Abstract
Huntington's disease (HD) is an untreatable neurological disorder caused by selective and progressive degeneration of the caudate nucleus and putamen of the basal ganglia. Although the etiology of HD pathology is not fully understood, the observed loss of neuronal cells is thought to occur primarily through apoptosis. Furthermore, there is evidence in HD that cell death is mediated through mitochondrial pathways, and mitochondrial deficits are commonly associated with HD. We have previously reported that treatment with tauroursodeoxycholic acid (TUDCA), a hydrophilic bile acid, prevented neuropathology and associated behavioral deficits in the 3-nitropropionic acid rat model of HD. We therefore examined whether TUDCA would also be neuroprotective in a genetic mouse model of HD. Our results showed that systemically administered TUDCA led to a significant reduction in striatal neuropathology of the R6/2 transgenic HD mouse. Specifically, R6/2 mice began receiving TUDCA at 6 weeks of age and exhibited reduced striatal atrophy, decreased striatal apoptosis, as well as fewer and smaller size ubiquitinated neuronal intranuclear huntingtin inclusions. Moreover, locomotor and sensorimotor deficits were significantly improved in the TUDCA-treated mice. In conclusion, TUDCA is a nontoxic, endogenously produced hydrophilic bile acid that is neuroprotective in a transgenic mouse model of HD and, therefore, may provide a novel and effective treatment in patients with HD.
Huntington's disease (HD) is a heritable disorder caused by abnormal expansion of the trinucleotide (CAG) repeat sequence in exon 1 of the gene (Htt) encoding the huntingtin protein (1). This expansion results in the selective death of neurons in the caudate and putamen, with secondary pre- and postsynaptic cell loss. Mitochondrial insufficiency is a prominent feature of HD neuropathology. Mitochondrial compromise in HD is demonstrated by abnormal energy metabolite concentrations and utilizations (2–5), impaired striatal mitochondrial respiratory chain complex II/III activity (6–8), increased stress-induced mitochondrial depolarization (9), and increased free radical production and associated oxidative damage (6, 10, 11). Mice transgenic (Tg) for mutant Htt exon 1 exhibit reduced striatal aconitase and mitochondrial complex IV activity (12), and have benefited from energy subsidizing treatments (13–15).
Mitochondrial toxicity results in impairment of intra- and extracellular signaling pathways, which are affected in HD (16, 17). Moreover, mitochondria play an important role in the mediation of apoptotic pathways. Mitochondrial membrane perturbation results in the release of cytochrome c that initiates a caspase-mediated apoptotic cascade. In fact, it has been demonstrated that lymphoblasts isolated from HD patients are more susceptible to apoptotic signals that are mediated through mitochondrial pathways (9). Apoptotic cells are elevated in HD neostriatum (18–20), and their frequency is positively correlated with the length of CAG repeats (10). Caspases, which are crucial for the initiation and execution of apoptosis, are elevated and activated in HD brain (21, 22). Finally, expression of a dominant negative caspase-1 mutant (21), or minocycline inhibition of caspase-1 and caspase-3 (23), resulted in extended life span and delayed onset of neuropathology and behavioral deficits in Tg HD mice.
Tauroursodeoxycholic acid (TUDCA) is a hydrophilic bile acid that is normally produced endogenously in humans at very low levels. TUDCA is formed in the conjugation pathway of ursodeoxycholic acid (UDCA), which is commonly used as a bile acid replacement therapy for the treatment of certain cholestatic syndromes. Recent reports have shown that hydrophilic bile acids, such as UDCA and TUDCA, can prevent hepatic cytotoxicity through several mechanisms. For example, TUDCA prevents the production of reactive oxygen species and thus acts as an antioxidant (24–26). Additionally, TUDCA mitigates mitochondrial insufficiency and toxicity, and prevents apoptosis, in part, by inhibiting Bax translocation from cytosol to the mitochondria. In hepatocytes, this inhibition results in reduced mitochondrial membrane perturbation, release of cytochrome c, and activation of downstream caspases (24, 27–30). TUDCA reduced cytotoxicity in neurons through similar mechanisms, as well as mitochondrial pathways that are independent of the permeability transition (26, 31).
TUDCA prevented striatal degeneration and ameliorated locomotor and cognitive deficits in the in vivo 3-nitropropionic acid (3-NP) rat model of HD (32). However, the Tg mouse models of HD result from genetic rather than chemical alterations, involve chronic versus acute pathophysiology, and therefore may more accurately reflect the true pathophysiology of HD. Thus, we examined the effects of TUDCA in the well-characterized R6/2 Tg mouse model of HD, containing a trinucleotide CAG expansion (≈150 repeats) of Htt exon 1. The mice exhibit severe neuropathophysiology and associated neurodegeneration with concomitant sensorimotor deficits, and typically die at ≈14 weeks of age (33, 34). In addition, they exhibit prominent cerebral atrophy and formation of neuronal intranuclear inclusions (NII) (35). Here, we report that TUDCA treatment of R6/2 mice led to a marked reduction in striatal cell apoptosis and degeneration. In addition, intracellular inclusions were significantly reduced, and the TUDCA-treated mice showed improved locomotor and sensorimotor abilities.
Materials and Methods
Drug Administration.
