Abstract
1. Recordings of afferent discharges from external intercostal muscle spindles were made from in-continuity dorsal roots of anaesthetized, paralysed cats and the afferents were characterized as described in the preceding paper (Kirkwood & Sears, 1982).
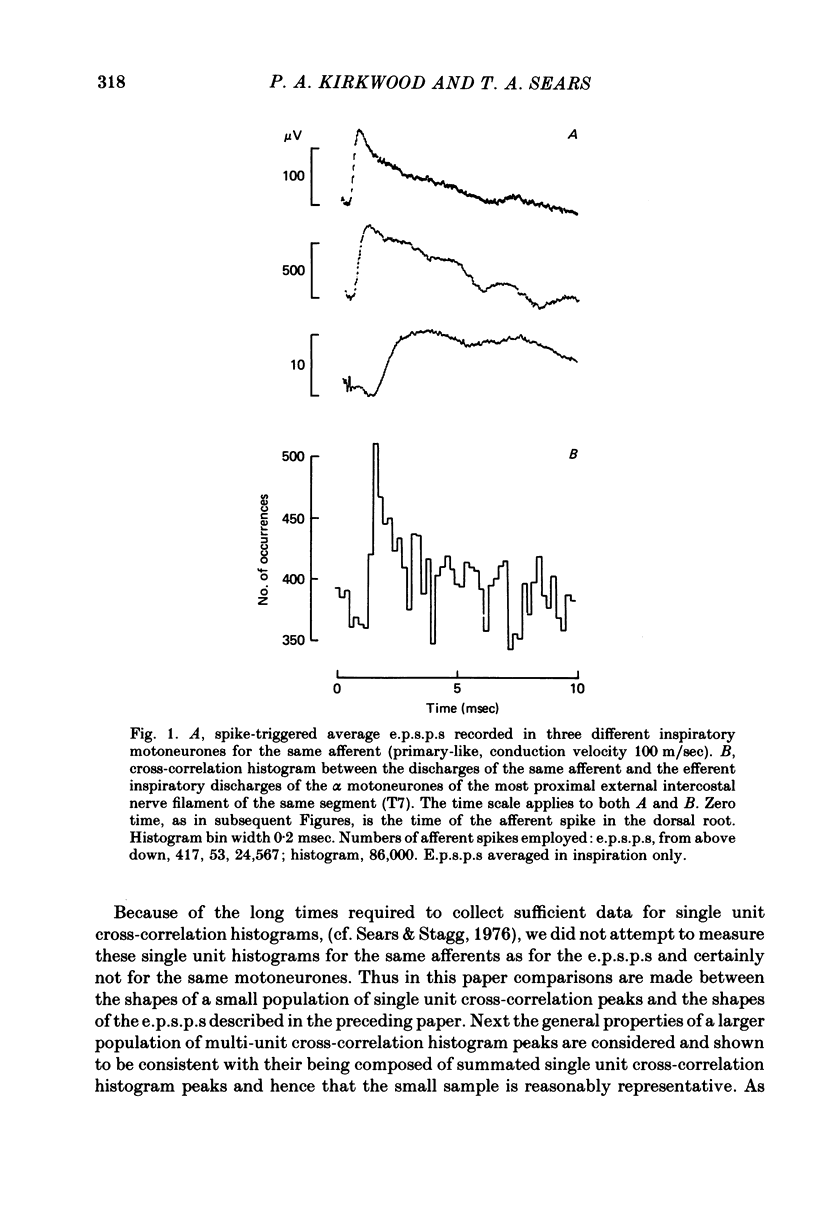
2. The strengths and time courses of the raised probabilities of firing of external intercostal motoneurones evoked by the synaptic actions of impulses in the afferents were measured by constructing cross-correlation histograms from the discharges of the individual afferents and the discharges of the motoneurones, which were simultaneously recorded from the cut central ends of intercostal nerve filaments. Other dorsal roots, apart from the rootlet containing the afferent fibre, were cut to prevent afferent synchronization from affecting the results.
