Abstract
Endometrial adenocarcinoma is the most common gynecologic malignancy in the United States. However, reliable diagnostic or prognostic tumor markers have not been identified for endometrial cancer. In this study, we examined whether urokinase plasminogen activator receptor (UPAR), a glycosyl-phosphatidylinositol-linked membrane protein, is a candidate diagnostic or prognostic marker for patients with cancer of the endometrium. Sixty-five surgically excised, formalin-fixed endometrial tissue specimens were accessioned through the Department of Pathology Registry at the University of California, Los Angeles, and analyzed for UPAR expression by using immunohistochemical techniques. A retrospective review was also performed to determine stage and histopathologic grade of disease, recurrence, and mortality. No expression of UPAR protein was present in seven patients with benign neoplasia of the endometrium. UPAR protein expression highly correlated with stage of disease (ungrouped Spearman correlation = 0.625, P < 0.0001): 40% of patients with stage I, 66% of patients with stage II, 100% of patients with stage III, and 85% with stage IV demonstrated the highest level of UPAR expression. Moreover, high UPAR expression positively correlated with grade of disease (ungrouped Spearman correlation = 0.71, P < 0.0001): 29% of grade 1 specimens, 57% of grade 2, and over 90% of specimens with grade 3, the majority representing uterine papillary serous carcinoma and mixed malignant mesodermal tumor. Finally, UPAR protein expression also positively correlated with rate of recurrence and mortality in patients with adenocarcinoma of the endometrium (ungrouped P = 0.034). Our data suggest that UPAR is a useful prognostic marker for biologically aggressive forms of endometrial cancer.
Endometrial cancer is the most common malignancy of the female genital tract in the United States (1). More than 36,100 new cases of endometrial cancer were diagnosed in the year 2000 with more than 6,500 deaths reported in the same year (1). Clinical parameters such as the stage of disease, nuclear grade, histologic subtype, and tumor size seem to correlate with outcome of disease. Unfortunately, no reliable prognostic tumor markers have been identified to date.
A prognostic marker needs to be an independent factor that not only guides treatment but also has an impact on patient survival. These criteria are rarely satisfied because most candidate prognostic markers fail to distinguish between tumors that require adjuvant treatment and those that do not. For example, tumor suppressor gene(s) (e.g., p53), oncogene(s) (e.g., HER-2/neu, K-ras), and DNA repair gene(s) (e.g., hMLH1), microsatellite instability, and/or protein-tyrosine phosphatase (PTEN) mutation and/or ras mutation have played a role in endometrial cancer (2–9). However, none of these genes have proven to be clinically useful prognostic markers for endometrial cancer. Therefore, we sought to identify potential molecular difference(s) that correlated with recurrence and mortality rates in patients with endometrial cancer to identify a prognostic marker for aggressive forms of endometrial cancer.
Recently, Foca et al. (10) demonstrated that urokinase plasminogen activator receptor (UPAR) mRNA levels correlated with the invasive potential of endometrial carcinomas and showed a 33-fold increase in UPAR mRNA levels in advanced clinical stage endometrial tumors compared with normal endometrial tissue. Furthermore, the increase in UPAR mRNA levels also correlated linearly with the progression of disease stage. UPAR is a glycosyl-phosphatidylinositol-linked membrane protein lacking transmembrane and cytosolic domains (11, 12) that participates in the localization of plasminogen activation to the cell surface. This cell surface activity facilitates cellular movement by proteolytic extracellular matrix degradation for tumor cell invasion, chemotaxis, and cellular adhesion (13–17).
In the current study, we examined whether UPAR protein expression correlated with (i) the grade and stage of endometrial cancer and (ii) recurrence and mortality rate in patients with endometrial cancer. Our goal, ultimately, was to determine whether UPAR protein could be used as a candidate prognostic marker for patients with cancer of the endometrium.
Materials and Methods
Patients and Tissue Specimens.