All animal experiments were performed according to procedures approved by the Institutional Review Board's Animal Care and Use Committee. Six-week-old male wild-type (wt) (n = 28) and R6/2 (n = 28) mice (The Jackson Laboratory) were separated into equal groups, and received either TUDCA (500 mg/kg, s.c.) (Calbiochem) or vehicle (0.15 M NaHCO3, 1 ml/kg, s.c.) (Sigma) once every 3 days under 1.5% halothane anesthesia. We have previously shown that this dosing regimen increases TUDCA levels in the brain ≈7-fold (36). Administration of the drug and/or vehicle continued for the duration of the experiments.
Behavioral Analysis.
General locomotor activity was quantified by using an open field apparatus, consisting of a walled base (24 in × 24 in) divided into 25 equal squares. At 10 weeks of age, mice were placed in the center, and the number of central squares entered was recorded over 5 min. Square crossings were counted only if both forepaws were placed in the square. Between each test, the apparatus was thoroughly cleaned with 70% isopropyl alcohol. Mice were also examined for sensorimotor deficits at 10, 11, and 12 weeks of age by using the Rota-Rod (Ugo Basile, Camerio, Italy) rotational treadmill. For training, animals were placed on the apparatus (15 rpm) for a maximum of 60 sec three consecutive times. The following day, the 10-week-old mice were placed on the Rota-Rod (5 rpm), and latency to fall was recorded, with a maximum latency of 600 sec per trial. This was repeated once for each animal. Subsequent identical tests were conducted at 15, 25, and 35 rpm. The entire protocol was repeated at ages 11 and 12 weeks.
Tissue Preparation and Staining.
Animals were killed for histology at 13 weeks of age. Mice were anesthetized with 5% chloral hydrate, followed by transcardial perfusion with PBS and 4% formaldehyde. Brains were then removed and postfixed for 1 hr in 4% formaldehyde, and then cryopreserved in 30% sucrose. Before sectioning, brain depth (dorsoventral axis) was measured by using calipers placed perpendicularly at the level of the anterior portion of the optic chiasm. Striatal sections (15 μm) were prepared for analysis from vehicle and TUDCA-treated mice. Apoptotic cells were identified by using the ApopTag in situ detection kit (Intergen, Purchase, NY). General anatomic evaluation and volumetric analysis was performed on 30-μm sections mounted on SuperFrost Plus (Fisher Scientific) microscope slides and stained according to the Nissl protocol. NII were detected by using a polyclonal antibody to ubiquitin (1:1000) (DAKO). Sections were incubated in blocking solution (5% normal serum) for 1 hr, followed by overnight incubation with primary antibody in 2% normal serum. After washing, sections were incubated with biotinylated secondary antibody followed by peroxidase labeling using the ABC kit and incubation with diaminobenzidine substrate (Vector Laboratories).
Cellular and Anatomic Quantifications.
Quantitation of apoptosis was performed in a blinded fashion by counting apoptotic cells as a subset of total cells within a defined area of the striatum in four sections, 240 μm apart, from each animal. Unbiased stereologic volumetric analysis was performed according to the Cavalieri method (37). Volumes were calculated by the formula V = T × a/p × ΣPi, where V is the volume of interest, T is the distance between sections (in mm), a/p is the area associated with each point (in mm2), and Pi is the number of points hitting the object in each section. NII were quantified in four striatal sections separated by 240 μm, and this analysis was performed in preselected areas from each section. Quantitation of inclusion size was performed by using imagepro plus (Media Cybernetics, Silver Spring, MD).
Results
TUDCA Prevents Striatal Apoptosis.
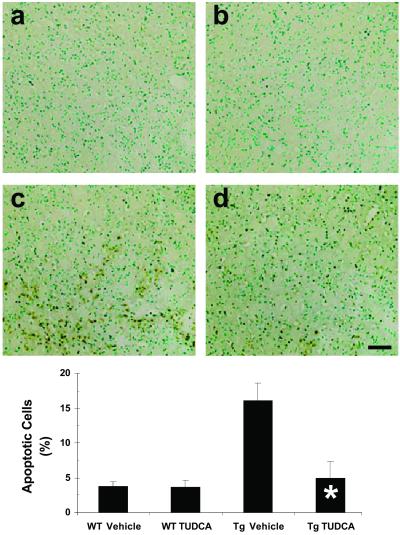
Mutant, N-terminal huntingtin fragments are strong inducers of caspase activation and apoptosis (38–40). Therefore, we tested whether TUDCA treatment of R6/2 mice would result in a reduction in striatal apoptosis. Terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) was used to identify apoptotic cells in mice killed at 13 weeks of age. Analysis showed that striatal sections from TUDCA-treated Tg mice contained fewer TUNEL-positive cells compared with mice that received vehicle alone (Fig. 1). In untreated R6/2 sections, 16.1% ± 2.3% of total striatal cells were TUNEL positive, compared with 5.0% ± 2.3% in TUDCA-treated Tg mice (P < 0.01), and 3.8% ± 0.6% and 3.7% ± 1.0% in vehicle- and TUDCA-treated wt mice, respectively (P < 0.001). Interestingly, previous studies in R6/2 mice detected little or no TUNEL immunoreactivity in Tg or wt brain tissues.
Fig 1.
TUDCA reduces striatal apoptosis in HD Tg mice. Representative striatal photomicrographs of TUNEL-stained sections are shown for each treatment group. Quantitation of apoptotic cells is indicated in the bar graph. Control Tg HD mice (c) exhibited significantly increased proportions of apoptotic vs. total cells compared with vehicle (a) and TUDCA-treated (b) wt control mice. TUDCA-treated R6/2 mouse striata (d) contained significantly fewer apoptotic cells compared with untreated Tg mice. Sections are counterstained with methyl green. *, P < 0.01 for Tg TUDCA vs. vehicle. (Scale bar, 100 μm.)
