Abstract
Insulin resistance and β cell toxicity are key features of type 2 diabetes. One leading hypothesis suggests that these abnormalities result from excessive flux of nutrients through the UDP–hexosamine biosynthetic pathway leading to “glucose toxicity.” How the products of the hexosamine pathway mediate these effects is not known. Here, we show that transgenic overexpression of an enzyme using UDP-GlcNAc to modify proteins with O-GlcNAc produces the type 2 diabetic phenotype. Even modest overexpression of an isoform of O-GlcNAc transferase, in muscle and fat, leads to insulin resistance and hyperleptinemia. These data support the proposal that O-linked GlcNAc transferase participates in a hexosamine-dependent signaling pathway that is linked to insulin resistance and leptin production.
Insulin resistance is a prominent feature of type 2 diabetes. Clinically, insulin resistance is most easily characterized by a failure of insulin to stimulate normal glucose uptake into its target tissues of striated muscle and fat. Although insulin resistance appears to play a primary pathogenic role in type 2 diabetes (1, 2), such resistance can also be acquired as a result of excess nutrient delivery to muscle cells (3). Furthermore, it has been suggested that insulin resistance provides an adaptive mechanism designed to protect muscle from excess nutrient loading and to favor shunting of excess calories for long-term storage as fat (4). Thus, insulin resistance would be a consequence of overfeeding that can appear well before glucose intolerance or diabetes (1). In experimental models, insulin resistance is induced within a few hours by excess glucose (3).
Evidence has accumulated that glucose flux through the hexosamine biosynthetic pathway may provide a nutrient-sensing mechanism that is responsible for glucose-induced insulin resistance (5). For example, we have reported that targeted overexpression of the rate-limiting enzyme for hexosamine synthesis in the striated muscle and fat of transgenic mice leads to insulin resistance (6). This insulin resistance was phenotypically similar to that observed in human type 2 diabetes. Specifically, the insulin resistance was characterized by decreased insulin-dependent recruitment of GLUT4 to the plasma membrane and was reversed by the thiazolidinedione antidiabetic drug troglitazone (7). Significantly, glucose also up-regulates the ob gene via the hexosamine pathway, which leads to enhanced leptin expression (8, 9). Insulin resistance caused by free fatty acids has also been suggested to be sensed through the hexosamine pathway (10). These data support the function of the hexosamine biosynthetic pathway as a central nutrient sensor for both glucose and free fatty acids.
How the products of the hexosamine pathway might exert nutrient sensing or regulate signal transduction is not known. A leading hypothesis suggests that the terminal metabolite of the pathway, UDP-GlcNAc, is used as a substrate by the recently cloned O-linked GlcNAc transferase (OGT) (11–14). O-linked glycosylation by GlcNAc modifies the serine and threonine residues of cytosolic and nuclear proteins and, like phosphorylation, can change the function of such proteins as Sp1 and endothelial nitrogen oxide synthase (15, 16). Given the relationship between the hexosamine pathway and the development of insulin resistance, we sought to determine whether these effects were mediated through the O-glycosylation pathway. Here, we have expressed OGT under control of the GLUT4 promoter and demonstrated that overexpression of the glycosyltransferase induces insulin resistance and hyperleptinemia.
Methods
Transgenic Animals.
The human GLUT4 promoter, containing the transcription initiation site, was used to target transgenic expression of human OGT to adipose tissue and cardiac and skeletal muscle. A 2.1-kb EcoRI–ApaI fragment from the modified pHSS6 plasmid (17) was ligated upstream of human OGT into Lv4F-cytomegalovirus (CMV)-sport partially digested with EcoRI and ApaI (11). The 5.8-kb transgene construct was excised from CMV-sport by using KpnI and PvuI, purified and microinjected into one-cell mouse embryos. These embryos were reimplanted into pseudopregnant FVB female mice at the National Institutes of Health. Heterozygous transgenic mice and control nontransgenic animals from the same litters were used in experiments that were approved by the Laboratory Animal Use Committees at the National Institutes of Health, University of Utah Medical Center, and the Salt Lake City Veterans Affairs Medical Center.
