Abstract
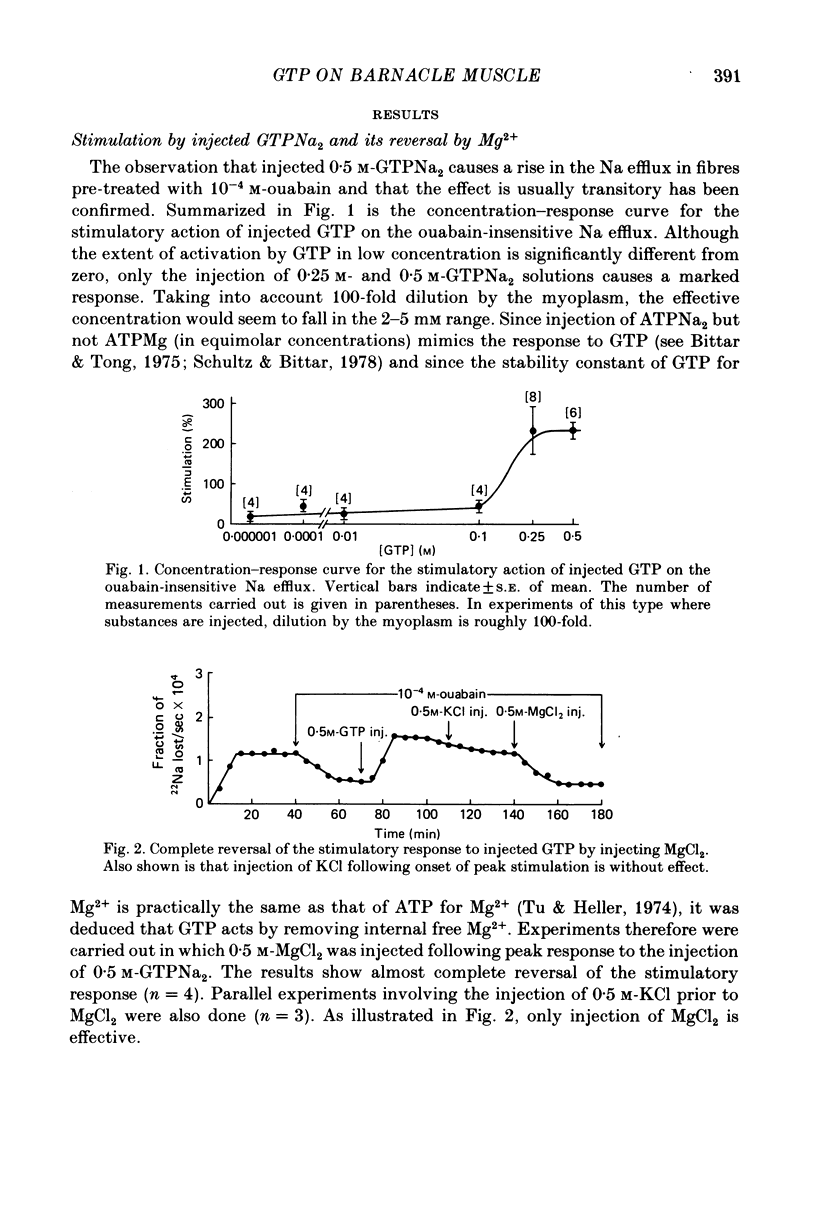
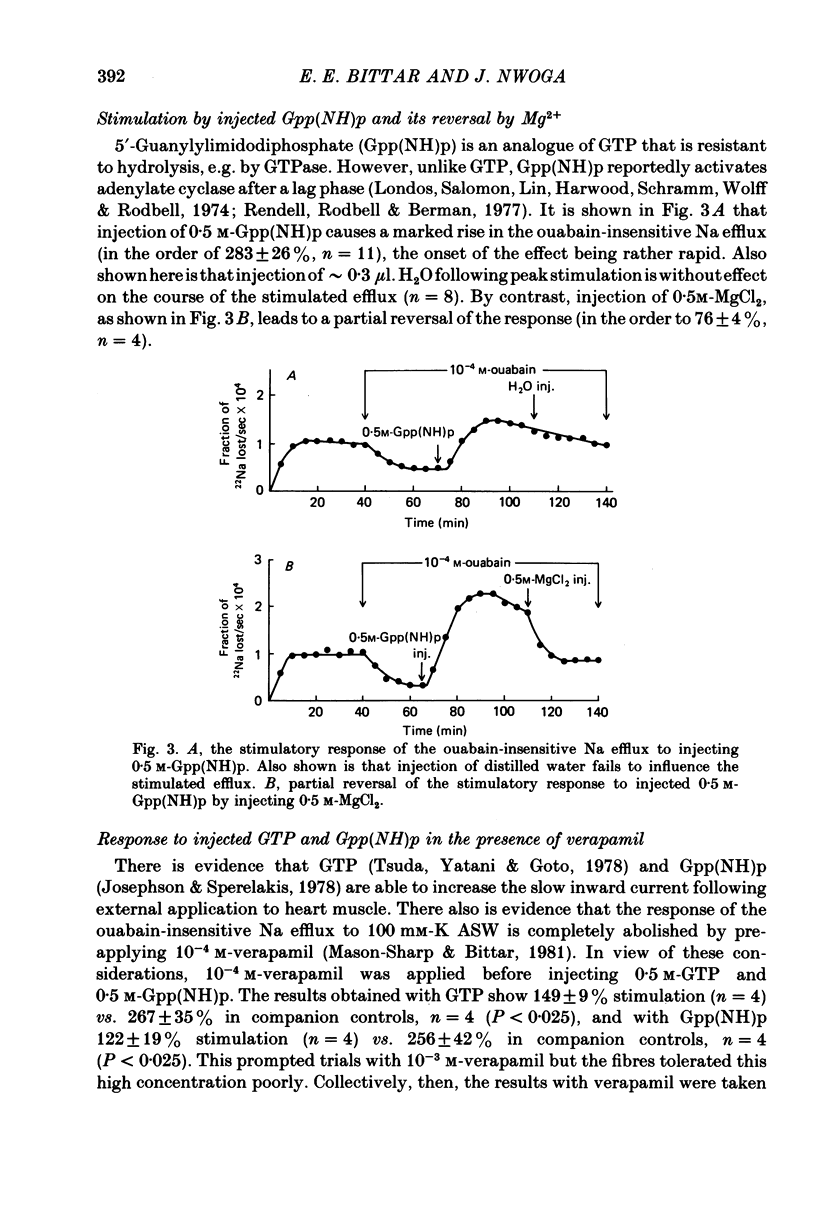
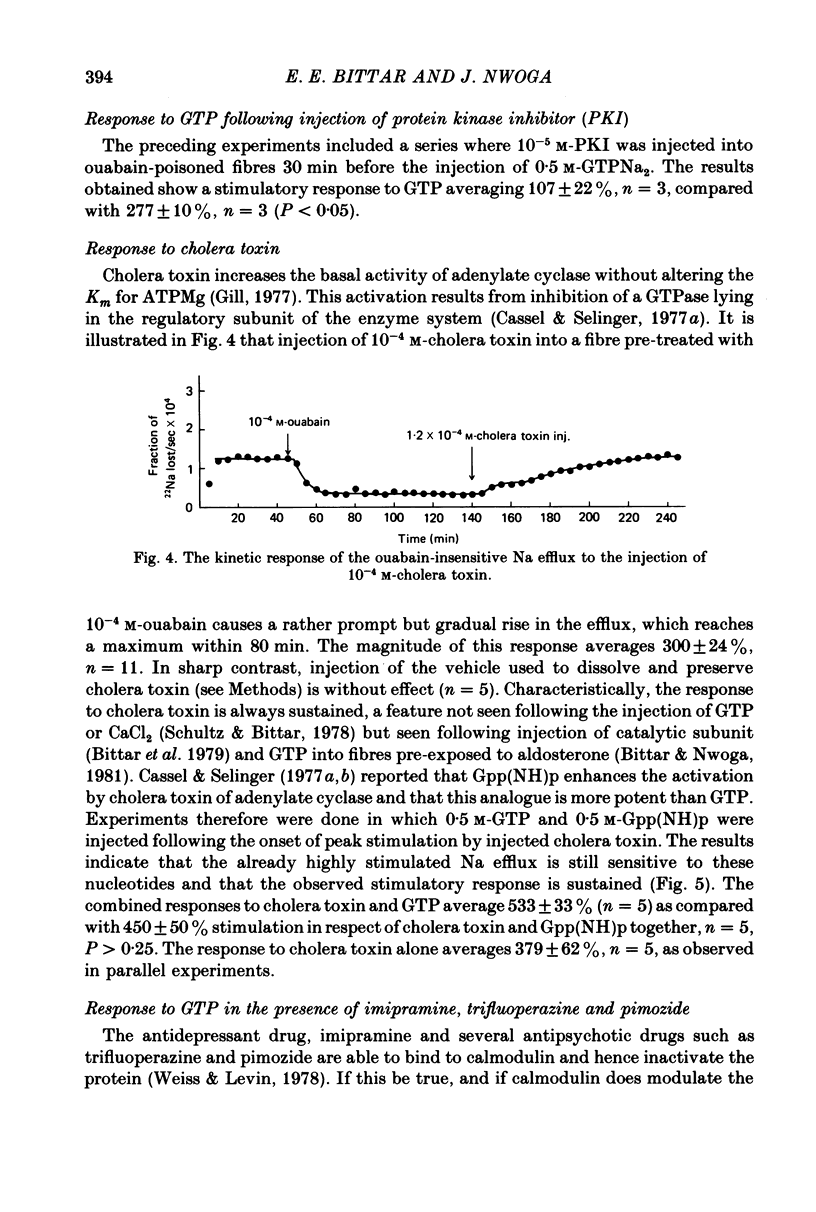
1. A study has been made of the mechanism by which injected disodium GTP stimulates the ouabain-insensitive Na efflux in single muscle fibres from the barnacle, Balanus nubilus. 2. Injection of GTPNa2 causes a stimulatory response which is usually transitory and almost completely reversed by injecting MgCl2 (but not KCl). 3. Injected 5'-guanylylimidodiphosphate, Gpp(NH)p, mimics this action of GTP but the reversal seen with injected Mg2+ is less pronounced. 4. (i) Pre-treatment of these fibres with verapamil reduces the size of the stimulatory response to GTP and Gpp(NH)p. (ii) Pre-injection of protein kinase inhibitor (PKI) or regulatory subunits reduces the response as well. (iii) Pre-treatment with imipramine or trifluoperazine reduces the response to injected GTP; in combination with verapamil, a greater reduction in response is seen. 5. Injection of EDTA leads to a stimulatory response which is transitory. This response is largely abolished by verapamil. 6. Injection of cholera toxin leads to a sustained stimulatory rather than a transitory response. GTP or Gpp(NH)p when injected following peak stimulation by cholera toxin leads to a moderate sustained stimulation. 7. These results support the view that the stimulatory response to injected GTPNa2 is the result of activation of Ca2+ channels and of increased availability of GTPMg and that these two conditions bring about activation of adenylate cyclase and hence activation of cyclic AMP-protein kinase by newly formed cyclic AMP.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashley C. C., Ellory J. C. The efflux of magnesium from single crustacean muscle fibres. J Physiol. 1972 Nov;226(3):653–674. doi: 10.1113/jphysiol.1972.sp010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett V., Mong L., Cuatrecasas P. Mechanism of activation of adenylate cyclase by Vibrio cholerae enterotoxin. Relations to the mode of activation by hormones. J Membr Biol. 1975 Nov 7;24(2):107–129. doi: 10.1007/BF01868618. [DOI] [PubMed] [Google Scholar]
