Abstract
Three key steps of cytochrome c biogenesis in many Gram-negative bacteria, the uptake of heme by the heme chaperone CcmE, the covalent attachment of heme to CcmE, and its subsequent release from CcmE to an apocytochrome c, have been achieved in vitro. apo-CcmE from Escherichia coli preferentially bound to ferric, with high affinity (Kd, 200 nM), rather than ferrous heme. The preference for ferric heme was confirmed by competition with 8-anilino-1-naphthalenesulfonate, which bound to a hydrophobic pocket in apo-CcmE. Reduction under certain conditions of the ferric heme–CcmE complex, which has characteristics of a b-type cytochrome, resulted in covalent attachment of heme to the protein. The resulting in vitro-produced holo-CcmE was identical to the in vivo-produced holo-CcmE, proving that unmodified Fe-protoporphyrin IX is incorporated into CcmE. Only noncovalent binding of mesoheme to CcmE was observed, thus implicating at least one vinyl group in covalent binding of heme to CcmE. Heme transferred in vitro from holo-CcmE to apocytochrome c, provided the heme was reduced. The necessity for reduced holo-CcmE might explain the role of the heme chaperone, i.e., prevention of reaction of ferric heme with apocytochrome and thus avoidance of incorrect side products. In addition, an AXXAH mutant of the CXXCH binding motif in the apocytochrome c was unable to accept heme from holo-CcmE. These in vitro results mimic, and thus have implications for, the molecular pathway of heme transfer during c-type cytochrome maturation in many species of bacteria in vivo.
Heme-containing proteins are ubiquitous in nature and have a variety of functions varying from oxygen transport and storage to catalysis and electron transfer. These proteins can be classified according to the attachment and type of heme present. c-type cytochromes contain Fe-protoporphyrin IX that is covalently attached via two thioether bonds between the heme vinyl groups and two cysteine residues found in a CXXCH binding motif in the protein. This class of proteins, found in almost all organisms, is involved mainly in electron transport (1–5). In many eukaryotes, the mitochondrial enzyme heme lyase catalyses the attachment of the heme group to the apocytochrome. Heme lyases have a high specificity and do not act on bacterial proteins (6).
In many Gram-negative bacteria, heme attachment to cytochromes c occurs via a system that involves the products of at least 12 genes (7, 8), namely the cytochrome c maturation (Ccm) proteins A–H (9, 10) as well as DsbA and DsbB (11, 12), which are involved in disulfide bond formation in the periplasm, and DipZ (DsbD) and TrxA (13, 14), which provide reductant to the periplasm. In Escherichia coli, c-type cytochromes are synthesized under anaerobic conditions and are found in the periplasm or in the cytoplasmic membrane with the heme groups on the periplasmic side (15). Heme synthesis occurs in the cytoplasm, whereas heme attachment to apocytochromes occurs in the oxidizing environment of the periplasm. The apocytochromes are transported to the periplasm via the general type II secretion (sec) pathway (16). Some studies have suggested that the proteins CcmA, B, and C form the heme transporter (17); there are several other lines of evidence that suggest otherwise (18–20) and that these proteins may transport some other component required by the Ccm system. The protein CcmC, however, seems essential for the delivery of heme to the membrane-anchored protein CcmE and is sufficient for this function under some conditions (21). CcmC interacts directly with CcmE via a tryptophan-rich motif forming the first part of the periplasmic heme delivery system (21). CcmE has been identified unexpectedly as a heme chaperone in this pathway and has been shown, remarkably, to bind heme covalently via a conserved histidine residue (22). CcmD is a small membrane protein that seems to stabilize CcmE in the membrane (23). CcmF and CcmH have been proposed to function as a heme lyase (24), catalyzing the covalent attachment of heme to apocytochrome c, and CcmG is a thioredoxin-like protein that is postulated to reduce disulfide bonds in apocytochromes (25). However, remarkably little is known overall about this widespread assembly system for a common class of cell proteins, the c-type cytochromes.
CcmE has been shown to transfer its covalently bound heme to apocytochromes in vivo (22). However, the nature of the bond formed between the heme and the histidine residue of CcmE (His-130 in E. coli) as well as the mechanism of heme attachment and subsequent release are unknown. Attempts to identify the nature of the bond have failed (22). CcmE has been identified in several species of bacteria as well as in Arabidopsis mitochondria (26). The protein could have several possible roles such as heme storage and control of regio- and stereochemical attachment of heme to apocytochromes as well as catalysis of covalent heme binding to these proteins. To investigate these processes and their mechanisms, we have studied the interaction of CcmE with heme in vitro, the conditions under which covalent attachment occurs, as well as the transfer of heme from CcmE to an apocytochrome c in vitro. CcmE has been found to have even more remarkable properties than were evident upon its first description (22).
Materials and Methods
Strains and Plasmids.
E. coli strain DH5α was used as the cloning host, and the strain JM109(DE3) was used for protein expression. The expression vector was constructed by amplifying the ccmE gene by PCR from the plasmid pEC86 (27), which was kindly provided by L. Thöny-Meyer (ETH Zürich, Switzerland), by using Pfx DNA polymerase (Invitrogen). The primers (5′-AAAACCATGGATTCGAATATCGATCTCTTTTATGC-3′, forward, and 5′-AAAACTCGAGTGATGCTGGGTCCTTATAAACACTC-3′, reverse) were designed to introduce NcoI and XhoI sites at either end of the gene to facilitate cloning into the expression vector pET22-b (Novagen). The membrane anchor region of CcmE was removed in the cloning procedure to produce the soluble periplasmic region of the protein starting from Ser-32 (hereafter referred to as CcmE′). The vector includes a cleavable pelB signal sequence for periplasmic targeting of the protein as well as a poly-His affinity tag at the C terminus.
