Abstract
We have developed an antibody fusion protein (anti-rat TfR IgG3-Av) with the ability to deliver different molecules into cancer cells. It consists of avidin genetically fused to the CH3 region of a human IgG3 specific for the rat transferrin receptor. It forms strong, noncovalent interactions with biotinylated molecules such as glucose oxidase and β-galactosidase, and delivers them into the rat myeloma cell line Y3-Ag1.2.3 through receptor-mediated endocytosis. Importantly, the β-galactosidase retains activity after internalization. Furthermore, we have unexpectedly discovered that anti-rat TfR IgG3-Av, but not a recombinant anti-rat TfR IgG3 or a nonspecific IgG3-Av, possesses proapoptotic activities against Y3-Ag1.2.3 and the rat T cell lymphoma cell line C58 (NT) D.1.G.OVAR.1. These activities were not observed in two rat cell lines of nonhematopoietic lineage (bladder carcinoma BC47 and gliosarcoma 9L). Anti-human TfR IgG3-Av also demonstrated proapoptotic activity against the human erythroleukemia cell line K562. Studies showed that anti-rat TfR IgG3-Av exists as a dimer, suggesting that cross-linking of the surface transferrin receptor may be responsible for the cytotoxic activity. These findings demonstrate that it is possible to transform an antibody specific for a growth factor receptor that does not exhibit inhibitory activity into a drug with significant intrinsic cytotoxic activity against selected cells by fusing it with avidin. The antitumor activity may be enhanced by delivering biotinylated therapeutics into cancer cells. Further development of this technology may lead to effective therapeutics for in vivo eradication of hematological malignancies, and ex vivo purging of cancer cells in autologous transplantation.
The primary function of transferrin (Tf) is to transport iron through the blood (1) and deliver it to cells through the transferrin receptor (TfR) (1). After binding the TfR on the cell surface, Tf is internalized into an acidic compartment where iron dissociates and the apo-Tf is returned to the cell surface where ligand-receptor dissociation occurs. Because of its pivotal role in iron uptake, the TfR is more abundantly expressed in rapidly dividing cells than quiescent cells (2–4). In normal tissues, constitutive expression of the TfR is limited to the liver, epidermis, intestinal epithelium, vascular endothelium of brain capillary, and certain populations of blood cells in the bone marrow (5–9). In contrast, high levels of TfR expression have been identified on many tumors (5, 10–15). In fact, studies have shown that the TfR is expressed more abundantly in malignant tissues than their normal counterparts (5, 13, 16, 17). Therefore, the TfR expressed on tumor cells should be suitable for the delivery of therapeutics into cancer cells by receptor-mediated endocytosis.
Both Ab specific for the TfR and Tf have been used to target cytotoxic molecules to tumors (18–22). Immunotoxins and Tf-toxin conjugates can be constructed either by chemically conjugating the Ab or Tf to the toxins, or by genetically fusing the two moieties. Chemical conjugates have many drawbacks, including a lack of homogeneity (23, 24). On the other hand, use of fusion proteins requires that a different protein be constructed for each application, which is cumbersome, and sometimes there is a decrease in activity of one or both covalently conjugated partners. It would, therefore, be desirable to develop a universal delivery system that eliminates the need to make a specific construct for each application.
We previously reported the production of anti-rat TfR IgG3-Av and showed that it can deliver biotinylated molecules across the blood–brain barrier (BBB) and into the brain through TfR-mediated endocytosis and transcytosis across brain capillary endothelial cells (25). In the present study, we describe an application of anti-rat TfR IgG3-Av as a universal delivery system to deliver different biotinylated compounds into cells expressing the TfR. In addition, we have found that anti-rat TfR IgG3-Av and anti-human TfR IgG3-Av have intrinsic proapoptotic activities against selected cells.
Materials and Methods
Antibodies and Antibody Fusion Proteins.
Anti-rat TfR IgG3-Av and anti-human TfR IgG3-Av were constructed by substituting the variable regions of the heavy and light chains of anti-dansyl (5-dimethylamino naphthalene-1-sulfonyl chloride) IgG3-CH3-Av (26) with the variable regions of anti-rat TfR IgG2a monoclonal Ab OX26 (6) and anti-human TfR IgG1 monoclonal Ab 128.1 (27), respectively. Anti-rat TfR IgG3-Av and anti-human TfR IgG3-Av were expressed in the murine myelomas P3×63Ag8.653 and Sp2/0-Ag14, respectively, as H2L2 molecules of the expected sizes. Recombinant anti-rat TfR IgG3 containing the variable regions of OX26 and recombinant anti-dansyl IgG3 have been described (28, 29). Ab and Ab fusion proteins were purified from culture supernatants by using protein G immobilized on Sepharose 4B fast flow (Sigma). Purity was assessed by Coomassie blue staining of SDS/PAGE gels. All protein concentrations were determined by the bicinchoninic acid based protein assay (BCA Protein Assay, Pierce) and ELISA. OX26 and 128.1 were kindly supplied by William M. Pardridge (University of California, Los Angeles) and Phillip M. Friden (Alkermes, Cambridge, MA). The murine IgG1 anti-human IgG3 monoclonal Ab HP6050 was a gift from Robert G. Hamilton (John Hopkins University, Baltimore). Goat anti-human IgG was purchased from Zymed.
