Abstract
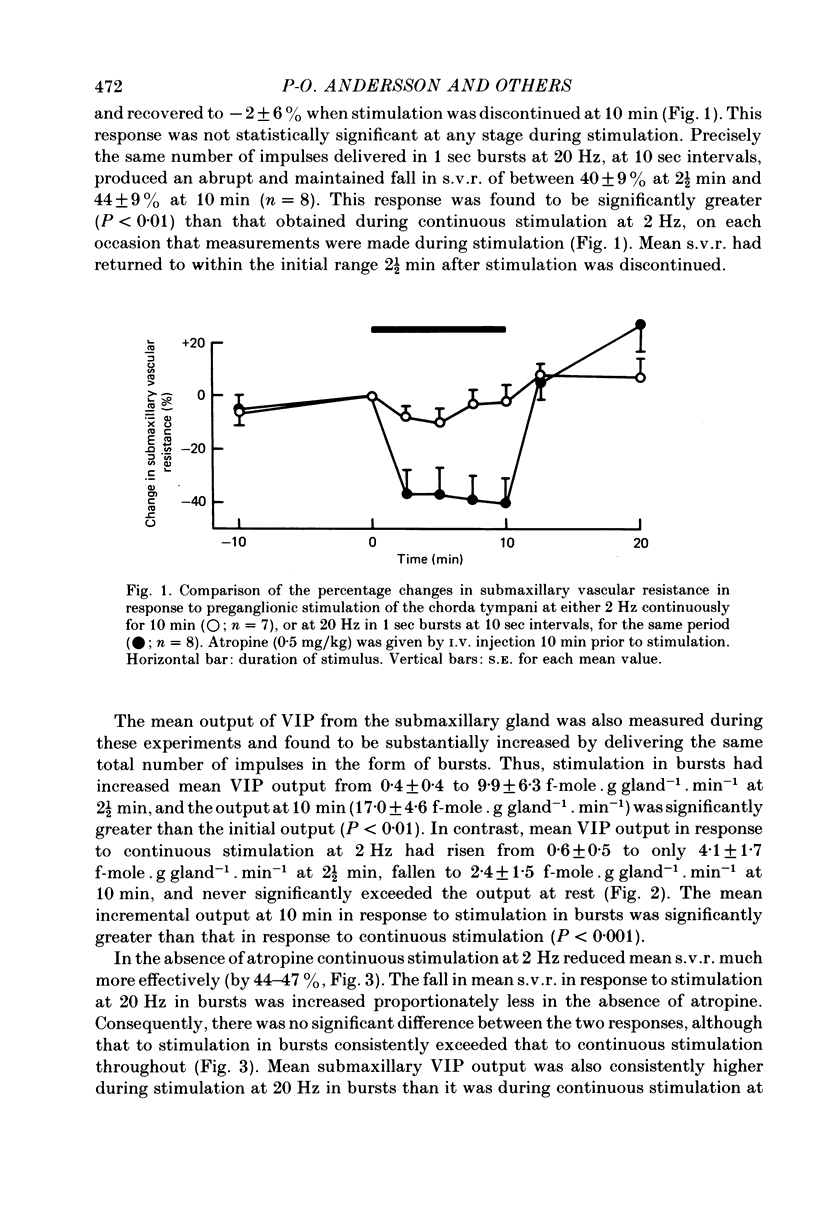
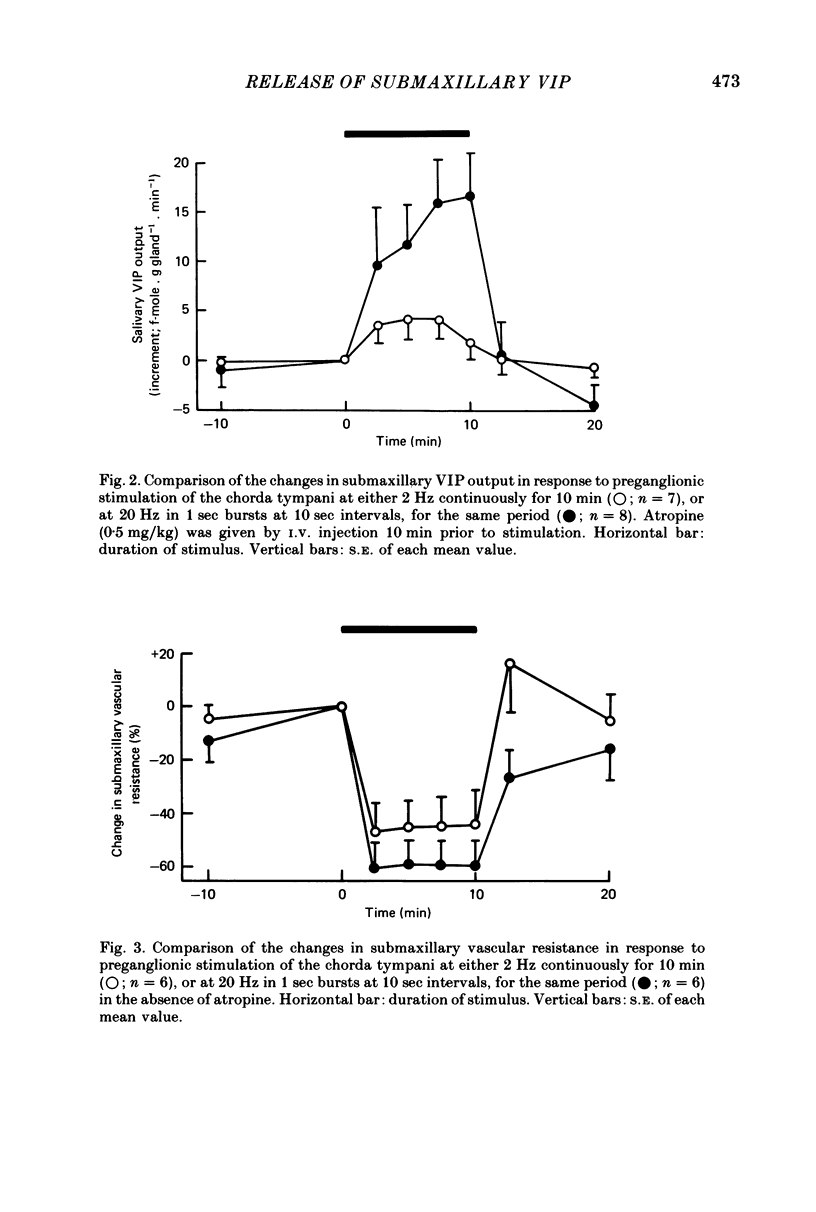
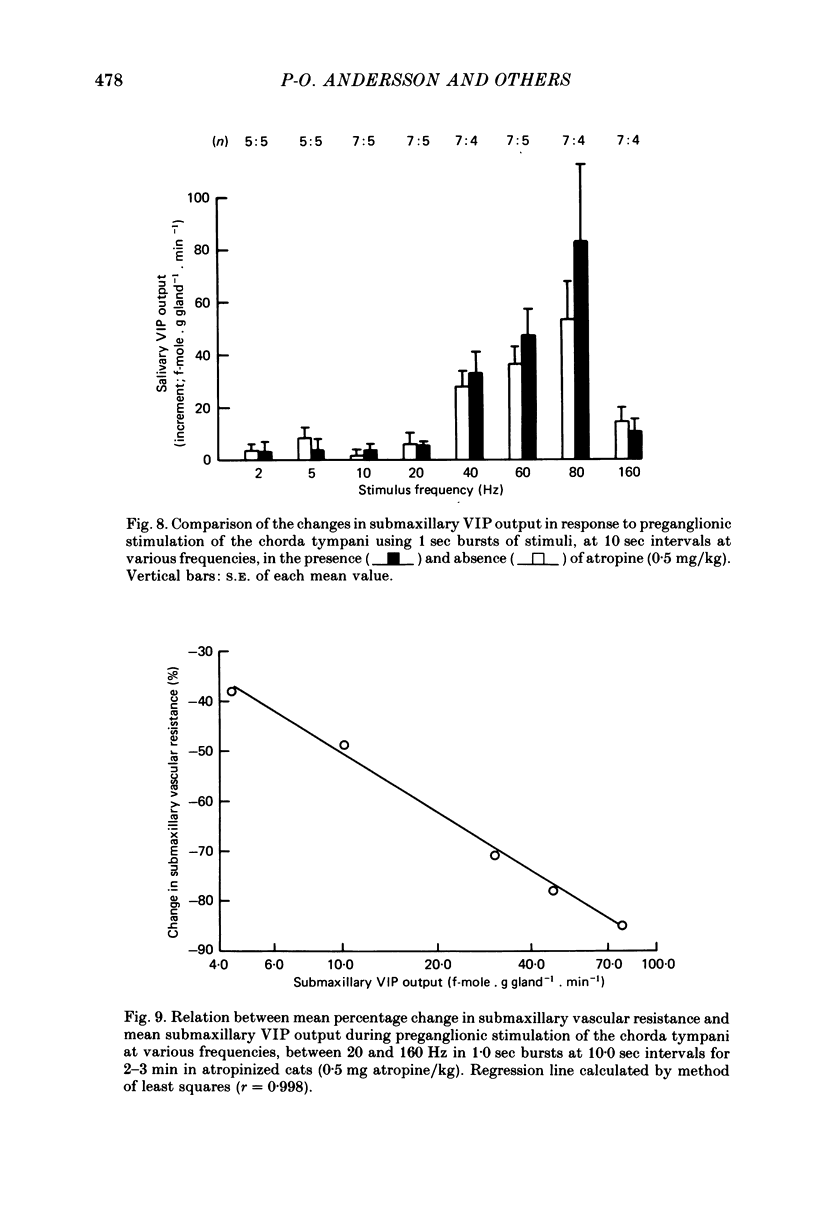
1. The effects of continuous preganglionic stimulation of the chorda