Expression and Purification of CcmE′.
For expression of holo-CcmE′, the expression vector was cotransformed with the plasmid pEC86 (27), which expresses the Ccm proteins A–H. The coexpression of these proteins was found to be essential for production of holo-CcmE′. High levels of apo-CcmE′ were expressed in the periplasm in the absence of pEC86. E. coli cultures were grown aerobically in LB medium to midexponential phase and induced with 1 mM isopropyl β-D-thiogalactoside for 5 h at 37°C. Cells were harvested and resuspended in 50 mM Tris⋅HCl, pH 7.4/250 mM NaCl. The periplasmic fraction was prepared by adding polymyxin B sulfate to the cell suspension to a final concentration of 1 mg/ml followed by incubation at 37°C for 30 min and centrifugation at 10,000 × g for 20 min. The periplasmic extract was applied to a Ni2+-chelating Sepharose column equilibrated with 50 mM Tris⋅HCl, pH 7.4. The column was washed with 10 column volumes of the same buffer, and the protein was eluted from the column with 100 mM imidazole, pH 7.4. To separate holo- from apo-CcmE′ the mixture was applied to a SOURCE phehydrophobic-interaction FPLC column (Amersham Pharmacia) equilibrated in 50 mM Tris⋅HCl, pH 8.0/30% ammonium sulfate. Elution was performed by using a gradient from 30 to 0% ammonium sulfate.
Protein Characterization.
SDS/PAGE (15% acrylamide) was performed by using the discontinuous buffer system (28). Staining for covalently bound heme was carried out according to the method of Goodhew et al. (29). Visible absorption spectra were recorded on a Perkin–Elmer Lambda 2 spectrophotometer by using 5 μM protein in 50 mM sodium phosphate buffer, pH 7.0. Pyridine hemochrome spectra were obtained according to the method of Bartsch (30) by using 5 μM protein in 19% (vol/vol) pyridine/0.15 M NaOH. Electrospray ionization mass spectra were recorded on a Micromass (Manchester, U.K.) Bio-Q II-ZS triple quadrupole atmospheric pressure mass spectrometer equipped with an electrospray interface. Samples (10 μl) were introduced into the electrospray source via a loop injector as a solution (20 pmol⋅μl−1 in 1:1 water/acetonitrile/1% formic acid) at a flow rate of 10 μl⋅min−1. Quantitative analysis of the proportion of CcmE′ containing heme was determined by HPLC on a C18 column equilibrated with 20:80 acetonitrile/water (0.1% trifluoroacetic acid) and an elution gradient of 20–80% acetonitrile and detection at 280 and 408 nm. Equilibrium analytical ultracentrifugation experiments were performed (with a Beckman–Coulter Optima XL-A instrument) to establish the self-association state of the protein in the concentration range of 16–65 μM.
Addition of Heme to apo-CcmE′.
Hemin (Sigma) or mesoheme (Frontier Scientific, Carnforth, U.K.) was added from a stock solution (1 mM) in dimethyl sulfoxide (DMSO) to an apo-CcmE′ solution in 50 mM sodium phosphate buffer, pH 7.0. To achieve quantitative loading of CcmE′ with heme, 1 equivalent of protein was incubated with 1.1 equivalents of ferric heme at room temperature. Excess heme was removed by passing the heme–protein-containing solution through a desalting column. Heme was reduced with disodium dithionite (Sigma). Potassium cyanide was added to a final concentration of 2 mM from a concentrated stock solution in 200 mM phosphate buffer, pH 7.0. Potassium ferricyanide was added to oxidize reduced samples. The half-life of the b-type heme– or mesoheme–CcmE′ complex after reduction was measured by the decrease of the Soret bands at 425 and 407.5 nm for heme and mesoheme, respectively, over a time period of 5 min. Kinetic data were analyzed by using TABLECURVE (Jandel, San Rafael, CA). Fluorescence measurements were made by using a Perkin–Elmer LS 50B fluorimeter with excitation at 280 nm and emission from 285 to 350 nm (slit widths, 5 nm). The dissociation constant for heme was determined by measuring the quenching of the intrinsic protein fluorescence with increasing heme concentrations (100 μM and 1 mM stocks in DMSO) and standard double-reciprocal plot analysis. ANS [8-anilino-1-naphthalenesulfonate (Sigma)/1 mM stock in 50 mM sodium phosphate buffer, pH 7.0] binding was examined by protein fluorescence quenching as described for heme as well as by the enhancement of ANS fluorescence (31), which was measured by excitation at 380 nm and emission from 420 to 540 nm. The displacement of ANS by heme was measured by the decrease in ANS fluorescence at 480 nm after the addition of aliquots of heme (100 μM and 1 mM in DMSO) to a mixture of protein and ANS (25 and 750 μM, respectively). Fluorescence intensities were corrected for the values of free ANS and heme. For studies with ferrous heme, dithionite was added to both the protein–ANS solution and the heme stocks (in buffer/DMSO, 50:50).
Covalent Attachment of Heme to CcmE′.