- Bittar E. E., Demaille J., Fischer E. H., Schultz R. Mode of stimulation by injection of cyclic AMP and external acidification of the sodium efflux in barnacle muscle fibres. J Physiol. 1979 Nov;296:277–289. doi: 10.1113/jphysiol.1979.sp013005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittar E. E. Effect of inhibitors and uncouplers on the Na pump of the Maia muscle fibre. J Physiol. 1966 Nov;187(1):81–103. doi: 10.1113/jphysiol.1966.sp008077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittar E. E., Hift H., Huddart H., Tong E. The effects of caffeine on sodium transport, membrane potential, mechanical tension and ultrastructure in barnacle muscle fibres. J Physiol. 1974 Oct;242(1):1–34. doi: 10.1113/jphysiol.1974.sp010691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittar E. E., Nwoga J. Stimulation by injected guanosine triphosphate of the sodium efflux in barnacle muscle fibres pre-exposed to aldosterone. J Physiol. 1981;313:499–511. doi: 10.1113/jphysiol.1981.sp013678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittar E. E., Schultz R., Harkness C. Influence of insulin on sodium efflux in barnacle muscle fibers. J Membr Biol. 1977 Jun 6;34(2-3):203–222. doi: 10.1007/BF01870300. [DOI] [PubMed] [Google Scholar]
- Bittar E. E., Sharp D. M. Stimulation by cyclic GMP of sodium efflux in barnacle muscle fibres. J Physiol. 1979 Aug;293:135–151. doi: 10.1113/jphysiol.1979.sp012882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittar E. E., Tallitsch R. B. Stimulation by aldosterone of the sodium efflux in barnacle muscle fibres: effects of RNA inhibitors and spironolactone. J Physiol. 1975 Sep;250(2):331–346. doi: 10.1113/jphysiol.1975.sp011057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittar E. E., Tong E. Sensitivity of the sodium efflux in barnacle muscle fibers to the microinjection of ATP. Life Sci. 1975 Jan 15;16(2):289–296. doi: 10.1016/0024-3205(75)90027-2. [DOI] [PubMed] [Google Scholar]