DNA and RNA Analysis.
Founders were screened by Southern blot analysis and PCR. EcoRI digests of chromosomal DNA (10 μg) isolated from mouse tails were transferred to nylon membranes (DuPont). The human OGT probe was obtained by digesting Lv4F-cytomegalovirus-sport with EcoRV/XbaI (2.8 kb) and labeled with [α-32P]dCTP using a random prime labeling kit (Pharmacia). Membranes were hybridized as previously described (11). PCR primers (5′-CAGCCAAACTCTAAACCCCA-3′, 5′-TGCAAACCACCATGTTCAGT-3′) were constructed to make a 329-bp product extending from the GLUT4 promoter into the human OGT sequence. The expected PCR product could be screened with BamHI, which cleaves it into 178- and 151-bp fragments.
Quantification of mRNA was accomplished by reverse transcription followed by quantitative PCR (RT-PCR). Skeletal muscle was dissected, frozen in liquid nitrogen, pulverized under liquid nitrogen, and homogenized in Trizol Reagent. RNA was isolated according to the manufacturer's protocol (Invitrogen). First-strand cDNA synthesis was carried out by using 600 ng of total RNA, 250 ng of random hexamer primers, and Maloney murine leukemia virus reverse transcriptase (Invitrogen). Real-time PCR was performed with a rapid thermal cycler (LightCycler, Roche Diagnostics). PCR reactions included: 4 ng of template/0.5 μM each primer/200 μM each dNTP/50 mM Tris, pH 8.3/500 μg/ml of nonacetylated BSA/3 mM MgCl2/0.04 units/μl of platinum TaqDNA polymerase (Invitrogen)/1:30,000 dilution of SYBR green I fluorescent dye (Molecular Probes). Mouse leptin primers 5′-CGGTTCCTGTGGCTTTGG-3′ and 5′-GGTCTGAGGCAGGGAGCA-3′ amplified a 345-bp product. For transgene expression measurements, human OGT primers 5′-CGGGCTATCGAACTACAACCA-3′ and 5′-CCCATATTAGAGTAGGCATCAGCAAAG-3′ amplified a 356-bp product. Mouse cyclophilin-A primers 5′-AGCACTGGAGAGAAAGGATTTGG-3′ and 5′-TCTTCTTGCTGGTCTTGCCATT-3′ amplified a 349-bp product. Amplification used 32–40 four-step cycles, with 20°C/s temperature change between steps. Steps were 95°C, 0 s; 58°C, 0 s; 72°C, 11 s; and 78°C, 1 s, with fluorescence detected during the fourth step. The cycle number corresponding to the second derivative maximum was determined for all cDNA amplifications of five-point dilutions of a control cDNA. Analyses of the melting curves and visualization of the DNA products on agarose TAE gel confirmed the presence of predicted DNA product and absence of nonspecific product. Results were normalized to levels of cyclophilin A (18).
OGT Assay.
Muscle was dissected from transgenic and control littermates, frozen, pulverized in liquid nitrogen, and extracted with 10 mM Hepes at pH 7.4 containing 10 mM MgCl2, 5% glycerol, and protease inhibitors (Complete Mini, Roche Diagnostics). After thorough mixing, the preparation was centrifuged (20,000 × g for 30 min). Supernatant fluids were assayed as described (19) by using 0.1 μmol of the casein kinase II acceptor peptide PGGSTPVSSANMM (DNA/peptide facility, University of Utah, Salt Lake City, UT).
Glucose, Insulin, Free Fatty Acid, and Triglyceride Levels in Serum.
Glucose levels were measured by a glucometer and levels were also independently performed by Analytics (Gaithersburg, MD). Insulin concentrations were measured by using the Linco sensitive rat insulin RIA kit (Linco Research, St. Charles, MO). Leptin levels were measured by RIA (Analytics) by using mouse leptin as a standard. Levels of UDP-N-acetylhexosamines were assayed by HPLC as described (20).