Low molecular weight thiol-containing compounds were added to a 20 μM solution of the quantitatively formed b-type ferric heme–CcmE′ complex. DTT (Avocado, Heysham, U.K.), propane-1,3-dithiol (Avocado), cysteine (Sigma), or 2-mercaptoethanesulfonic acid (Sigma) were added from 100 mM stock solutions to give final concentrations of 5 mM in the protein solution. Noncovalently bound heme was removed by treating the heme–protein solution (in 50 mM sodium phosphate buffer, pH 7.0) with 1 M imidazole overnight at 4°C in the dark followed by extensive dialysis. Solutions were deoxygenated by thoroughly sparging with humidified argon. Reactions were carried out in the dark.
Apocytochrome Production.
Apocytochrome from Hydrogenobacter thermophilus was produced as described by Fisher et al. (32). After the removal of the silver cations by the addition of DTT, the protein was dialyzed extensively against 25 mM sodium acetate, pH 5.0. C11A/C14A apocytochrome was obtained as described by Tomlinson and Ferguson (33). Ellman's reagent was used according to Riddles et al. (34).
Heme Transfer from holo-CcmE′ to Apocytochrome.
H. thermophilus apocytochrome c552 (10 μM) or the apo-C11A/C14A variant (33) and an equimolar amount of E. coli holo-CcmE′ were incubated in 50 mM sodium phosphate buffer, pH 7.0 at 25°C for 18 h. Samples were reduced by the addition of disodium dithionite to a final concentration of 5 mM. Alternatively, samples were reduced with disodium dithionite and DTT, both to a final concentration of 2 mM. Solutions were sparged thoroughly with humidified argon, and reactions were carried out in the dark.
Results
In vivo-produced apo-CcmE′ was shown to be highly pure by SDS/PAGE (Fig. 1, lane 2), and complete cleavage of the periplasmic targeting sequence was confirmed by determining the mass of the purified protein by electrospray mass spectrometry [molecular mass: calculated 15,516 Da, observed 15,517 (±1) Da]. Similarly, in vivo-produced holo-CcmE′ was of high purity (Fig. 1, lane 1); however, HPLC analysis showed that no more than one fifth of the protein contained covalently bound heme. Hydrophobic-interaction FPLC was used to separate the apo from the holo form, resulting in 100% holo-CcmE′. The molecular mass of holo-CcmE′ was shown by mass spectrometry to be 16,132 (±1) Da [calculated mass for apoprotein (15,516 Da) plus heme (616 Da) = 16,132 Da], ruling out the possibility that a modified form of heme was incorporated into the heme chaperone. The visible absorption spectrum of the reduced holo-CcmE′ and its pyridine hemochrome (Table 1) were essentially identical to those reported previously (22). The data from the analytical ultracentrifugation experiment show that CcmE′ is present as a monomer in solution (data not shown).
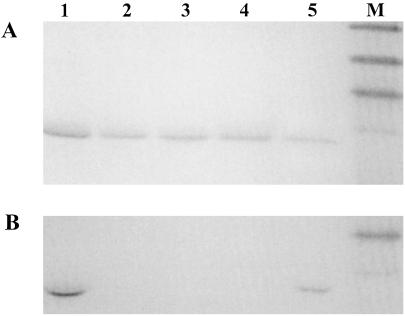
Figure 1.
SDS/PAGE analysis of CcmE′ with covalently or noncovalently bound heme, or mesoheme. (A) Coomassie blue-stained. (B) Activity stained for covalently bound heme. Lane 1, the in vivo-produced holo form of CcmE′ from E. coli produced in the presence of plasmid pEC86; lane 2, apo-CcmE′ from E. coli produced in the absence of plasmid pEC86; lane 3, the oxidized b-type cytochrome obtained after the addition of ferric heme to apo-CcmE′; lane 4, the b-type cytochrome analogue formed from apoprotein and Fe-mesoporphyrin, shown after incubation with DTT (5 mM) for 14 h; lane 5, in vitro-produced holo-CcmE′ obtained from the reaction of b-type cytochrome with DTT (5 mM) after 14 h; lane M, prestained molecular mass markers from top: 47.5, 32.5, 25, and 16.5 kDa. Protein (200–300 pmol) was applied to each lane of the gel for Coomassie staining, and 20–30 pmol were applied for heme staining.
Table 1.
Absorption maxima obtained for visible spectra of forms of CcmE′
| Form of CcmE | Oxidized, nm | Reduced, nm | Pyridine hemochrome (α band), nm | ||||
|---|---|---|---|---|---|---|---|
| b-type heme–CcmE′ complex | 533 | 413 | 560 | 530 | 425 | 556 | |
| b-type cyanide-heme–CcmE′ complex | 543 | 421 | 562 | 533 | 429 | — | |
| Mesoheme–CcmE′ complex | 522 | 403 | 549 | 521 | 412 | 546 | |
| In vivo holo-CcmE′ | 528 | 408 | 555 | 526 | 421 | 651 | 550.7 |
| In vivo holo-CcmE (given in ref. 22) | 554.5 | 524.5 | 426.5 | 550.5 | |||
| In vitro holo-CcmE′* | 529 | 408 | 554 | 525 | 419 | 650 | 551 |
Reduced spectra were recorded after the addition of disodium dithionite.