TUDCA Reduces Striatal Degeneration.
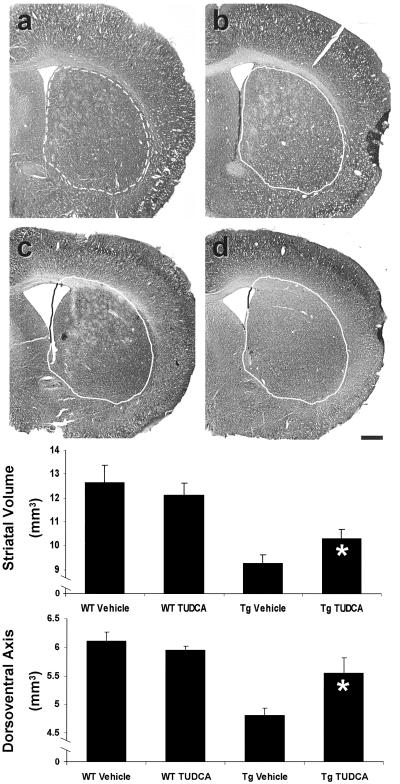
Neuropathology of human HD is characterized by extensive atrophy of the neostriatum. In addition, the brains of late stage R6/2 mice are significantly smaller compared with littermate controls (33, 35). Although selective cell death in R6/2 Tg mice occurs late in the course of illness, we examined whether TUDCA, by preventing neuronal degeneration and death, would also reduce the amount of striatal atrophy. Before sectioning, general brain size (dorsoventral axis) was calculated at the level of the anterior portion of the optic chiasm. Control R6/2 mice exhibited prominent atrophy (4.8 ± 0.12 mm) compared with control (6.1 ± 0.16 mm) and TUDCA-treated (6.0 ± 0.06 mm) wt mice. Measurements from TUDCA-treated R6/2 mice (5.6 ± 0.27 mm) showed significantly less cerebral atrophy compared with untreated Tg animals (P < 0.05) (Fig. 2). To determine the effects of TUDCA on atrophy, striatal volumes were calculated by using Nissl-stained sections from control and TUDCA-treated mice. Striatal volume was significantly reduced in untreated Tg mice (9.26 ± 0.3 mm3) when compared with wt controls (vehicle, 12.65 ± 0.7 mm3; TUDCA, 12.16 ± 0.5 mm3) (P < 0.05). Although TUDCA-treated R6/2 mice (10.34 ± 0.4 mm3) also had significantly decreased striatal volumes compared with wt controls, the reduction was far less (≈45%) than that seen in the untreated Tg animals (P < 0.05), particularly when comparing ventricular dilation (Fig. 2).
Fig 2.
TUDCA significantly reduces cerebral and striatal atrophy in R6/2 HD mice. Representative Nissl-stained striatal sections are shown for each treatment group. The untreated wt striatum (a) is outlined (broken line) for reference and superimposed (solid line) on striata from each of the other groups (b–d). Quantitation of striatal volume and dorsoventral axis measurements are represented graphically. Control R6/2 (c) striatal volume was reduced compared with wt vehicle (a) and TUDCA (b) controls. TUDCA-treated mouse striatal volume (d) was significantly larger than untreated R6/2 mice. Dorsoventral axis measurements were used to quantitate cerebral atrophy. In TUDCA-treated mice, distance of the cerebral dorsoventral axis was significantly larger than Tg controls. *, P < 0.05 for Tg TUDCA vs. vehicle. (Scale bar, 500 μm.)
Neuronal Intranuclear Inclusions Are Decreased in TUDCA-Treated Mice.
The formation of intracellular inclusions composed primarily of huntingtin and ubiquitin is a common pathological hallmark of HD. Similar aggregates are also found in the Tg HD mouse models (35, 41). Cytoplasmic inclusions are composed of full-length and N-terminal huntingtin fragments, whereas nuclear aggregates consist primarily of the cleaved fragments and mediate increased cytotoxicity (35, 39, 42–45). The R6/2 mouse strain is Tg for N-terminal Htt and, therefore, does not require caspase cleavage of full-length huntingtin for nuclear translocation and enhanced toxicity. Thus, we determined whether TUDCA treatment could inhibit mutant huntingtin aggregation by analyzing both the size and number of NII, which were identified immunohistochemically by using an antibody specific for ubiquitin. A significant reduction in striatal NII was observed in TUDCA-treated (155 ± 7/high power field) vs. untreated (189 ± 8/high power field) Tg HD mice (P < 0.05) (Fig. 3). Moreover, TUDCA decreased the average area of individual inclusions. Striatal NII in TUDCA-treated mice (37.8 ± 0.4 μm2) were significantly smaller than untreated Tg controls (40.1 ± 0.4 μm2) (P < 0.05). NII were not present in vehicle and TUDCA-treated wt animals. Thus, TUDCA treatment reduced the size and the overall number of NII in the R6/2 mice, thereby significantly improving a hallmark of HD pathology.
Fig 3.