Glucose Tolerance Test and Determination of Glucose Disposal Rates.
All experiments were performed in weight-matched nonsedated transgenic and littermate control mice. After a 12-h fast, a glucose load of 1 mg/g of body weight was administered i.p. Tail-vein blood was sampled for blood glucose determination (Miles Elite glucometer) before and 5, 15, 30, 60, 90, and 120 min after glucose administration. Insulin sensitivity for glucose disposal was assessed by using the hyperinsulinemic–euglycemic clamp technique previously described (6). Animals, fasted overnight, were infused with recombinant human insulin (HumulinR, Lilly Research Laboratories, Indianapolis) through a right internal jugular venous catheter at a rate of 20 milliunits/kg/min, while 50% dextrose was infused by a variable infusion pump (Harvard Apparatus). Whole-blood samples (3 μl) were collected every 5–10 min from tail bleeds, and glucose concentration was measured by glucometer.
Results
Overexpression of OGT in Skeletal Muscle and Fat.
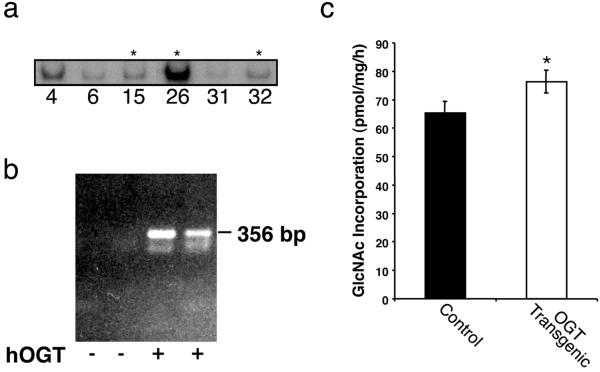
A total of eight founder mice were identified by PCR and Southern analysis. The results obtained for representative lines (indicated by the numbers) are shown in Fig. 1a. The bulk of our subsequent studies were done on the three lines indicated by the asterisks in Fig. 1a. There was no relationship between GLUT4-OGT copy number and OGT expression or transgenic phenotype. On the basis of the Southern analysis and expression levels, five founder mice (15, 18, 23, 26, and 32) were chosen to breed into a C57BL6 background. Presence of the OGT transgene mRNA in muscle (Fig. 1b, third lane) and fat (fourth lane) was verified in these lines by RT-PCR. OGT mRNA was not detected in liver or kidney (not shown). The RT-PCR products were not seen in the absence of reverse transcriptase, indicating that the signals were not the result of contamination by genomic DNA. Modest increases in the levels of total OGT activity were detected in skeletal muscle extracts by enhanced GlcNAc labeling of a synthetic peptide in vitro (Fig. 1c). A modest increase in activity was observed in all of the lines. Failure to detect robust activity in the transgenic lines suggests that such expression may be toxic. As would be expected, the concentration of UDP-GlcNAc, the substrate for OGT, was not altered in transgenic muscle and epididymal fat pads (not shown).
Fig 1.
OGT expression in control and transgenic mice. (a) Southern analysis of OGT transgenics (asterisks indicate three of the transgenic lines chosen for subsequent analysis). Isolated DNA from the founder mice was probed with an [α-32P]-labeled DNA fragment corresponding to 2.8 kb of the OGT coding region. (b) Presence of OGT cDNA products after RT-PCR of muscle and fat tissue. Quantification of human OGT mRNA transgene expression in muscle (lane 3) and fat (lane 4) was perfomed by RT-PCR. Lanes 1 and 2 represent control animals containing no transgene. (c) OGT activity in muscle of control and transgenic mice. Muscle from control and transgenic littermates was assayed for OGT activity by using casein kinase II as an acceptor peptide and measuring GlcNAc incorporation. (P < 0.05).
General Characteristics of the Transgenic Mice.