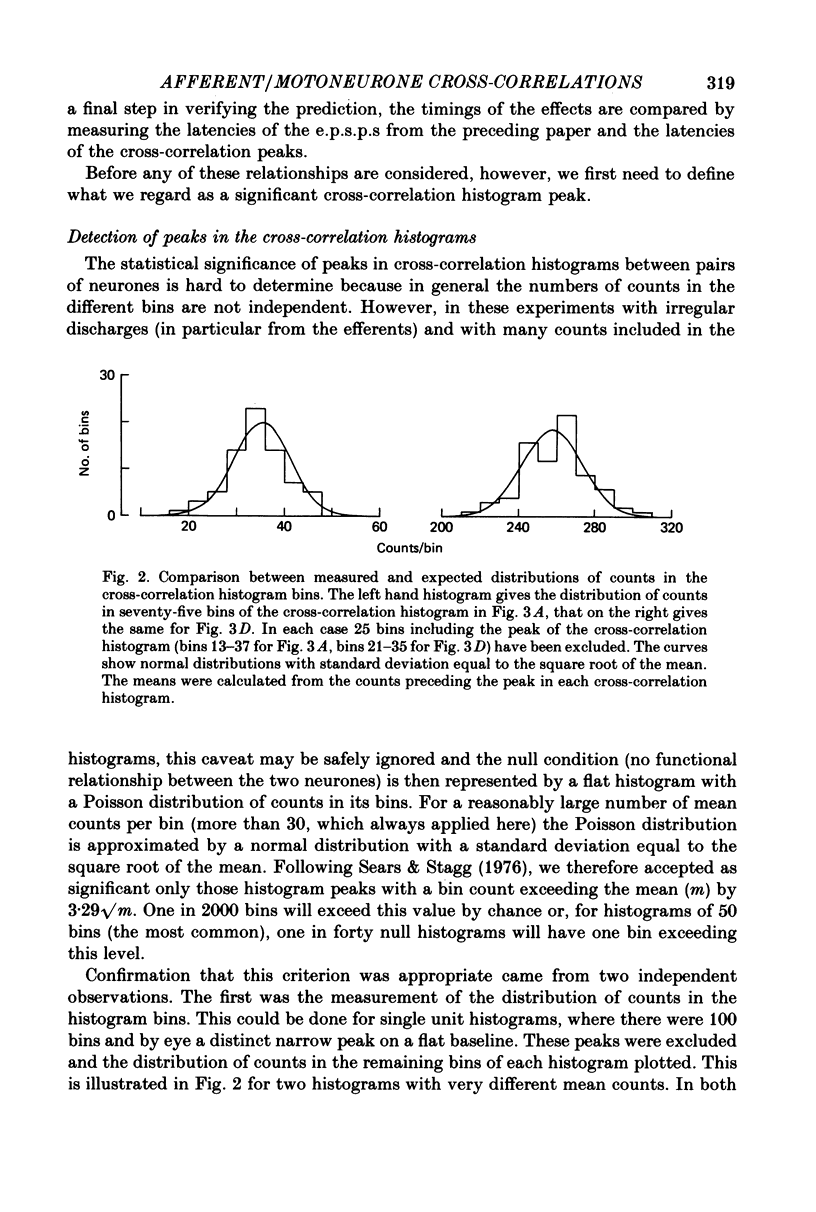
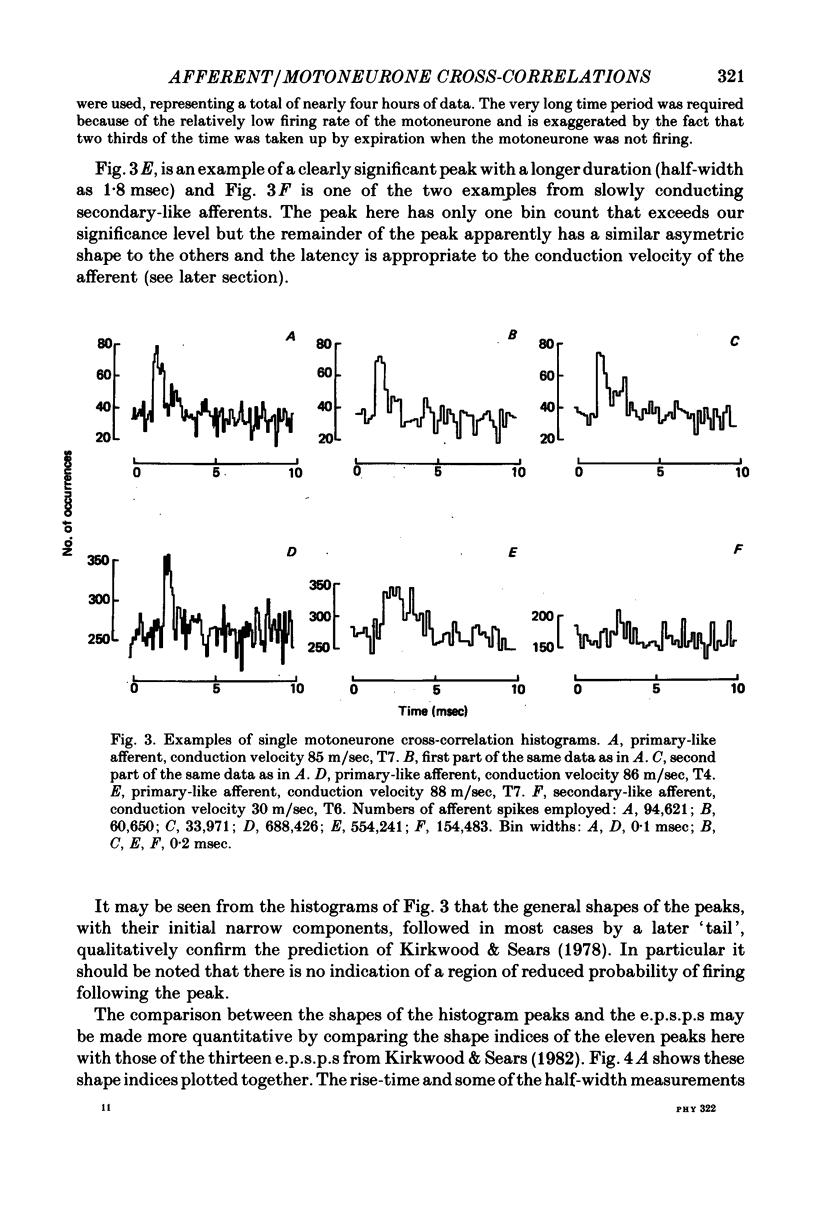
3. Cross-correlation histograms involving single efferent discharges showed narrow peaks (mean half-width 0·99 msec) at monosynaptic latencies.