TUDCA treatment reduces the size and number of ubiquitinated neuronal intranuclear inclusions (NII) in R6/2 mice. Representative striatal sections are shown for each treatment group, with quantitation of the number and size of striatal NII in bar graphs below. No aggregates were identified in vehicle (a) and TUDCA-treated (b) wt mice. In contrast, vehicle (c) and TUDCA-treated (d) R6/2 mouse striatum contained extensive aggregate formation. Quantitative analysis of TUDCA-treated Tg mice revealed significantly fewer NII compared with R6/2 control animals. Furthermore, NII in TUDCA-treated R6/2 mice were significantly smaller than those in Tg controls (Inset; c and d). hpf, high power field. *, P < 0.05 for Tg TUDCA vs. vehicle. (Scale bar, 100 μm; Inset bar, 20 μm.)
TUDCA Ameliorates Locomotor and Sensorimotor Deficits.
Impairment of motor abilities in R6/2 mice is well characterized (34). Therefore, we used measures of sensorimotor (Rota-Rod) and locomotor (open field) behavior to test whether TUDCA-treated R6/2 mice demonstrated improved motor capacity. In open field experiments, 10-week-old R6/2 mice exhibited reduced locomotor activity (28.3 ± 3.9 squares per 5 min) when compared with vehicle-treated (37.8 ± 4.4 squares per 5 min) and TUDCA-treated (38.3 ± 5.1 squares per 5 min) wt mice (P = 0.06) (Fig. 4). TUDCA-treated R6/2 mice (41.5 ± 4.9 squares per 5 min) were significantly more active than the Tg vehicle controls (P < 0.05), but did not differ from wt mice. Thus, the aged R6/2 mice exhibited pronounced hypoactivity that was reduced with TUDCA.
Fig 4.
Quantitation of total squares entered in an open field task as a measurement of R6/2 mouse hypoactivity. Control Tg mice were significantly less active compared with vehicle and TUDCA-treated wt mice. However, TUDCA-treated R6/2 mice were significantly improved compared with vehicle controls. *, P < 0.05 for Tg TUDCA vs. vehicle.
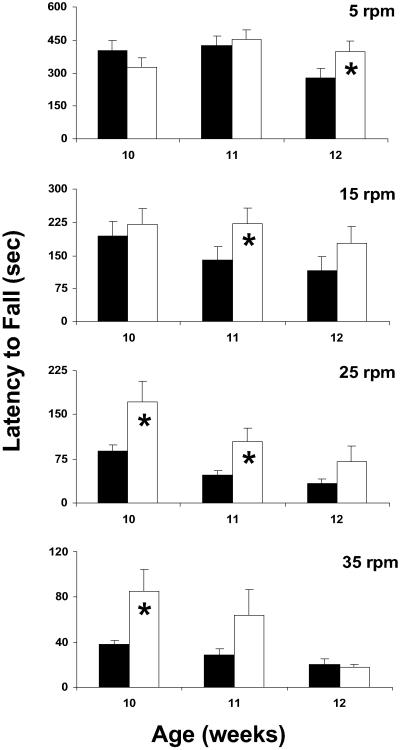
R6/2 mice exhibit impaired Rota-Rod performance that is inversely proportional to the age of the mouse and the rotational velocity of the apparatus (34). We examined whether neuroprotection by TUDCA would reduce the degree of impairment in these animals. To evaluate sensorimotor abilities longitudinally, we conducted Rota-Rod tests at weeks 10, 11, and 12. Moreover, to identify differences in performance on simple, intermediate, and difficult tasks, four rotational velocities were used for each time point. Wild-type animals treated with either vehicle or TUDCA were subjected to Rota-Rod behavior during the first trial, and mastered the task at each rotational speed (data not shown). Therefore, wt mice were not subjected to Rota-Rod behavioral analysis in subsequent trials. In general, TUDCA-treated R6/2 mice (TU) performed better than vehicle Tg controls (Tg) on the Rota-Rod, and this effect was most prominent earlier in the course of the disease (Fig. 5). Specifically, 10-week-old TUDCA mice performed better at 35 rpm (TU, 85 ± 19 sec; Tg, 38 ± 4 sec; P < 0.05) and at 25 rpm (TU, 171 ± 36 sec; Tg, 89 ± 11 sec; P < 0.05). TUDCA-treated 11-week-old animals showed improved Rota-Rod performance at 25 rpm (TU, 104 ± 24 sec; Tg, 47 ± 7 sec; P < 0.05) and at 15 rpm (TU, 222 ± 36 sec; Tg, 139 ± 31 sec; P < 0.05), and 12-week-old animals were better at 5 rpm (TU, 395 ± 50 sec; Tg, 279 ± 44 sec; P < 0.05). These results suggest that TUDCA improves sensorimotor deficits in HD mice, and age and task difficulty influence the observed degree of behavioral recovery.
Fig 5.
TUDCA treatment improves Rota-Rod performance in an age- and speed-dependent fashion. Control and TUDCA-treated Tg mice were subjected to the Rota-Rod behavioral task as a measure of sensorimotor ability. Four different speeds with increasing task difficulty were used. Twelve-week-old TUDCA-treated R6/2 mice (white bars) exhibited significant improvement compared with control Tg mice (black bars) at slow (5 rpm) rotational velocity, whereas 11-week-old TUDCA-treated animals performed significantly better at 15 rpm. At 25 rpm, 10- and 11-week-old TUDCA-treated R6/2 mice were markedly better than control Tg animals, whereas at the highest speed (35 rpm), 10-week-old TUDCA-treated mice exhibited significant sensorimotor improvement. *, P < 0.05 for Tg TUDCA vs. vehicle.
