Abstract
Kaposi sarcoma-associated herpesvirus (KSHV) is an oncogenic DNA virus that causes Kaposi sarcoma and AIDS-related primary effusion lymphoma (PEL). Here we show that KSHV lytic cycle replication in PEL cells induces G1 cell cycle arrest, presumably to facilitate the progression of viral DNA replication. Expression of a KSHV-encoded early lytic protein referred to as RAP or K8 is induced within 12–24 h after the onset of lytic cycle induction in host PEL cells, and coincides with increased levels of both the endogenous C/EBPα and p21CIP-1 proteins in the nucleus of the same cells. The KSHV RAP protein binds to C/EBPα in vitro and stimulates C/EBPα-induced expression from both the C/EBPα and p21 promoters in cotransfected cells. A recombinant adenovirus expressing the RAP protein induced the expression of both the C/EBPα and p21 proteins in primary human fibroblasts, and flow cytometric analysis revealed a dramatic inhibition of G1 to S cell cycle progression in the same cells. All of these effects were abolished in cells that lack C/EBPα or by deletion of the basic/leucine zipper region in RAP that interacts with C/EBPα. Therefore, C/EBPα is essential for the p21-mediated inhibition of G1 to S-phase progression by RAP in KSHV-infected host cells.
Kaposi sarcoma-associated herpesvirus (KSHV), which causes classical endemic and AIDS-associated forms of Kaposi sarcoma (KS) (1), as well as primary effusion lymphoma (PEL) in patients with AIDS (2), is related to Epstein–Barr virus (EBV) and can infect human primary dermal microvascular endothelial cells (3).
KSHV undergoes two alternative phases of infection, namely a quiescent latent state or a productive lytic cycle. During KSHV latency, a few viral genes are expressed from extrachromosomal episomes. However, during the lytic phase of viral infection, the full KSHV genome expression pattern is activated and the cells undergo productive viral DNA replication. Although the KSHV lytic cycle is not directly associated with neoplastic transformation, it is required for the release of infectious particles and the spread of the KSHV infections. KSHV and herpesviruses in general encode many of their own viral DNA replication and nucleotide synthesis machinery components, which partially obviate the need for cellular S-phase-associated replication machinery (4, 5). Nevertheless, the ability to block the cell cycle in G0/G1 also seems to be an important early step in the lytic cycle for most herpesviruses (6), possibly to prevent competition with host-cell DNA synthesis for limited free nucleotides and to provide nuclear spaces for progeny viral DNA accumulation.
CCAAT/enhancer-binding proteins belong to the family of cellular bZIP DNA-binding nuclear transcription factors including also c-JUN, c-FOS, and CREB (7), and they play important roles as the determining factors for adipocyte, granulocyte, and neutrophil differentiation (8–11). The C/EBPα gene encodes a 42-kDa protein that can positively autoregulate its own gene promoter (12–14), and it controls differentiation and G1 cell cycle arrest through three reported mechanisms: (i) by up-regulating the expression of the cdk inhibitor-p21CIP (15, 16); (ii) by inhibiting E2F transcription (17); and (iii) by directly inhibiting cdk2 and cdk4 (18, 19).
Recently, we found that a KSHV-encoded early nuclear protein, known as replication-associated protein (RAP or K8), which is related to the bZIP family and associates with viral DNA replication compartments (4), also physically interacts in vitro with both the C/EBPα and C/EBPβ proteins and enhances C/EBPα-mediated up-regulation of both the C/EBPα and p21 gene promoters in transient reporter gene assays (F.Y.W., C.-J. Chiou, and G.S.H., unpublished work). In the present study, we demonstrate that both KSHV lytic cycle progression and RAP protein expressed from a recombinant adenovirus vector induce the C/EBPα and p21 cell cycle arrest pathway.
Materials and Methods
Cells and Virus.
PELs containing latent KSHV (BCBL-1 and JSC-1) (20), EBV-positive Burkitt lymphoma cells (Akata), and herpesvirus-negative DG-75 transformed B cells were grown in 10% FBS RPMI medium 1640. Human diploid fibroblast cells (HF), HeLa cells, 3T3-L1 cells, C/EBPα (−/−) mouse embryonic fibroblast cells (MEF) (16), and MDA-MB 468 breast epithelial tumor cells were grown in 10% FBS DMEM. Plasmid pSG5-RAP and pMSV-C/EBPα encode RAP and C/EBPα, respectively. Recombinant adenoviruses, Ad-RAP (amino acids 1–237) and Ad-RAPΔ169–237 (amino acids 1–168), were generated with the Cre-lox recombination system as described elsewhere (21). Recombinant adenoviruses were generated by cotransfecting Ψ5 viral DNA with pFYW28 (full length RAP) or with pFYW29 (RAPΔ169–237) into CRE8 cells. Human diploid fibroblast, C/EBPα (−/−) MEF or MDA-MB 468 cells (American Type Culture Collection) were seeded at low confluence in two-well slide chambers for immunofluorescence assay (IFA) and infected with adenovirus vectors at a multiplicity of infection of 0.5 (22). Induction of the KSHV lytic cycle in PEL cells (BCBL-1 and JSC-1) with phorbol 12-tetradecanoate 13-acetate (TPA) (20 ng/ml) was performed as described (3).