Cell Lines.
Y3-Ag1.2.3, a myeloma from the Lou strain of rats resistant to azaguanine, was a generous gift from Vernon T. Oi (Stanford University, Palo Alto, CA) (30). C58 (NT) D.1.G.OVAR.1, a rat T cell lymphoma from the WFu strain resistant to 0.1 mM 6-thioguanine and 1 mM ouabain (31), was purchased from American Type Culture Collection. BC47 is a rat bladder carcinoma (32) kindly provided by H Tanoguchi (Keio University, Tokyo). The 9L gliosarcoma (33, 34) was a generous gift from J. Laterra (Johns Hopkins University). The human erythroleukemia cell line K562 (35) was kindly provided by J. Larrick (Palo Alto Institute of Molecular Medicine, Mountain View, CA). Cells were cultured at 37°C, 5% CO2 in DMEM (GIBCO/BRL) supplemented with 5% CS (HyClone); C58 (NT) D.1.G.OVAR.1 required 10% CS.
Conjugation of Glucose Oxidase (GOX) with Biotin and FITC.
GOX (Sigma) is a 186-kDa protein that generates H2O2 by using glucose as a substrate. 2 mg of GOX dissolved in PBS was incubated at a 1:10 molar ratio with EZ-Link NHS-LC-Biotin (Pierce) on ice for 2.5 h and then dialyzed into dialysis buffer (50 mM Tris base/150 mM NaCl at pH 7.8). The biotinylated GOX (b-GOX) was conjugated with FITC (b-GOX-FITC) following a procedure suggested by the manufacturer using the Alexa Fluor 488 Protein Labeling Kit (Molecular Probes).
Internalization of Antibody Fusion Proteins Complexes.
Ab fusion proteins complexed with biotinylated FITC (b-FITC) (1:10 molar ratio) or b-GOX-FITC (1:2 molar ratio) in dialysis buffer for 3 h at room temperature, b-FITC or b-GOX-FITC were incubated with 106 cells for 3 h on ice. The cells were washed and incubated for 45 min (b-GOX-FITC experiment) or 2.5 h (b-FITC experiment) on ice, or at 37°C, resuspended in 2% paraformaldehyde, and analyzed by flow cytometry using a FACScan (Becton Dickinson) equipped with a blue laser excitation of 15 mW at 488 nm. Alternatively, the cells were treated twice for 45 min with a protease solution (50 μg/ml chymotrypsin and 50 μg/ml proteinase K in HBSS with 0.35 g/liter of NaHCO3, pH 7.8) to remove surface-bound fusion proteins before analysis. The percentage of internalized protein was calculated for cells incubated at 37°C using the following equation: (mean fluorescence intensity treated with proteases − background) × 100/(mean fluorescence intensity not treated with proteases − background).
All solutions used for incubation in the b-GOX-FITC experiment contained 100 μg/ml of catalase (Sigma). Some cells in the b-FITC experiment were mounted on glass slides by using ProLong Antifade Kit (Molecular Probes) and analyzed on a Leica TCS-SP confocal microscope (Leica, Heidelberg).
Ab fusion proteins complexed with biotinylated β-galactosidase (b-β-gal) (Sigma) were added to 106 cells and incubated at 37°C for 3 h. The cells were then washed and prepared for the detection of intracellular β-gal activity following a procedure suggested by the manufacturer, using the DetectaGene Green CMFDG lacZ Gene Expression Kit (Molecular Probes).
Proliferation Assays.
BC47 and 9L, which are adherent cells, were plated 1 day before treatment at 5 × 103 cells per well. Y3-Ag1.2.3 (104 per well) or C58 (NT) D.1.G.OVAR.1 (5 × 104 per well) were placed in wells on the day of treatment. Cells were treated with dialysis buffer, Ab, or Ab fusion proteins in 96-well plates (Becton Dickinson) for 48 h at 37°C. After 24 h, [methyl-3H]thymidine (ICN) was added to a final concentration of 4 μCi/ml (1 Ci = 37 GBq). Cells were cultured for an additional 24 h and then harvested onto glass fiber filters by using a 11050 Micro Cell Harvester (Skatron, Lier, Norway) and counted in a 1205 Betaplate Liquid Scintillation Counter (Wallac, Gaithersburg, MD). Each value presented is the mean of quadruplicate samples expressed as % of the [3H]thymidine incorporation in cells treated with anti-dansyl-IgG3 for the cross-linking assay or dialysis buffer for other assays. In the cross-linking assay, anti-rat TfR IgG3 was mixed at 1:5 molar ratio with monoclonal anti-human IgG3 or with polyclonal anti-human IgG in DMEM containing 5% CS for 2 h at room temperature before addition to cells. K562 cells (5 × 103 per well) were treated with dialysis buffer or different concentrations of anti-human TfR IgG3-Av in 96-well plates for 72 h at 37°C. The cells were then cultured in the presence of [methyl-3H]thymidine for 24 h and harvested as described above.