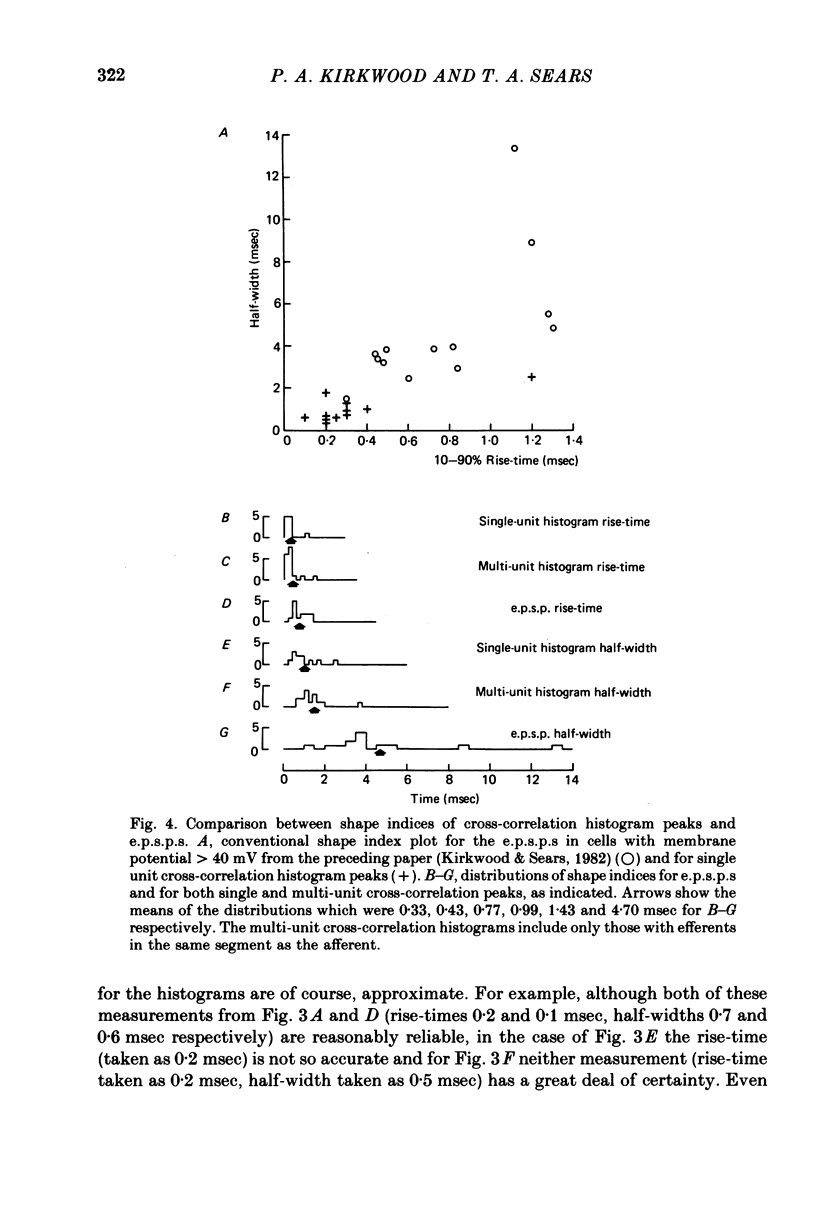
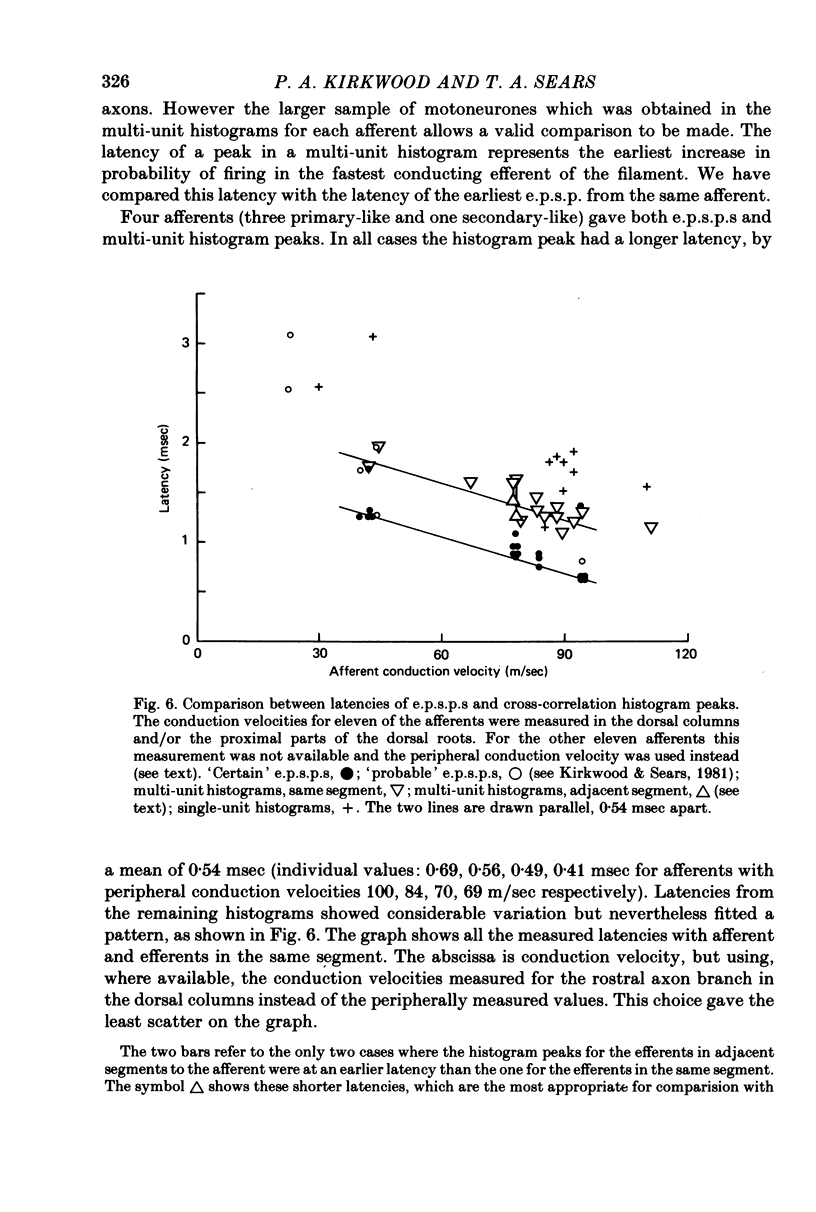
4. This mean half-width is slightly longer than the mean rise-time of the e.p.s.p.s described in the preceding paper, but much shorter than the mean half-width of those e.p.s.p.s. These measurements, together with the shapes of individual histogram peaks thus confirm the prediction of Kirkwood & Sears (1978) that the shape of such a peak should be approximated by the time differential of the underlying e.p.s.p. plus the e.p.s.p. time course itself. Amplitudes of the peaks were also consistent with the predictions of Kirkwood & Sears (1978).
