Abstract
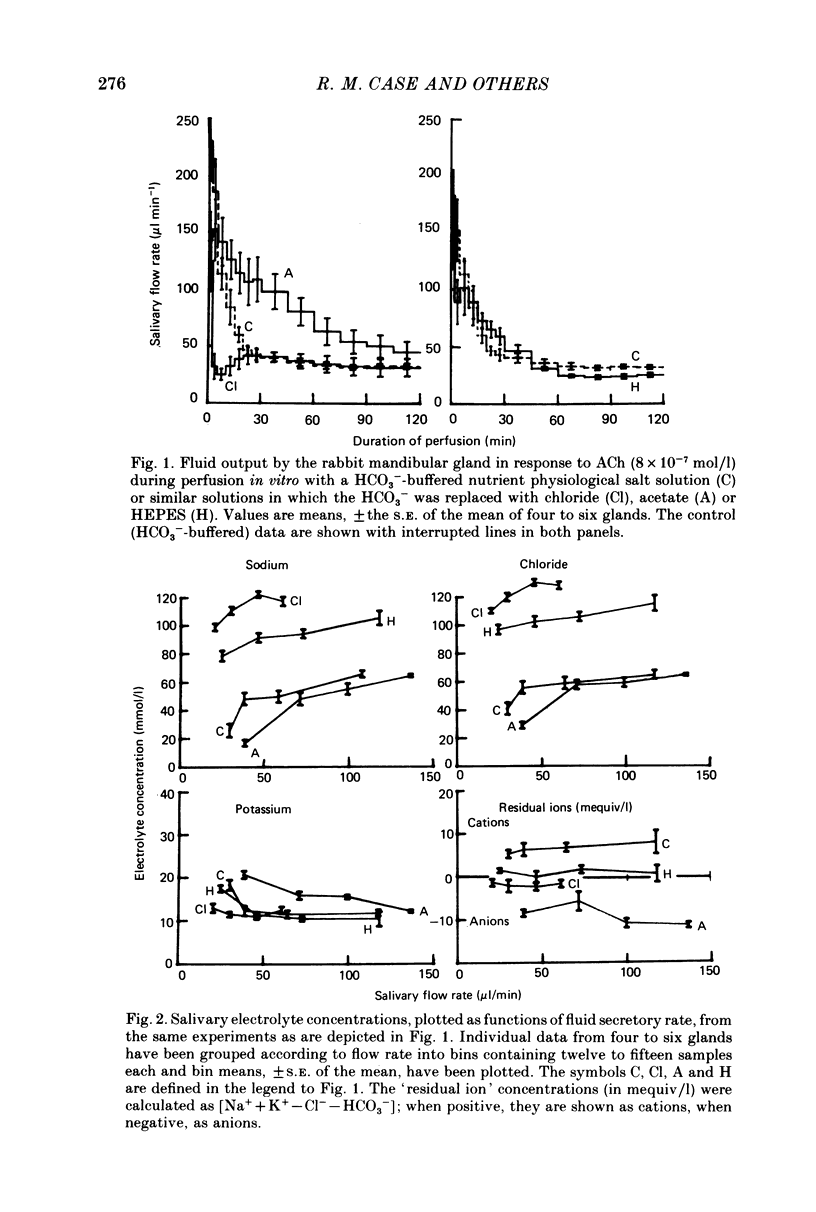
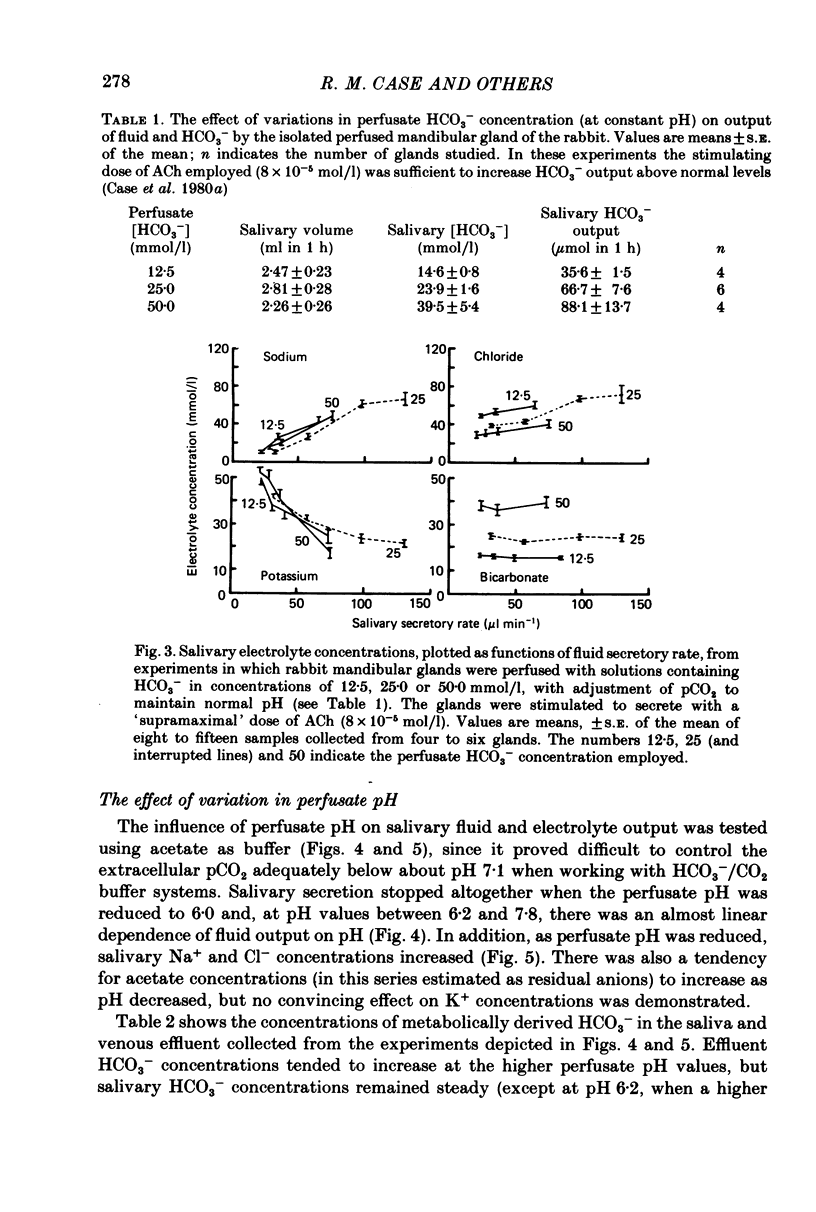
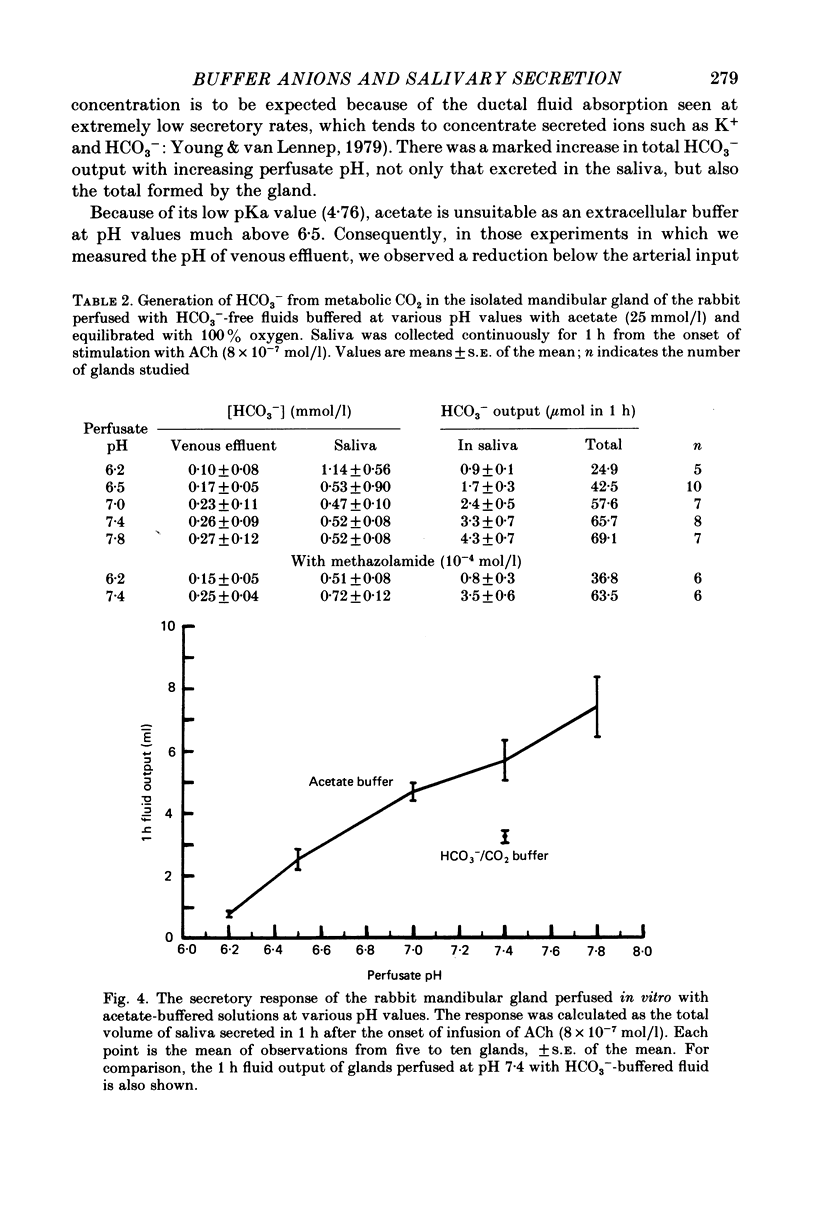
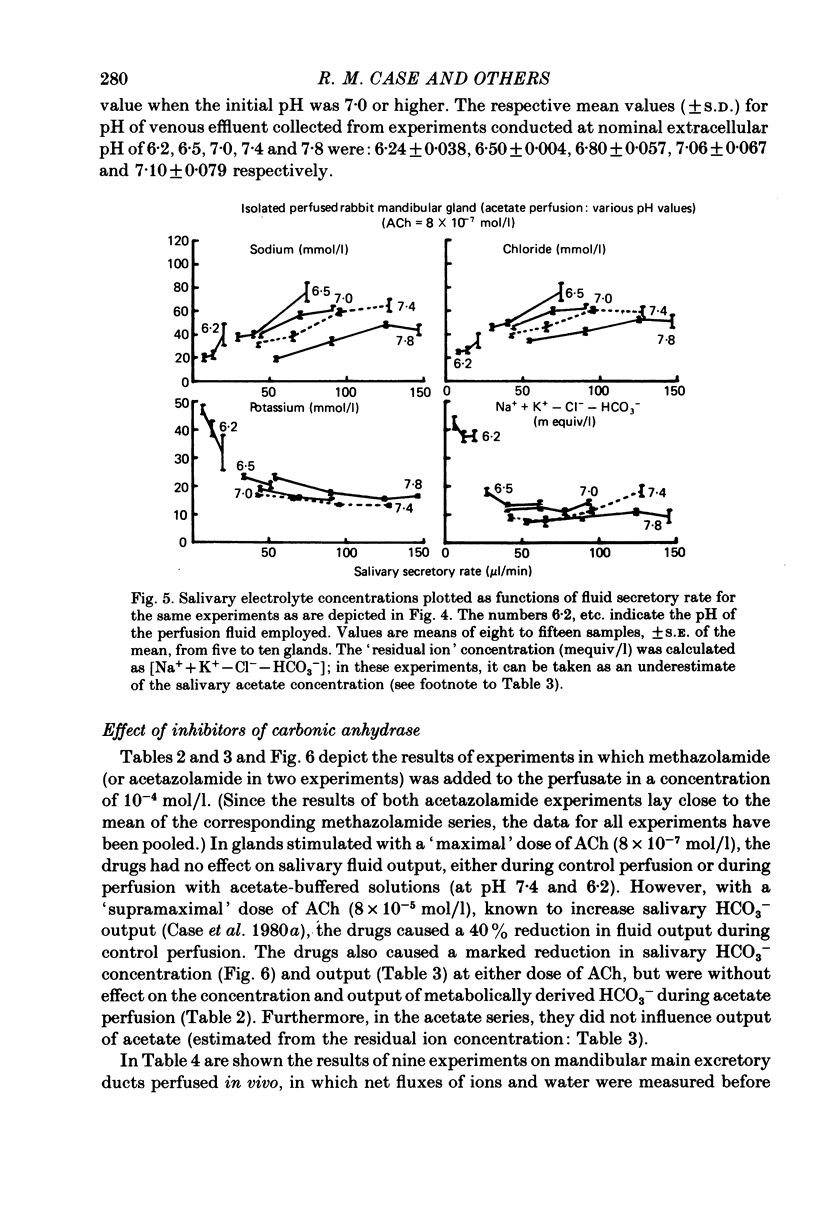
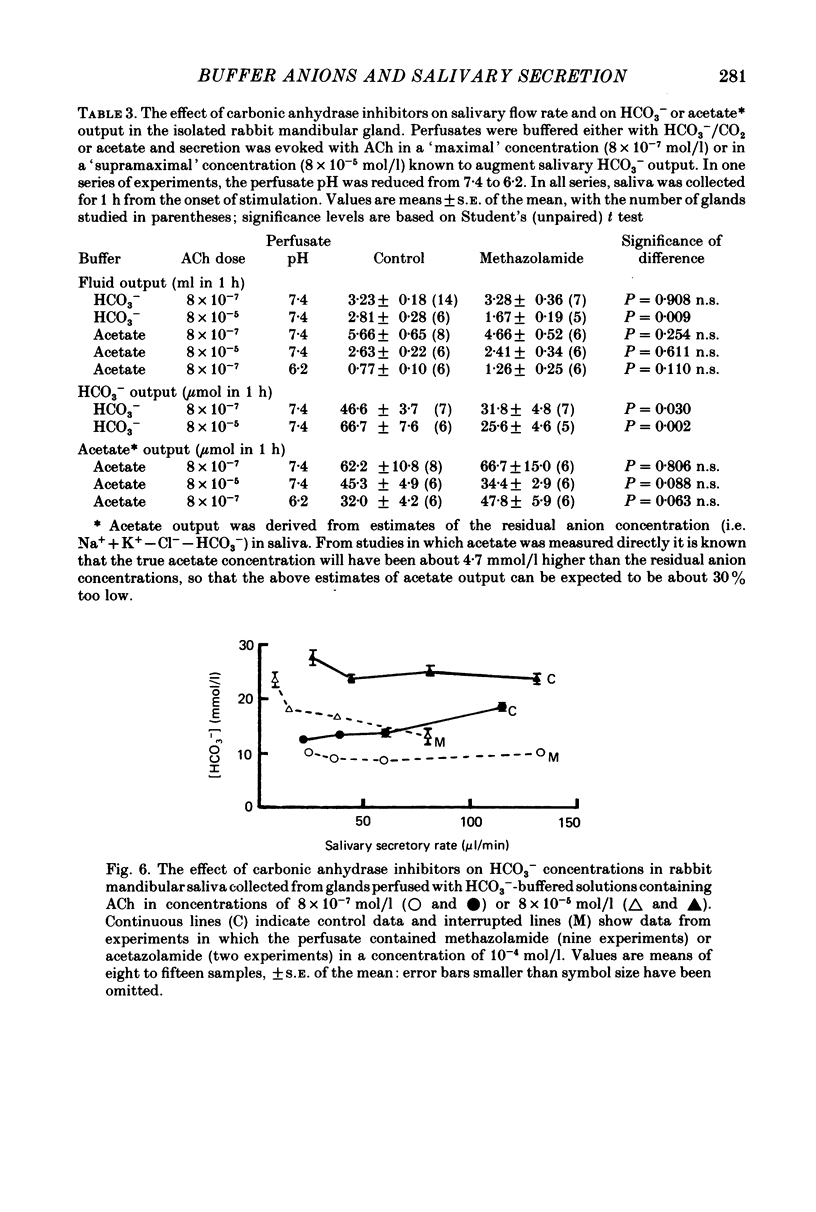
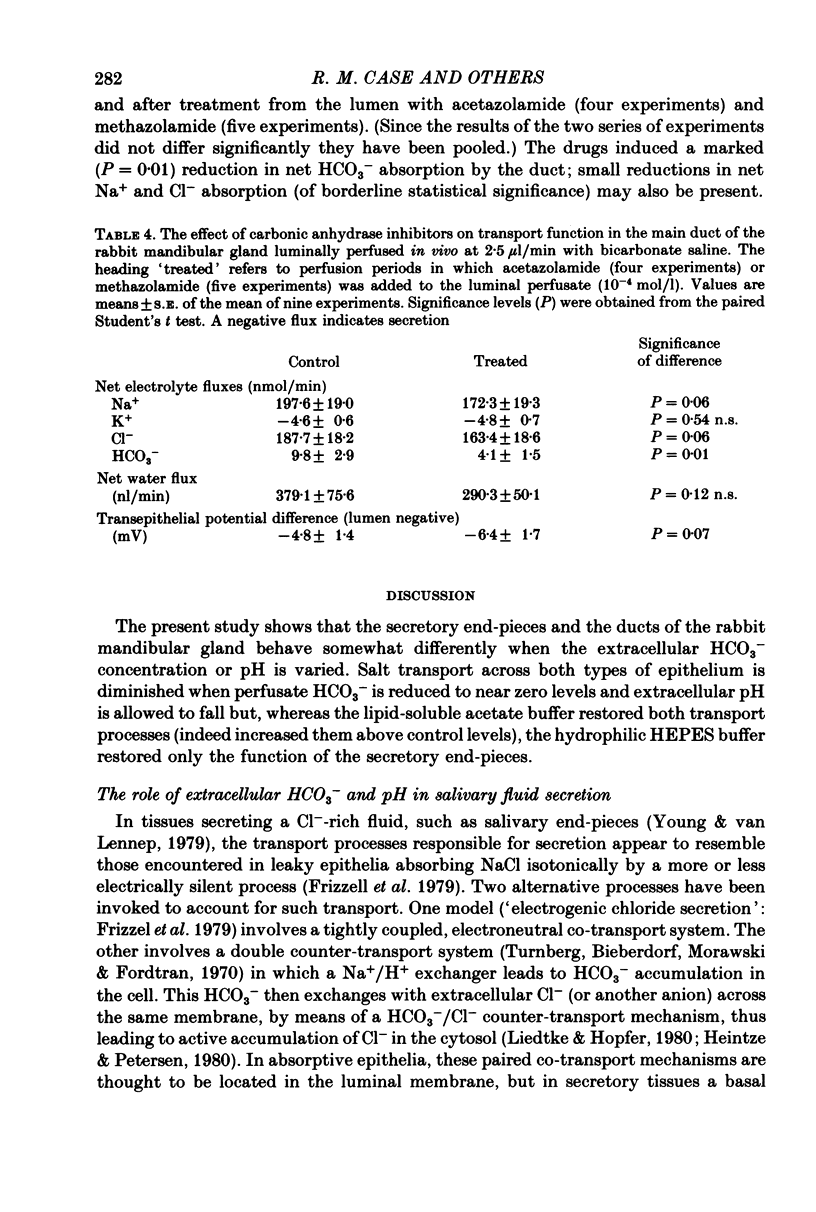
1. The role of extracellular HCO3- and H+ in the formation of primary saliva and its subsequent modification by the glandular ducts has been investigated in the isolated perfused mandibular salivary gland of the rabbit. 2. Variation of extracellular HCO3- concentration between 12.5 and 50.0 mmol/l was without effect on salivary flow rate or on Na+ and K+ excretion, even though salivary HCO3- (and Cl-) content altered with changes in the extracellular concentration of the two anions. 3. Complete replacement of perfusate HCO3- by Cl- reduced fluid secretion by 34% and almost abolished ductal Na+ absorption. However, when extracellular pH was controlled by replacing HCO3- with the hydrophilic HEPES buffer, fluid secretion but not ductal Na+ absorption was restored to normal. 