Abstract
1. The abdominal slow flexor muscle was isolated from the crayfish (Cambarus clarkii) and placed in 150 microliters. Harreveld solution. The concentrations of glutamate and aspartate in this solution were measured by mass fragmentography. 2. Application of black widow spider venom (BWSV) produced a marked increase in the frequency of miniature excitatory post-synaptic potentials (m.e.p.s.p.s). During the high frequency discharge of m.e.p.s.p.s, the glutamate content in the solution was significantly increased. There was an approximately linear relationship between the increase in the glutamate efflux produced by BWSV and the variance of the membrane potential fluctuation during high frequency discharge of m.e.p.s.p.s. 3. In most cases, the efflux of aspartate during control rest periods was smaller than that of glutamate. During the discharge of m.e.p.s.p.s produced by BWSV, the increase in the aspartate efflux was very small compared to glutamate. 4. Nerve stimulation caused a significant increase in the efflux of glutamate, but the change in the aspartate efflux was very small and not significant. 5. Application of methylene blue increased the frequency of m.e.p.s.p.s and glutamate efflux, but little, if any, increase was found in aspartate efflux. 6. It is concluded that glutamate is preferentially released from nerve terminals in a quantal fashion.
Full text
PDF

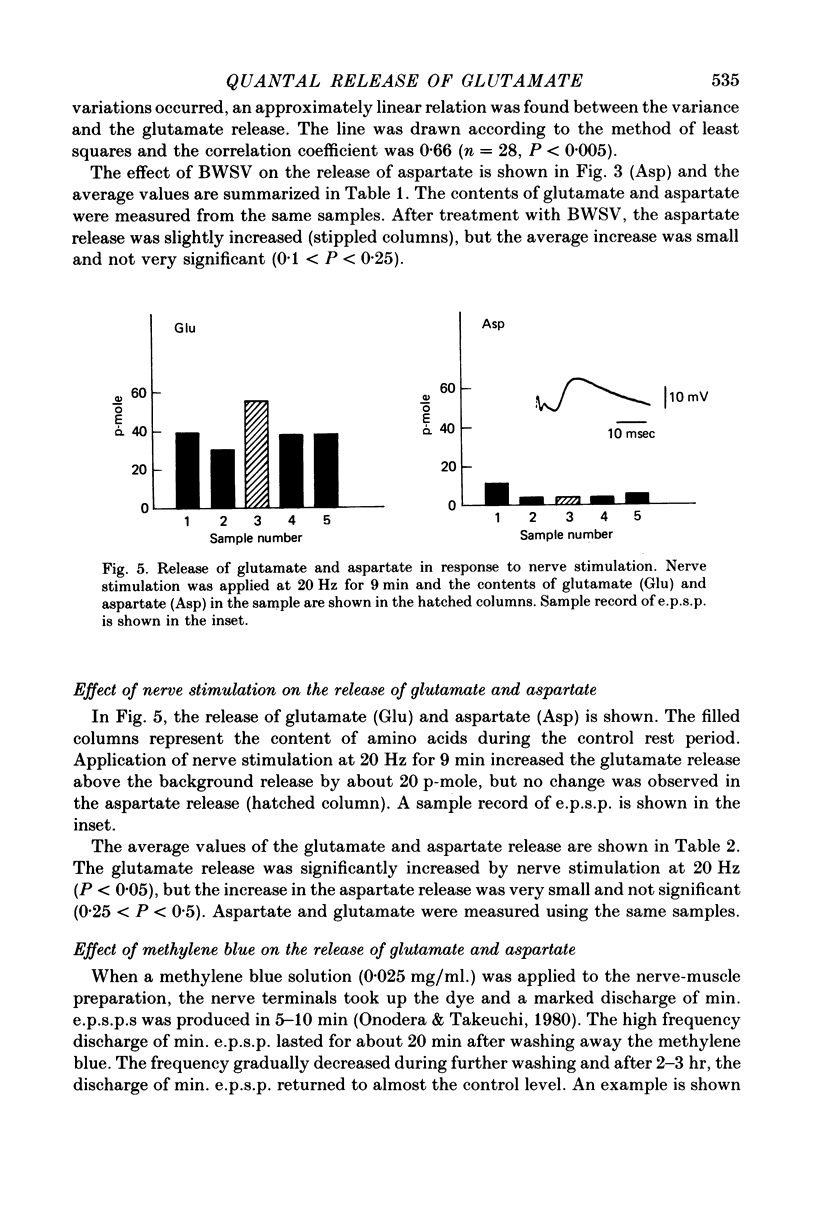
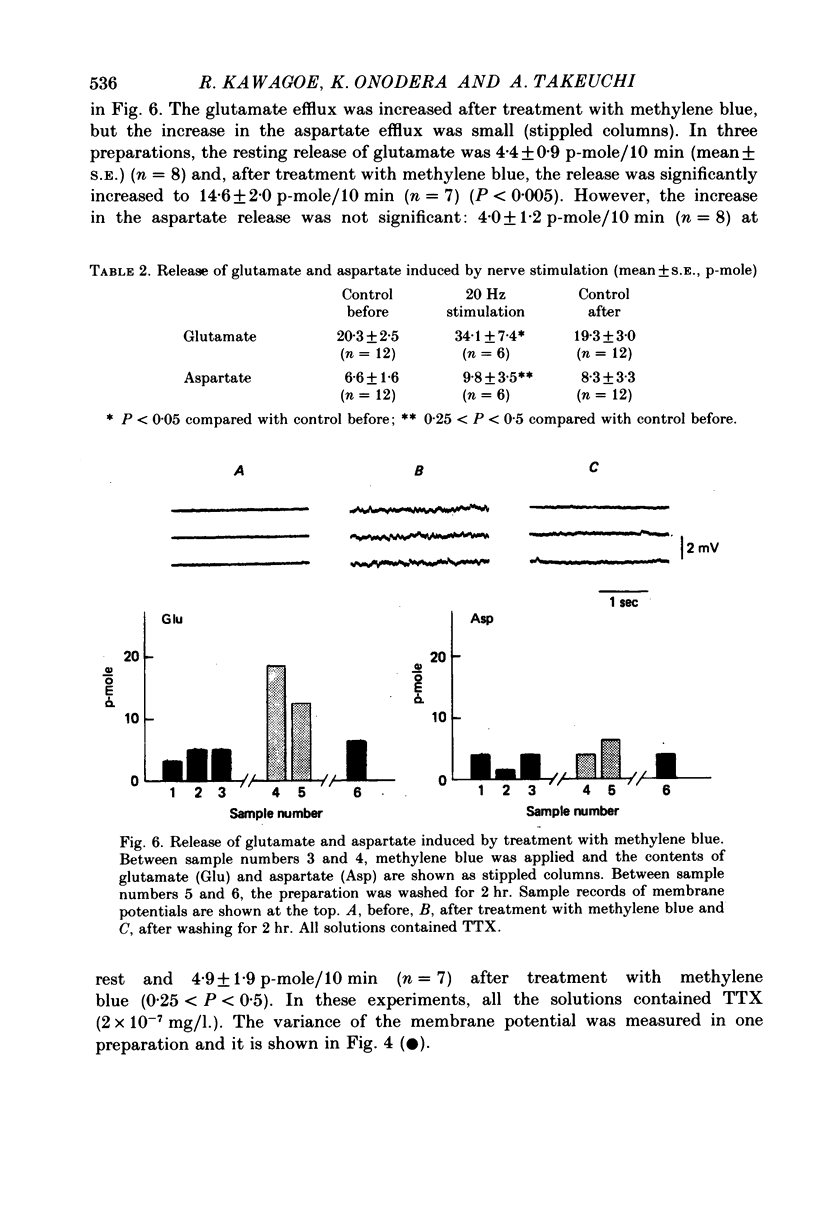



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Crawford A. C., McBurney R. N. The synergistic action of L-glutamate and L-aspartate at crustacean excitatory neuromuscular junctions. J Physiol. 1977 Jul;268(3):697–709. doi: 10.1113/jphysiol.1977.sp011877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S. G., Neal H., Usherwood P. N. Action of black widow spider venom on an aminergic synapse. Nature. 1973 Feb 2;241(5388):353–354. doi: 10.1038/241353a0. [DOI] [PubMed] [Google Scholar]
- DUDEL J., KUFFLER S. W. The quantal nature of transmission and spontaneous miniature potentials at the crayfish neuromuscular junction. J Physiol. 1961 Mar;155:514–529. doi: 10.1113/jphysiol.1961.sp006644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daoud A., Miller R. Release of glutamate and other amino acids from arthropod nerve-muscle preparations. J Neurochem. 1976 Jan;26(1):119–123. [PubMed] [Google Scholar]
- Dudel J. Aspartate and other inhibitors of excitatory synaptic transmission in crayfish muscle. Pflugers Arch. 1977 May 6;369(1):7–16. doi: 10.1007/BF00580803. [DOI] [PubMed] [Google Scholar]