tympani at 2 Hz for 10 min were compared with those of stimulation at 20 Hz in 1 sec bursts at 10 sec intervals for the same period in cats treated with atropine (0·5 mg/kg). Both the fall in mean submaxillary vascular resistance (s.v.r.) and the rise in mean vasoactive intestinal peptide (VIP) output from the gland were increased significantly (P < 0·01; P < 0·02) when the same total number of impulses were delivered in the form of bursts at the higher frequency.
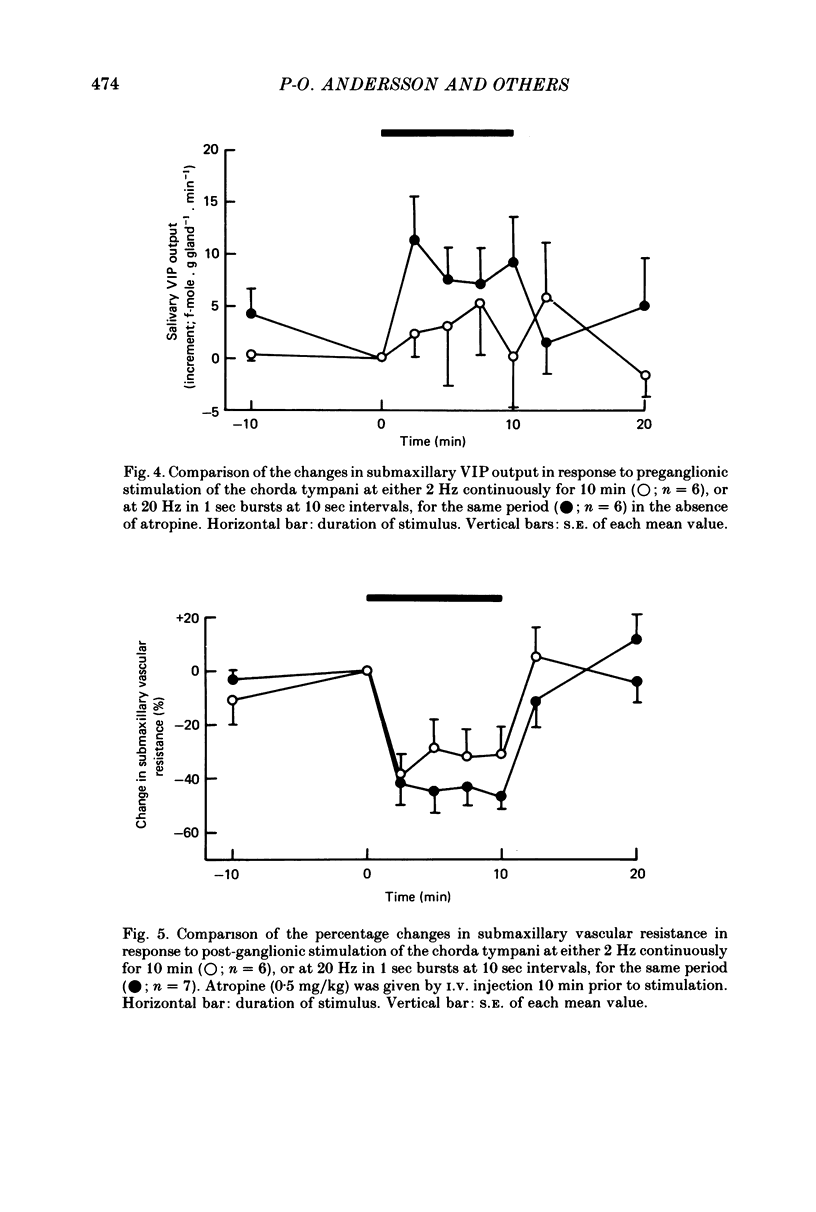
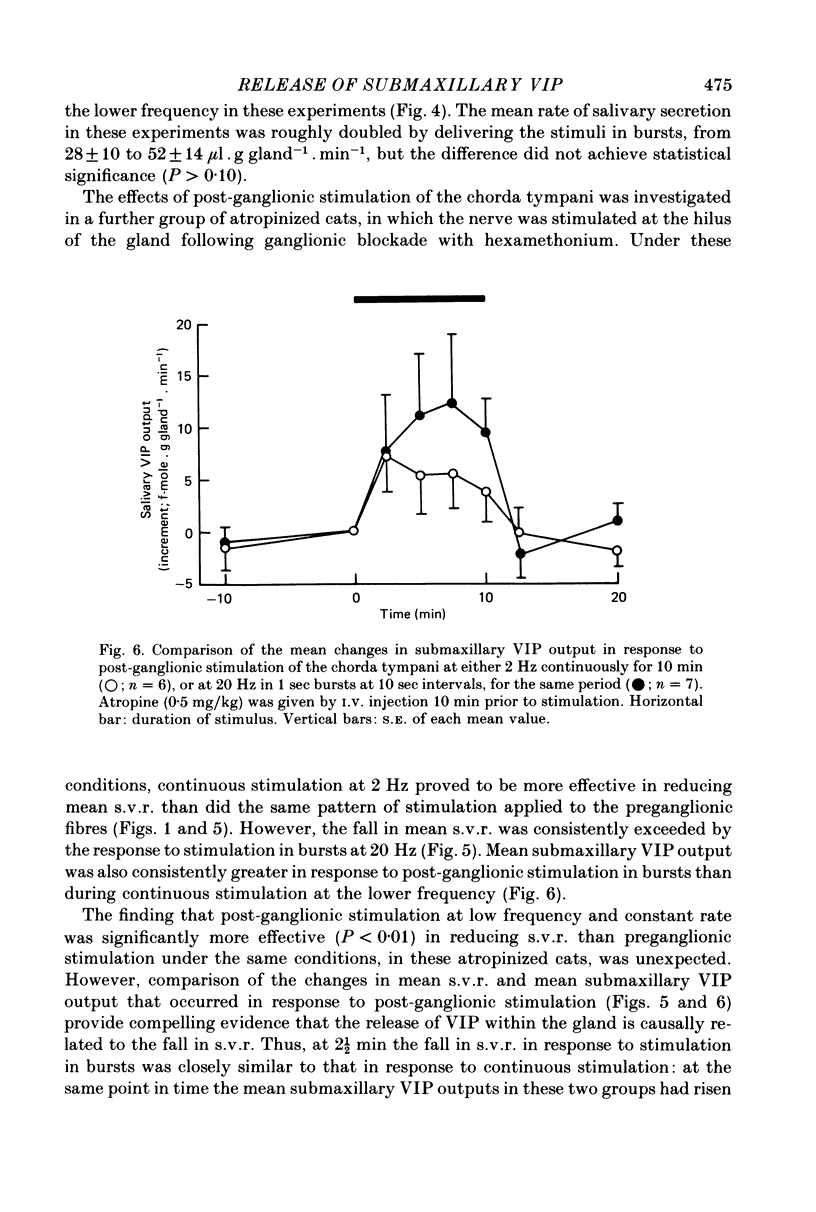
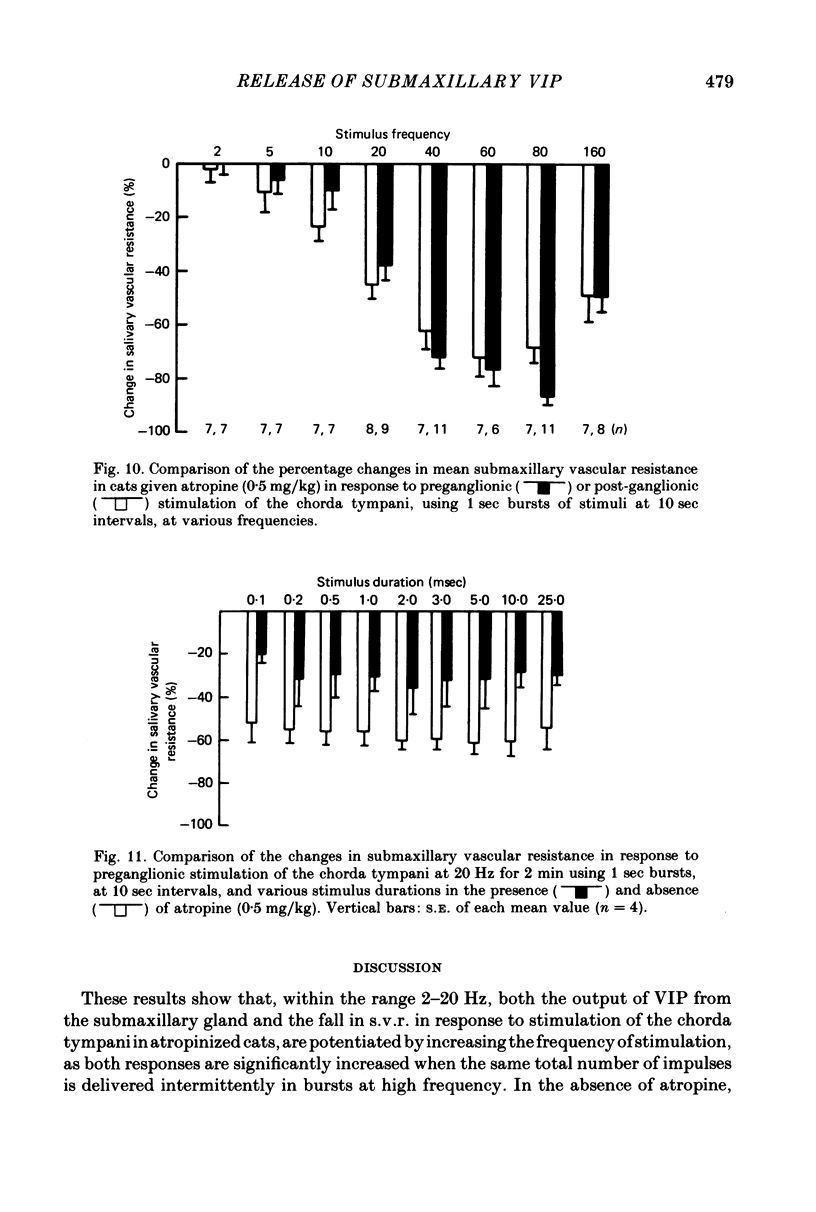
2. Both responses were also consistently increased by stimulating the post-ganglionic innervation in bursts in atropinized cats, or by stimulating the preganglionic innervation in bursts in the absence of atropine.