These data were collected for samples produced by three different methods as described. Oxidized absorption maxima were obtained by treatment of the ferric heme–CcmE complex with either cysteine or 2-mercaptoethanesulfonic acid. Reduced absorption maxima were acquired by the addition of ferrous heme to apo-CcmE or treatment of the ferric heme–CcmE with either DTT or propane-1,3-dithiol.
Addition of Heme to apo-CcmE′.
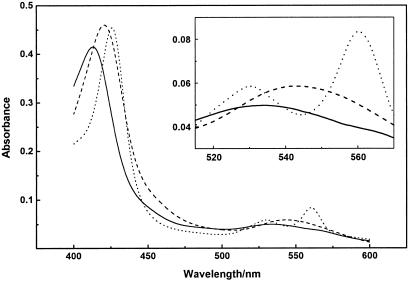
The addition of oxidized heme to apo-CcmE′ resulted in a change in the visible spectrum compared with that of ferric heme within the mixing time. The species formed was stable at room temperature for several hours. The remarkable stability of this noncovalent complex was apparent from the HPLC data. Under the HPLC conditions described, apo-CcmE′ eluted at 50% acetonitrile/water, whereas the heme–CcmE′ complex eluted at 55% acetonitrile/water, without separation of the heme from the protein. The characteristics of the spectrum suggest the presence of a high-spin Fe-center with a Soret band at 413 nm (Fig. 2 and Table 1). This observation was substantiated by the addition of cyanide, which led to a shift of the Soret band to 421 nm and an appearance of spectral characteristics of a low-spin six-coordinate Fe-center (Fig. 2 and Table 1), which is interpreted as binding of cyanide to the ferric iron (35). After reduction of the oxidized heme–CcmE′ complex in the absence of cyanide with disodium dithionite, the visible spectrum instantaneously showed characteristics of a b-type cytochrome with six-coordinate low-spin heme iron (Fig. 2 and Table 1). The pyridine hemochrome spectrum at this stage had an α-band at 556 nm, which is characteristic of heme with two free vinyl groups and shows that the heme was not modified immediately after its addition to apo-CcmE′.
Figure 2.
Absorption spectra of the b-type cytochrome formed after the addition of heme to E. coli apo-CcmE′, showing the oxidized spectrum (——), the reduced spectrum obtained immediately after the addition of disodium dithionite (- - -), and the spectrum obtained after treating the ferric b-type complex with 2 mM potassium cyanide (– – –). Absorption spectra were recorded by using ≈5 μM b-type cytochrome in 50 mM sodium phosphate buffer, pH 7.0.
When the quantitatively loaded heme–CcmE′ complex was reduced with disodium dithionite, the initial absorption peaks of the reduced b-type cytochrome subsequently decreased in intensity, and peaks that are characteristic of free ferrous heme appeared (data not shown). These data reflect the dissociation of the ferrous heme–CcmE′ complex, which was confirmed by separation of protein and heme by size-exclusion chromatography. The half-life of this reduced b-type cytochrome species was ≈35 sec. Reoxidation of the ferrous heme and CcmE′ mixture by the addition of potassium ferricyanide resulted in the quantitative reformation of the ferric b-type complex, showing the reversibility of the process. This observation implies that apo-CcmE, unusually, favors the coordination of ferric heme.
The affinity of apo-CcmE′ for heme was measured by protein fluorescence quenching, and the binding isotherms appeared to show two transitions, suggesting that there are two binding sites with different affinities for heme (data not shown). Dissociation constants of 200 nM and 10 μM were determined from double-reciprocal plots, corresponding to a high-affinity and a low-affinity binding site, respectively. Binding of the hydrophobic ligand ANS was examined, and it also appeared to bind to two sites on the protein as observed for heme, although with substantially lower affinities (Kd, 2 and 100 μM). To confirm that the ANS, which is known to bind to the heme pocket in apomyoglobin (31), bound to the same site(s) as heme, the bound ANS was shown to be displaced after heme addition. The observation that ANS has two binding sites shows that the presence of a low-affinity heme-binding site is unlikely to be caused by nonspecific heme stacking in a single binding site, although the low affinity suggests that it may not be physiologically relevant.
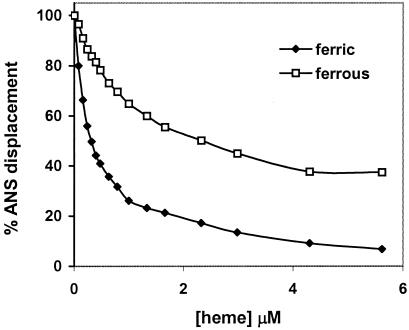
The ability of heme to displace ANS from its binding site(s) was used to confirm the preference of the protein for ferric heme. The fluorescence emission spectrum of ANS (excitation at 380 nm) in the presence of apo-CcmE′ showed a maximum at 480 nm, which is characteristic of the dye bound to a nonpolar binding site (31). The addition of ferric heme resulted in displacement of the bound ANS from the protein (as shown in Fig. 3) at a maximum molar equivalence of ≈0.5 heme per protein. The displacement curve is consistent with the presence of two binding sites, with the ANS on the high-affinity site being displaced at lower heme concentrations. It was found that ferrous heme also was able to displace bound ANS but to a significantly lower extent than ferric heme (Fig. 3), again showing the preference of CcmE′ for ferric heme.
Figure 3.