- Freeman A. R. Polyfunctional role of glutamic acid in excitatory synaptic transmission. Prog Neurobiol. 1976;3(2):137–153. doi: 10.1016/0301-0082(76)90012-5. [DOI] [PubMed] [Google Scholar]
- Fritz L. C., Atwood H. L., Jahromi S. S. Lobster neuromuscular junctions treated with black widow spider venom: correlation between ultrastructure and physiology. J Neurocytol. 1980 Oct;9(5):699–721. doi: 10.1007/BF01205034. [DOI] [PubMed] [Google Scholar]
- Futamachi K. J. Acetylcholine: possible neuromuscular transmitter in Crustacea. Science. 1972 Mar 24;175(4028):1373–1375. doi: 10.1126/science.175.4028.1373. [DOI] [PubMed] [Google Scholar]
- Gerschenfeld H. M. Chemical transmission in invertebrate central nervous systems and neuromuscular junctions. Physiol Rev. 1973 Jan;53(1):1–119. doi: 10.1152/physrev.1973.53.1.1. [DOI] [PubMed] [Google Scholar]
- Gorio A., Hurlbut W. P., Ceccarelli B. Acetylcholine compartments in mouse diaphragm. Comparison of the effects of black widow spider venom, electrical stimulation, and high concentrations of potassium. J Cell Biol. 1978 Sep;78(3):716–733. doi: 10.1083/jcb.78.3.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths D. J., Smyth T., Jr Action of black widow spider venom at insect neuromuscular junctions. Toxicon. 1973 Jul;11(4):369–374. doi: 10.1016/0041-0101(73)90035-4. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The statistical nature of the acetycholine potential and its molecular components. J Physiol. 1972 Aug;224(3):665–699. doi: 10.1113/jphysiol.1972.sp009918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. Transmitter leakage from motor nerve endings. Proc R Soc Lond B Biol Sci. 1977 Feb 11;196(1122):59–72. doi: 10.1098/rspb.1977.0029. [DOI] [PubMed] [Google Scholar]
- Kawagoe R., Onodera K., Takeuchi A. Release of glutamate from the crayfish neuromuscular junction. J Physiol. 1981 Mar;312:225–236. doi: 10.1113/jphysiol.1981.sp013625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai N., Mauro A., Grundfest H. Effect of black widow spider venom on the lobster neuromuscular junctions. J Gen Physiol. 1972 Dec;60(6):650–664. doi: 10.1085/jgp.60.6.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe J., Marder E. Identification and effects of neural transmitters in invertebrates. Annu Rev Pharmacol Toxicol. 1976;16:245–268. doi: 10.1146/annurev.pa.16.040176.001333. [DOI] [PubMed] [Google Scholar]
- Kerkut G. A., Wheal H. V. Proceedings: The excitatory effects of aspartate and glutamate on the crustacean neuromuscular junction. Br J Pharmacol. 1974 May;51(1):136P–137P. [PMC free article] [PubMed] [Google Scholar]