Assays for Apoptosis.
Fifty thousand cells were treated with anti-rat TfR IgG3-Av for 48 h and 5,000 cells were treated with anti-human TfR IgG3-Av for 96 h. The cells were then washed with ice-cold PBS. The Annexin V/propidium iodide (PI) assay or terminal deoxynucleotidyltransferase-mediated dUTP end labeling (TUNEL) assay was conducted following procedures suggested by the manufacturer, using the Vybrant Apoptosis Assay Kit #2 and APO-BrdU TUNEL assay Kit (Molecular Probes), respectively.
FPLC.
Purified Ab and fusion proteins were analyzed in 0.5 M NaCl/20 mM phosphate solution, pH 6.5, using two consecutive analytical Superose 6 HR 10/30 columns (Amersham Pharmacia Biotech) at a flow rate of 0.25 ml/min. The injection volume of 100 μl contained 50 μg of protein.
Statistical Analysis.
Statistical analysis of the experimental findings was made using a two-tailed Student's t test. Results were regarded as significant if P values were ≤0.05.
Results
TfR-Mediated Endocytosis of Anti-Rat TfR IgG3-Av Complexed with Biotinylated FITC.
To test the ability of anti-rat TfR IgG3-Av to trigger receptor-mediated endocytosis after binding to the TfR, Y3-Ag1.2.3 cells were incubated with anti-dansyl IgG3-Av (Fig. 1 A–D) or anti-rat TfR IgG3-Av (Fig. 1 E–H) complexed with biotinylated FITC (b-FITC) on ice (Fig. 1 A, B, E, and F) or at 37°C (Fig. 1 C, D, G, and H) for 2.5 h. Cells were then incubated on ice with buffer (PBS) (Fig. 1 A, C, E, and G) or treated with protease (Fig. 1 B, D, F, and H) before flow cytometry analysis. No cell-associated fluorescence was seen under any conditions following treatment with anti-dansyl IgG3-Av-b-FITC (Fig. 1 A–D) or b-FITC (data not shown). In contrast, anti-rat TfR IgG3-Av-b-FITC was bound to the cells (Fig. 1 E–H). Because endocytosis ceases at low temperature, following incubation on ice, the anti-rat TfR IgG3-Av-b-FITC remained at the cell surface and was removed by proteases (Fig. 1 E and F). In contrast, after 2.5 h incubation at 37°C, cells treated with proteases showed only a modest decrease in the fluorescence intensity with 80% of the cell-associated anti-rat TfR IgG3-Av-b-FITC internalized and protected from protease degradation (Fig. 1 G and H).
Fig 1.
Flow cytometry demonstrating the internalization of biotinylated FITC complexed with anti-rat TfR IgG3-Av. Eight micrograms of anti-dansyl IgG3-Av (A–D) or anti-rat TfR IgG3-Av (E–H) was complexed with b-FITC and incubated with Y3-Ag1.2.3 cells for 2.5 h on ice (A, B, E, and F), or at 37°C (C, D, G, and H). Cells were then analyzed by flow cytometry either directly (A, C, E, and G) or following protease treatment (B, D, F, and H).
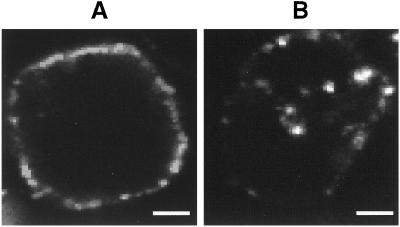
Confocal microscopy also indicated that anti-rat TfR IgG3-Av-b-FITC was taken up into the cells. When images of single cells were captured with a focal plane across the midsection of the cell, in cells treated with anti-rat TfR IgG3-Av-b-FITC on ice, the majority of the Ab fusion protein was found bound at the cell surface (Fig. 2A). In contrast, following incubation at 37°C, the Ab fusion protein was internalized, possibly accumulating in endocytotic vesicles in the cytoplasm (Fig. 2B).
Fig 2.
Internalization of biotinylated FITC complexed with anti-rat TfR IgG3-Av detected by confocal microscopy. Y3-Ag1.2.3 cells incubated with anti-rat TfR IgG3-Av-b-FITC conjugates on ice (A) and at 37°C (B) were analyzed by confocal microscopy. Representative cells are shown. (Scale bar, 2 μm.)
TfR-Mediated Endocytosis of Biotinylated Proteins Complexed with Anti-Rat TfR IgG3-Av.
To determine whether anti-rat TfR IgG3-Av could deliver cytotoxic proteins into cancer cells, Y3-Ag1.2.3 cells were incubated with anti-dansyl IgG3-Av or anti-rat TfR IgG3-Av plus biotinylated GOX-FITC (b-GOX-FITC) under conditions similar to those described in Fig. 1. After incubation for 45 min at 37°C, 60% of the cell-associated anti-rat TfR IgG3-Av-b-GOX-FITC complex was internalized, whereas anti-dansyl IgG3-Av-b-GOX-FITC or b-GOX-FITC alone did not bind to the cells (see Fig. 8, which is published as supporting information on the PNAS web site, www.pnas.org). This study demonstrated that anti-rat TfR IgG3-Av is capable of delivering a 186-kDa protein into Y3-Ag1.2.3 cells by TfR-mediated endocytosis.