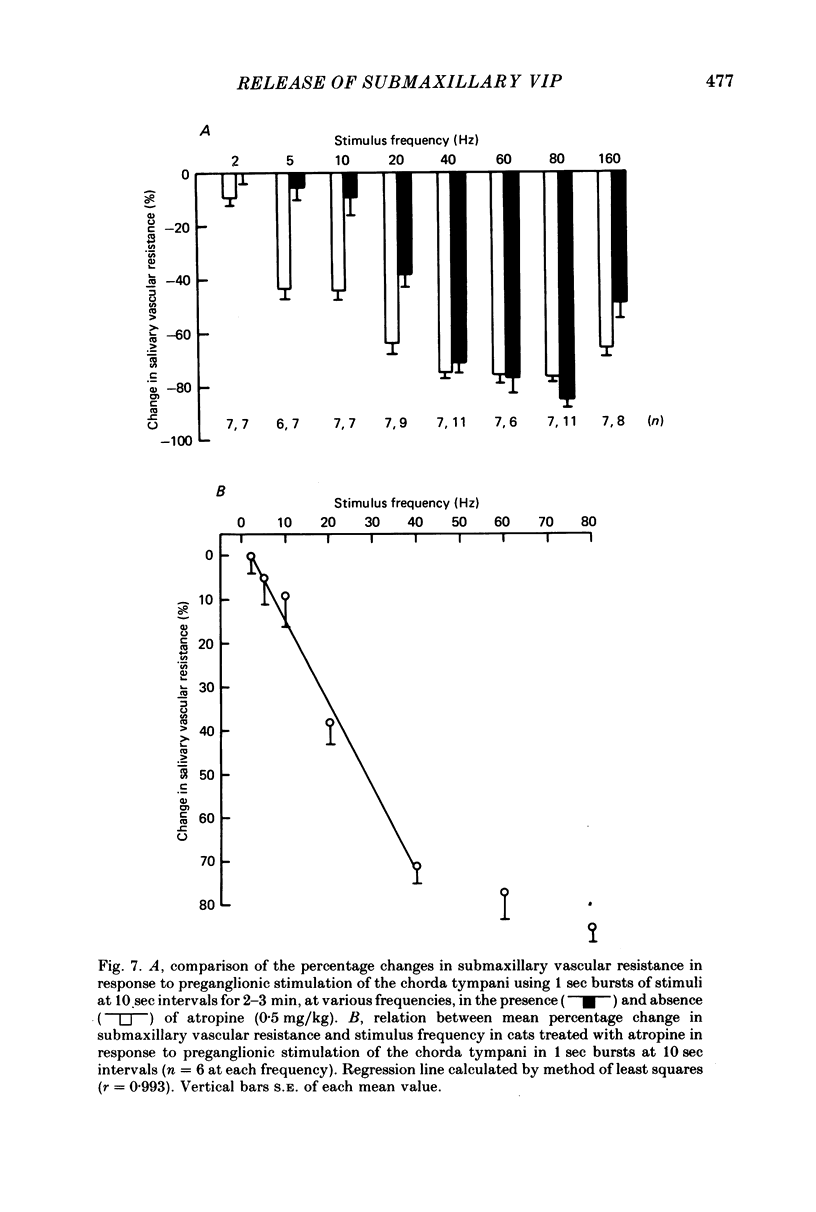
3. The effects of stimulation of the chorda tympani in 1 sec bursts at 10 sec intervals for 2-3 min were investigated over the frequency range 2-160 Hz. The change in mean s.v.r. under these conditions was significantly reduced by administration of atropine at frequencies between 2 and 20 Hz, but not at higher frequencies. In atropinized cats, the fall in mean s.v.r. was linearly related to stimulus frequency (r = 0·993) over the range 2-60 Hz and a maximal response was obtained during stimulation at 80 Hz. In the same experiments the fall in mean s.v.r. was linearly related to log mean VIP output over the frequency range 20-160 Hz (r = 0·998).
4. Closely similar changes in mean s.v.r occurred in response to both pre- and post-ganglionic stimulation at all frequencies tested between 2 and 160 Hz in atropinized cats when the stimuli were delivered in bursts. The changes in mean s.v.r. were not significantly affected by varying the duration of individual stimuli over the range 0·1-25·0 msec.
