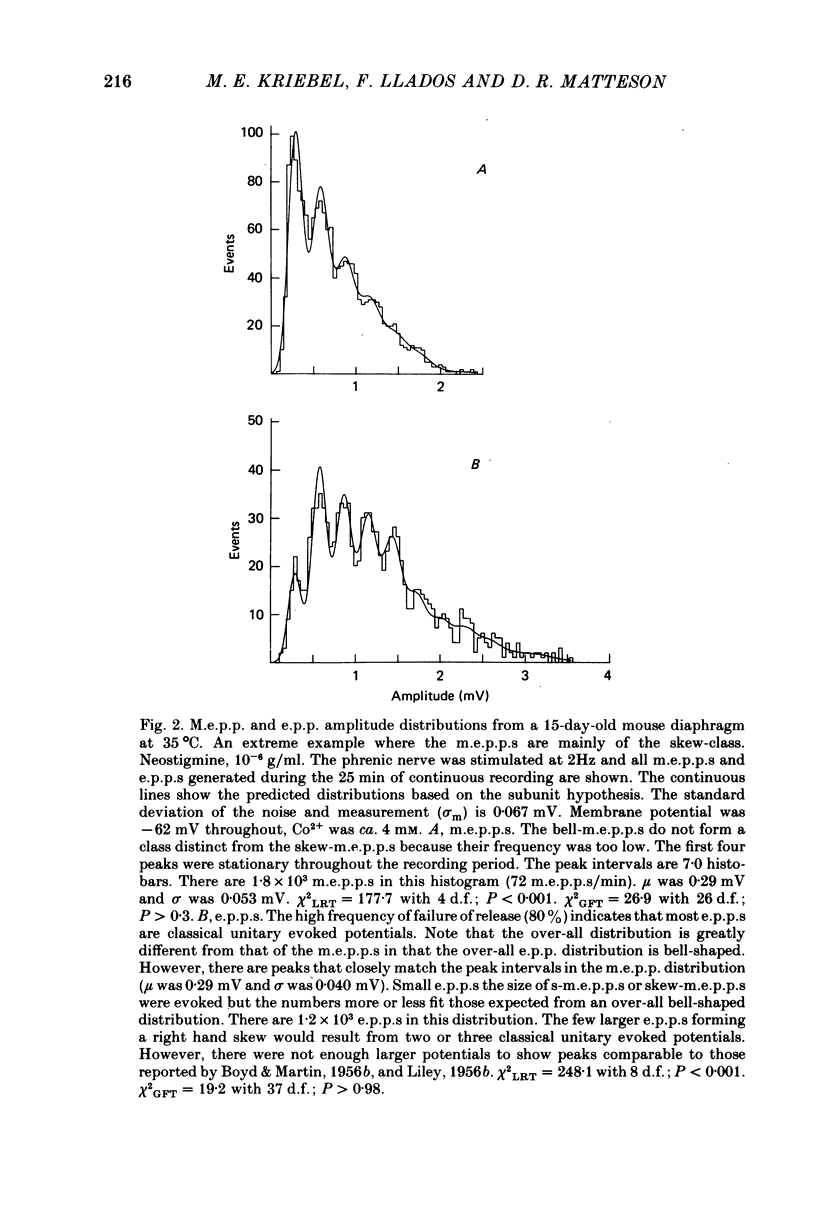
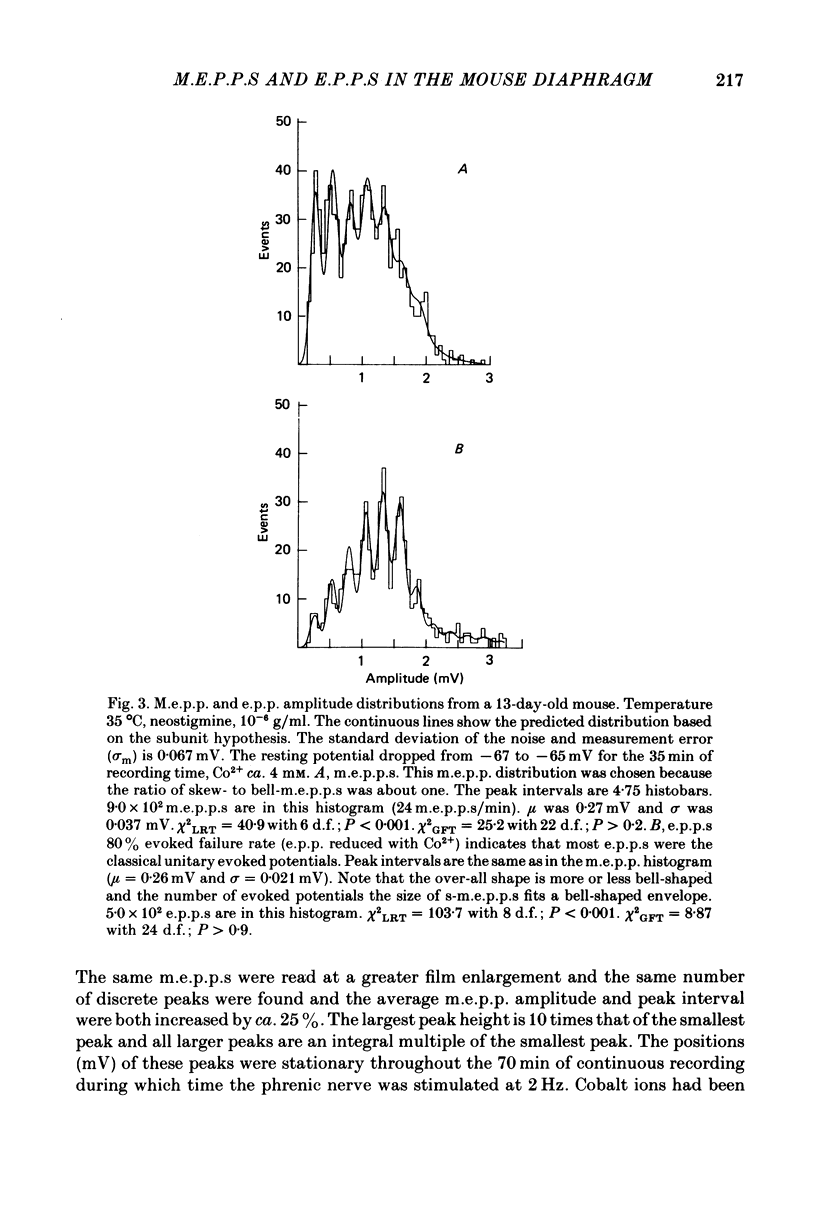
1. Two classes of miniature end-plate potentials (m.e.p.p.s) were recorded from diaphragm neuromuscular junctions. Amplitude histograms of both classes had multiple peaks that were integral multiples of the smallest peak (s-m.e.p.p.s). The smaller m.e.p.p.s formed the first three or four peaks of histograms and the number of m.e.p.p.s (skew-m.e.p.p.s) in each peak decreased, forming an over-all skewed distribution. The larger m.e.p.p.s (bell-m.e.p.p.s) formed a more-or-less bell-shaped distribution. The distribution of m.e.p.p.s varied from mainly skew- to mainly bell-m.e.p.p.s. In young adult mice the number of subunits composing the classical m.e.p.p.s varied between ten and fifteen at room temperature; at higher temperatures the range was from three to ten subunits.
2. End-plate potentials (e.p.p.s) were reduced with cobalt ions (ca. 4 mm) until most nerve impulses failed to release transmitter. The amplitudes of `unitary evoked potentials' were of the bell-m.e.p.p. class and histograms show integral multiple peaks that correspond to the peaks in histograms of the bell-m.e.p.p.s.
3. The peaks in both m.e.p.p. and unitary e.p.p. histograms remained in the same position throughout the recording period and became more distinct as the sample size increased.
4. The variance of the s-m.e.p.p. was estimated from the noise and measurement error and the variance of all peaks in the histograms. Most variance of the first peak (s-m.e.p.p.) was due to noise and measurement error.