Discussion
TUDCA is a unique bile acid that acts as a potent anti-apoptotic agent. In addition to its antioxidant properties (24), it inhibits mitochondrial processes, such as mitochondrial permeability transition, cytochrome c release, Bax translocation, and caspase activation (29, 30). We have shown that administration of TUDCA, a nontoxic, endogenously produced, hydrophilic bile acid, to a genetic mouse model of HD significantly reduced striatal neurodegeneration and ameliorated locomotor and sensorimotor deficits. This study extends our previous reports in pharmacologic animal models of HD and suggests that TUDCA may be a viable therapeutic option for the treatment of HD (32).
TUDCA treatment of the R6/2 Tg mice reduced striatal atrophy and the number of apoptotic cells and resulted in fewer and smaller size striatal NII. Although it is not entirely clear how TUDCA reduced huntingtin aggregation, the ability to detoxify mutant huntingtin is an important consideration in the treatment of HD (46, 47). The expanded protein is implicated in multiple pathophysiological pathways, including increased oxidative stress (11), mitochondrial toxicity (48), intracellular signaling disruption (16), and extracellular neurotrophic impairment (17). Aberrant mutant huntingtin interaction with its effector proteins provides a likely mechanism, as caspase activation is a consequence of abnormal interactions between mutant huntingtin and its effector proteins. It has been shown that reduced binding affinity of mutant huntingtin to Hip-1 results in the activation of Hip-1/Hippi/caspase-8-mediated apoptotic cascades (49). Huntingtin contains intrinsic caspase cleavage sites, and polyglutamine expansions make striatal neurons more likely to generate active caspases (50, 51). Also, the rate of caspase cleavage is positively correlated with CAG repeat length (52); truncated huntingtin is more toxic than its full-length counterpart, and more readily redistributes to the nucleus and forms nuclear aggregates (43, 53). Moreover, caspase cleavage-resistant huntingtin exhibits lower toxicity and aggregate formation than the unaltered protein (50).
Caspase-mediated apoptosis may not be the predominant mechanism of HD pathophysiology. In fact, there is evidence to show that HD symptomatology occurs before extensive striatal cell death (54–57), and apoptosis tends to be an acute, rather than chronic, process. In fact, in the initial characterization of the R6/2 Tg HD mouse, no significant cell death was detected. Thus, it is possible that apoptosis contributes significantly only at the later stages of the disease, as observed in this study. The slowly developing vulnerability of striatal neurons to mutant huntingtin could be caused by a direct, abnormal interaction between mutant huntingtin and the mitochondria that causes a progressive accumulation of cytotoxic molecules. It could also be caused by huntingtin aggregate-mediated impairment of intracellular trafficking or signaling, with subsequent cytoplasmic and mitochondrial insult, leading to elevated cytotoxicity. In either case, as caspase activity increases and huntingtin is cleaved at higher frequencies, toxic N-terminal huntingtin fragments are generated that accelerate cytotoxicity through further activation of caspases and impairment of transcriptional activity after nuclear translocation (44, 58). Thus, by reducing mutant huntingtin, stabilizing mitochondria, and preventing apoptotic pathways, TUDCA may be acting at multiple levels to protect vulnerable cells in the caudate and putamen, and may reduce HD pathology by derailing pathophysiologic mechanisms early in the process.
TUDCA-treated HD mice exhibited reduced striatal neuropathology, with associated improvement of motor abilities. Improved performance in TUDCA-treated animals depended on task difficulty. Younger TUDCA-treated mice performed better at more difficult rotational speeds, whereas older mice were less impaired at slower velocities. This finding supports the progressive nature of the pathology in the Tg mice, and demonstrates their accuracy to the human condition. Our results also underscore the relationship between task difficulty and ability. We chose several rotational speeds based on the notion that TUDCA would improve Rota-Rod performance in Tg mice, but might not be evident at exceptionally easy or difficult tasks. Although wt mice mastered each rotational velocity, the TUDCA-treated mice were not able to achieve those levels. This result may be caused by the delayed TUDCA treatment, which did not begin until the animals were 6 weeks old.
Although prominent behavioral deficits do not present before the eighth week of age (34), a significant degree of subcellular pathology occurs before mice become symptomatic. For example, in 4- to 6-week-old presymptomatic R6/2 mice, expression of certain striatal signaling genes, as well as proteins important for neurotransmission, is significantly reduced (54–57). In addition, marked striatal and cortical neuron atrophy is detectable at 6 weeks of age (42). Thus, significant pathophysiology and neurodegeneration had occurred before TUDCA administration. Further studies are required to establish the most efficient dosing schedule and route of administration of TUDCA to significantly prolong the life span of Tg HD mice.
The present study extends results showing a neuroprotective effect of TUDCA in the 3-NP rat model of HD (32). Although the 3-NP model is a well-studied and accepted animal model of the disease (59, 60), it is unknown whether the mechanism of neurotoxicity of 3-NP is representative of HD pathophysiology. HD patients exhibit signs of mitochondrial stress and insufficiency (48), and 3-NP toxicity is mediated by irreversible inhibition of succinate dehydrogenase, which is a key metabolic enzyme. However, Tg animal models of HD may represent more accurate models because they bridge the gap between genetic mutation and mitochondrial pathophysiology. We chose the R6/2 mouse model because it is a well-characterized genetic animal model of HD, and because the severity of symptomatology and disease progression provides a basis with which to evaluate therapeutic efficacy in fulminant HD.