- Brinley F. J., Jr, Scarpa A., Tiffert T. The concentration of ionized magnesium in barnacle muscle fibres. J Physiol. 1977 Apr;266(3):545–565. doi: 10.1113/jphysiol.1977.sp011781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CALDWELL P. C., WALSTER G. STUDIES ON THE MICRO-INJECTION OF VARIOUS SUBSTANCES INTO CRAB MUSCLE FIBRES. J Physiol. 1963 Nov;169:353–372. doi: 10.1113/jphysiol.1963.sp007261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassel D., Selinger Z. Catecholamine-induced release of [3H]-Gpp(NH)p from turkey erythrocyte adenylate cyclase. J Cyclic Nucleotide Res. 1977 Feb;3(1):11–22. [PubMed] [Google Scholar]
- Cassel D., Selinger Z. Mechanism of adenylate cyclase activation by cholera toxin: inhibition of GTP hydrolysis at the regulatory site. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3307–3311. doi: 10.1073/pnas.74.8.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill D. M. Mechanism of action of cholera toxin. Adv Cyclic Nucleotide Res. 1977;8:85–118. [PubMed] [Google Scholar]
- Goldberg N. D., Haddox M. K. Cyclic GMP metabolism and involvement in biological regulation. Annu Rev Biochem. 1977;46:823–896. doi: 10.1146/annurev.bi.46.070177.004135. [DOI] [PubMed] [Google Scholar]
- Helmer P., Rusy B., Bittar E. E. Sensitivity to halothane of the sodium efflux in single barnacle muscle fibers. J Pharmacol Exp Ther. 1981 May;217(2):248–253. [PubMed] [Google Scholar]
- Josephson I., Sperelakis N. 5'-guanylimidodiphosphate stimulation of slow Ca2+ current in myocardial cells. J Mol Cell Cardiol. 1978 Dec;10(12):1157–1166. doi: 10.1016/0022-2828(78)90360-7. [DOI] [PubMed] [Google Scholar]
- Kato K., Kobayashi M., Sato S. Inactivation and reactivation of phosphoprotein phosphatase of rabbit skeletal muscle. Role of ATP and divalent metal ions. J Biochem. 1975 Apr;77(4):811–815. doi: 10.1093/oxfordjournals.jbchem.a130787. [DOI] [PubMed] [Google Scholar]
- Li H. C., Hsiao K. J., Chan W. W. Purification and properties of phosphoprotein phosphatases with different substrate and divalent cation specificities from canine heart. Eur J Biochem. 1978 Mar;84(1):215–225. doi: 10.1111/j.1432-1033.1978.tb12159.x. [DOI] [PubMed] [Google Scholar]