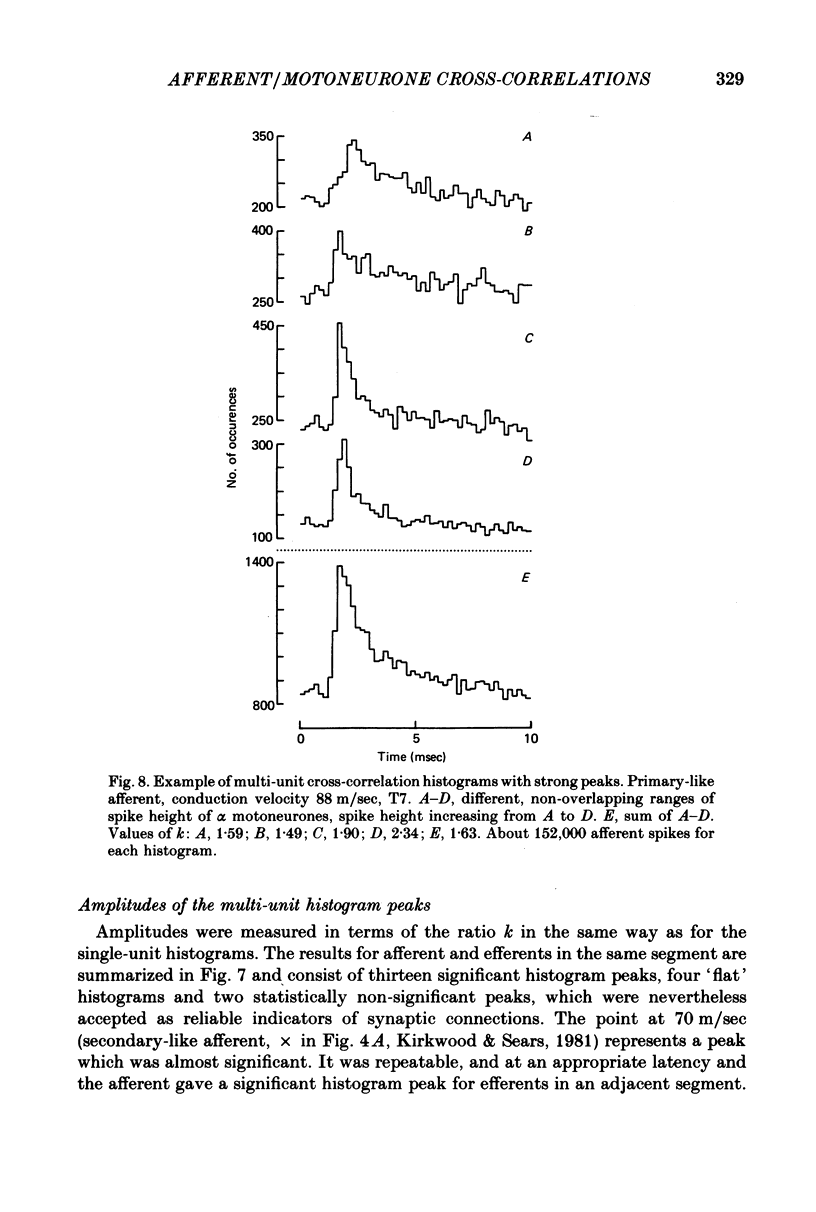
5. The cross-correlation histograms involving multi-unit efferent discharges were entirely consistent with those involving single efferent discharges and were used to demonstrate the patterns of projections of individual afferents to different groups of intercostal motoneurones.
6. Individual afferents excited α motoneurones of all spike sizes in the intercostal filament recordings but excitation of γ motoneurones was not detected.
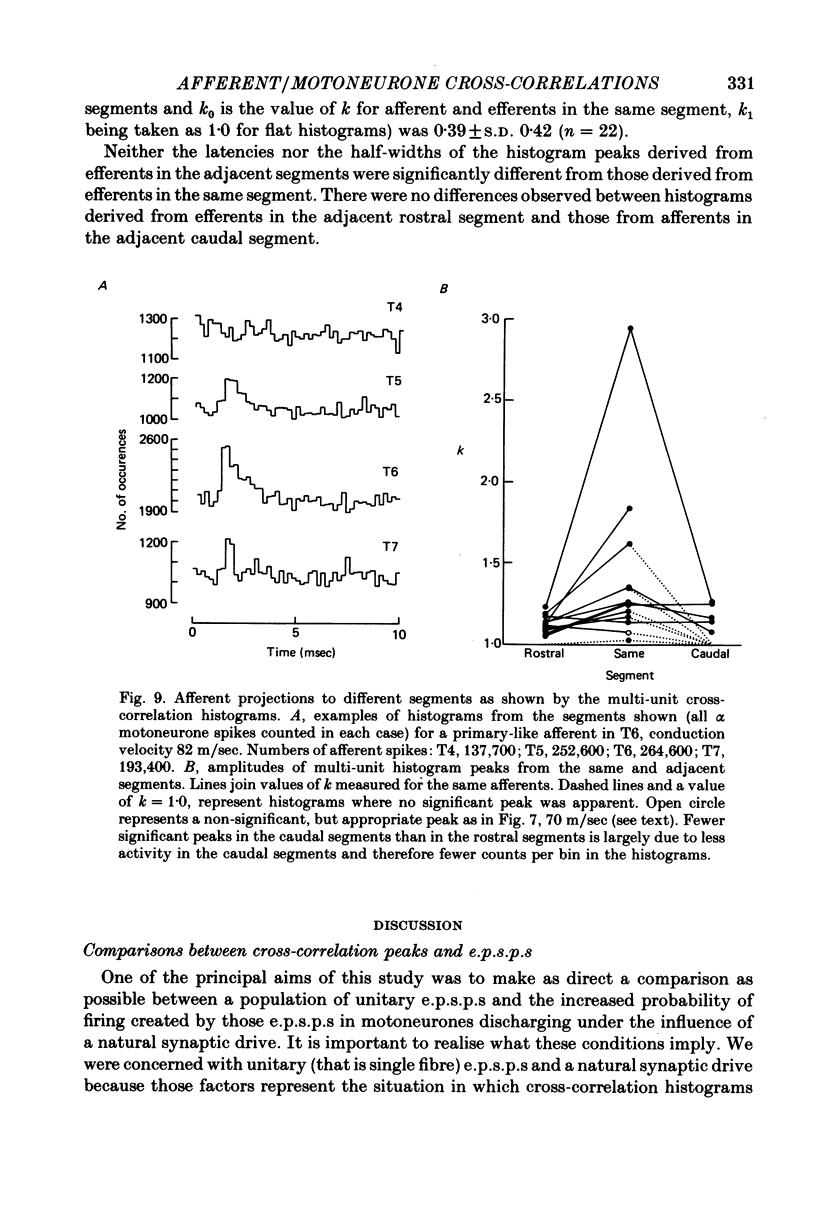
7. Individual afferents excited motoneurones of the same segment and both adjacent segments, but no projection beyond these was detected.