Displacement of ANS from its complex with apo-CcmE′ by ferric and ferrous heme. The percentage displacement was calculated from the decrease in ANS fluorescence after the addition of heme to a solution of protein and ANS (excitation wavelength, 380 nm; emission wavelength, 480 nm).
Mesoheme has ethyl substituents in the position of the vinyl groups in the protoporphyrin IX analogue. When ferric mesoheme was added to apo-CcmE′, uptake was observed by measuring the visible absorption spectrum, which gave rise to bands at 522 and 403 nm (Table 1). After the addition of disodium dithionite to the quantitatively formed mesoheme–protein complex, the absorption spectrum of the reduced mesoheme–CcmE′ complex was observed (Table 1). Subsequently, just as when heme itself was studied, the mesoheme dissociated from the protein, with a half-life of the mesoheme–protein complex of ≈30 sec, as judged from the same criteria used with heme (see above). The subsequent addition of potassium ferricyanide led to the reformation of the oxidized mesoheme–CcmE′ complex, showing the reversibility of this process, which depends on the oxidation state of the iron.
Covalent Attachment of Heme to CcmE′.
In the presence of a 5-fold excess of apo-CcmE′ protein relative to ferrous heme, a condition that increased the fraction of heme in this oxidation state bound to the protein, the α-band shifted from 560 to 554 nm in the visible spectrum of the b-type cytochrome over a time period of several hours. A shift in the β- and Soret bands was observed also, giving spectral characteristics of in vivo-formed reduced holo-CcmE′ (Table 1).
After the addition of dithiols (DTT or propane-1,3-dithiol) to the (1:1) quantitative complex of ferric heme and CcmE′, reduction of the b-type CcmE′ occurred without apparent dissociation of the heme–protein complex. Furthermore, the characteristic cytochrome bands shifted over time, just as observed after the addition of ferrous heme to an excess of CcmE′. The change of the spectral feature was complete after ≈14 h; again similarity to the spectrum of reduced in vivo-formed holo-CcmE′ was evident (Table 1).
The addition of monothiols (2-mercaptoethanesulfonic acid or cysteine) to the quantitative ferric heme–CcmE′ complex resulted, over time, in a shift in the visible spectrum toward the (oxidized) in vivo-formed holo-CcmE′ spectral characteristics (Table 1). Neither compound was able to yield a sufficiently reducing environment to give rise to reduced visible spectra.
The reaction mixtures containing any of the monothiol or dithiol compounds, as described above, were passed through a desalting column after 14 h of incubation, and any weakly associated heme was removed by dialysis against imidazole. Analysis of the protein confirmed, remarkably, that the spectral features, including the pyridine hemochrome spectrum, in either oxidation state, of the proteins prepared in vitro, were identical to those of the in vivo-produced CcmE′ (Table 1). Interestingly, a broad band was observed in the visible spectrum of the protein with a maximum of ≈650 nm (Table 1). The same band was present in the spectrum of the in vivo-produced holo-CcmE′. In agreement with the preference for ferric over ferrous heme of the protein, this spectral feature may indicate the ligation of a tyrosine to the heme iron (35). This ligation could be provided by one of the three highly conserved tyrosine residues in the protein (Tyr-38, -95-or -134). In addition, SDS/PAGE analysis followed by a staining method for covalently attached heme proved the heme to be covalently attached to the CcmE′ protein (Fig. 1, lane 5), whereas the b-type CcmE′ and the mesoheme–CcmE′ complex are shown not to bind heme covalently (Fig. 1, lanes 3 and 4, respectively). HPLC analysis showed that less than 50% of the initial b-type heme–CcmE′ complex could be converted into the covalent holo-CcmE′ form.
CcmE′ does not form a covalent bond with either reduced mesoheme or if the mesoheme–CcmE′ complex is treated with the low molecular weight thiol-containing compounds used. These data show that at least one vinyl group of Fe-protoporphyrin IX is involved in forming the covalent bond between heme and CcmE′.
Transfer of Heme from in Vivo-Produced holo-CcmE′ to Apocytochrome.
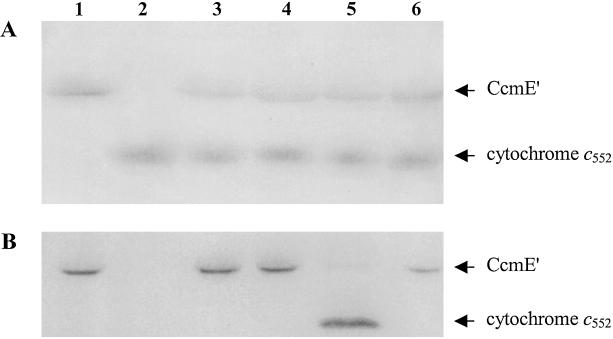
Apocytochrome c552 from H. thermophilus, which is known to be a substrate for the Ccm system (36), was shown to be pure by SDS/PAGE (Fig. 4, lane 2). The presence of free cysteines in the CXXCH motif of the apocytochrome was confirmed by analysis with Ellman's reagent (34), which showed two free thiol moieties per protein. After incubation of in vivo-produced holo-CcmE′ from E. coli with H. thermophilus apocytochrome c, holocytochrome c552 characteristics were observed after the addition of dithionite. Thus, under these reducing conditions the visible spectrum shifted toward that of the cytochrome c552 spectrum. SDS/PAGE analysis showed that heme transfer occurred in the presence of reductant, leading to the formation of holocytochrome c552 (Fig. 4, lane 5), but this was not the case in the absence of reductant (Fig. 4, lane 4). Under oxidizing conditions no change of the spectral characteristics was observed. Incubating holo-CcmE′ alone under reductive conditions showed that heme remained bound to the heme chaperone as observed by visible spectroscopy and SDS/PAGE (Fig. 4, lane 1). Incubation of the C11A/C14A apocytochrome with CcmE′ under the same reducing conditions did not lead to a transfer of heme from holo-CcmE′, as determined from the visible spectrum, and retention of heme by CcmE′, as shown by SDS/PAGE (Fig. 4, lane 6). The C11A/C14A apocytochrome is not able to form thioether bonds, because it lacks the cysteine residues that are involved in forming the covalent bonds to the heme prosthetic group. However, it is known that this mutant is capable of forming a b-type cytochrome (33). The heme-transfer reaction from holo-CcmE′ to apocytochrome c552 (Fig. 4, lane 5) took place over several hours. For the SDS/PAGE analysis shown, the time interval between the starting point (Fig. 4, lane 3) and the end point was 18 h.