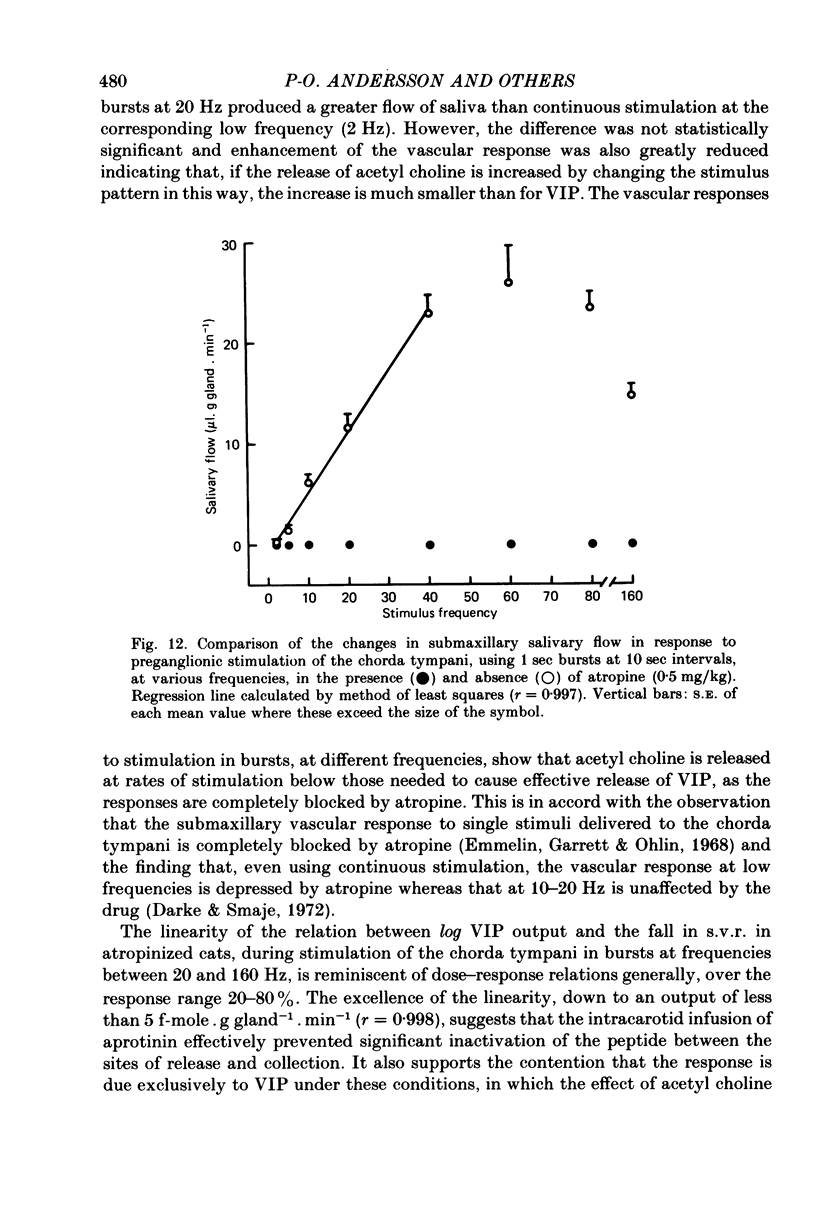
5. The flow of submaxillary saliva was also linearly related to stimulus frequency over the range 2-40 Hz (r = 0·997) when the stimuli were delivered in bursts in the absence of atropine and secretion of saliva was maximal at 60 Hz.