Transgenic mice had normal weights compared with their nontransgenic littermate controls, both at 2–4 and 17 mo of age (Table 1). Epidydimal fat pads were normal in both weight and appearance. No differences in muscle histology were noted on light microscopic examination of sections stained by hematoxylin/eosin (not shown). Fasting glucose values were also indistinguishable.
Table 1.
Characteristics of male control and OGT-transgenic mice
| Control | OGT transgenic | |
|---|---|---|
| Weight (2–4 mo), g | 22.9 ± 2.1 | 23.2 ± 2.4 |
| Weight (17 mo), g | 42.3 ± 0.6 | 42.2 ± 0.7 |
| Epidydimal fat pad weight, mg | 128 ± 6 | 121 ± 11 |
| Fasting glucose, mM | 5.0 ± 0.3 | 5.5 ± 0.2 |
| GLUT4 expression | 1.32 ± 0.08 | 1.49 ± 0.43 |
Results are the means ± SE for 7–12 determinations per group for weights and fasting glucose, and three determinations per group for GLUT4 expression. GLUT4 expression is given in arbitrary units of density from films of immunoblots.
The GLUT4 promoter was used in these studies to direct OGT expression to the target tissues of striated muscle and fat. An important control experiment was performed to ensure that GLUT4-dependent expression did not have an adverse effect on expression of GLUT4 itself, because GLUT4 is essential for normal insulin-stimulated glucose disposal in these tissues. Levels of GLUT4 protein, assessed by immunoblotting, did not differ in the transgenic animals (Table 1). Therefore, use of the GLUT4 promoter did not appear to diminish the activity of the endogenous promoter or associated transcription factors.
Insulin Resistance in Transgenic Animals.
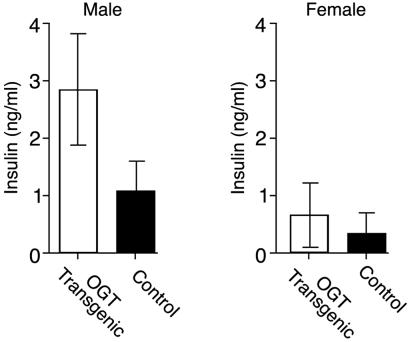
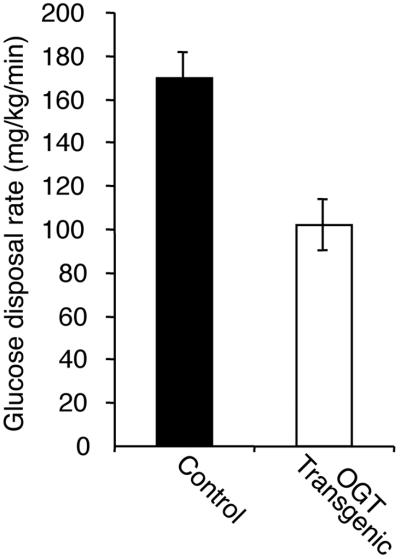
Similar to previously generated mice that overexpressed glutamine:fructose amido transferase (GFAT) in insulin responsive tissues, insulin levels of the OGT overexpressing mice in the random fed state were also significantly higher than in the controls (Fig. 2, P < 0.02). Although both male and female transgenic mice showed elevated serum insulin levels, the hyperinsulinemia was more pronounced in males. Despite the hyperinsulinemia, both males and females had normal glucose tolerance. Insulin resistance was confirmed by using the hyperinsulinemic–euglycemic clamp technique. Transgenic male mice had maximal glucose disposal rates that were 46% lower than controls (Fig. 3, P < 0.01), whereas the females exhibited no statistical differences. These studies were performed on mice of equal weights.
Fig 2.
Insulin levels in random-fed control and OGT transgenic mice. Insulin levels were measured in over 100 control and transgenic littermates. Measurements were performed by using a rat insulin RIA. Differences between serum insulin levels were statistically significant (P < 0.02).
Fig 3.