To determine whether anti-rat TfR IgG3-Av could deliver a larger protein into Y3-Ag1.2.3 cells and whether the internalized protein remains active, b-β-gal (464 kDa) was added to anti-rat TfR IgG3-Av or anti-dansyl IgG3-Av and incubated with Y3-Ag1.2.3 cells. Using a membrane-permeable, fluorogenic β-gal substrate that only detects intracellular β-gal activity (36), flow cytometry showed that the b-β-gal was transported by anti-rat TfR IgG3-Av into the cells and remained functional (Fig. 3, bold line). Cells treated with the anti-dansyl IgG3-Av-b-β-gal complex (Fig. 3, narrow line) showed no internal β-gal activity.
Fig 3.
Internalization of biotinylated β-gal complexed with anti-rat TfR IgG3-Av. Y3-Ag1.2.3 cells were incubated at 37°C, for 3 h with 0.1 μg of anti-dansyl IgG3-Av (narrow line) or anti-rat TfR IgG3-Av (bold line) bound to biotinylated β-gal (1:1 molar ratio). After washes, intracellular β-gal activity was detected using a membrane permeable, fluorogenic β-gal substrate, and flow cytometry.
Anti-Rat TfR IgG3-Av Exhibits Intrinsic Cytotoxic Activity on Selected Rat Cancer Cell Lines.
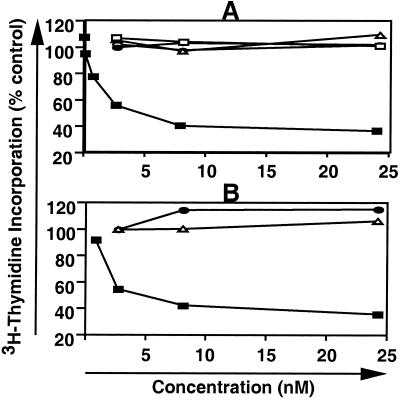
Although anti-rat TfR IgG3-Av was originally designed to deliver molecules into cancer cells, we observed that cells treated with the molecule appeared to undergo apoptosis with cell shrinkage, budding, and the formation of clusters of apoptotic bodies (data not shown). To further investigate this phenomenon, we incubated Y3-Ag1.2.3 cells with varying concentrations of anti-rat TfR IgG3-Av, anti-dansyl IgG3-Av, recombinant anti-rat TfR IgG3, anti-rat TfR IgG2a (OX26), and recombinant anti-dansyl IgG3 (Fig. 4A), and then monitored cell proliferation by thymidine incorporation. Anti-rat TfR IgG3, anti-rat TfR IgG2a, anti-dansyl IgG3, and anti-dansyl IgG3-Av showed no inhibition of proliferation. In contrast, anti-rat TfR IgG3-Av showed 50% inhibition of proliferation (IC50) at 4.5 nM. Statistical analysis of the three highest concentrations of anti-rat TfR IgG3-Av and anti-dansyl IgG3-Av showed that the anti-rat TfR IgG3-Av was a potent inhibitor of proliferation (P ≤ 0.002). Similar results were obtained in two independent studies of Y3-Ag1.2.3, as well as in C58 (NT) D.1.G.OVAR.1 cells (data not shown). Growth inhibition was observed only when the anti-rat TfR variable regions and the avidin moiety were present in the same molecule. Although the fusion protein was able to inhibit the growth of two cancer cell lines of hematopoietic lineage, under the same condition, it did not inhibit the growth of the rat gliosarcoma 9L or the rat bladder carcinoma BC47 (Fig. 4B). Anti-dansyl IgG3-Av, anti-rat TfR IgG3, anti-dansyl IgG3, and anti-rat TfR IgG2a also did not inhibit the proliferation of BC47 and 9L (data not shown).
Fig 4.
Antiproliferative effect of antibody fusion proteins on selected rat cancer cell lines. (A) Y3-Ag 1.2.3 cells were treated with anti-rat TfR IgG3-Av (▪), anti-dansyl IgG3-Av (□), anti-rat TfR IgG2a (▵), anti-rat TfR IgG3 (•), or anti-dansyl IgG3 (○) at various concentrations for 24 h. The cells were then cultured in the presence of [3H]thymidine for an additional 24 h, harvested, and [3H]thymidine incorporation was determined. Each value is the mean of quadruplicate assays expressed as the % control mean (controls are cells treated with buffer alone). (B) Y3-Ag 1.2.3 (▪), BC47 (•), and 9L (▵) cells were treated with various concentrations of anti-rat TfR IgG3-Av for 24 h and processed as described in A.
Anti-Rat TfR IgG3-Av Induces Apoptosis in Selected Rat Cancer Cell Lines.