Paraffin-embedded endometrial tissue samples from 65 patients were obtained from the Department of Pathology, University of California, Los Angeles. The seven samples of nonneoplastic endometrial tissue were obtained from hysterectomy specimens performed for prolapsed uterus and leiomyomata. A total of 58 endometrial carcinomas were evaluated: 40 were endometrioid-subtype adenocarcinomas of various grades, 12 were uterine papillary serous carcinoma (UPSC), and 6 were mixed malignant mesodermal tumor (MMMT). Among patients with endometrioid adenocarcinoma, 21 had grade 1, 11 had grade 2, and 8 had grade 3 disease (Table 1).
Table 1.
Distribution of FIGO stage, histologic subtype, and grade in 58 patients with endometrial cancer (endometrioid, UPSC, and MMMT)
| FIGO stage | Grade 1 | Grade 2 | Grade 3 | Total |
|---|---|---|---|---|
| I | 17 (53%) | 10 (31%) | 5 (16%) | 32 |
| II | 3 (50%) | 1 (17%) | 2 (33%) | 6 |
| III | 1 (14%) | 1 (14%) | 5 (72%) | 7 |
| IV | 0 (0%) | 2 (15%) | 11 (85%) | 13 |
| Total | 21 | 14 | 23 | 58 |
| Endometrioid total | 21 | 11 | 8 | 40 |
Summary of the clinical data for the 58 patients with a diagnosis of endometrial cancer (endometrioid, n = 40; UPSC, n = 12; MMMT, n = 6). The correlation of grade with stage was statistically significant (ungrouped Spearman correlation = 0.588, P < 0.001). The data extracted included histologic type of endometrial cancer and FIGO stage. Specimens from patients with a diagnosis of endometrioid adenocarcinoma were graded: well differentiated (grade 1), moderately differentiated (grade 2), or poorly differentiated (grade 3).
Immunohistochemical Procedure.
Tissue localization of UPAR was evaluated by using a polyclonal goat anti-human UPAR antibody (R&D Systems, no. AF807). Paraffin tissue sections were cut at 2-μm thickness, mounted on Snow coat X-tra slides (Surgipath, Richmond, IL), and baked overnight at 60°C. Slides were deparaffinized in xylene, rehydrated, and incubated in 3% hydrogen peroxide/absolute methanol for 10 min to block endogenous activity. The sections were pretreated in 1% Tween 20 (Sigma) and prepared in PBS at 37°C for 30 min. Slides were then sequentially incubated in primary goat anti-UPAR antibody diluted 1:50 for 16 h at 4°C, rabbit anti-goat IgG (Dako) diluted 1:50 for 15 min, followed by Envision+ (goat anti-rabbit, Peroxidase, Dako) for 30 min. The slides were further incubated in diaminobenzidine and hydrogen peroxide solution for detecting UPAR signal. Slides were counterstained with hematoxylin. In immunodepletion experiments, the goat anti-human UPAR antibody was mixed with purified recombinant human UPAR protein (R&D Systems, no. 807-UK) at a ratio of 1 μg to 50 μg in 0.4 ml of buffer and incubated for 16 h at 4°C.
UPAR Measurement by Immunohistochemistry.
The immunohistochemical localization of UPAR protein was scored in a semiquantitative fashion, which incorporated the analysis of both the intensity and the percentage of distribution of specific staining. The evaluations were recorded as percentages of positive staining which were denoted as 0 (no staining), 1+ (weak staining but detectable above control), 2+ (distinct), and 3+ (intense staining). We categorized staining intensities of 1+ or 2+ into the low group and 3+ into the high group to help focus on the most abundant UPAR protein staining. Evaluation of tissue sampling was performed by two qualified observers, independently and blinded.
Clinical Data.
Human Subject Protection Committee approval was obtained from UCLA Medical Center. A retrospective review of clinical data pertaining to 58 patients with a diagnosis of endometrial cancer, who underwent primary surgical staging at UCLA Medical Center, was performed (Table 1). All patients were staged according to the FIGO classification for endometrial cancer (18). Specimens with a diagnosis of endometrioid adenocarcinoma were graded: well differentiated (grade 1), moderately differentiated (grade 2), or poorly differentiated (grade 3). The data extracted included histologic subtype of endometrial cancer, FIGO stage and histopathologic grade of disease, recurrence rate of disease, and time interval to recurrence, disease-free and overall survival. The median age of our study population was 66 years (range, 36–93). The median follow-up time was 12.8 months (range, 1–78 months).