Immunoflurescence and Immunohistochemistry.
Procedures for BrdUrd labeling, IFA, and fluorescence microscopy were all performed as described (4). Phosphonoacetic acid (PAA) (400 μg/ml) was maintained in the RPMI medium 1640 throughout the 48-h lytic induction of BCBL-1 cultures; BrdUrd pulse-labeling (45 min) of BCBL-1 cells was performed at 0 and 48 h after lytic induction in the presence of PAA. For IFA, BrdUrd pulse-labeling of 3T3-L1 cells was performed for 45 min at 48 h after transfection. For IFA in adenovirus vector infections, BrdUrd pulse-labeling of HF and C/EBPα (−/−) MEF cells was performed for 45 min at 30 h. Sheep anti-BrdUrd primary Ab (20-BS17, Fitzgerald, Concord, MA) was used to detect BrdUrd incorporation, and various secondary donkey- or goat-derived FITC or rhodamine-conjugated IgG (Jackson Pharmaceuticals, West Grove, PA) were used to detect primary Abs. Mounting solution with 4′,6-diamidino-2-phenylindole (DAPI) (Vector Shield) was used to visualize cellular DNA. Immunohistochemistry was performed on KSHV-uninduced or -induced paraffin-embedded PEL cells (BCBL-1 and JSC-1) by using reagents from DAKO according to procedures described (23). Primary Abs included rabbit anti-RAP antiserum (N-terminal epitope) (1:800) (4); mouse anti-human p21 mAb (sc-817, 1:200, Santa Cruz Biotechnology); mouse anti-mouse p21 mAb (sc-6246, 1:200, Santa Cruz Biotechnology), mouse anti-p16 mAb (NA29–100 μg/1:100, Oncogene), rabbit anti-C/EBPα antiserum (1:800) (14), and goat anti-C/EBPα (sc-9314, 1:100, Santa Cruz Biotechnology).
DNA Transfection and Western Blot Analysis.
Calcium phosphate DNA transfection was performed in HeLa cells by using either 0.5 or 4 μg of pFYW01 equilibrated with 4.5 or 1 μg of pSG5 as carrier DNA per well in a six-well plate, and the cells were harvested after 40 h (4). Transfections of 3T3-L1 cells were performed with FuGENE 6 Transfection Reagents (Roche Diagnostics) by using 2 μg of pMSV-C/EBPα DNA in a two-well chamber slide. Cell lysate preparations and Western blot analyses were performed as described (24). Primary Abs included RAP PAb (1:1000), C/EBPα and β PAb (1:2,000), mouse anti-human p21 mAb (1:1,000), and β-actin mAb (1:5,000).
FITC Channel-Gated Flow Cytometric Analysis.
Primary HF cells were seeded in T175 flasks at 2 × 106 cells per ml, infected with the adenovirus vector at 40% confluence at a multiplicity of infection of 0.5 and harvested at 48 h after infection. Washed and resuspended cells were then incubated in succession, in 2% paraformaldehyde for 20 min at room temperature, in permeabilization solution (0.5% Saponin, 1% BSA, 1× PBS) for 30 min at room temperature, in 1% BSA/PBS containing 5% goat blocking serum for 30 min at room temperature, in 1% BSA/PBS with rabbit anti-RAP PAb incubating for 1 h at 37°C , and in 1% BSA/PBS with secondary anti-rabbit IgG conjugated with FITC (Jackson Pharmaceuticals) for 30 min at 37°C. After washing, the cells were fixed again in 2% paraformaldehyde for 20 min at room temperature, then resuspended in Hoescht solution (10 ng/ml Hoescht/0.5% Nonidet P-40/3.5% formaldehyde/1× PBS) at 4°C. FITC channel-gated flow cytometric analyses were performed with a BDLSR–Becton Dickinson FACScan (10,000 FITC-positive events per sample), and the results were analyzed with the CELL QUEST program.
Results
Expression of C/EBPα Is Increased After Lytic Cycle Induction in PEL Cells Coincident with Increased Expression of p21 and KSHV RAP.
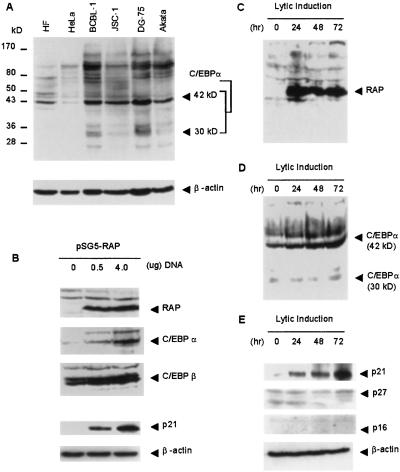
Transcription of the C/EBPα gene can be activated both by autoactivation by C/EBPα itself and by transactivation by C/EBPβ (12–14). Relevant to the lytic cycle, C/EBPα can both stabilize p21 cdk2 inhibitor protein and up-regulate expression of the p21 gene (15, 16). We have previously shown that KSHV-encoded RAP can also stimulate transactivation of both the C/EBPα and p21 gene promoters by either C/EBPα or C/EBPβ up to 4- to 8-fold in Vero cells, although RAP alone had little effect (F.Y.W., C.-J. Chiou, and G.S.H., unpublished work). We initially examined endogenous C/EBPα levels in two different KSHV-positive PEL cell lines, BCBL-1 and JSC-1, and in two different forms of Burkitt lymphoma cell lines, the EBV-negative DG-75 and EBV-positive Akata, by immunoblotting after gel electrophoresis of cell lysates (Fig. 1A). Full-length 42-kDa form of C/EBPα was detected in most of the cell lines. Relatively low levels of C/EBPα were detected in HeLa cells.