Glucose disposal rates assessed by the hyperinsulinemic–euglycemic clamp technique. Transgenic (n = 4) and control (n = 5) male mice were infused with maximally active insulin concentrations and their glucose disposal rates determined (P < 0.01, control vs. transgenic). Weights of the mice in the two groups were equivalent (control 24.6 ± 2.1 g, transgenic 25.4 ± 3.0 g).
Leptin Up-Regulation.
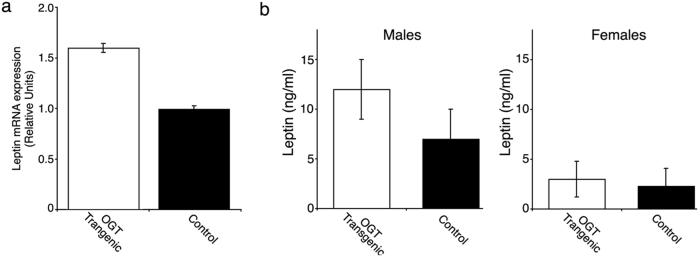
It has been previously demonstrated that the ob gene and the levels of its product leptin are regulated by hexosamines (8). If this regulation occurred through the O-glycosylation pathway, OGT transgenic mice should have a similar degree of leptin up-regulation. Our data support this hypothesis (Fig. 4). A 70% increase in leptin mRNA (Fig. 4a; P < 0.0005) was seen in the transgenic male mice, paralleled by a similar increase in serum leptin levels (Fig. 4b).
Fig 4.
Leptin mRNA (a) and protein (b) levels in control and OGT mice. (a) Leptin mRNA was quantified by using total RNA from fat pads from three wild type and four transgenic, random-fed, male mice. Shown are means ± SE. from duplicate determinations from each mouse (P < 0.0005). (b) Serum leptin levels were assayed by RIA as described in Methods.
Discussion
Excess nutrient intake plays a major role in the pathogenesis of type 2 diabetes and in the higher cardiovascular mortality associated with diabetes and obesity. How these nutrient fluxes lead to changes in cell metabolism and gene expression, however, is not known. Many aspects of glucose sensing have been shown to occur as a result of the metabolic conversion of glucose to hexosamines (6, 21–26). In the presence of chronic hexosamine excess, these varied pathways can lead to many of the hallmarks of type 2 diabetes, such as obesity, hypertriglyceridemia, β cell failure, and hyperglycemia (25, 26). The relevant end product of the hexosamine pathway, UDP-GlcNAc, is the precursor for intracellular glycosylation by OGT. As is shown here, even a modest (approximately 20%) overexpression of an isoform of this enzyme (OGT) in fat and muscle under the control of the GLUT4 promoter leads to insulin resistance and hyperleptinemia. The hyperinsulinemia and hyperleptinemia observed in the OGT transgenics is intriguing because the mice are neither overtly hyperglycemic nor obese. A likely explanation is that both hyperinsulinemia and hyperleptinemia result from a disregulated hexosamine-signaling pathway. Convincing evidence suggests that insulin increases leptin synthesis and secretion in a fashion dependent on the hexosamine pathway (8, 9, 27–30). Our findings provide strong evidence that OGT can mediate the effects attributed to the hexosamine pathway. Therefore, OGT appears to be the terminal step in a hexosamine-signaling pathway; signal transduction occurs by O-GlcNAc modification of numerous target proteins in a manner similar to phosphorylation. The upstream components of this pathway are highly regulated. For example, mammalian GFAT is feedback inhibited by UDP-GlcNAc, such that levels of this metabolite are maintained under tight metabolic control. In yeast, where OGT is absent, GFAT does not exhibit such feedback inhibition. Given the tight regulation of hexosamine metabolism, it is perhaps not surprising that only modest levels of OGT overexpression were tolerated in the transgenic lines that we have generated (Fig. 1).
The existence of intracellular O-linked GlcNAc was recognized relatively recently (31–33). Since then, numerous proteins have been shown to carry O-GlcNAc residues as identified in nuclear pore proteins, components of the transcriptional machinery, and signal transduction proteins (34–37). Cloning of the O-linked GlcNAc transferase (11, 12, 38) and O-GlcNAcase has now provided a means for exploring the function of O-GlcNAc addition. Unfortunately, targeted gene disruption results in embryonic lethality (39), thereby leaving the precise function of OGT unknown.