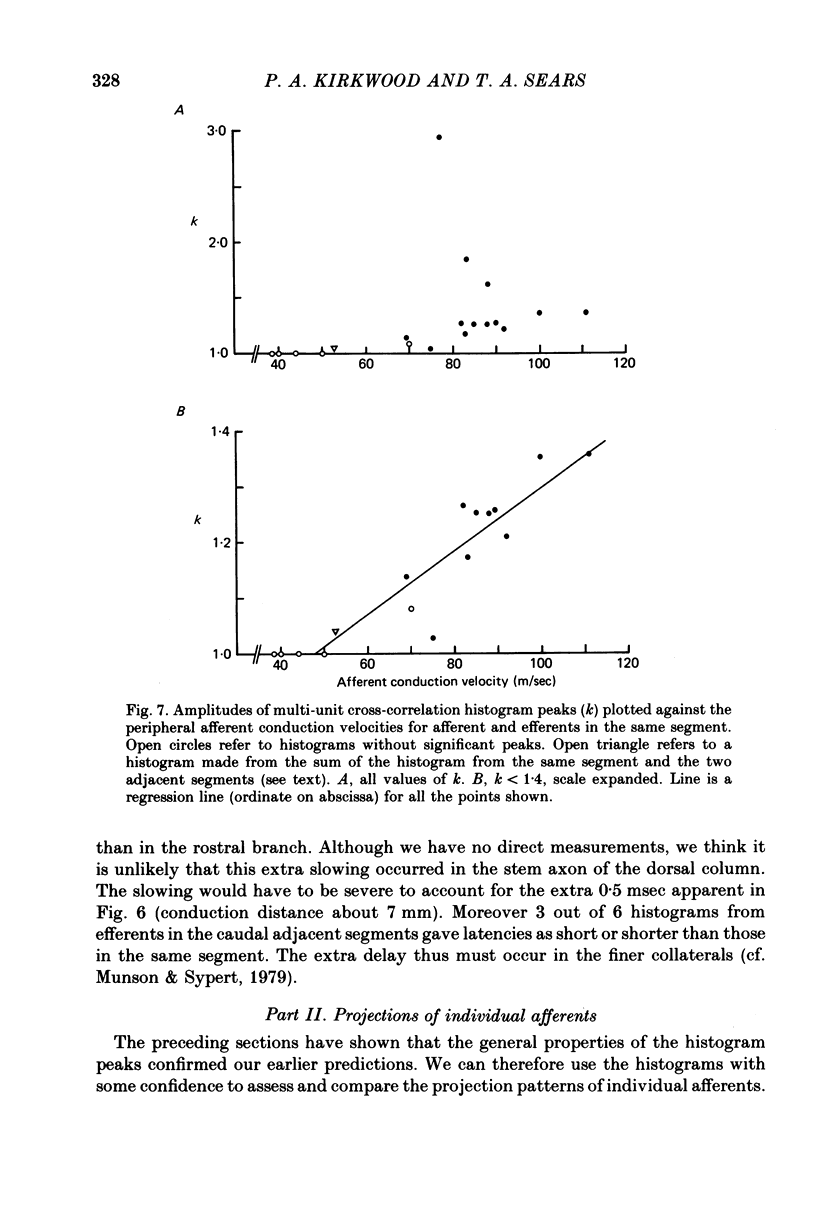
8. The amplitude of the multi-unit cross-correlation peak was taken as a measure of the over-all strength of the connexions to a given segment. When this amplitude was measured for motoneurones of the same segment as the afferent, for sixteen out of nineteen afferents there was a highly significant positive correlation with the conduction velocity of the afferent. The remaining three afferents gave a strength of connexion which appeared to be much stronger than would be expected from the rest of the population of afferents.