4. Complete replacement of exogenous HCO3- with acetate increased fluid secretion by 110% and also stimulated ductal Na+ absorption. This effect did not appear to be related to changes in cell pH and remains unexplained. Acetate entered the saliva in concentrations comparable to those seen for HCO3- in control experiments. 5. Salivary secretion showed an almost linear dependence on extracellular pH, rising from 14% of control (pH 7.4) levels at pH 6.2 to 130% at pH 7.8. Ductal Na+ absorption showed similar pH dependence. 6. Carbonic anhydrase inhibitors did not affect fluid secretion rates (except when supramaximal doses of ACh were used to evoke secretion) but they did cause a large reduction in salivary HCO3- output. In glands perfused with acetate rather than HCO3-, carbonic anhydrase inhibitors had no effect on excretion of fluid, acetate or metabolically derived HCO3-. Duct perfusion studies suggested that the effect of the inhibitors on HCO3- output was at the site of primary secretion rather than at the ductal site of HCO3- transport.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blair-West J. R., Fernley R. T., Nelson J. F., Wintour E. M., Wright R. D. The effect of carbonic anhydrase inhibitors on the anionic composition of sheep's parotid saliva. With an appendix on uncatalysed carbon dioxide-water kinetics by P. T. McTigue. J Physiol. 