Statistical Analysis.
Associations between ordinal scores (e.g., UPAR protein expression vs. stage of disease, UPAR protein expression vs. grade of disease) were assessed by using Spearman correlations. Survival or recurrence over time was estimated by using Kaplan–Meier methods; P values for survival or recurrence comparisons were computed by using the log-rank test. All statistics were computed by using SAS software version 8 (SAS Institute, Cary, NC). Statistical analyses of data were calculated both for ungrouped and grouped statistical data. The ungrouped values were obtained from statistical analysis of stage 0, Ia, Ib, Ic, 2a… 4b or grade 1, 2, 3, as a function of UPAR protein expression 0, 1+, 2+, 3+. The grouped values were derived from statistical analysis of stages I and II or grades 1, 2, 3 as a function of low (1+, 2+) or high (3+) UPAR protein expression.
Results
UPAR Protein Expression Is Abundant and Specific in Adenocarcinoma of the Endometrium.
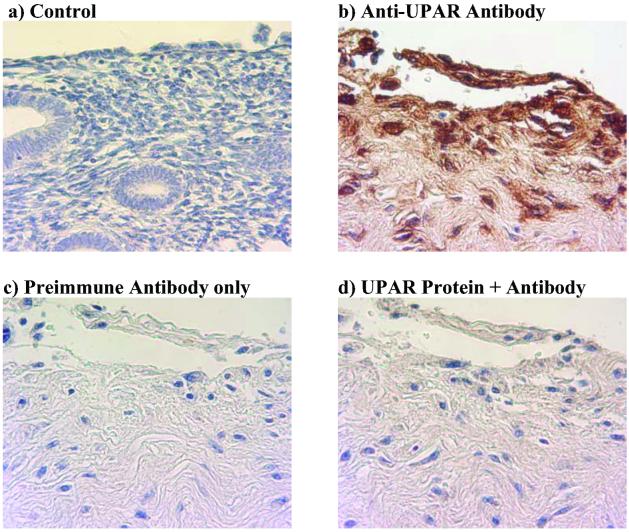
UPAR protein is not detected in normal endometrium (Fig. 1a), whereas UPAR protein is highly expressed in specimens representing poorly differentiated (grade 3) endometrioid adenocarcinoma (Fig. 1b). The specificity of the UPAR antibody was confirmed by staining the sections with preimmune serum (Fig. 1c) and also by immunodepletion of the anti-UPAR antiserum with recombinant UPAR protein (Fig. 1d). UPAR protein staining was not observed in any of the seven benign specimens (data not shown).
Fig 1.
UPAR protein expression is abundant and specific in adenocarcinoma of the endometrium. Immunohistochemistry was performed on paraffin-embedded tissue sections by using a polyclonal goat anti-human UPAR antibody and counter stained with hematoxylin. Normal endometrium tissue does not express UPAR protein (a), whereas UPAR protein is abundantly expressed in specimens representing poorly differentiated (grade 3) endometrioid adenocarcinoma (b). UPAR protein expression is absent in the preimmune antibody-only control (c) and blocked when recombinant UPAR protein is preincubated with UPAR antibody before staining (d). (Original magnification: ×400.)
UPAR Protein Expression Correlated with the Histologic Grade of Endometrial Cancer.