9. The strength of connexion to adjacent segments was generally lower than to the same segment.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appenteng K., O'Donovan M. J., Somjen G., Stephens J. A., Taylor A. The projection of jaw elevator muscle spindle afferents to fifth nerve motoneurones in the cat. J Physiol. 1978 Jun;279:409–423. doi: 10.1113/jphysiol.1978.sp012353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett E. F., Barrett J. N., Crill W. E. Voltage-sensitive outward currents in cat motoneurones. J Physiol. 1980 Jul;304:251–276. doi: 10.1113/jphysiol.1980.sp013323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley K., Somjen G. G. Accommodation in motoneurones of the rat and the cat. J Physiol. 1961 Apr;156(1):75–92. doi: 10.1113/jphysiol.1961.sp006659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvin W. H., Stevens C. F. Synaptic noise and other sources of randomness in motoneuron interspike intervals. J Neurophysiol. 1968 Jul;31(4):574–587. doi: 10.1152/jn.1968.31.4.574. [DOI] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., LUNDBERG A. The convergence of monosynaptic excitatory afferents on to many different species of alpha motoneurones. J Physiol. 1957 Jun 18;137(1):22–50. doi: 10.1113/jphysiol.1957.sp005794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES R. M., LUNDBERG A. Integrative pattern of Ia synaptic actions on motoneurones of hip and knee muscles. J Physiol. 1958 Dec 4;144(2):271–298. doi: 10.1113/jphysiol.1958.sp006101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellaway P. H., Trott J. R. Autogenetic reflex action on to gamma motoneurones by stretch of triceps surae in the decerebrated cat. J Physiol. 1978 Mar;276:49–66. doi: 10.1113/jphysiol.1978.sp012219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu T. C., Schomburg E. D. Electrophysiological investigation of the projection of secondary muscle spindle afferents in the cat spinal cord. Acta Physiol Scand. 1974 Jul;91(3):314–329. doi: 10.1111/j.1748-1716.1974.tb05687.x. [DOI] [PubMed] [Google Scholar]
- Kirkwood P. A. On the use and interpretation of cross-correlations measurements in the mammalian central nervous system. J Neurosci Methods. 1979 Aug;1(2):107–132. doi: 10.1016/0165-0270(79)90009-8. [DOI] [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A. Excitatory post-synaptic potentials from single muscle spindle afferents in external intercostal motoneurones of the cat. J Physiol. 1982 Jan;322:287–314. doi: 10.1113/jphysiol.1982.sp014038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A., Stagg D. Proceedings: Synchronized firing of respiratory motoneurones during spontaneous breathing in the anaesthetized cat. J Physiol. 1974 May;239(1):11P–13P. [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A. The synaptic connexions to intercostal motoneurones as revealed by the average common excitation potential. J Physiol. 1978 Feb;275:103–134. doi: 10.1113/jphysiol.1978.sp012180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A., Westgaard R. H. Recurrent inhibition of intercostal motoneurones in the cat. J Physiol. 