1980 Feb;299:29–44. doi: 10.1113/jphysiol.1980.sp013108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case R. M., Conigrave A. D., Novak I., Young J. A. Electrolyte and protein secretion by the perfused rabbit mandibular gland stimulated with acetylcholine or catecholamines. J Physiol. 1980 Mar;300:467–487. doi: 10.1113/jphysiol.1980.sp013173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case R. M., Hotz J., Hutson D., Scratcherd T., Wynne R. D. Electrolyte secretion by the isolated cat pancreas during replacement of extracellular bicarbonate by organic anions and chloride by inorganic anions. J Physiol. 1979 Jan;286:563–576. doi: 10.1113/jphysiol.1979.sp012637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case R. M., Scratcherd T., Wynne R. D. The origin and secretion of pancreatic juice bicarbonate. J Physiol. 1970 Sep;210(1):1–15. doi: 10.1113/jphysiol.1970.sp009193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton J., Martinez J. R., Martinez A. M., Young J. A. Fluid and electrolyte secretion from the isolated, perfused submandibular and sublingual glands of the rat. Arch Oral Biol. 1981;26(7):555–561. doi: 10.1016/0003-9969(81)90017-0. [DOI] [PubMed] [Google Scholar]
- Cremaschi D., Hénin S., Meyer G. Stimulation by HCO3- of Na+ transport in rabbit gallbladder. J Membr Biol. 1979 May 21;47(2):145–170. doi: 10.1007/BF01876114. [DOI] [PubMed] [Google Scholar]
- Frizzell R. A., Field M., Schultz S. G. Sodium-coupled chloride transport by epithelial tissues. Am J Physiol. 1979 Jan;236(1):F1–F8. doi: 10.1152/ajprenal.1979.236.1.F1. [DOI] [PubMed] [Google Scholar]
- Funder J., Ussing H. H., Wieth J. O. The effects of CO2 and hydrogen ions on active Na transport in the isolated frog skin. Acta Physiol Scand. 1967 Sep;71(1):65–76. doi: 10.1111/j.1748-1716.1967.tb03710.x. [DOI] [PubMed] [Google Scholar]
- Gentile D. E., Brodsky W. A. Effect of ambient pH on sodium transport across isolated turtle bladders. Am J Physiol. 1969 Sep;217(3):652–660. doi: 10.1152/ajplegacy.1969.217.3.652. [DOI] [PubMed] [Google Scholar]
- Gilliland E. L., Glazer G. Parallel secretion of enzymes by the rabbit pancreas. J Physiol. 