Figure 4.
SDS/PAGE analysis of the transfer of heme from in vivo-produced holo-CcmE′ to H. thermophilus apocytochrome c552. (A) Coomassie blue-stained. (B) Activity stained for covalently bound heme. Lane 1, the in vivo-produced holo form of CcmE′ from E. coli after 18 h of incubation under reducing conditions; lane 2, wild-type apocytochrome c552, which had the covalently bound heme removed by treatment with silver sulfate; lane 3, in vitro incubation of holo-CcmE′ with apocytochrome c552 immediately after mixing; lane 4, incubation of holo-CcmE′ with apocytochrome c552 in the absence of reductant for 18 h; lane 5, reaction of holo-CcmE′ with apocytochrome c552 in the presence of reductant for 18 h; lane 6, incubation for 18 h of holo-CcmE′ with the C11A/C14A mutant of H. thermophilus apocytochrome c552 in the presence of reductant. Protein (300–400 pmol) was applied to each lane of the gel for Coomassie staining, and 30–40 pmol were applied for heme staining.
Discussion
We have shown that apo-CcmE′ takes up heme to form a complex resembling a b-type cytochrome. Heme- and ANS-binding data show that CcmE′ has a structured binding pocket in the apo form, a feature that has not been reported previously. ANS has been shown to bind to heme-binding pockets in other proteins (31). The observation that apo-CcmE′ is more efficient at taking up ferric rather than ferrous heme contrasts with the usual behavior of heme proteins and suggests that heme could be presented to CcmE by CcmC in the ferric state in vivo.
The covalent attachment of heme to CcmE′ was shown to occur between ferrous heme and CcmE′ in the presence of a 5-fold excess of protein. Interestingly, these data correlate with the observation that only 10–25% of the CcmE protein is isolated in the holo form, i.e., with covalently attached heme, in vivo (8). Alternatively, the addition of low molecular weight thiol-containing compounds to the ferric heme–CcmE′ complex was found to facilitate attachment of the heme to this heme chaperone. By proving that the final product of this reaction is identical to the in vivo-produced holo-CcmE′, it is clear that in the presence of an appropriate potential reductant, covalent bond formation between heme and CcmE can occur. This result suggests that either the periplasm or the Ccm proteins have to supply the appropriate reductant, plausibly in the form of a thiol-containing species, to the heme–CcmE complex such that attachment of the heme to the heme chaperone can occur. Furthermore, it is apparent that in vivo formation of holo-CcmE can occur by reaction with Fe-protoporphyrin IX, which has not been modified before attachment to the protein. The covalent attachment of mesoheme to CcmE′ did not take place when the noncovalent complex between CcmE′ and mesoheme was treated with the selected compounds. The implication from these data is that the covalent attachment of the heme-binding protein residue, which was shown to be a histidine by Thöny-Meyer and coworkers (22), requires a vinyl group of the Fe-protoporphyrin IX. In this context the absorbance maximum at 551 nm in the pyridine hemochrome spectrum of holo-CcmE′ can be interpreted as arising from the presence of one intact vinyl group and one vinyl group that has reacted in a previously undescribed way. The alternative of both vinyl groups having reacted with one histidine residue does not seem very plausible. Thus the maximum at 551 nm is only coincidentally similar to that for the pyridine hemochrome spectrum of a c-type cytochrome in which both vinyl groups have been saturated.
The implications from these in vitro experiments are that ferric heme can be taken up by CcmE in the periplasm in vivo followed by reduction of the heme to yield the covalently bound holo-heme–CcmE complex. A central aspect for future investigations will be the determination of the nature of the bond between the CcmE protein and the heme prosthetic group, which has proved intractable (22, 24).