Recent studies on the metabolic consequences of elevated glucose/hexosamine levels have provided a body of impressive data supporting the existence of a hexosamine-signaling pathway. First, enrichment of OGT in the pancreatic β cells and the physiologic increase in the levels of O-GlcNAc residues present in these cells suggested that OGT may participate in glucose sensing and/or the regulation of insulin secretion (40). Second, transcription factor glycosylation appears to regulate gene expression. For example, transcription of the gene for transforming growth factor-α (TGFα) was shown to be glucose- and glucosamine-responsive; that responsiveness mapped to Sp1 sites in the TGFα promoter (41). Sp1 was the first of a large number of Pol II transcription factors shown to carry the O-GlcNAc modification (35). Addition of O-GlcNAc has been shown to affect rates of proteasomal degradation and transcriptional activity of Sp1 (15, 42). The effects of glucose on regulation of plasminogen activator inhibitor 1 and transforming growth factor-β, proteins that are thought to play a role in the complications of diabetes, have also been shown to correlate with the degree of Sp1 glycosylation (43). Third, O-linked GlcNAc modification of endothelial nitrogen oxide synthase (eNOS) was recently demonstrated; these findings were used to explain the nonresponsiveness of eNOS to insulin stimulation in diabetes (16). O-glycosylation may also mediate the effects of superoxide production from mitochondria in response to hyperglycemia (44). Finally, a number of important targets of OGT have been identified that may mediate more rapid responses to nutrient excess. We have previously shown that a key regulatory enzyme, glycogen synthase kinase, is a substrate for OGT (36). Glycogen synthase activity has been shown to increase with glucosamine treatment (45). In addition, components of GLUT4 vesicles may be glycosylated in response to elevated hexosamine signaling (46, 47). These and other substrates yet to be defined may mediate a rapid response to elevated flux through the hexosamine-signaling pathway terminating in OGT.
O-linked glycosylation appears to be ideally suited for a role in nutrient sensing. Generation of UDP-GlcNAc and intracellular levels of O-linked GlcNAc are responsive to extracellular glucose (48), lipid, and amino acid (glutamine) fluxes (10), suggesting a more general nutrient-sensing role. The data presented here demonstrate a central role for OGT in the insulin and leptin-signaling cascades. The findings further suggest a more general role for glycan-dependent signaling in nutrient sensing and the pathogenesis of type II diabetes mellitus.
Acknowledgments
We acknowledge Trent Smith for his assistance in data collection. This work was supported by the Research Service of the Veterans Affairs Medical Center, Salt Lake City, UT, by the Ben B. and Iris M. Margolis Foundation, and by the National Institutes of Health [DK42356 and Z01DK60200-01 (intramural)].