1980 Jun;303:33–41. doi: 10.1113/jphysiol.1980.sp013268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez C. F., Shamoo Y. E., Brodsky W. A. The accelerating effect of serosal HCO3- on Na+ transport in short-circuited turtle bladders. Biochim Biophys Acta. 1969;193(2):403–418. doi: 10.1016/0005-2736(69)90200-4. [DOI] [PubMed] [Google Scholar]
- Knauf H. The minimum requirements for the maintenance of active sodium transport across the isolated salivary duct epithelium of the rabbit. Pflugers Arch. 1972;333(4):326–336. doi: 10.1007/BF00586212. [DOI] [PubMed] [Google Scholar]
- Maren T. H. Carbonic anhydrase: chemistry, physiology, and inhibition. Physiol Rev. 1967 Oct;47(4):595–781. doi: 10.1152/physrev.1967.47.4.595. [DOI] [PubMed] [Google Scholar]
- Maren T. H. Current status of membrane-bound carbonic anhydrase. Ann N Y Acad Sci. 1980;341:246–258. doi: 10.1111/j.1749-6632.1980.tb47176.x. [DOI] [PubMed] [Google Scholar]
- Martin C. J., Frömter E., Gebler B., Knauf H., Young J. A. The effects of carbachol on water and electrolyte fluxes and transepithelial electrical potential differences of the rabbit submaxillary main duct perfused in vitro. Pflugers Arch. 1973 Jun 26;341(2):131–142. doi: 10.1007/BF00587320. [DOI] [PubMed] [Google Scholar]