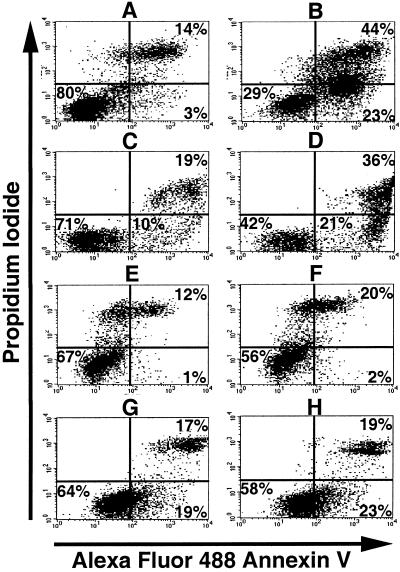
To confirm that cell death mediated by anti-rat TfR IgG3-Av was through apoptosis, cells treated with anti-rat TfR IgG3-Av were assayed for the translocation of phosphatidylserine from the inner to the outer leaflet of the plasma membrane by using the annexin V affinity assay (37). Dead cells can be stained by PI, which enters cells with disrupted plasma membrane and binds to DNA. Following treatment with anti-rat TfR IgG3-Av, Y3-Ag1.2.3 (Fig. 5 A and B), and C58 (NT) D.1.G.OVAR.1 (Fig. 5 C and D), cells showed an increase in the number of dead cells (annexin V/PI bright) compared with cells treated with buffer (compare Fig. 5 A and C with 5 B and D). In Y3-Ag1.2.3 cells, a large population that stained brightly with annexin V but less brightly with PI appeared after treatment with anti-rat TfR IgG3-Av. Furthermore, even the population that did not stain with PI showed stronger staining with annexin V, suggesting that apoptosis had been initiated in all cells (Fig. 5 A and B). Increased genomic DNA fragmentation in all Y3-Ag1.2.3 cells treated with anti-rat TfR IgG3-Av was also seen when using the TUNEL assay (data not shown). After similar treatment of BC47 (Fig. 5 E and F) and 9L (Fig. 5 G and H), significant numbers of cells undergoing apoptosis were not observed.
Fig 5.
Anti-rat TfR IgG3-Av induces apoptosis in selected rat cancer cell lines. Y3-Ag1.2.3 (A and B), C58 (NT) D.1.G.OVAR.1 (C and D), BC47 (E and F), and 9L (G and H) cells were incubated with buffer alone (A, C, E, and G), or 9 nM of anti-rat TfR IgG3-Av (B, D, F, and H) for 48 h. The cells were then washed, stained with Alexa Fluor 488 Annexin V and PI, and analyzed by flow cytometry. The percentage of cells located in each quadrant is shown at the corner.
Anti-Rat TfR IgG3-Av Exists as a Noncovalent Dimer.
Avidin is a tetramer comprised of four noncovalently linked monomers. Each molecule of anti-rat TfR IgG3-Av contains only two avidin moieties. FPLC analysis was performed under nondenaturing conditions to determine whether anti-rat TfR IgG3-Av forms a dimeric structure with four avidin moieties (Fig. 6). Under these conditions, anti-rat TfR IgG3 eluted at the position expected given its size (173 kDa), but anti-rat TfR IgG3-Av appeared to have a molecular mass of ≈400 kDa, corresponding to a noncovalent dimer composed of two fusion protein monomers of 200 kDa. The fact that anti-rat TfR IgG3 does not dimerize suggests that noncovalent interactions among the avidin molecules led to dimerization.
Fig 6.
Noncovalent association of anti-rat TfR IgG3-Av results in a dimeric structure. Anti-rat TfR IgG3 (173 kDa) and anti-rat TfR IgG3-Av (200 kDa for monomer) were analyzed by FPLC. As a standard the profile of dimeric IgA (360 kDa) and monomeric IgG (150 kDa) separated under identical conditions is shown. Fraction size is 1 ml.
Recombinant Anti-Rat TfR IgG3 Cross-Linked with Secondary Antibodies Inhibits Cell Growth.
To further investigate whether dimerization or multimerization of anti-rat TfR is associated with a growth inhibitory effect, we incubated Y3-Ag1.2.3 cells with 23.7 nM of anti-dansyl IgG3, anti-rat TfR IgG3 alone, anti-rat TfR IgG3-Av, or anti-rat TfR IgG3 cross-linked by secondary Ab. Y3-Ag1.2.3 cells treated with anti-rat TfR IgG3 incorporated 94.1% as much thymidine as control cells treated with anti-dansyl IgG3. In contrast, cells treated with anti-rat TfR IgG3-Av, or anti-rat TfR IgG3 premixed with either a monoclonal or polyclonal anti-human IgG had lower levels of thymidine incorporation: 23.6%, 25.8%, and 27.1%, respectively, of the amount incorporated by control cells treated with anti-dansyl IgG3 (P < 0.0001 compared with controls). Therefore, multimeric forms of anti-rat TfR inhibit growth of Y3-Ag1.2.3.
Anti-Human TfR IgG3-Av Exhibits Intrinsic Cytotoxic Activity on a Human Cancer Cell Line.