In HD patients and animal models of HD, mitochondrial insufficiency and apoptosis are significant indicators of disease progression that could potentially be reduced by TUDCA. Previous studies with TUDCA have focused on the antiapoptotic and cytoprotective effects in hepatic systems, and most recently in acute stroke (61). This study, combined with previous work in the 3-NP rat model (32), supports the continued evaluation of TUDCA for the treatment of HD. Furthermore, a multitude of neurological disorders, including Friedreich's ataxia, amyotrophic lateral sclerosis, Parkinson's disease, and Alzheimer's disease, are mediated in some fashion through mitochondrial perturbation (48). TUDCA may, therefore, exhibit neuroprotective properties in other chronic neurological conditions.
Acknowledgments
We thank Jerry Sedgewick and the Biomedical Image and Processing Laboratory, Susana Solá, Anna Abt, Steve Spellman, Wei-Jun Wang, and Bill Kaemmerer for technical assistance, and Betsy T. Kren for critical review of the manuscript. This work was supported by the Lyle French Fund, Fundação para a Ciência e Tecnologia Postdoctoral PRAXISXXI/BPD/11849/97 and PRAXIS/C/SAU/14311/98 (to C.M.P.R.), and National Institute of Mental Health Predoctoral National Research Service Award 5F30MH12157-03 (to C.D.K.).
Abbreviations
HD, Huntington's disease
NII, neuronal intranuclear inclusions
3-NP, 3nitropropionic acid
TUDCA, tauroursodeoxycholic acid
Tg, transgenic
wt, wild type
TUNEL, terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling
References
- 1.Huntington's Disease Collaborative Research Group (1993) Cell 72, 971-983. [DOI] [PubMed] [Google Scholar]
- 2.Koroshetz W. J., Jenkins, B. G., Rosen, B. R. & Beal, M. F. (1997) Ann. Neurol. 41, 160-165. [DOI] [PubMed] [Google Scholar]
- 3.Jenkins B., Koroshetz, W. J., Beal, M. F. & Rosen, B. R. (1993) Neurology 43, 2689-2695. [DOI] [PubMed] [Google Scholar]
- 4.Kuwert T., Lange, H. W., Langer, K.-J., Herzog, H., Aulich, A. & Feinendegen, L. E. (1990) Brain 113, 1405-1423. [DOI] [PubMed] [Google Scholar]
- 5.Lodi R., Schapira, A. H., Manners, D., Stules, P., Wood, N. W., Taylor, D. J. & Warner, T. T. (2000) Ann. Neurol. 48, 72-76. [PubMed] [Google Scholar]
- 6.Browne S. E. & Beal, M. F. (1997) Ann. Neurol. 41, 646-653. [DOI] [PubMed] [Google Scholar]
- 7.Gu M., Gash, M. T., Mann, V. M., Javoy-Agid, F., Cooper, J. M. & Schapira, A. H. (1996) Ann. Neurol. 39, 385-389. [DOI] [PubMed] [Google Scholar]
- 8.Tabrizi S. J., Cleeter, M. W., Xuereb, J., Taanman, J. W., Cooper, J. M. & Schapira, A. H. (1999) Ann. Neurol. 45, 25-32. [DOI] [PubMed] [Google Scholar]
- 9.Sawa A., Wiegand, G. W., Cooper, J., Margolis, R. L., Sharp, A. H., Lawler, J. F., Jr., Greenamyre, J. T., Snyder, S. H. & Ross, C. A. (1999) Nat. Med. 5, 1194-1198. [DOI] [PubMed] [Google Scholar]
- 10.Butterworth N. J., Williams, L., Bullock, J. Y., Love, D. R., Faull, R. L. & Dragunow, M. (1998) Neuroscience 87, 49-53. [DOI] [PubMed] [Google Scholar]
- 11.Browne S. E., Ferrante, R. J. & Beal, M. F. (1999) Brain Pathol. 9, 147-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tabrizi S. J., Workman, J., Hart, P. E., Mangiarini, L., Mahal, A., Bates, G., Cooper, J. M. & Schapira, A. H. (2000) Ann. Neurol. 47, 80-86. [DOI] [PubMed] [Google Scholar]
- 13.Ferrante R. J., Andreassen, O. A., Jenkins, B. G., Dedeoglu, A., Kuemmerle, S., Kubilus, J. K., Kaddurah-Daouk, R., Hersch, S. M. & Beal, M. F. (2000) J. Neurosci. 20, 4389-4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferrante R. J., Andreassen, O. A., Dedeoglu, A., Ferrante, K. L., Jenkins, B. G., Hersch, S. M. & Beal, M. F. (2002) J. Neurosci. 22, 1592-1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andreassen O. A., Dedeoglu, A., Ferrante, R. J., Jenkins, B. G., Ferrante, K. L., Thomas, M., Friedlich, A., Browne, S. E., Schilling, G., Borchelt, D. R., et al. (2001) Neurobiol. Dis. 8, 479-491. [DOI] [PubMed] [Google Scholar]