Fig 1.
Correlation between RAP, C/EBPα, and p21 protein expression. (A) Western immunoblot showing the levels of endogenous C/EBPα protein in different human primary, tumor, and lymphoma cells. (B) Western immunoblot showing increased expression of the C/EBPα and p21 proteins in HeLa cells after transfection with the pSG5-RAP cDNA expression plasmid. (C–E) Western immunoblot analysis illustrating the levels of RAP, C/EBPα, p21, p27, and p16 protein in JSC-1 cells at 0, 24, 48, and 72 h after TPA induction.
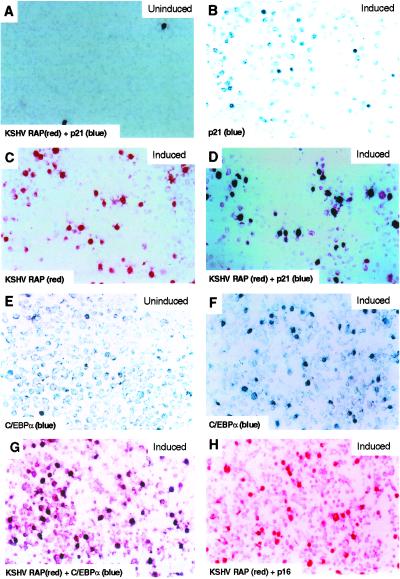
The KSHV-encoded RAP protein was not detected in KSHV-latent JSC-1 PEL cells, but was significantly induced at 24 h after the onset of lytic cycle induction by TPA and its expression persisted throughout the lytic cycle (Fig. 1C). The level of C/EBPα increased steadily between 24 and 72 h after TPA treatment of JSC-1 cells (Fig. 1D). Similarly, the fraction of BCBL-1 cells expressing nuclear C/EBPα increased from 4.9% before lytic induction to 19.7% after TPA treatment (Fig. 2F), coinciding with a dramatic increase in cells expressing RAP from 0.9% before (Fig. 2A) to 17% after lytic induction (Fig. 2C). Eighty-five percent of RAP-positive BCBL-1 cells also expressed C/EBPα as detected by double-label IHC (Fig. 2G), confirming that most lytically infected cells displayed increased C/EBPα expression. Likewise, 81% of the C/EBPα-positive cells were also RAP-positive (Fig. 2F).
Fig 2.
Immunohistochemical evidence for induction of p21 and C/EBPα protein expression only in lytically induced BCBL-1 cells that expressed the KSHV RAP protein. (A) In uninduced cultures, expression of RAP (Vecta Stain-red chromogen) and p21 (DAKO-True Blue chromogen) expression could only be detected in very few spontaneously lytic cell nuclei by double staining. (B and C) Increased numbers of cells expressing both p21 (blue) and KSHV RAP (red) 48 h after lytic reactivation by TPA induction. (D) Double-staining depicting colocalization of nuclear RAP (red) and p21 (blue) in a large fraction of the cells after TPA induction. (E) Low expression of C/EBPα protein (blue) in uninduced latent PEL cell culture. (F) Increased expression of C/EBPα (blue) in lytically activated cells after TPA induction. (G) Double-staining for KSHV RAP and C/EBPα in TPA-treated cells, showing induced C/EBPα (blue) expression colocalized in the nucleus predominantly in the same cells that were also expressing RAP (red). (H) No p16 protein (blue) expression was detected even in RAP-positive (red) cells after TPA induction.
Increased expression of both the RAP and C/EBPα proteins also proved to correlate with the strongly heightened expression of p21 detected by Western immunoblots between 24 and 72 h in the TPA-induced JSC-1 cells (Fig. 1E). Similarly, only 1.0% of BCBL-1 cells expressed p21 before lytic induction (Fig. 2A), whereas after induction, 18% of the cells expressed nuclear p21 (Fig. 2B). Again, double-label IHC revealed that 94% of the RAP protein-positive BCBL-1 cells also expressed p21 protein and 98% of p21-positive cells were also RAP-positive (Fig. 2D). Two other cdk inhibitor proteins associated with G1 cell cycle arrest—i.e., p16 and p27—were absent both by Western blotting (Fig. 1E) and IHC (Fig. 2H, and data not shown) in lytically induced BCBL-1 PEL cells, including those cells that were positive for RAP and C/EBPα. Analogous experiments performed with DG-75 lymphoma cells as negative controls revealed no induction of either the C/EBPα or p21 proteins after TPA treatment (data not shown), suggesting that KSHV lytic infection was responsible for the up-regulation of both of these proteins. These results imply that, unlike the EBV lytic cycle trigger protein ZTA (also known as BELF1 or Zebra), which reportedly induces both p21 and p27 protein expression (25), KSHV RAP protein induces G1 cell cycle arrest only through the p21 pathway.