Abbreviations
OGT, O-linked GlcNAc transferase
RT-PCR, reverse transcription–PCR
References
- 1.Kahn B. B. & Flier, J. S. (2000) J. Clin. Invest. 106, 473-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kruszynska Y. T. & Olefsky, J. M. (1996) J. Invest. Med. 44, 413-428. [PubMed] [Google Scholar]
- 3.Rossetti L., Giaccari, A. & DeFronzo, R. A. (1990) Diabetes Care 13, 610-630. [DOI] [PubMed] [Google Scholar]
- 4.McClain D. A. & Crook, E. D. (1996) Diabetes 45, 1003-1009. [DOI] [PubMed] [Google Scholar]
- 5.Rossetti L. (2000) Endocrinology 141, 1922-1925. [DOI] [PubMed] [Google Scholar]
- 6.Hebert L. F. J., Daniels, M. C., Zhou, J., Crook, E. D., Turner, R. L., Simmons, S. T., Neidigh, J. L., Zhu, J. S., Baron, A. D. & McClain, D. A. (1996) J. Clin. Invest. 98, 930-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooksey R. C., Hebert, L. F. J., Zhu, J. H., Wofford, P., Garvey, W. T. & McClain, D. A. (1999) Endocrinology 140, 1151-1157. [DOI] [PubMed] [Google Scholar]
- 8.Wang J., Liu, R., Hawkins, M., Barzilai, N. & Rossetti, L. (1998) Nature (London) 393, 684-688. [DOI] [PubMed] [Google Scholar]
- 9.McClain D. A., Alexander, T., Cooksey, R. C. & Considine, R. V. (2000) Endocrinology 141, 1999-2002. [DOI] [PubMed] [Google Scholar]
- 10.Hawkins M., Barzilai, N., Liu, R., Hu, M., Chen, W. & Rossetti, L. (1997) J. Clin. Invest. 99, 2173-2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lubas W. A., Frank, D. W., Krause, M. & Hanover, J. A. (1997) J. Biol. Chem. 272, 9316-9324. [DOI] [PubMed] [Google Scholar]
- 12.Kreppel L. K., Blomberg, M. A. & Hart, G. W. (1997) J. Biol. Chem. 272, 9308-9315. [DOI] [PubMed] [Google Scholar]
- 13.Hanover J. A. (2001) FASEB J. 15, 1865-1876. [DOI] [PubMed] [Google Scholar]
- 14.Wells L., Vosseller, K. & Hart, G. W. (2001) Science 291, 2376-2378. [DOI] [PubMed] [Google Scholar]
- 15.Yang X., Su, K., Roos, M. D., Chang, Q., Paterson, A. J. & Kudlow, J. E. (2001) Proc. Natl. Acad. Sci. USA 98, 6611-6616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Du X. L., Edelstein, D., Dimmeler, S., Ju, Q., Sui, C. & Brownlee, M. (2001) J. Clin. Invest. 108, 1341-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buse J. B., Yasuda, K., Lay, T. P., Seo, T. S., Olson, A. L., Pessin, J. E., Karam, J. H., Seino, S. & Bell, G. I. (1992) Diabetes 41, 1436-1445. [DOI] [PubMed] [Google Scholar]
- 18.Simpson D. A., Feeney, S., Boyle, C. & Stitt, A. W. (2000) Mol. Vis. 6, 178-183. [PubMed] [Google Scholar]
- 19.Comer F. I. & Hart, G. W. (2001) Biochemistry 40, 7845-7852. [DOI] [PubMed] [Google Scholar]
- 20.Daniels M. C., Ciaraldi, T. P., Nikoulina, S., Henry, R. R. & McClain, D. A. (1996) J. Clin. Invest. 97, 1235-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marshall S., Bacote, V. & Traxinger, R. R. (1991) J. Biol. Chem. 266, 4706-4412. [PubMed] [Google Scholar]
- 22.Robinson K. A., Sens, D. A. & Buse, M. G. (1993) Diabetes 42, 1333-1346. [DOI] [PubMed] [Google Scholar]
- 23.Baron A. D., Zhu, J. S., Zhu, J. H., Weldon, H., Maianu, L. & Garvey, W. T. (1995) J. Clin. Invest. 96, 2792-2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rossetti L., Hawkins, M., Chen, W., Gindi, J. & Barzilai, N. (1995) J. Clin. Invest. 96, 132-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang J., Neidigh, J. L., Cooksey, R. C. & McClain, D. A. (2000) Diabetes 49, 1492-1499. [DOI] [PubMed] [Google Scholar]