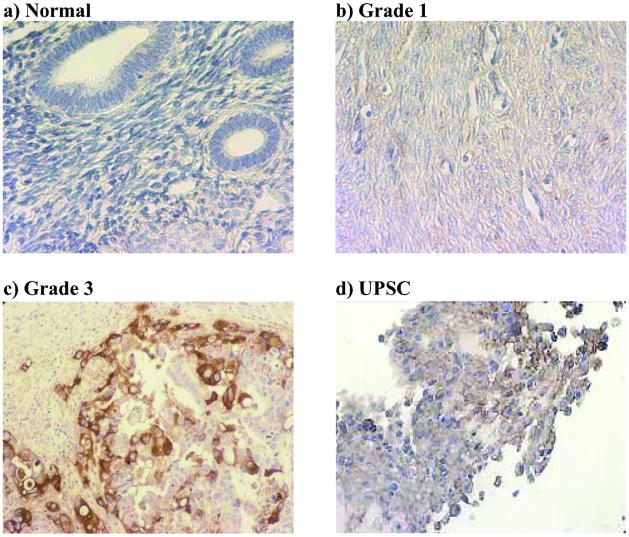
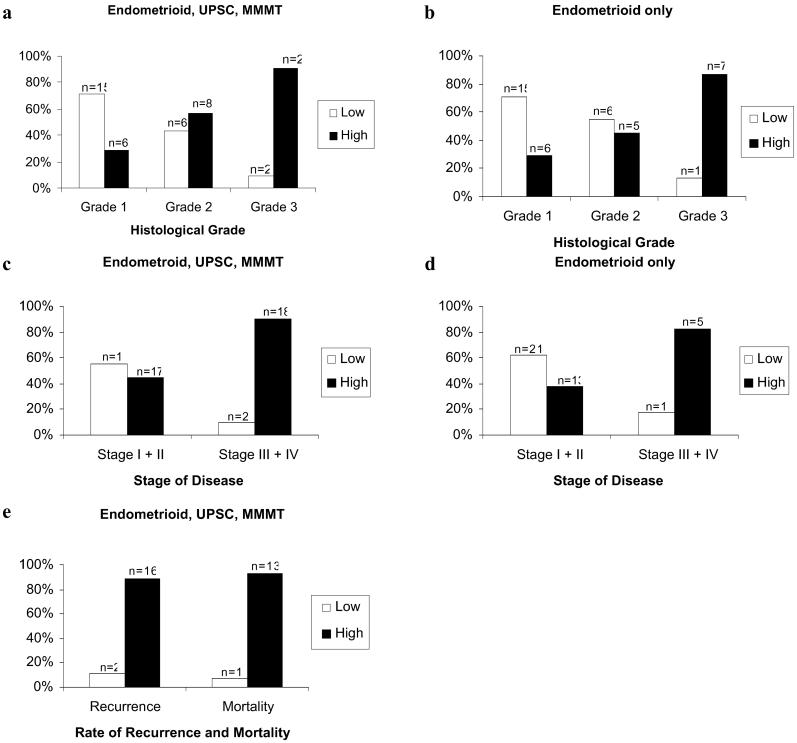
UPAR protein is not expressed in normal endometrium (Fig. 2a) and minimally expressed in well differentiated (grade 1) endometrioid adenocarcinoma (Fig. 2b). In contrast, UPAR protein is highly expressed in specimens from patients with poorly differentiated (grade 3) endometrioid adenocarcinoma (Fig. 2c), and an extremely aggressive subtype of endometrial cancer, UPSC (Fig. 2d). UPAR protein expression correlated with grade of disease in endometrioid subtypes of endometrial carcinoma (ungrouped Spearman correlation = 0.71, P < 0.0001; grouped Spearman correlation = 0.440, P < 0.0015; Table 2). Highest levels of UPAR protein expression were noted in 29% of grade 1 specimens, 57% of grade 2, and 91% of specimens with grade 3 (Fig. 3a). In patients with endometrioid adenocarcinoma only (excluding patients with UPSC and MMMT cancers), highest levels of UPAR protein expression were noted in 29% of grade 1 specimens, 45% of grade 2, and 87% of specimens with grade 3 (Fig. 3b).
Fig 2.
The degree of UPAR protein expression correlates with histologic grade in specimens from patients with adenocarcinoma of the endometrium. Immunohistochemistry was performed on paraffin-embedded tissue sections by using a polyclonal goat anti-human UPAR antibody and counter stained with hematoxylin. UPAR protein is not expressed in normal endometrium (a) and minimally expressed in well differentiated (grade 1) endometrioid adenocarcinoma (b). However, UPAR protein is abundantly expressed in specimens from patients with poorly differentiated (grade 3) endometrioid adenocarcinoma (c) and UPSC, a more aggressive subtype of endometrial cancer (d). (Original magnification: ×400.)