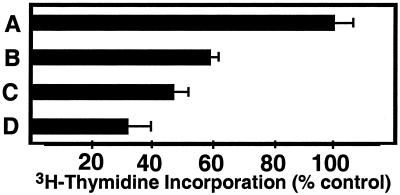
Anti-rat TfR IgG3-Av is specific only for the TfR expressed on rat cells (6, 25). To determine whether anti-human TfR IgG3-Av also possesses cytotoxic activity against human cancer cells, we constructed a fusion protein virtually identical to anti-rat TfR IgG3-Av but with specificity for the human TfR. The fusion protein binds biotin and triggers receptor-mediated endocytosis after binding to the TfR on human erythroleukemia cell line K562 (data not shown). Anti-human TfR IgG3-Av inhibited the growth of K562 cells in a dose-dependent manner (Fig. 7). In contrast, the proliferation of K562 cells was not inhibited by anti-dansyl IgG3-Av or anti-human TfR IgG1 (128.1) (data not shown). In addition, flow cytometry analysis using the Annexin V/PI assay confirmed that anti-human TfR IgG3-Av induces apoptosis in K562 cells (see Fig. 9, which is published as supporting information on the PNAS web site, www.pnas.org).
Fig 7.
Antiproliferative effect of anti-human TfR IgG3-Av on K562 cells. K562 cells were treated with buffer (A), 25.9 nM (B), 51.9 nM (C), or 104 nM (D) of anti-human TfR IgG3-Av for 72 h. The cells were then cultured in the presence of [3H]thymidine for another 24 h before being harvested. The amount of [3H]thymidine incorporation is expressed as the % the incorporation seen when cells were treated with buffer.
Discussion
The original goal of the present study was to determine whether anti-rat TfR IgG3-Av could be used to deliver different molecules to cancer cells. Anti-rat TfR IgG3-Av contains the variable regions of the mouse monoclonal Ab OX26, which binds specifically to the rat TfR without blocking Tf binding (6). Chemical conjugation of OX26 with recombinant ricin A chain yielded an immunotoxin exhibiting strong inhibition of protein synthesis in Y3-Ag1.2.3, NBT II, and 9L cell lines (38, 39). These findings suggested that anti-rat TfR IgG3-Av could be used to deliver molecules into rat cancer cells through receptor-mediated endocytosis, and we have now demonstrated that anti-rat TfR IgG3-Av can deliver different biotinylated molecules into Y3-Ag1.2.3 cells. We found that 60% of the cell-associated anti-rat TfR IgG3-Av–b-GOX-FITC complex was internalized after 45 min at 37°C. This efficiency of uptake is comparable to that observed for 125I-Tf in the human leukemia cell line HL-60 (53% in 45 min; ref. 27), and for anti-PECAM -125I-GOX in PECAM-positive human umbilical vein endothelial cells (HUVECs) (60% in 90 min; ref. 40). Anti-rat TfR IgG3-Av was able to deliver proteins as large as β-gal into a cell with the intracellular β-gal retaining enzymatic activity, suggesting that at least a fraction escaped lysosomal degradation.
Unexpectedly, anti-rat TfR IgG3-Av was found to induce apoptosis in two rat malignant cell lines [Y3-Ag1.2.3 and C58 (NT) D.1.G.OVAR.1] of hematological origin. This effect requires both the specificity for TfR and the avidin moiety. FPLC analysis showed that anti-rat TfR IgG3-Av exists as a noncovalent dimer, leading us to speculate that the cytotoxic activity of the molecule may be, at least in part, due to its dimeric structure. In fact, we found that whereas anti-rat TfR IgG3 alone did not have any inhibitory activity, anti-rat TfR IgG3 cross-linked with secondary Ab exhibited an antiproliferative activity comparable to that of anti-rat TfR IgG3-Av.
A correlation between the valence of anti-TfR and effects on cells expressing the TfR has been described (41). Divalent anti-TfR IgG increases the rate of TfR internalization and degradation, resulting in a decrease in TfR expression and cell growth rate in certain cases. On the other hand, treatment with multivalent anti-TfR, such as IgM (valence = 10–12), causes extensive receptor cross-linking which inhibits internalization and leads to severe growth inhibition (41). Dimeric (tetravalent) anti-rat TfR IgG3-Av would be expected to cause less extensive TfR cross-linking than anti-TfR IgM because of its lower valence and, unlike IgM, anti-rat TfR IgG3-Av was able to efficiently deliver biotinylated molecules via receptor-mediated endocytosis. The inhibition of growth by anti-rat TfR IgG3-Av may reflect a combination of a partial block of iron uptake and TfR down-regulation. Earlier studies showed that the addition of ferric citrate as an iron supplement can completely prevent apoptosis in cells treated with anti-TfR (42). Preliminary data by our group showed that addition of 50 μM of ferric citrate greatly attenuated the growth inhibitory effect of anti-rat TfR IgG3-Av on Y3-Ag 1.2.3 (unpublished data), supporting a role for iron deprivation in the cell growth arrest observed.