- 26.Veerababu G., Tang, J., Hoffman, R. T., Daniels, M. C., Hebert, L. F. J., Crook, E. D., Cooksey, R. C. & McClain, D. A. (2000) Diabetes 49, 2070-2078. [DOI] [PubMed] [Google Scholar]
- 27.Coleman R. A. & Herrmann, T. S. (1999) Diabetologia 42, 639-646. [DOI] [PubMed] [Google Scholar]
- 28.Considine R. V., Cooksey, R. C., Williams, L. B., Fawcett, R. L., Zhang, P., Ambrosius, W. T., Whitfield, R. M., Jones, R., Inman, M., Huse, J. & McClain, D. A. (2000) J. Clin. Endocrinol. Metab. 85, 3551-3556. [DOI] [PubMed] [Google Scholar]
- 29.Considine R. V. (2001) Rev. Endocr. Metab. Disord. 2, 357-363. [DOI] [PubMed] [Google Scholar]
- 30.Zhang P., Klenk, E. S., Lazzaro, M. A., Williams, L. B. & Considine, R. V. (2002) Endocrinology 143, 99-106. [DOI] [PubMed] [Google Scholar]
- 31.Torres C. R. & Hart, G. W. (1984) J. Biol. Chem. 259, 3308-3317. [PubMed] [Google Scholar]
- 32.Holt G. D. & Hart, G. W. (1986) J. Biol. Chem. 261, 8049-8057. [PubMed] [Google Scholar]
- 33.Hanover J. A., Cohen, C. K., Willingham, M. C. & Park, M. K. (1987) J. Biol. Chem. 262, 9887-9894. [PubMed] [Google Scholar]
- 34.Snow C. M., Senior, A. & Gerace, L. (1987) J. Cell. Biol. 104, 1143-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jackson S. P. & Tjian, R. (1988) Cell 55, 125-133. [DOI] [PubMed] [Google Scholar]
- 36.Lubas W. A. & Hanover, J. A. (2000) J. Biol. Chem. 275, 10983-10988. [DOI] [PubMed] [Google Scholar]
- 37.Comer F. I. & Hart, G. W. (2000) J. Biol. Chem. 275, 29179-29182. [DOI] [PubMed] [Google Scholar]
- 38.Gao Y., Wells, L., Comer, F. I., Parker, G. J. & Hart, G. W. (2001) J. Biol. Chem. 276, 9838-9845. [DOI] [PubMed] [Google Scholar]
- 39.Shafi R., Iyer, S. P., Ellies, L. G., O'Donnell, N., Marek, K. W., Chui, D., Hart, G. W. & Marth, J. D. (2000) Proc. Natl. Acad. Sci. USA 97, 5735-5739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hanover J. A., Lai, Z., Lee, G., Lubas, W. A. & Sato, S. M. (1999) Arch. Biochem. Biophys. 362, 38-45. [DOI] [PubMed] [Google Scholar]
- 41.McClain D. A., Paterson, A. J., Roos, M. D., Wei, X. & Kudlow, J. E. (1992) Proc. Natl. Acad. Sci. USA 89, 8150-8154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Han I. & Kudlow, J. E. (1997) Mol. Cell. Biol. 17, 2550-2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goldberg H. J., Scholey, J. & Fantus, I. G. (2000) Diabetes 49, 863-871. [DOI] [PubMed] [Google Scholar]
- 44.Du X. L., Edelstein, D., Rossetti, L., Fantus, I. G., Goldberg, H., Ziyadeh, F., Wu, J. & Brownlee, M. (2000) Proc. Natl. Acad. Sci. USA 97, 12222-12226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ciaraldi T. P., Carter, L., Nikoulina, S., Mudaliar, S., McClain, D. A. & Henry, R. R. (1999) Endocrinology 140, 3971-3980. [DOI] [PubMed] [Google Scholar]
- 46.Hawkins M., Angelov, I., Liu, R., Barzilai, N. & Rossetti, L. (1997) J. Biol. Chem. 272, 4889-4895. [DOI] [PubMed] [Google Scholar]
- 47.Thomson M. J., Williams, M. G. & Frost, S. C. (1997) J. Biol. Chem. 272, 7759-7764. [DOI] [PubMed] [Google Scholar]
- 48.Yki-Jarvinen H., Virkamaki, A., Daniels, M. C., McClain, D. & Gottschalk, W. K. (1998) Metabolism 47, 449-455. [DOI] [PubMed] [Google Scholar]