- Martin D. W. The effect of the bicarbonate ion on the gallbladder salt pump. J Membr Biol. 1974;18(3-4):219–230. doi: 10.1007/BF01870113. [DOI] [PubMed] [Google Scholar]
- Petersen O. H., Ueda N. Secretion of fluid and amylase in the perfused rat pancreas. J Physiol. 1977 Jan;264(3):819–835. doi: 10.1113/jphysiol.1977.sp011696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K. The physiology, pharmacology, and biochemistry of the eccrine sweat gland. Rev Physiol Biochem Pharmacol. 1977;79:51–131. doi: 10.1007/BFb0037089. [DOI] [PubMed] [Google Scholar]
- Sewell W. A., Young J. A. Secretion of electrolytes by the pancreas of the anaestetized rat. J Physiol. 1975 Nov;252(2):379–396. doi: 10.1113/jphysiol.1975.sp011149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson C. H., Solomon A. K. Micropuncture analysis of the cellular mechanisms of electrolyte secretion by the in vitro rabbit pancreas. J Gen Physiol. 1975 Jan;65(1):22–45. doi: 10.1085/jgp.65.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnberg L. A., Bieberdorf F. A., Morawski S. G., Fordtran J. S. Interrelationships of chloride, bicarbonate, sodium, and hydrogen transport in the human ileum. J Clin Invest. 