1981;319:111–130. doi: 10.1113/jphysiol.1981.sp013895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox C. K. Cross-correlation functions for a neuronal model. Biophys J. 1974 Aug;14(8):567–582. doi: 10.1016/S0006-3495(74)85936-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox C. K., Poppele R. E. Correlation analysis of stimulus-evoked changes in excitability of spontaneously firing neurons. J Neurophysiol. 1977 May;40(3):616–625. doi: 10.1152/jn.1977.40.3.616. [DOI] [PubMed] [Google Scholar]
- Lüscher H. R., Ruenzel P., Fetz E., Henneman E. Postsynatpic population potentials recorded from ventral roots perfused with isotonic sucrose: connections of groups Ia and II spindle afferent fibers with large populations of motoneurons. J Neurophysiol. 1979 Jul;42(4):1146–1164. doi: 10.1152/jn.1979.42.4.1146. [DOI] [PubMed] [Google Scholar]
- Mendell L. M., Henneman E. Terminals of single Ia fibers: location, density, and distribution within a pool of 300 homonymous motoneurons. J Neurophysiol. 1971 Jan;34(1):171–187. doi: 10.1152/jn.1971.34.1.171. [DOI] [PubMed] [Google Scholar]
- Munson J. B., Fleshman J. W., Sypert G. W. Properties of single-fiber spindle group II EPSPs in triceps surae motoneurons. J Neurophysiol. 1980 Oct;44(4):713–725. doi: 10.1152/jn.1980.44.4.713. [DOI] [PubMed] [Google Scholar]
- Munson J. B., Sypert G. W. Latency-rise time relationship in unitary postsynaptic potentials. Brain Res. 1978 Aug 4;151(2):404–408. doi: 10.1016/0006-8993(78)90897-1. [DOI] [PubMed] [Google Scholar]
- Munson J. B., Sypert G. W. Properties of single central Ia afferent fibres projecting to motoneurones. J Physiol. 1979 Nov;296:315–327. doi: 10.1113/jphysiol.1979.sp013007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROMANES G. J. The motor cell columns of the lumbo-sacral spinal cord of the cat. J Comp Neurol. 1951 Apr;94(2):313–363. doi: 10.1002/cne.900940209. [DOI] [PubMed] [Google Scholar]
- SEARS T. A. EFFERENT DISCHARGES IN ALPHA AND FUSIMOTOR FIBRES OF INTERCOSTAL NERVES OF THE CAT. J Physiol. 1964 Nov;174:295–315. doi: 10.1113/jphysiol.1964.sp007488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEARS T. A. THE FIBRE CALIBRE SPECTRA OF SENSORY AND MOTOR FIBRES IN THE INTERCOSTAL NERVES OF THE CAT. J Physiol. 1964 Jul;172:150–161. doi: 10.1113/jphysiol.1964.sp007409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwindt P. C. Membrane-potential trajectories underlying motoneuron rhythmic firing at high rates. J Neurophysiol. 1973 May;36(3):434–439. doi: 10.1152/jn.1973.36.3.434. [DOI] [PubMed] [Google Scholar]
- Sears T. A., Stagg D. Short-term synchronization of intercostal motoneurone activity. J Physiol. 1976 Dec;263(3):357–381. doi: 10.1113/jphysiol.1976.sp011635. [DOI] [PMC free article] [PubMed] [Google Scholar]
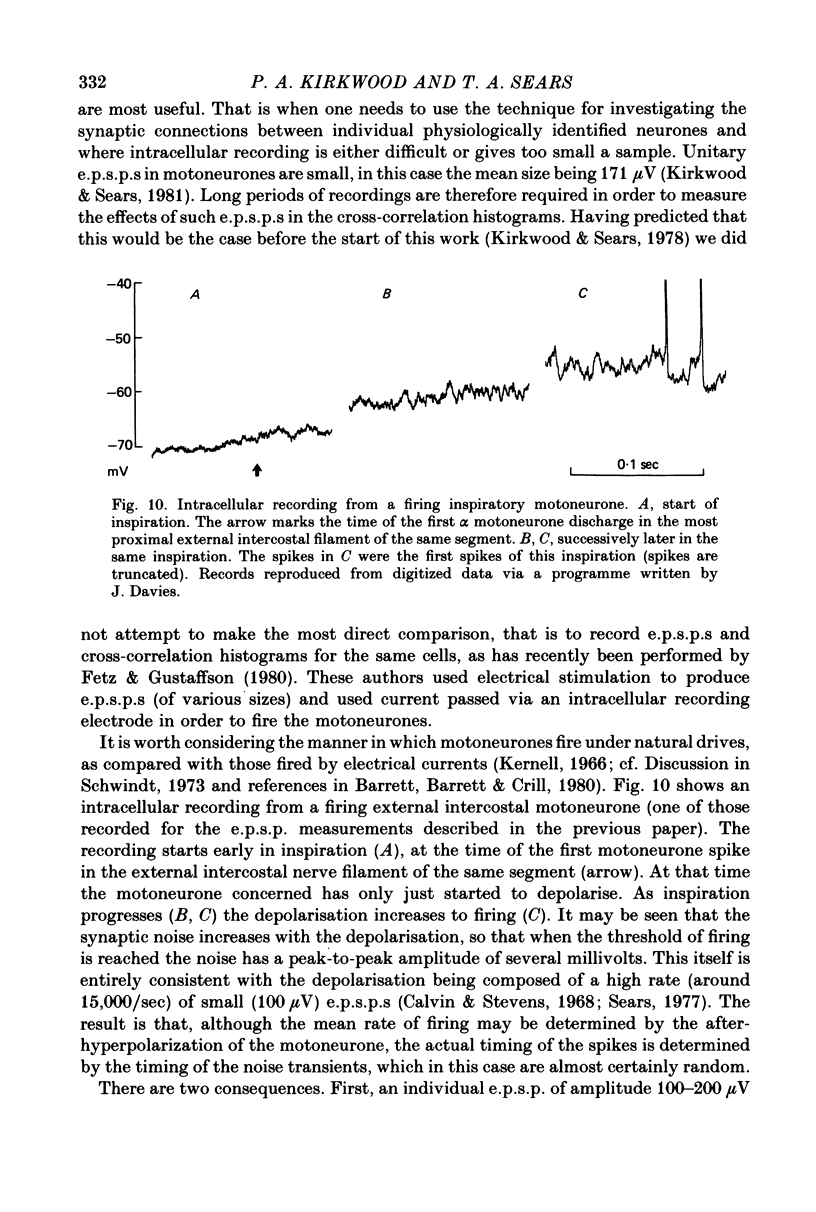
- Sears T. A. The respiratory motoneuron and apneusis. Fed Proc. 1977 Sep;36(10):2412–2420. [PubMed] [Google Scholar]