Transient Expression of RAP in DNA-Transfected HeLa Cells Induces Endogenous C/EBPα and p21 Protein Expression.
Untreated HeLa cells express high levels of C/EBPβ, but only low levels of C/EBPα (Fig. 1B). However, after transfection with a mammalian cell expression plasmid encoding the KSHV RAP protein, expression of the 42-kDa form of C/EBPα was induced in a dose-responsive manner (Fig. 1B). Furthermore, the increase in C/EBPα level coincided with strongly induced expression of the p21 protein (Fig. 1B). Therefore, by removing RAP from the context of the KSHV genomes in lytically induced PEL cell lines, which are complicated by many other viral factors and by TPA treatment, we have shown that RAP protein alone directly induces both C/EBPα and p21 protein levels in HeLa cells, and therefore is probably responsible for the effect in induced PEL cells.
KSHV Lytically Infected BCBL-1 Cells Do Not Undergo S-Phase Progression.
Although it is known that S-phase progression in EBV-lytically infected cells is inhibited during viral DNA replication (6), whether a similar situation applies also to KSHV lytic infection has not been addressed. Because it is difficult to distinguish between cellular DNA replication during S phase and KSHV lytic DNA genome replication by using the BrdUrd-incorporation assay, which detects newly synthesized DNA in S-phase cells, we used PAA to inhibit KSHV viral DNA synthesis specifically. After 48 h of TPA induction of the KSHV lytic cycle in BCBL-1 cells in the presence of PAA, 12% of the cells were positive for RAP and thus undergoing lytic cycle progression; however, 94% of those cells did not incorporate BrdUrd. In contrast, of the 26% of the cells that were in S phase as judged by incorporation of BrdUrd, most were not in the lytic cycle and fewer than 4% were positive for RAP (Fig. 3 D–F). Therefore, like the lytic cycles of EBV and other herpesviruses, events in the KSHV lytic cycle seem to inhibit host G1 to S-phase cell cycle progression and host-cell DNA synthesis. However, despite this correlation, it cannot be assumed that inhibition of S-phase progression in KSHV-lytically infected BCBL-1 cells was caused by RAP itself, because other lytically induced viral proteins could also be responsible. Thus, we assessed the linkage between RAP protein expression and cell cycle arrest by using recombinant replication-defective adenovirus vectors that encode the isolated wild-type intact or mutant RAP protein genes.
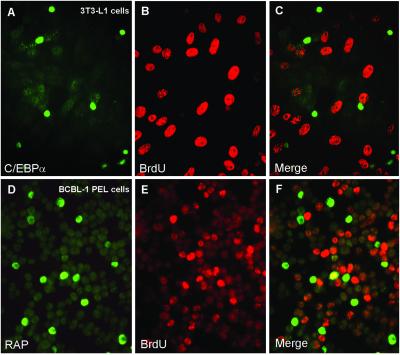
Fig 3.
Inhibition of S-phase progression by the C/EBPα and KSHV RAP proteins as detected by double-label IFA. (A and B) C/EBPα-mediated cell cycle arrest occurs in 3T3-L1 cells transfected with the pMSV-C/EBPα expression plasmid as demonstrated by the absence of BrdUrd pulse-labeling of newly synthesized DNA (B, rhodamine, red), in all of the cells expressing the C/EBPα protein (A, FITC, green). (C) Merge of the two frames. (D and E) At 48 h after TPA treatment in the presence of PAA to inhibit viral DNA synthesis, 15% of the BCBL-1 cells expressed the lytically induced RAP protein (D, green), but BrdUrd incorporation (E, red) revealed that very few (less than 4%) of the RAP-positive cells were undergoing cellular S phase. PAA treatment (400 μg/ml) for 48 h had no effect on cellular DNA replication during S phase as shown by positive BrdUrd incorporation (E, red). (F) Merge of the two frames.
An Adenovirus Vector Expressing the RAP Protein Inhibits G1 to S Cell Cycle Progression in Infected Fibroblast Cells Through the Induction of C/EBPα and p21.