The observation that heme can be transferred from holo-CcmE′ to an apocytochrome c in vitro is in agreement with previous in vivo studies (22). However, more detailed conclusions can be drawn from the in vitro experiments on the behavior of two principal proteins involved in cytochrome c maturation, CcmE and the apocytochrome c itself. First, the oxidation state during this process is crucial. As previous work has shown, ferrous heme is required for attachment to a thiol group, because side products can be formed between ferric heme and a cysteine residue in a heme-binding motif of an apocytochrome (37, 38). Mitochondrial cytochrome c assembly also has been shown to require reducing conditions (39–41). Our data prove that in vitro transfer of heme from holo-CcmE′ to apocytochrome also requires reduced heme. Therefore, one role of CcmE might be to prevent ferric heme from reacting with apocytochrome by taking up ferric heme itself and storing it in a covalently bound form. Second, because release of heme from CcmE to apocytochrome can occur only after reduction, it is possible that the Ccm protein(s) involved in the transfer has to supply reductant. Candidates for supplying reductant are CcmG/H and DipZ. Third, the in vitro heme transfer between the two proteins is slower than in vivo heme transfer. Therefore, one or more of the Ccm gene products must be catalyzing the transfer of the heme from holo-CcmE to apocytochrome. The most likely candidates for this role are CcmF and CcmH, as suggested previously (24). However, it is clear from the data in the present work that the heme transfer can occur unaided by any other gene product(s). Fourth, the fact that the AXXAH mutant of the CXXCH binding motif in the cytochrome was not capable of taking up heme from holo-CcmE′ highlights the requirement for the binding motif of c-type cytochromes. The mechanism of the heme transfer therefore is concluded to involve the regio- and stereospecific reaction of the cysteine thiols of the apocytochrome with the α-carbon of the Fe-porphyrin substituents in the position of the vinyl groups of Fe-protoporphyrin IX. Furthermore, the data with the AXXAH mutant are in agreement with the observation that cytochrome b562 in the periplasm incorporates heme from a source other than CcmE (41).
It is clear that the complete pathway of c-type cytochrome biogenesis in Gram-negative bacteria can be elucidated only by more detailed studies involving more of the Ccm apparatus and the combined function of the Ccm gene products. However, the data presented in this work provide considerable insight into the main steps of heme incorporation into the heme chaperone CcmE, the covalent attachment of the heme to CcmE and its subsequent release to the apocytochrome c. The purpose of the covalent bond between heme and the heme chaperone, CcmE, remains unclear, although it might be mechanistically coupled to the breaking of the bond between heme and CcmE and the formation of the thioether bonds between heme with apocytochrome.
Acknowledgments
C.W.H. and J.M.S. gratefully acknowledge funding from the Biotechnology and Biological Sciences Research Council (BBSRC). We thank Mark Bushell, Matthew Ellington, James Allen, Lin Hong, Russell Wallis, and Neil Oldham for help and advice. This work was supported by U.K. BBSRC Grants C11888 and C13443 (to S.J.F.). O.D. is gratefully receiving a University of Oxford scholarship in association with a W.R. Miller award of St. Edmund Hall (Oxford). S.J.F. is a W.R. Miller Fellow of St. Edmund Hall.
Abbreviations
- Ccm
cytochrome c maturation protein
- ANS
8-anilino-1-naphthalenesulfonate
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Pettigrew G W, Moore G R. Cytochromes c: Biological Aspects. New York: Springer; 1987. [Google Scholar]
- 2.Moore G R, Pettigrew G W. Cytochromes c: Evolutionary, Structural, and Physicochemical Aspects. New York: Springer; 1990. [Google Scholar]
- 3.Scott R A, Mauk A G. Cytochrome c: A Multidisciplinary Approach. Mill Valley, CA: Univ. Sci. Books; 1995. [Google Scholar]
- 4.Barker P D, Ferguson S J. Structure Fold Des. 1999;7:R281–R290. doi: 10.1016/s0969-2126(00)88334-3. [DOI] [PubMed] [Google Scholar]
- 5.Page M D, Sambongi Y, Ferguson S J. Trends Biochem Sci. 1998;23:103–108. doi: 10.1016/s0968-0004(98)01173-6. [DOI] [PubMed] [Google Scholar]
- 6.Sanders C, Lill H. Biochim Biophys Acta. 2000;1459:131–138. doi: 10.1016/s0005-2728(00)00122-5. [DOI] [PubMed] [Google Scholar]
- 7.Thöny-Meyer L. Microbiol Mol Biol Rev. 1997;61:337–376. doi: 10.1128/mmbr.61.3.337-376.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thöny-Meyer L, Fabianek R A, Schulz H, Enggist E, Hennecke H. Biospektrum. 1999;5:185–191. [Google Scholar]