Table 2.
Relation between UPAR protein expression and clinicopathologic data
| Adenocarcinoma of the endometrium
|
Endometrioid | Endometrioid, UPSC, MMMT | ||||||
|---|---|---|---|---|---|---|---|---|
| UPAR protein expression | ||||||||
| Low | High | Low | High | |||||
| n = 22 | % | n = 18 | % | n = 23 | % | n = 35 | % | |
| Grade 1 | 15 | 71% | 6 | 29% | 15 | 71% | 6 | 29% |
| Grade 2 | 6 | 55% | 5 | 45% | 6 | 43% | 8 | 57% |
| Grade 3 | 1 | 13% | 7 | 87% | 2 | 9% | 21 | 91% |
| Stage I + II | 21 | 62% | 13 | 38% | 21 | 55% | 17 | 45% |
| Stage III + IV | 1 | 17% | 5 | 83% | 2 | 10% | 18 | 90% |
UPAR protein expression correlates with the grade and stage of disease in patients with adenocarcinoma of the endometrium and is statistically significant (grade vs. UPAR protein expression: ungrouped Spearman correlation = 0.710, P = 0.0001; grouped Spearman correlation = 0.440, P = <0.0015; stage vs. UPAR protein expression: ungrouped Spearman correlation = 0.625, P < 0.0001, grouped Spearman correlation = 0.559, P = <0.001). There was no UPAR protein staining observed in any of the seven benign specimens.
Fig 3.
UPAR protein expression correlates with grade and stage of disease and increases with rate of recurrence and mortality in specimens from patients with adenocarcinoma of the endometrium. An increasing proportion of specimens exhibited a high level of UPAR protein expression in progressing from grade 1 (well differentiated) to grade 3 (poorly differentiated) disease for endometrial adenocarcinoma (a, grouped Spearman correlation = 0.559, P = <0.001) and for patients with endometrioid adenocarcinoma exclusively (b, grouped Spearman correlation = 0.419, P = <0.01). An increased proportion of specimens demonstrates a high UPAR protein expression with advanced stage of disease in patients with endometrial adenocarcinoma (c, grouped Spearman correlation = 0.440, P = <0.0015) and in patients with endometrioid adenocarcinoma exclusively (d, grouped Spearman correlation = 0.324, P = <0.04). UPAR protein expression increases with rate of recurrence and mortality in patients with adenocarcinoma of the endometrium (e).
UPAR Protein Expression Correlated with the Stage of Endometrial Cancer.
UPAR protein expression also correlated with stage of disease (ungrouped Spearman correlation = 0.625, P < 0.0001; grouped Spearman correlation = 0.559, P < 0.001,): 45% of patients with stages I and II and 90% of patients with stages III and IV demonstrated the highest level of UPAR expression (Fig. 3c and Table 2). Of patients with endometrioid adenocarcinoma only (excluding patients with UPSC and MMMT cancers), 38% of patients with stages I and II and 83% of patients with stages III and IV demonstrated the highest level of UPAR expression (Fig. 3d and Table 2). The median follow-up was 12.8 months.
UPAR Protein Expression Correlated with Endometrial Cancer Recurrence Rate and Mortality Rate.
At follow-up, 11% recurrence and 7% mortality was found in patients whose tumors expressed low levels of UPAR expression, and 89% recurrence and 93% mortality was noted when tumors of patients expressed high levels of UPAR protein (Fig. 3e). The P value for mortality or recurrence rates as a function of UPAR protein expression is P = 0.034 by the log-rank test. Thus, increasing UPAR protein expression is associated with an increased recurrence and mortality rate.