Despite the fact that anti-rat TfR IgG3-Av inhibited the growth of the two hematological malignant cell lines tested, similar treatment did not inhibit the growth of the rat gliosarcoma 9L and bladder carcinoma BC47, cell lines of mesenchymal and epithelial origin, respectively. Low or no expression of the TfR is unlikely to explain the result seen with 9L, which has been successfully treated with an anti-TfR immunotoxin (38). Instead, these findings are consistent with previous studies showing that hematopoietic cells are generally more sensitive to the antiproliferative effects of anti-TfR monoclonal Abs than other cell types (43). The reasons for these differences are not well understood, but may reflect the capacity of individual cell type to respond to iron deprivation (43). Alternatively, an iron uptake pathway independent of the Tf-TfR system has been demonstrated in a murine cell (44), and it is possible that different cells vary in their dependence on the Tf-TfR system for iron.
The cytotoxic effects of anti-TfR IgG3-Av are not limited to hematopoietic cells of rat origin but were also observed when an anti-human TfR IgG3-Av was used to treat the human cell line K562. Anti-human TfR IgG3-Av is similar in structure to anti-rat TfR IgG3-Av, and would be expected to form dimers. Indeed, we found that anti-human TfR IgG3-Av induces apoptosis in K562 cells. In the U.S., more than 100,000 new cases of blood-related cancers are diagnosed every year (see The Leukemia and Lymphoma Society's web page, www.leukemia.org). Leukemia and lymphoma cells, which are of hematopoietic origin, overexpress the TfR compared with normal tissues (6, 9, 14, 15) and may be especially sensitive to the cytotoxic effect of anti-TfR. Furthermore, it is possible that tumor-specific cytotoxicity can be enhanced by using anti-human TfR IgG3-Av to deliver biotinylated cytotoxic molecules. Because anti-TfR IgG3-Av contains an intact Fc, it may also be effective in recruiting humoral and cellular effector mechanisms to kill tumor cells. In addition to in vivo applications, anti-human TfR IgG3-Av may also be used to eliminate residual tumor cells following standard ex vivo purging procedures (6).
One concern is that nonspecific cytotoxicity may be associated with the in vivo use of anti-human TfR IgG3-Av alone or in association with biotinylated cytotoxic agents. However, treatment of mice challenged with SL-2 leukemic cells twice weekly for up to 4 weeks with 3 mg of an IgM anti-mouse TfR produced no evidence of gross toxicity or cellular damage (45). In addition, when Tf-cytosine arabinoside conjugates were administered i.v. for the treatment of SL-2 leukemia, all of the control animals succumbed to the disease in 2 weeks, whereas 70% of the treated mice remained free of tumor until the termination of experiment at the 20th week (19). These results suggest that systemic administration of anti-human TfR IgG3-Av alone or conjugated with toxins may have antitumor activity with limited nonspecific toxicity. Furthermore, systemic toxicity may be minimized and high local concentrations of drug achieved by regional therapy of tumor-containing cavities, such as the brain. Direct intratumoral injection of Tf-diphtheria toxin conjugate showed efficacy with little systemic toxicity in patients with brain tumors (22). An alternative approach to eliminating toxicity to normal cells would be to use anti-human TfR IgG3-Av to deliver DNA encoding a “suicide gene” driven by a cancer-specific promoter (46).
An important issue in the use of anti-human TfR IgG3-Av as a therapeutic agent is its immunogenicity. Although this will not be a problem for ex vivo purging of cancer cells, one component of the fusion protein that can possibly elicit an immune response during in vivo use is the avidin moiety. However, its immunogenicity may be reduced because nearly everyone has been exposed to chicken avidin by eating eggs, and therefore may be tolerant to this oral antigen (47). Although a humoral immune response against the murine variable regions of the fusion protein may occur during treatment in individuals with a normal immune system, many patients undergoing chemotherapy for hematological malignancies are immune compromised and many chimeric antibodies with non-human variable regions have been used successfully in the clinic.
In conclusion, our results have demonstrated that anti-rat TfR IgG3-Av can be used as a vehicle for delivering different biotinylated compounds into cancer cells. In addition, both anti-rat TfR IgG3-Av and anti-human TfR IgG3-Av have been shown to possess intrinsic cytotoxic activity against malignant cell lines of hematopoietic origin. These findings demonstrate that it is possible to transform an Ab specific for a growth factor receptor (such as TfR) that does not exhibit growth inhibitory activity into a drug system with significant cytotoxic activity against selected cells by fusing it with avidin. Further development of this approach may lead to more effective therapeutics for in vivo eradication of hematological cancers and other malignancies, and for ex vivo purging of cancer cells in autologous transplantation.
Supplementary Material
Acknowledgments
We thank Dr. Koteswara Chintalacharuvu and Dr. Vladimir Muzykantov for useful suggestions, and Dr. Donald Morrison for assistance with statistical analysis. Dr. Matt Schibler at the Carol Moss Spivak Cell Imaging Facility [University of California, Los Angeles (UCLA), Brain Research Institute] assisted with confocal microscopy, and the Janis V. Giorgi Flow Cytometry Laboratory (UCLA Jonsson Comprehensive Cancer Center) provided assistance with flow cytometry. The Ng and Lai families provided unwavering support. This work was supported in part by National Cancer Institute/National Institutes of Health Grant CA86915, Susan G. Komen Breast Cancer Foundation Grant 9855, Department of Defense Breast Cancer Research Program Grant Proposal BC980134 (Award DAMD17-99-1-9098), and Cancer Center Core Grant CA-16042 (UCLA). P.P.N. was supported by U.S. Public Health Service National Research Service Award GM07185. The communicating member is part of the scientific advisory board of Lexrite Labs.