- 16.Nucifora F. C., Sasaki, M., Peters, M. F., Huang, H., Cooper, J. K., Yamada, M., Takahashi, H., Tsuji, S., Troncoso, J., Dawson, V. L., et al. (2001) Science 291, 2423-2428. [DOI] [PubMed] [Google Scholar]
- 17.Zuccato C., Ciammola, A., Rigamonti, D., Leavitt, B. R., Goffredo, D., Conti, L., MacDonald, M. E., Friedlander, R. M., Silani, V., Hayden, M. R., et al. (2001) Science 293, 493-498. [DOI] [PubMed] [Google Scholar]
- 18.Thomas L. B., Gates, D. J., Richfield, E. K., O'Brien, T. F., Schweitzer, J. B. & Steindler, D. A. (1995) Exp. Neurol. 133, 265-272. [DOI] [PubMed] [Google Scholar]
- 19.Dragunow M., Faull, R. L., Lawlor, P., Beilharz, E. J., Singleton, K., Walker, E. B. & Mee, E. (1995) NeuroReport 6, 1053-1057. [DOI] [PubMed] [Google Scholar]
- 20.Portera-Cailliau C., Hedreen, J. C., Price, D. L. & Koliatsos, V. E. (1995) J. Neurosci. 15, 3775-3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ona V. O., Li, M., Vonsattel, J. P., Andrews, L. J., Khan, S. Q., Chung, W. M., Frey, A. S., Menon, A. S., Li, X. J., Stieg, P. E., et al. (1999) Nature (London) 399, 263-267. [DOI] [PubMed] [Google Scholar]
- 22.Sanchez I., Xu, C.-J., Juo, P., Kakizaka, A., Blenis, J. & Yuan, J. (1999) Neuron 22, 623-633. [DOI] [PubMed] [Google Scholar]
- 23.Chen M., Ona, V. O., Li, M., Ferrante, R. J., Fink, K. B., Zhu, S., Bian, J., Guo, L., Farrell, L. A., Hersch, S. M., et al. (2000) Nat. Med. 6, 797-801. [DOI] [PubMed] [Google Scholar]
- 24.Rodrigues C. M. P., Fan, G., Wong, P. Y., Kren, B. T. & Steer, C. J. (1998) Mol. Med. 4, 165-178. [PMC free article] [PubMed] [Google Scholar]
- 25.Rodrigues C. M. P. & Steer, C. J. (2001) Expert Opin. Invest. Drugs 10, 1243-1253. [DOI] [PubMed] [Google Scholar]
- 26.Rodrigues C. M. P., Solá, S., Brito, M. A., Brondino, C. D., Brites, D. & Moura, J. J. G. (2001) Biochem. Biophys. Res. Commun. 281, 468-474. [DOI] [PubMed] [Google Scholar]
- 27.Benz C., Angermuller, S., Otto, G., Sauer, P., Stremmel, W. & Stiehl, A. (2000) Eur. J. Clin. Invest. 30, 203-209. [DOI] [PubMed] [Google Scholar]
- 28.Benz C., Angermuller, S., Tox, U. U., Kloters-Plachky, P., Riedel, H. D., Sauer, P., Stremmel, W. & Stiehl, A. (1998) J. Hepatol. 1, 99-106. [DOI] [PubMed] [Google Scholar]
- 29.Rodrigues C. M. P., Fan, G., Ma, X., Kren, B. T. & Steer, C. J. (1998) J. Clin. Invest. 101, 2790-2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodrigues C. M. P., Ma, X., Linehan-Stieers, C., Fan, G., Kren, B. T. & Steer, C. J. (1999) Cell Death Differ. 6, 842-854. [DOI] [PubMed] [Google Scholar]
- 31.Rodrigues C. M. P., Linehan-Stieers, C., Keene, C. D., Ma, X., Kren, B. T., Low, W. C. & Steer, C. J. (2000) J. Neurochem. 75, 2368-2379. [DOI] [PubMed] [Google Scholar]
- 32.Keene C. D., Rodrigues, C. M. P., Eich, T., Linehan-Stieers, C., Abt, A., Kren, B. T., Steer, C. J. & Low, W. C. (2001) Exp. Neurol. 171, 351-360. [DOI] [PubMed] [Google Scholar]
- 33.Mangiarini L., Sathasivam, K., Seller, M., Cozens, B., Harper, A., Hetherington, C., Lawton, M., Trottier, Y., Lehrach, H., Davies, S. W., et al. (1996) Cell 87, 493-506. [DOI] [PubMed] [Google Scholar]
- 34.Carter R. J, Lione, L. A., Humby, T., Mangiarini, L., Mahal, A., Bates, G. P., Dunnett, S. B. & Morton, A. J. (1999) J. Neurosci. 19, 3248-3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davies S. W., Turmaine, M., Cozens, B. A., DiFiglia, M., Sharp, A. H., Ross, C. A., Scherzinger, E., Wanker, E. E., Mangiarini, L. & Bates, G. P. (1997) Cell 90, 537-548. [DOI] [PubMed] [Google Scholar]
- 36.Kaemmerer W. F., Rodrigues, C. M. P., Steer, C. J. & Low, W. C. (2001) Neuroscience 103, 713-724. [DOI] [PubMed] [Google Scholar]
- 37.Howard C. V., Howard, V. & Reed, M. G., (1998) Unbiased Stereology: Three-Dimensional Measurement in Microscopy (BIOS Scientific, New York).