5. The integral peaks in the m.e.p.p. and `unitary evoked potential' histograms are predicted with a probability density model based on the estimated variance of the s-m.e.p.p. and the assumption that larger potentials are composed of subunits the size of s-m.e.p.p.s. The data and model support the hypothesis that m.e.p.p.s and unitary potentials are composed of subunits.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOYD I. A., MARTIN A. R. Spontaneous subthreshold activity at mammalian neural muscular junctions. J Physiol. 1956 Apr 27;132(1):61–73. doi: 10.1113/jphysiol.1956.sp005502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOYD I. A., MARTIN A. R. The end-plate potential in mammalian muscle. J Physiol. 1956 Apr 27;132(1):74–91. doi: 10.1113/jphysiol.1956.sp005503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Pettigrew A. G. The formation of synapses in reinnervated and cross-reinnervated striated muscle during development. J Physiol. 1974 Sep;241(2):547–573. doi: 10.1113/jphysiol.1974.sp010671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan S. Sub-miniature end-plate potentials at untreated frog neuromuscular junctions. J Physiol. 1976 Jun;258(1):145–155. doi: 10.1113/jphysiol.1976.sp011411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke J. D., Quastel D. M. Transmitter release by mammalian motor nerve terminals in response to focal polarization. J Physiol. 1973 Jan;228(2):377–405. doi: 10.1113/jphysiol.1973.sp010092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S. G., Lundh H., Thesleff S. Effects of botulinum toxin on neuromuscular transmission in the rat. J Physiol. 1976 Aug;260(1):177–203. doi: 10.1113/jphysiol.1976.sp011510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M. J., Miledi R. Characteristics of transmitter release at regenerating frog neuromuscular junctions. J Physiol. 1974 Jun;239(3):571–594. doi: 10.1113/jphysiol.1974.sp010583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M., Miledi R. Lack of correspondence between the amplitudes of spontaneous potentials and unit potentials evoked by nerve impulses at regenerating neuromuscular junctions. Nat New Biol. 1971 Jul 28;232(30):126–128. doi: 10.1038/newbio232126a0. [DOI] [PubMed] [Google Scholar]
- Heuser J. E., Reese T. S., Dennis M. J., Jan Y., Jan L., Evans L. Synaptic vesicle exocytosis captured by quick freezing and correlated with quantal transmitter release. J Cell Biol. 1979 May;81(2):275–300. doi: 10.1083/jcb.81.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. Estimates of quantal content during 'chemical potentiation' of transmitter release. Proc R Soc Lond B Biol Sci. 1979 Aug 31;205(1160):369–378. doi: 10.1098/rspb.1979.0070. [DOI] [PubMed] [Google Scholar]
- Kriebel M. E., Gross C. E. Multimodal distribution of frog miniature endplate potentials in adult denervated and tadpole leg muscle. J Gen Physiol. 1974 Jul;64(1):85–103. doi: 10.1085/jgp.64.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriebel M. E., Llados F., Matteson D. R. Spontaneous subminature end-plate potentials in mouse diaphragm muscle: evidence for synchronous release. J Physiol. 1976 Nov;262(3):553–581. doi: 10.1113/jphysiol.1976.sp011610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriebel M. E. Small mode miniature end plate potentials are increased and evoked in fatigued preparations and in high Mg2+ saline. Brain Res. 1978 Jun 16;148(2):381–388. doi: 10.1016/0006-8993(78)90726-6. [DOI] [PubMed] [Google Scholar]
- LILEY A. W. An investigation of spontaneous activity at the neuromuscular junction of the rat. J Physiol. 1956 Jun 28;132(3):650–666. doi: 10.1113/jphysiol.1956.sp005555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LILEY A. W. The quantal components of the mammalian end-plate potential. J Physiol. 1956 Sep 27;133(3):571–587. doi: 10.1113/jphysiol.1956.sp005610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llados F., Matteson D. R., Kriebel M. E. beta-Bungarotoxin preferentially blocks one class of miniature endplate potentials. Brain Res. 1980 Jun 23;192(2):598–602. doi: 10.1016/0006-8993(80)90914-2. [DOI] [PubMed] [Google Scholar]
- Matteson D. R., Kreibel M. E., Llados F. A statistical model supports the subunit hypothesis of quantal relsease. Neurosci Lett. 1979 Dec;15(2-3):147–152. doi: 10.1016/0304-3940(79)96104-4. [DOI] [PubMed] [Google Scholar]
- Miller D. C., Weinstock M. M., Magleby K. L. Is the quantum of transmitter release composed of subunits? Nature. 1978 Jul 27;274(5669):388–390. doi: 10.1038/274388a0. [DOI] [PubMed] [Google Scholar]
- Wernig A., Stirner H. Quantum amplitude distributions point to functional unity of the synaptic 'active zone'. Nature. 1977 Oct 27;269(5631):820–822. doi: 10.1038/269820a0. [DOI] [PubMed] [Google Scholar]