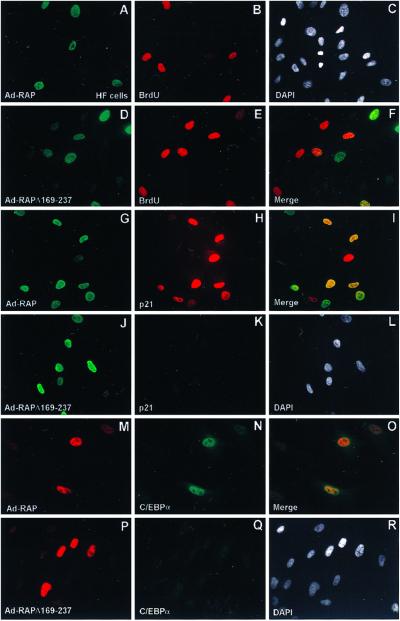
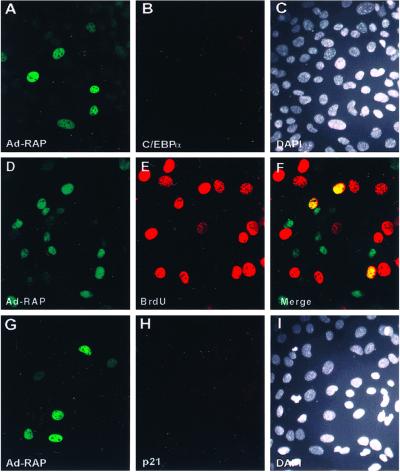
To confirm the reported ability of C/EBPα to arrest the cell cycle, we transfected a C/EBPα mammalian expression plasmid into mouse 3T3-L1 cells and pulsed-labeled the cells with BrdUrd. As shown in Fig. 3 A–C, expression of C/EBPα and the incorporation of BrdUrd in the same cell were mutually exclusive, being detected in <1% of the cell population. Thus, S-phase progression did not occur in C/EBPα-positive cells. To verify more definitively that RAP protein itself causes G1 cell cycle arrest mediated through C/EBPα, we conducted IFA and flow cytometric cell cycle analysis in HF. Because HF cells have very low DNA-transfection efficiency, we constructed recombinant Ad5ΔE1A/E1B adenovirus vectors that express either the wild-type 237-aa RAP protein (Ad-RAP) or a bZIP domain-deleted RAP mutant (Ad-RAPΔ169–237). HF cells were infected with the adenovirus vectors at low multiplicity (multiplicity of infection 0.5) to prevent cytotoxicity. After infecting with wild-type Ad-RAP, 21% of the cells expressed RAP and 29% of the cells were positive for BrdUrd (S phase), but only <1% of the cells were double-positive for both RAP and BrdUrd, indicating that S-phase progression was blocked in cells expressing the intact RAP protein (Fig. 4 A–C). In contrast, in cells infected with Ad-RAPΔ169–237, 13% were positive for mutant RAP protein in the nucleus, and 19% of the cells were positive for BrdUrd, but more than 10% of the cells were double-positive for both mutant RAP expression and BrdUrd (Fig. 4 D–F), indicating that no correlation exists between mutant RAP protein expression and cell cycle arrest. Among the HF cells that were positive for wild-type RAP, 82% of them were double-positive by IFA for both RAP and endogenous p21 protein (Fig. 4 G–I), whereas for those infected with Ad-RAPΔ169–237, mutant RAP expression and p21 induction were not correlated (Fig. 4 J–L). Similarly, of the HF cells positive for wild-type RAP (27%), 75% were double-positive for endogenous C/EBPα (Fig. 4 M–O); however, for those infected with Ad-RAPΔ169–237, expression of C/EBPα was not induced above the basal level (Fig. 4 P–R). Taken together, the IFA data strongly suggest that RAP alone is sufficient to inhibit S-phase cell cycle progression directly although induction of the C/EBPα and p21 proteins.
Fig 4.
KSHV RAP expressed by an adenovirus vector (Ad-RAP) induces C/EBPα and p21 protein expression in HF cells. (A and B) Double-label IFA showing the absence of BrdUrd incorporation (rhodamine, red) in most cells expressing the wild-type RAP protein (FITC, green), and (C) DAPI nuclear staining of the whole cell population. (D and E) Double-label IFA showing normal BrdUrd incorporation and S-phase patterns (red) in Ad-RAP(Δ169–237) expressing cells (green), and (F) merge illustrating that S phase occurs in RAP(Δ169–237)-positive cells. (G and H) Double-label IFA showing the induced expression of wild-type RAP (green) and p21 (red) in the same cell population, and (I), merge showing that p21 is induced only in RAP positive cells. (J and K) Double-label IFA showing RAP(Δ169–237) protein expression (green) and the absence of induced p21 protein (red) in the same cell population, and (L) merge showing that p21 expression is not up-regulated in Ad-RAP(Δ169–237)-positive cells. (M, N) Double-label IFA illustrating expression of RAP (red) and up-regulation of C/EBPα (green) in the same cell population, and (O) merge illustrating coexpression of C/EBPα only in RAP-positive cells. (P and Q) Double-label IFA showing RAP(Δ169–237) expression (red) and the absence of enhanced C/EBPα expression (green) in the same cell population, and (R) merge illustrating that RAP(Δ169–237) does not induce C/EBPα expression.