1970 Mar;49(3):557–567. doi: 10.1172/JCI106266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich K. J., Radtke H. W., Rumrich G. The role of bicarbonate and other buffers on isotonic fluid absorption in the proximal convolution of the rat kidney. Pflugers Arch. 1971;330(2):149–161. doi: 10.1007/BF00643031. [DOI] [PubMed] [Google Scholar]
- Wright E. M. Effect of bicarbonate and other buffers on choroid plexus Na+/K+pump. Biochim Biophys Acta. 1977 Aug 1;468(3):486–489. doi: 10.1016/0005-2736(77)90297-8. [DOI] [PubMed] [Google Scholar]
- Wright E. M. Mechanisms of ion transport across the choroid plexus. J Physiol. 1972 Oct;226(2):545–571. doi: 10.1113/jphysiol.1972.sp009997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J. A., Case R. M., Conigrave A. D., Novak I. Transport of bicarbonate and other anions in salivary secretion. Ann N Y Acad Sci. 1980;341:172–190. doi: 10.1111/j.1749-6632.1980.tb47171.x. [DOI] [PubMed] [Google Scholar]
- Young J. A., Schneyer C. A. Composition of saliva in mammalia. Aust J Exp Biol Med Sci. 1981 Feb;59(1):1–53. doi: 10.1038/icb.1981.1. [DOI] [PubMed] [Google Scholar]