Discussion
Prognostic tumor markers provide information that is independent of conventional predictors of outcome such as grade and stage of the tumor. Endometrial cancer is the most common gynecologic malignancy in the United States and is associated with more than 6,500 deaths per annum (1). The candidate tumor markers currently in use for endometrial cancer, including p53, HER-2/neu, K-ras, and hMLH1, do not correlate with stage of disease, nuclear grade, and histologic subtype (2–5). Furthermore, no prognostic markers correlate with recurrence rate and mortality rate in endometrial cancer.
UPAR is a glycosyl-phosphatidylinositol-anchored glycoprotein that, by regulating membrane-associated plasmin activity, facilitates cellular movement for tumor-cell invasion, chemotaxis, and cellular adhesion (13–17). UPAR protein exists in two forms, as the glycosyl-phosphatidylinositol-anchored glycoprotein (50–60 kDa) present on the surface of most cells, and a soluble form of UPAR (sUPAR), produced after cleavage of UPAR by urokinase (35 kDa), present in very low levels in the serum of healthy individuals. UPAR activation ultimately leads to the degradation of the extracellular matrix, which seems to be necessary for functions as diverse as the local invasion and metastasis of tumor cells and for nerve growth factor-induced PC12 cell neurite outgrowth (19–21). Pacheco et al. (22), using Northern blot analysis, measured the coexpression of the matrix metalloprotease MMP-9, UPA, and UPAR mRNAs in biopsy samples from patients with breast cancer and found that increased UPAR mRNA levels were associated with poor prognosis. In addition, it has been reported that sUPAR is a useful prognostic marker for patients with ovarian, breast, prostate, and colon cancer (23–27), although sUPAR has no known physiological function.
Sier et al. (27) assayed the ascites and the serum of patients with ovarian carcinoma for the sUPAR and compared the results with serum concentrations of an established diagnostic marker, CA-125. Most of the patients with ovarian cancer had enhanced preoperative serum levels of sUPAR compared with healthy controls, and high preoperative levels of sUPAR were associated with decreased patient survival, whereas CA-125 had no prognostic implication (27). Kruger et al. (28) showed that the overexpression of sUPAR impairs proteolysis, tumor growth, and metastatic potential of breast carcinoma cells in vivo. McCabe et al. (24) observed markedly elevated sUPAR levels in serum samples from patients with prostate cancer compared with healthy individuals and showed that elevated sUPAR levels correlated with mortality rates. Moreover, sUPAR levels in preoperative plasma from patients with colorectal cancer also independently predicted survival (10). In contrast, sUPAR has not yet been shown to correlate with the outcome of endometrial cancer.
Foca et al. (10) demonstrated that UPAR mRNA levels correlated with the invasive potential of endometrial carcinomas. A 33-fold increase in UPAR mRNA levels was observed, from normal endometria to advanced clinical stage carcinomas and showed a near linear increase with each progression in clinical stage (10). Tecimer et al. (29) assessed UPA, UPAR, and PAI-1 levels in extracts of endometrial cancer tissue by using ELISA and correlated their expression levels with tumor histology, stage and grade of disease, and recurrence rate. Although PAI-1 was a predictor of survival, in these studies UPAR did not show any significant correlation (29). More recently, Nordengren et al. (30) quantified UPAR protein by ELISA in homogenates of 274 samples of endometrial cancer tissue and found no correlation with patient survival; however, only patients with FIGO surgical stage I–II were used in their study. We believe that immunohistochemistry was the ideal technique for our study. We wanted to assess the pattern of UPAR protein expression in samples from patients with endometrial cancer. Immunohistochemistry was selected for the following reasons: (i) to permit the use of accessible banked paraffin-embedded tissue, (ii) to identify which endometrial cell type (e.g., normal, premalignant, well differentiated, or poorly differentiated adenocarcinoma) was expressing the UPAR protein, and (iii) to avoid the risk of misinterpreting tissue-sample ELISA data inherent to the analysis of heterogeneous frozen tumor tissue. Although we advocate the use of ELISA for measuring UPAR levels in patient serum, frozen tissue samples labeled as “poorly differentiated” may have foci of normal, premalignant, or well differentiated tumor cells present in the sample, leading to “dilution” of UPAR protein in the ELISA assay. A false decrease in levels of UPAR protein may result. Alternatively, tissue samples labeled as “well differentiated” may have been “contaminated” with foci of poorly differentiated tumor cells resulting in a false increase in levels of UPAR protein on ELISA analysis. In either scenario, the ELISA data may not permit or may conceal a real and statistically significant underlying difference between study conditions.