- Londos C., Salomon Y., Lin M. C., Harwood J. P., Schramm M., Wolff J., Rodbell M. 5'-Guanylylimidodiphosphate, a potent activator of adenylate cyclase systems in eukaryotic cells. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3087–3090. doi: 10.1073/pnas.71.8.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason-Sharp D., Bittar E. E. Stimulation by high external potassium of the sodium efflux in barnacle muscle fibers. J Membr Biol. 1981 Feb 28;58(3):213–226. doi: 10.1007/BF01870907. [DOI] [PubMed] [Google Scholar]
- Rendell M. S., Rodbell M., Berman M. Activation of hepatic adenylate cyclase by guanyl nucleotides. Modeling of the transient kinetics suggests an "excited" state of GTPase is a control component of the system. J Biol Chem. 1977 Nov 25;252(22):7909–7912. [PubMed] [Google Scholar]
- Schulman H., Greengard P. Ca2+-dependent protein phosphorylation system in membranes from various tissues, and its activation by "calcium-dependent regulator". Proc Natl Acad Sci U S A. 1978 Nov;75(11):5432–5436. doi: 10.1073/pnas.75.11.5432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz R., Bittar E. E. Studies of the mode of stimulation by external acidification and raising the internal free calcium concentration of the sodium efflux in barnacle muscle fibers. Pflugers Arch. 1978 Apr 25;374(1):31–38. doi: 10.1007/BF00585694. [DOI] [PubMed] [Google Scholar]
- Tsuda Y., Yatani A., Goto M. Effects of exogenously applied guanosine triphosphate on membrane current and tension of bullfrong atrial muscle. J Mol Cell Cardiol. 1978 Sep;10(9):813–826. doi: 10.1016/0022-2828(78)90391-7. [DOI] [PubMed] [Google Scholar]
- Weiss B., Levin R. M. Mechanism for selectively inhibiting the activation of cyclic nucleotide phosphodiesterase and adenylate cyclase by antipsychotic agents. Adv Cyclic Nucleotide Res. 1978;9:285–303. [PubMed] [Google Scholar]
- Wolff D. J., Brostrom C. O. Properties and functions of the calcium-dependent regulator protein. Adv Cyclic Nucleotide Res. 1979;11:27–88. [PubMed] [Google Scholar]