6. It is concluded that release of VIP from the submaxillary gland of the cat is optimal in response to stimulation of the chorda tympani at relatively high frequencies, when the impulses are delivered in bursts. Flow of saliva which is mediated by acetyl choline, may also be potentiated under the same conditions.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bloom S. R., Edwards A. V. The relationship between release of vasoactive intestinal peptide in the salivary gland of the cat in response to parasympathetic stimulation and the atropine resistant vasodilatation [proceedings]. J Physiol. 1979 Oct;295:35P–36P. [PubMed] [Google Scholar]
- Bloom S. R., Edwards A. V. Vasoactive intestinal peptide in relation to atropine resistant vasodilatation in the submaxillary gland of the cat. J Physiol. 1980 Mar;300:41–53. doi: 10.1113/jphysiol.1980.sp013150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darke A. C., Smaje L. H. Dependence of functional vasodilatation in the cat submaxillary gland upon stimulation frequency. J Physiol. 1972 Oct;226(1):191–203. doi: 10.1113/jphysiol.1972.sp009980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delius W., Hagbarth K. E., Hongell A., Wallin B. G. Manoeuvres affecting sympathetic outflow in human skin nerves. Acta Physiol Scand. 1972 Feb;84(2):177–186. doi: 10.1111/j.1748-1716.1972.tb05168.x. [DOI] [PubMed] [Google Scholar]
- Emmelin N., Garrett J. R., Ohlin P. Neural control of salivary myoepithelial cells. J Physiol. 1968 May;196(2):381–396. doi: 10.1113/jphysiol.1968.sp008513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmelin N., Holmberg J. Impulse frequency in secretory nerves of salivary glands. J Physiol. 1967 Jul;191(1):205–214. doi: 10.1113/jphysiol.1967.sp008245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagbarth K. E., Hallin R. G., Hongell A., Torebjörk H. E., Wallin B. G. General characteristics of sympathetic activity in human skin nerves. Acta Physiol Scand. 1972 Feb;84(2):164–176. doi: 10.1111/j.1748-1716.1972.tb05167.x. [DOI] [PubMed] [Google Scholar]
- Hallin R. G., Torebjörk H. E. Single unit sympathetic activity in human skin nerves during rest and various manoeuvres. Acta Physiol Scand. 1974 Nov;92(3):303–317. doi: 10.1111/j.1748-1716.1974.tb05749.x. [DOI] [PubMed] [Google Scholar]
- Lincoln D. W., Wakerley J. B. Electrophysiological evidence for the activation of supraoptic neurones during the release of oxytocin. J Physiol. 1974 Oct;242(2):533–554. doi: 10.1113/jphysiol.1974.sp010722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg J. M., Anggård A., Fahrenkrug J., Hökfelt T., Mutt V. Vasoactive intestinal polypeptide in cholinergic neurons of exocrine glands: functional significance of coexisting transmitters for vasodilation and secretion. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1651–1655. doi: 10.1073/pnas.77.3.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miolan J. P., Roman C. Décharge des fibres vagales efférentes destinées au cardia du chien. J Physiol (Paris) 1973;66(2):171–198. [PubMed] [Google Scholar]
- Miolan J. P., Roman C. Décharge unitaire des fibres vagales efférentes lors de la relaxation réceptive de l'estomac du chien. J Physiol (Paris) 1974;68(6):692–704. [PubMed] [Google Scholar]
- Mitchell S. J., Bloom S. R. Measurement of fasting and postprandial plasma VIP in man. Gut. 1978 Nov;19(11):1043–1048. doi: 10.1136/gut.19.11.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaud L. P. Tuberoinfundibular neurons in the basomedial hypothalamus of the rat: electrophysiological evidence for axon collaterals to hypothalamic and extrahypothalamic areas. Brain Res. 1976 Mar 19;105(1):59–72. doi: 10.1016/0006-8993(76)90922-7. [DOI] [PubMed] [Google Scholar]
- Uddman R., Fahrenkrug J., Malm L., Alumets J., Håkanson R., Sundler F. Neuronal VIP in salivary glands: distribution and release. Acta Physiol Scand. 1980 Sep;110(1):31–38. doi: 10.1111/j.1748-1716.1980.tb06626.x. [DOI] [PubMed] [Google Scholar]
- Wharton J., Polak J. M., Bryant M. G., Van Noorden S., Bloom S. R., Pearse A. G. Vasoactive intestinal polypeptide (VIP)-like immunoreactivity in salivary glands. Life Sci. 1979 Jul 16;25(3):273–280. doi: 10.1016/0024-3205(79)90295-9. [DOI] [PubMed] [Google Scholar]