Our data show that UPAR protein expression highly correlates with the histologic grade and stage of disease of patients with endometrial cancer. Moreover, our data demonstrated that UPAR protein expression correlates with recurrence and mortality rate in patients with endometrial cancer, which suggests that UPAR protein measurement can yield promising prognostic information for patients with endometrial cancers.
Acknowledgments
This work was supported by the Joan English Fund for Women's Cancer Research (I.D. no. 95213) and National Institutes of Health/National Institute of Child Health and Human Development Obstetrics and Gynecology Training Grant HD01281:03.
Abbreviations
UPAR, urokinase plasminogen activator receptor
UPSC, uterine papillary serous carcinoma
MMMT, mixed malignant mesodermal tumor
FIGO, International Federation of Gynecology and Obstetrics
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Greenlee R. T., Murray, T., Bolden, S. & Wingo, P. A. (2000) CA Cancer J. Clin. 50, 7-33. [DOI] [PubMed] [Google Scholar]
- 2.Enomoto T., Fujita, M., Inoue, M., Rice, J. M., Nakajima, R., Tanazawa, O. & Nomura, T. (1993) Cancer Res. 53, 1883-1888. [PubMed] [Google Scholar]
- 3.Rasty G., Murray, R, Lu, L., Kubilis, P., Benrubi, G. & Masood, S. (1998) Ann. Clin. Lab. Sci. 28, 138-143. [PubMed] [Google Scholar]
- 4.Sasaki H., Nishii, H., Takahashi, H., Tada, A., Furusato, M., Terashima, Y., Siegal, G. P., Parker, S. L., Kohler, M. F. & Berchuck, A. (1993) Cancer Res. 53, 1906-1910. [PubMed] [Google Scholar]
- 5.Esteller M., Catasus, L., Matias-Guiu, X., Mutter, G. L., Prat, J., Baylin, S. B. & Herman, J. G. (1999) Am. J. Pathol. 155, 1767-1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sakamoto T., Murase, T., Urushibata, H., Kato, K, Takada, H., Imamura, T., Mori, H. & Wake, N. (1998) Gynecol. Oncol. 71, 53-58. [DOI] [PubMed] [Google Scholar]
- 7.Esteller M., Levine, R., Baylin, S. B., Ellenson, L. H. & Herman, J. G. (1998) Oncogene 16, 2413-2417. [DOI] [PubMed] [Google Scholar]
- 8.Levine R. L., Cargile, C. B., Blazes, M. S., van Rees, B., Kurman, R. J. & Ellenson, L. H. (1998) Cancer Res. 58, 3524-3528. [PubMed] [Google Scholar]
- 9.Mutter G. L., Wada, H., Faquin, W. C. & Enomoto, T. (1999) J. Clin. Pathol. Mol. Pathol. 52, 257-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foca C., Moses, E. K., Quinn, M. A. & Rice, G. E. (2000) Gynecol. Oncol. 79, 244-250. [DOI] [PubMed] [Google Scholar]
- 11.Ploug M., Ronne, E., Behrendt, N., Jensen, A. L., Blasti, F. & Dano, K. (1991) J. Biol. Chem. 266, 1926-1933. [PubMed] [Google Scholar]
- 12.Wang Y., Dang, J., Johnson, L. K., Selhamer, J. J. & Doe, W. F. (1995) Eur. J. Biochem. 227, 116-122. [DOI] [PubMed] [Google Scholar]
- 13.Ellis V., Behrendt, N. & Dano, K. (1991) J. Biol. Chem. 266, 12752-12758. [PubMed] [Google Scholar]