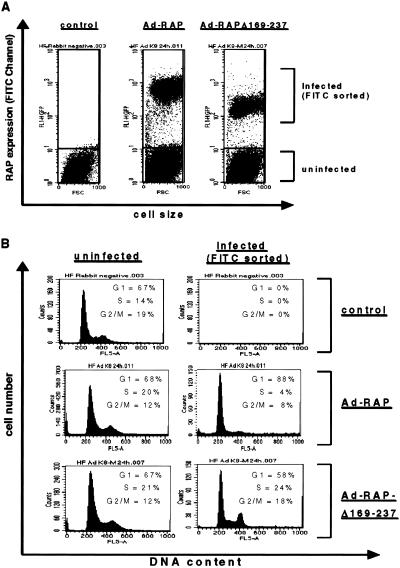
Finally, cell cycle analysis was performed by analytical fluorescence-activated cell sorting with anti-RAP polyclonal antibody to ascertain whether those Ad-vector infected HF cells that expressed RAP were indeed arrested in G1. For the gated fluorescence-positive cell population that expressed wild-type RAP, dramatic G1/S cell cycle arrest (87% G1, 4% S, 8% G2/M) was observed compared with the normal cell cycle profile of the nonexpressing cell population (68% G1, 20% S, 12% G2/M) in the same experiment (Fig. 5). Furthermore, among cells expressing the RAPΔ169–237 protein, a normal cell cycle profile with distinct G1 (58%), S (24%), and G2/M (18%) peaks was observed (Fig. 5). This result both showed that the Ad-vector alone failed to affect the cell cycle and further confirmed that wild-type RAP, but not RAPΔ169–237, could inhibit G1- to S-phase cell cycle progression in infected cells.
Fig 5.
Analytical fluorescence-activated cell sorting to demonstrate that the KSHV wild-type RAP protein but not mutant RAP causes G1/S cell cycle arrest in human HF cells. (A) RAP-positive cells in Ad-RAP (1–237) or Ad-RAP(Δ169–237) infected HF cell populations were sorted from the uninfected RAP-negative cells through an FITC-gated channel on the basis of the difference in their fluorescence signals. (B) Flow cytometric analysis showing that the wild-type RAP-expressing cells (FITC channel-gated) were predominantly arrested in G1 in comparison with either the uninfected cells from the same population, or the Ad-RAP(Δ169–237) mutant positive cells.
Recombinant Ad-RAP Fails to Induce p21 and Inhibit S-Phase Progression in C/EBPα Negative Cells.
Two separate experiments were conducted to determine whether KSHV RAP protein can induce p21 and cell cycle arrest in cells that either fail to express the C/EBPα protein or lack the C/EBPα gene all together. In the first case, a human epithelial breast cancer cell line MDA-MB 468 that lacks C/EBPα expression was used; and in the second case, C/EBPα (−/−) mouse embryonic fibroblasts (16) were used. For the (−/−) MEF cells infected with wild-type Ad-RAP, 17% were singly positive for RAP, 23% were singly positive for BrdUrd, and 16% were double-positive for both RAP and BrdUrd (Fig. 6 A–C). The results for MB 468 cells were similar (not shown), indicating that, unlike in Ad-RAP-infected HF cells, no correlation existed between RAP expression and the inhibition of S-phase progression in either of these cell types. Both the RAP-positive C/EBPα (−/−) MEF and MB 468 cells also failed to induce p21 protein expression (Fig. 6 G–I), although p21 could be induced in the MB 468 cells by mimosine treatment, which arrests cells in G1 (not shown). These findings proved that, although p21 was not affected in MB 468 or in (−/−) MEF cells, the absence of C/EBPα abolished the ability of RAP to induce p21 presumably because RAP itself cannot significantly activate the p21 gene promoter. Therefore, this experiment provided an important control showing that in C/EBPα-negative cells, intact wild-type RAP failed to inhibit S-phase progression, thereby confirming our model that RAP induces G1 cell cycle arrest through up-regulation of C/EBPα, which up-regulates p21 and inhibits cdk.
Fig 6.
The KSHV RAP protein cannot efficiently up-regulate p21 expression in C/EBPα knockout mouse embryonic fibroblast MEF (−/−) cells. (A and B) Double-label IFA showing the expression of RAP protein (FITC, green) in Ad-RAP-infected cells and the absence of C/EBPα protein (rhodamine, red) in the same cells. (C) DAPI nuclear staining of the whole cell population. (D and E) Double-label IFA of RAP protein expression (green) and BrdUrd incorporation (red) in Ad-RAP-infected cells. (F) Merge illustrating that S phase still proceeds in RAP-positive MEF (−/−) cells that lack C/EBPα. (G and H) Double-label IFA showing RAP protein expression (green) and the absence of mouse p21 induction (red) in RAP-positive (−/−) MEF cells after Ad-RAP infection. (I) DAPI nuclear staining of the same whole cell population.
Discussion
The use of very early functioning herpesvirus viral factors to elicit growth arrest ensures that cells are arrested before engaging in viral DNA replication as a way to eliminate cellular competition for resources. Although several candidate viral-encoded growth-arrest regulatory genes of herpesviruses, including IE2 and UL69 of HCMV, ICP0 of HSV, and ZTA of EBV, have been identified (6), their mechanism of action is unknown. Our previous studies showed that the KSHV RAP protein interacts with core components of the lytic DNA replication machinery and concentrates within replication compartments (4), suggesting that RAP has some direct involvement in viral DNA replication. Further analysis revealed that RAP and C/EBPα interact in vitro and that C/EBPα is also incorporated into KSHV viral DNA replication compartments in cotransfection assembly assays, but only in the presence of RAP (F.Y.W., C.-J. Chiou, and G.S.H., unpublished work). Therefore, we investigated possible biochemical and functional interactions between RAP and the C/EBPα family of proteins.