- Kuffler S. W., Yoshikami D. The number of transmitter molecules in a quantum: an estimate from iontophoretic application of acetylcholine at the neuromuscular synapse. J Physiol. 1975 Oct;251(2):465–482. doi: 10.1113/jphysiol.1975.sp011103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longenecker H. E., Jr, Hurlbut W. P., Mauro A., Clark A. W. Effects of black widow spider venom on the frog neuromuscular junction. Effects on end-plate potential, miniature end-plate potential and nerve terminal spike. Nature. 1970 Feb 21;225(5234):701–703. doi: 10.1038/225701a0. [DOI] [PubMed] [Google Scholar]
- McBride W. J., Shank R. P., Freeman A. R., Aprison M. H. Levels of free amino acids in excitatory, inhibitory and sensory axons of the walking limbs of the lobster. Life Sci. 1974 Mar 16;14(6):1109–1120. doi: 10.1016/0024-3205(74)90235-5. [DOI] [PubMed] [Google Scholar]
- Murayama K., Shindo N., Mineki R., Ohta K. Determination of glutamic acid and gamma-aminobutyric acid in Ringer's solution without desalination at the femtomole level by gas chromatography chemical ionization mass spectrometry. Biomed Mass Spectrom. 1981 Apr;8(4):165–169. doi: 10.1002/bms.1200080407. [DOI] [PubMed] [Google Scholar]
- Narahashi T., Moore J. W., Shapiro B. I. Condylactis toxin: interaction with nerve membrane ionic conductances. Science. 1969 Feb 14;163(3868):680–681. doi: 10.1126/science.163.3868.680. [DOI] [PubMed] [Google Scholar]
- Onodera K., Takeuchi A. Distribution and pharmacological properties of synaptic and extrasynaptic glutamate receptors on crayfish muscle. J Physiol. 1980 Sep;306:233–250. doi: 10.1113/jphysiol.1980.sp013394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shank R. P., Freeman A. R. Cooperative interaction of glutamate and aspartate with receptors in the neuromuscular excitatory membrane in walking limbs of the lobster. J Neurobiol. 1975 May;6(3):289–303. doi: 10.1002/neu.480060305. [DOI] [PubMed] [Google Scholar]
- Shank R. P., Freeman A. R., McBride W. J., Aprison M. H. Glutamate and aspartate as mediators of neuromuscular excitation in the lobster. Comp Biochem Physiol C. 1975 Jan 1;50(1):127–131. [PubMed] [Google Scholar]
- Sorenson M. M. The free amino acids in peripheral nerves and in isolated inhibitory and excitatory nerve fibres of Cancer magister. J Neurochem. 1973 Apr;20(4):1231–1245. doi: 10.1111/j.1471-4159.1973.tb00092.x. [DOI] [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. THE EFFECT ON CRAYFISH MUSCLE OF IONTOPHORETICALLY APPLIED GLUTAMATE. J Physiol. 1964 Mar;170:296–317. doi: 10.1113/jphysiol.1964.sp007332. [DOI] [PMC free article] [PubMed] [Google Scholar]