- 14.Moller L. B., Pollanen, J., Ronne, E., Pedersen, N. & Blasi, F. (1993) J. Biol. Chem. 268, 11152-11159. [PubMed] [Google Scholar]
- 15.Gyetko M. R., Todd, R. F., III, Wilkinson, C. C. & Sitrin, R. G. (1994) J. Clin. Invest. 93, 1380-1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xing R. H. & Rabbani, S. A. (1996) Int. J. Cancer 67, 423-429. [DOI] [PubMed] [Google Scholar]
- 17.Cantero D., Friess, H., Deflorin, J., Zimmermann, A., Brundler, M. A., Riesle, E., Korc, M. & Buchler, M. W. (1997) Br. J. Cancer 75, 388-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.International Federation of Gynecology and Obstetrics (1989) Int. J. Gynecol. Obstet. 28, 189-190. [Google Scholar]
- 19.Festuccia C., Dolo, V., Guerra, F., Violini, S., Muzi, P., Pavan, A. & Bologna, M. (1998) Clin. Exp. Metastasis 16, 513-528. [DOI] [PubMed] [Google Scholar]
- 20.Farias-Eisner R., Vician, L., Silver, A., Reddy, S., Rabbani, S. A. & Herschman, H. R. (2000) J. Neurosci. 20, 230-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farias-Eisner R., Vician, L., Reddy, S., Basconcillo, R., Rabbani, S. A., Wu, Y. Y., Bradshaw, R. A. & Herschman, H. R. (2001) J. Neurosci. Res. 63, 341-346. [DOI] [PubMed] [Google Scholar]
- 22.Pacheco M. M., Nishimoto, I. N., Mourao Neto, M., Mantovani, E. B. & Brentani, M. M. (2001) Int. J. Biol. Markers 16, 62-68. [DOI] [PubMed] [Google Scholar]
- 23.Stephens R. W., Pedersen, A. N., Nielsen, H. J., Hamers, M. J., Hoyer-Hansen, G., Ronne, E., Dybkjaer, E., Dano, K. & Brunner, N. (1997) Clin. Chem. 43, 1868-1876. [PubMed] [Google Scholar]
- 24.McCabe N. P., Angwafo, F. F., 3rd, Zaher, A, Selman, S. H., Kouinche, A. & Jankun, J. (2000) Oncol. Rep. 7, 879-882. [DOI] [PubMed] [Google Scholar]
- 25.Riisbro R., Stephens, R. W., Brunner, N., Christensen, I. J., Nielsen, H. J., Heilmann, L. & von Tempelhoff, G. F. (2001) Gynecol. Oncol. 82, 523-531. [DOI] [PubMed] [Google Scholar]
- 26.Stephens R. W., Nielsen, H. J., Christensen, I. J., Thorlacius-Ussing, O., Sorensen, S., Dano, K. & Brunner, N. (1999) J. Natl. Cancer Inst. 91, 869-874. [DOI] [PubMed] [Google Scholar]
- 27.Sier C. F., Stephens, R., Bizik, J., Mariani, A., Bassan, M., Pedersen, N., Frigerio, L., Ferrari, A., Dano, K., Brunner, N. & Blasi, F. (1998) Cancer Res. 58, 1843-1849. [PubMed] [Google Scholar]
- 28.Kruger A., Soeltl, R., Lutz, V., Wilhelm, O. G., Magdolen, V., Rojo, E. E., Hanntzpoulos, P. A., Graeff, H., Gansbacher, B. & Schmitt, M. (2000) Cancer Gene Ther. 7, 292-299. [DOI] [PubMed] [Google Scholar]
- 29.Tecimer C., Doering, D. L., Goldsmith, L. J., Meyer, J. S., Abdulhay, G. & Wittliff, J. L. (2001) Gynecol. Oncol. 80, 48-55. [DOI] [PubMed] [Google Scholar]
- 30.Nordengren J., Fredstorp Lidebring, M., Bendahl, P. O., Brunner, N., Ferno, M., Hogberg, T., Stephens, R. W., Willen, R. & Casslen, B. (2002) Int. J. Cancer 97, 379-385. [DOI] [PubMed] [Google Scholar]