C/EBPα is identified here as a new and important cellular player in the KSHV lytic cycle process. Our results lead to five principal conclusions. First, during KSHV infection of both lymphoblast cells and primary dermal microvascular endothelial cells, G1 cell cycle arrest occurs in all cells progressing into the lytic cycle and synthesizing viral DNA. Second, KSHV RAP is responsible for the induction of the p21 cdk inhibitor and hence the onset of G1 cell cycle arrest. Third, deletion of the leucine zipper region of the RAP protein (which is needed for interaction with C/EBPα) abolishes its ability to arrest cells at the G1 stage of the cell cycle. Fourth, the mechanism of RAP-mediated G1 cell cycle arrest and p21 induction involves and is mediated by the induction of C/EBPα. Fifth, the absence of C/EBPα in C/EBPα (−/−) MEF cells severely compromises the ability of RAP to induce p21 and cell cycle arrest.
The mechanism for the induction of C/EBPα by RAP in PEL cells may involve either up-regulation of C/EBPα transcription or posttranslational protein stabilization through direct interaction with C/EBPα, probably both. The up-regulation of p21 may be due to both the induction of C/EBPα (15, 16) and the synergy between RAP and C/EBPα-mediated transcriptional activation of the p21 promoter (F.Y.W., C.-J. Chiou, and G.S.H., unpublished work). However, it is not yet clear whether p21 is solely responsible for the G1 cell cycle arrest that we observed, because C/EBPα itself can directly cause cell cycle arrest by inhibiting cdk2 and thereby E2F function in the absence of p21 (17, 18).
The EBV-encoded ZTA lytic transactivator protein also inhibits G1 to S-phase cell cycle progression in lytically activated lymphoma cells by inducing the expression of the cyclin-dependent kinase inhibitors, p21CIP-1 and p27KIP (25, 26). The KSHV-encoded RAP protein is evolutionarily related to ZTA and also contains a leucine zipper dimerization domain, but has no amino acid homology. Unlike ZTA, RAP is not known either to bind to DNA or to function as a direct transcriptional activator (4, 27). Both RAP and ZTA have been suggested to interact with and stabilize p53 (25, 28), but ZTA can also induce p21 expression through an unknown p53-independent pathway. Furthermore, RAP up-regulates the human p21 gene promoter in reporter gene assays only when cotransfected with C/EBPα and still does so in the Hep3B cell line lacking p53 [F.Y.W., Q.T., H.C., C.A.R., S. Wang, C.F., G. Liao, M.F., C. Chiou, J.C., et al. (2002), unpublished work], implying the utilization of a p53-independent pathway. The leucine zipper dimerization domain of EBV ZTA is known to be essential for the function of ZTA in G1 arrest (29), and RAP displays similar properties, with its leucine zipper being critical for both transcriptional synergy with C/EBPα and for binding to C/EBPα (F.Y.W., C.-J. Chiou, and G.S.H., unpublished work), and for p21 induction and cell cycle arrest as shown here.
The strong inhibition of G1 to S cell cycle progression observed in HF cells suggests that the interaction and synergy between C/EBPα and RAP is sufficient to up-regulate both the C/EBPα and p21 proteins. We cannot, however, discount the possibility that RAP may cause G1 arrest through other mechanisms. Nevertheless, C/EBPα whose expression is induced by RAP is both necessary and sufficient for G1 arrest. However, herpesviruses are known to be highly redundant in manipulating the host machinery and frequently elicit more than a single pathway of action. Because the RAP protein can be phosphorylated by cyclin-dependent kinases (30), an examination of its potential ability to interact with cellular Cdks, pRB, or E2F are worthy of further investigation.
Acknowledgments
We thank Dr. Gretchen J. Darlington (Baylor College of Medicine) for kindly providing the MEF (−/−) cells, and we also thank Drs. Diane Hayward, Jian Huang, Gangling Liao, Shizhen Emily Wang, and Qizhi Zheng for valuable advice or technical assistance. These studies were funded by National Cancer Institute Research Grants R01 CA73585 and RO1 CA81400 (to G.S.H.), DK 38418 (to M.D.L.), and DK61355 (to Q.Q.T.) from the National Institutes of Health. F.Y.W. was a graduate student in Departments of Pharmacology and Molecular Sciences and in the Viral Oncology Program of the Sidney Kimmel Comprehensive Cancer Center at The Johns Hopkins University School of Medicine and was supported in part by the Anti-Cancer Drug Development Training Program (T32 CA09243).