- 38.Jana N. R., Zemskov, E. A., Wang, G. H. & Nukina, N. (2001) Hum. Mol. Genet. 10, 1049-1059. [DOI] [PubMed] [Google Scholar]
- 39.Li S.-H., Lam, S., Cheng, A. L. & Li, X.-J. (2000) Hum. Mol. Genet. 9, 2859-2867. [DOI] [PubMed] [Google Scholar]
- 40.Kim M., Lee, H.-S., LaForet, G., McIntyre, C., Martin, E. J., Chang, P., Kim, T. W., Williams, M., Reddy, P. H., Tagle, D., et al. (1999) J. Neurosci. 19, 964-973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sieradzan K. A., Mechan, A. O., Jones, L., Wanker, E. E., Nukina, N. & Mann, D. M. (1999) Exp. Neurol. 156, 92-99. [DOI] [PubMed] [Google Scholar]
- 42.Davies S. W., Turmaine, M., Cozens, B. A., Raza, A. S., Mahal, A., Mangiarini, L. & Bates, G. P. (1999) Phil. Trans. R. Soc. London 354, 971-979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hackam A. S., Singaraja, R., Wellington, C. L., Metzler, M., McCutcheon, K., Zhang, T., Kalchman, M. & Hayden, M. R. (1998) J. Cell Biol. 141, 1097-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hackam A. S., Singaraja, R., Zhang, T., Gan, L. & Hayden, M. R. (1999) Hum. Mol. Genet. 8, 25-33. [DOI] [PubMed] [Google Scholar]
- 45.Li H., Li, S. H., Cheng, A. L., Mangiarini, L., Bates, G. P. & Li, X. J. (1999) Hum. Mol. Genet. 8, 1227-1236. [DOI] [PubMed] [Google Scholar]
- 46.Leavitt B. R., Wellington, C. L. & Hayden, M. R. (1999) Semin. Neurol. 20, 385-395. [DOI] [PubMed] [Google Scholar]
- 47.Khoshnan A., Ko, J. & Patterson, P. H. (2002) Proc. Natl. Acad. Sci. USA 99, 1002-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beal M. F. (2000) Trends Neurosci. 23, 298-304. [DOI] [PubMed] [Google Scholar]
- 49.Gervais F. G., Singaraja, R., Xanthoudakis, S., Gutekunst, C. A., Leavitt, B. R., Metzler, M., Hackam, A. S., Tam, J., Vaillancourt, J. P., Houtzager, V., et al. (2002) Nat. Cell Biol. 4, 95-105. [DOI] [PubMed] [Google Scholar]
- 50.Wellington C. L., Singaraja, R., Ellerby, L., Savill, J., Roy, S., Leavitt, B., Cattaneo, E., Hackam, A., Sharp, A., Thornberry, N., et al. (2000) J. Biol. Chem. 275, 19831-19838. [DOI] [PubMed] [Google Scholar]
- 51.Wellington C. L. & Hayden, M. R. (2000) Clin. Gen. 57, 1-10. [DOI] [PubMed] [Google Scholar]
- 52.Goldberg Y. P., Nicholson, D. W., Rasper, D. M., Kalchman, M. A., Koide, H. B., Graham, R. K., Bromm, M., Kazemi-Esfarjani, P., Thornberry, N. A., Vaillancourt, J. P., et al. (1996) Nat. Gen. 13, 442-449. [DOI] [PubMed] [Google Scholar]
- 53.Martindale D., Hackam, A., Wieczorek, A., Ellerby, L., Wellington, C., McCutcheon, K., Singaraja, R., Kazemi-Esfarjani, P., Devon, R., Kim, S. U., et al. (1998) Nat. Gen. 18, 150-154. [DOI] [PubMed] [Google Scholar]
- 54.Luthi-Carter R., Strand, A., Peters, N. L., Solano, S. M., Hollingsworth, Z. R., Menon, A. S., Frey, A. S., Spektor, B. S., Penney, E. B., Schilling, G., et al. (2000) Hum. Mol. Genet. 9, 1259-1271. [DOI] [PubMed] [Google Scholar]
- 55.Bibb J. A., Yan, Z., Svenningsson, P., Snyder, G. L., Pleribone, V. A., Horiuchi, A., Nairn, A. C., Messer, A. & Greengard, P. (2000) Proc. Natl. Acad. Sci. USA 97, 6809-6814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cha J.-H. J., Kosinski, C. M., Kerner, J. A., Alsdorf, S. A., Mangiarini, L., Davies, S. W., Penney, J. B., Bates, G. P. & Young, A. B. (1998) Proc. Natl. Acad. Sci. USA 95, 6480-6485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cha J.-H. J., Frey, A. S., Alsdorf, S. A., Kerner, J. A., Kosinski, C. M., Mangiarini, L., Penney, J. B., Jr., Davies, S. W., Bates, G. P. & Young, A. B. (1999) Phil. Trans. R. Soc. London 354, 981-989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sawa A. (2001) J. Mol. Med. 79, 375-381. [DOI] [PubMed] [Google Scholar]
- 59.Borlongan C. V., Koutouzis, T. K. & Sanberg, P. R. (1997) Neurosci. Biobehav. Rev. 21, 289-293. [DOI] [PubMed] [Google Scholar]
- 60.Brouillet E., Conde, F., Beal, M. F. & Hantraye, P. (1999) Prog. Neurobiol. 59, 427-468. [DOI] [PubMed] [Google Scholar]
- 61.Rodrigues C. M. P., Spellman, S. R., Solá, S., Grande, A. W., Linehan-Stieers, C., Low, W. C. & Steer, C. J. (2002) J. Cereb. Blood Flow Metab. 22, 463-471. [DOI] [PubMed] [Google Scholar]