- 9.Thöny-Meyer L, Fischer F, Kunzler P, Ritz D, Hennecke H. J Bacteriol. 1995;177:4321–4326. doi: 10.1128/jb.177.15.4321-4326.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thöny-Meyer L. Biochim Biophys Acta. 2000;1459:316–324. doi: 10.1016/s0005-2728(00)00167-5. [DOI] [PubMed] [Google Scholar]
- 11.Metheringham R, Griffiths L, Crooke H, Forsythe S, Cole J. Arch Microbiol. 1995;164:301–307. doi: 10.1007/BF02529965. [DOI] [PubMed] [Google Scholar]
- 12.Metheringham R, Tyson K L, Crooke H, Missiakas D, Raina S, Cole J A. Mol Gen Genet. 1996;253:95–102. doi: 10.1007/pl00013815. [DOI] [PubMed] [Google Scholar]
- 13.Crooke H, Cole J. Mol Microbiol. 1995;15:1139–1150. doi: 10.1111/j.1365-2958.1995.tb02287.x. [DOI] [PubMed] [Google Scholar]
- 14.Reid E, Eaves D J, Cole J A. FEMS Microbiol Lett. 1998;166:369–375. doi: 10.1111/j.1574-6968.1998.tb13914.x. [DOI] [PubMed] [Google Scholar]
- 15.Iobbi-Nivol C, Crooke H, Griffiths L, Grove J, Hussain H, Pommier J, Mejean V, Cole J A. FEMS Microbiol Lett. 1994;119:89–94. doi: 10.1111/j.1574-6968.1994.tb06872.x. [DOI] [PubMed] [Google Scholar]
- 16.Thöny-Meyer L, Kunzler P. Eur J Biochem. 1997;246:794–799. doi: 10.1111/j.1432-1033.1997.t01-1-00794.x. [DOI] [PubMed] [Google Scholar]
- 17.Goldman B S, Beckman D L, Bali A, Monika E M, Gabbert K K, Kranz R G. J Mol Biol. 1997;268:724–738. doi: 10.1006/jmbi.1997.0992. [DOI] [PubMed] [Google Scholar]
- 18.Cook G M, Poole R K. Microbiology. 2000;146:527–536. doi: 10.1099/00221287-146-2-527. [DOI] [PubMed] [Google Scholar]
- 19.Page M D, Pearce D A, Norris H A, Ferguson S J. Microbiology. 1997;143:563–576. doi: 10.1099/00221287-143-2-563. [DOI] [PubMed] [Google Scholar]
- 20.Schulz H, Fabianek R A, Pellicioli E C, Hennecke H, Thöny-Meyer L. Proc Natl Acad Sci USA. 1999;96:6462–6467. doi: 10.1073/pnas.96.11.6462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ren Q, Thöny-Meyer L. J Biol Chem. 2001;276:32591–32596. doi: 10.1074/jbc.M103058200. [DOI] [PubMed] [Google Scholar]
- 22.Schulz H, Hennecke H, Thöny-Meyer L. Science. 1998;281:1197–1200. doi: 10.1126/science.281.5380.1197. [DOI] [PubMed] [Google Scholar]
- 23.Schulz H, Pellicioli E C, Thöny-Meyer L. Mol Microbiol. 2000;37:1379–1388. doi: 10.1046/j.1365-2958.2000.02083.x. [DOI] [PubMed] [Google Scholar]
- 24.Ren Q, Ahuja U, Thöny-Meyer L. J Biol Chem. 2002;277:7657–7663. doi: 10.1074/jbc.M110979200. [DOI] [PubMed] [Google Scholar]
- 25.Reid E, Cole J, Eaves D J. Biochem J. 2001;355:51–58. doi: 10.1042/0264-6021:3550051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spielewoy N, Schulz H, Grienenberger J M, Thöny-Meyer L, Bonnard G. J Biol Chem. 2001;276:5491–5497. doi: 10.1074/jbc.M008853200. [DOI] [PubMed] [Google Scholar]
- 27.Arslan E, Schulz H, Zufferey R, Kunzler P, Thöny-Meyer L. Biochem Biophys Res Commun. 1998;251:744–747. doi: 10.1006/bbrc.1998.9549. [DOI] [PubMed] [Google Scholar]
- 28.Laemmli U K. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 29.Goodhew C F, Brown K R, Pettigrew G W. Biochim Biophys Acta. 1986;852:288–294. [Google Scholar]
- 30.Bartsch R G. Methods Enzymol. 1971;23:344–363. [Google Scholar]
- 31.Stryer L. J Mol Biol. 1965;13:482–495. doi: 10.1016/s0022-2836(65)80111-5. [DOI] [PubMed] [Google Scholar]
- 32.Fisher W R, Taniuchi H, Anfinsen C B. J Biol Chem. 1973;248:3188–3195. [PubMed] [Google Scholar]
- 33.Tomlinson E J, Ferguson S J. Proc Natl Acad Sci USA. 2000;97:5156–5160. doi: 10.1073/pnas.090089397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Riddles P W, Blakeley R L, Zerner B. Methods Enzymol. 1983;91:49–60. doi: 10.1016/s0076-6879(83)91010-8. [DOI] [PubMed] [Google Scholar]
- 35.Abraham B D, Sono M, Boutaud O, Shriner A, Dawson J H, Brash A R, Gaffney B J. Biochemistry. 2001;40:2251–2259. doi: 10.1021/bi002121h. [DOI] [PubMed] [Google Scholar]
- 36.Karan E F, Russell B S, Bren K L. J Biol Inorg Chem. 2002;7:260–272. doi: 10.1007/s007750100292. [DOI] [PubMed] [Google Scholar]
- 37.Barker P D, Ferrer J C, Mylrajan M, Loehr T M, Feng R, Konishi Y, Funk W D, MacGillivray R T, Mauk A G. Proc Natl Acad Sci USA. 1993;90:6542–6546. doi: 10.1073/pnas.90.14.6542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Daltrop O, Allen J W A, Willis A C, Ferguson S J. Proc Natl Acad Sci USA. 2002;99:7872–7876. doi: 10.1073/pnas.132259099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nicholson D W, Neupert W. Proc Natl Acad Sci USA. 1989;86:4340–4344. doi: 10.1073/pnas.86.12.4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tong J, Margoliash E. J Biol Chem. 1998;273:25695–25702. doi: 10.1074/jbc.273.40.25695. [DOI] [PubMed] [Google Scholar]
- 41.Throne-Holst M, Thöny-Meyer L, Hederstedt L. FEBS Lett. 1997;410:351–355. doi: 10.1016/s0014-5793(97)00656-x. [DOI] [PubMed] [Google Scholar]