Abbreviations
KS, Kaposi sarcoma
KSHV, KS-associated herpesvirus
PEL, primary effusion lymphoma
DAPI, 4′,6-diamidino-2-phenylindole
EBV, Epstein–Barr virus
RAP, replication-associated protein
MEF, mouse embryonic fibroblast
PAA, phosphonoacetic acid
HF, human diploid fibroblast cells
IFA, immunofluorescence assay
TPA, phorbol 12-tetradecanoate 13-acetate
References
- 1.Chang Y., Cesarman, E., Pessin, M. S., Lee, F., Culpepper, J., Knowles, D. M. & Moore, P. S. (1994) Science 266, 1865-1869. [DOI] [PubMed] [Google Scholar]
- 2.Whitby D., Howard, M. R., Tenant-Flowers, M., Brink, N. S., Copas, A., Boshoff, C., Hatzioannou, T., Suggett, F. E., Aldam, D. M., Denton, A. S., et al. (1995) Lancet 346, 799-802. [DOI] [PubMed] [Google Scholar]
- 3.Ciufo D. M., Cannon, J. S., Poole, L. J., Wu, F. Y., Murray, P., Ambinder, R. F. & Hayward, G. S. (2001) J. Virol. 75, 5614-5626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu F. Y., Ahn, J. H., Alcendor, D. J., Jang, W. J., Xiao, J., Hayward, S. D. & Hayward, G. S. (2001) J. Virol. 75, 1487-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhong L. & Hayward, G. S. (1997) J. Virol. 71, 3146-3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flemington E. K. (2001) J. Virol. 75, 4475-4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Landschulz W. H., Johnson, P. F. & McKnight, S. L. (1988) Science 240, 1759-1764. [DOI] [PubMed] [Google Scholar]
- 8.Darlington G. J., Ross, S. E. & MacDougald, O. A. (1998) J. Biol. Chem. 273, 30057-30060. [DOI] [PubMed] [Google Scholar]
- 9.Lane M. D., Tang, Q. Q. & Jiang, M. S. (1999) Biochem. Biophys. Res. Commun. 266, 677-683. [DOI] [PubMed] [Google Scholar]
- 10.Zhang D. E., Zhang, P., Wang, N. D., Hetherington, C. J., Darlington, G. J. & Tenen, D. G. (1997) Proc. Natl. Acad. Sci. USA 94, 569-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang X., Scott, E., Sawyers, C. L. & Friedman, A. D. (1999) Blood 94, 560-571. [PubMed] [Google Scholar]
- 12.Christy R. J., Kaestner, K. H., Geiman, D. E. & Lane, M. D. (1991) Proc. Natl. Acad. Sci. USA 88, 2593-2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Timchenko N., Wilson, D. R., Taylor, L. R., Abdelsayed, S., Wilde, M., Sawadogo, M. & Darlington, G. J. (1995) Mol. Cell. Biol. 15, 1192-1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang Q. Q. & Lane, M. D. (1999) Genes Dev. 13, 2231-2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Timchenko N. A., Wilde, M., Nakanishi, M., Smith, J. R. & Darlington, G. J. (1996) Genes Dev. 10, 804-815. [DOI] [PubMed] [Google Scholar]
- 16.Timchenko N. A., Harris, T. E., Wilde, M., Bilyeu, T. A., Burgess-Beusse, B. L., Finegold, M. J. & Darlington, G. J. (1997) Mol. Cell. Biol. 17, 7353-7361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Slomiany B. A., D'Arigo, K. L., Kelly, M. M. & Kurtz, D. T. (2000) Mol. Cell. Biol. 20, 5986-5997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang H., Iakova, P., Wilde, M., Welm, A., Goode, T., Roesler, W. J. & Timchenko, N. A. (2001) Mol. Cell 8, 817-828. [DOI] [PubMed] [Google Scholar]
- 19.Harris T. E., Albrecht, J. H., Nakanishi, M. & Darlington, G. J. (2001) J. Biol. Chem. 276, 29200-29209. [DOI] [PubMed] [Google Scholar]
- 20.Cannon J. S., Ciufo, D., Hawkins, A. L., Griffin, C. A., Borowitz, M. J., Hayward, G. S. & Ambinder, R. F. (2000) J. Virol. 74, 10187-10193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hardy S., Kitamura, M., Harris-Stansil, T., Dai, Y. & Phipps, M. (1997) J. Virol. 71, 1842-1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu Y., Ahn, J.-H, Cheng, M., ApRhys, C. A., Chiou, C.-J., Zong, J. L., Matunis, M. J. & Hayward, G. S. (2001) J. Virol. 75, 10683-10695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poole L. J., Xu, Y.-X., Kim, P. S., Zheng, Q.-Z., Pevsner, J. & Hayward, G. S. (2002) J. Virol. 76, 3395-3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou S., Fujimuro, M., Hsieh, J. J., Chen, L., Miyamoto, A., Weinmaster, G. & Hayward, S. D. (2000) Mol. Cell. Biol. 20, 2400-2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cayrol C. & Flemington, E. K. (1996) EMBO J. 15, 2748-2759. [PMC free article] [PubMed] [Google Scholar]
- 26.Chang Y. N., Dong, D. L., Hayward, G. S. & Hayward, S. D. (1990) J. Virol. 64, 3358-3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin S. F., Robinson, D. R., Miller, G. & Kung, H. J. (1999) J. Virol. 73, 1909-1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodriguez A., Armstrong, M., Dwyer, D. & Flemington, E. (1999) J. Virol. 73, 9029-9038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cayrol C. & Flemington, E. (1996) J. Biol. Chem. 271, 31799-31802. [DOI] [PubMed] [Google Scholar]
- 30.Polson A. G., Huang, L., Lukac, D. M., Blethrow, J. D., Morgan, D. O., Burlingame, A. L. & Ganem, D. (2001) J. Virol. 75, 3175-3184. [DOI] [PMC free article] [PubMed] [Google Scholar]