Abbreviations
Tf, transferrin
TfR, Tf receptor
GOX, glucose oxidase
b-GOX-FITC, biotinylated GOX conjugated with FITC
b-FITC, biotinylated FITC
b-β-gal, biotinylated β-galactosidase
PI, propidium iodide
References
- 1.Dowlati A., Loo, M., Bury, T., Fillet, G. & Beguin, Y. (1997) Br. J. Cancer 75, 1802-1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Larrick J. W. & Cresswell, P. (1979) J. Supramol. Struct. 11, 579-586. [DOI] [PubMed] [Google Scholar]
- 3.Sutherland R., Delia, D., Schneider, C., Newman, R., Kemshead, J. & Greaves, M. (1981) Proc. Natl. Acad. Sci. USA 78, 4515-4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trowbridge I. S. & Omary, M. B. (1981) Proc. Natl. Acad. Sci. USA 78, 3039-3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gatter K. C., Brown, G., Trowbridge, I. S., Woolston, R. E. & Mason, D. Y. (1983) J. Clin. Pathol. 36, 539-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jefferies W. A., Brandon, M. R., Williams, A. F. & Hunt, S. V. (1985) Immunology 54, 333-341. [PMC free article] [PubMed] [Google Scholar]
- 7.Taetle R. & Honeysett, J. M. (1988) Blood 71, 1590-1595. [PubMed] [Google Scholar]
- 8.Jefferies W. A., Brandon, M. R., Hunt, S. V., Williams, A. F., Gatter, K. C. & Mason, D. Y. (1984) Nature (London) 312, 162-163. [DOI] [PubMed] [Google Scholar]
- 9.Lesley J., Domingo, D. L., Schulte, R. & Trowbridge, I. S. (1984) Exp. Cell Res. 150, 400-407. [DOI] [PubMed] [Google Scholar]
- 10.Lloyd J. M., O'Dowd, T., Driver, M. & Tee, D. E. (1984) J. Clin. Pathol. 37, 131-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raaf H. N., Jacobsen, D. W., Savon, S. & Green, R. (1993) Am. J. Clin. Pathol. 99, 232-237. [DOI] [PubMed] [Google Scholar]
- 12.Recht L. D., Griffin, T. W., Raso, V. & Salimi, A. R. (1990) Cancer Res. 50, 6696-6700. [PubMed] [Google Scholar]
- 13.Prost A. C., Menegaux, F., Langlois, P., Vidal, J. M., Koulibaly, M., Jost, J. L., Duron, J. J., Chigot, J. P., Vayre, P., Aurengo, A., et al. (1998) Int. J. Oncol. 13, 871-875. [DOI] [PubMed] [Google Scholar]
- 14.Habelshaw H. A., Lister, T. A. & Stansfeld, A. G. (1983) Lancet 1, 498-500. [DOI] [PubMed] [Google Scholar]
- 15.Beguin Y., Lampertz, S., Degroote, D., Igot, D., Malaise, M. & Fillet, G. (1993) Leukemia 7, 2019-2025. [PubMed] [Google Scholar]
- 16.Faulk W. P., Hsi, B. L. & Stevens, P. J. (1980) Lancet 2, 390-392. [DOI] [PubMed] [Google Scholar]
- 17.Shindelman J. E., Ortmeyer, A. E. & Sussman, H. H. (1981) Int. J. Cancer 27, 329-334. [DOI] [PubMed] [Google Scholar]
- 18.Mayers G. L., Raghavan, D., Hitt, S. & Glaves, D., (1998) Proceedings of the 89th Annual Meeting of the American Association for Cancer Research, pp. 63.
- 19.Mayers, G. L., Razeq, J. & Abu-Hadid, M. M. (1995) U.S. Patent Appl. 5,393,737.
- 20.Singh M., Atwal, H. & Micetich, R. (1998) Anticancer Res. 18, 1423-1427. [PubMed] [Google Scholar]
- 21.Laske D. W., Muraszko, K. M., Oldfield, E. H., DeVroom, H. L., Sung, C., Dedrick, R. L., Simon, T. R., Colandrea, J., Copeland, C., Katz, D., et al. (1997) Neurosurgery 41, 1039-1051. [DOI] [PubMed] [Google Scholar]
- 22.Laske D. W., Youle, R. J. & Oldfield, E. H. (1997) Nat. Med. 3, 1362-1368. [DOI] [PubMed] [Google Scholar]
- 23.Murphy J. R. (1988) Cancer Treat. Res. 37, 123-140. [DOI] [PubMed] [Google Scholar]
- 24.Pastan I. & FitzGerald, D. (1991) Science 254, 1173-1177. [DOI] [PubMed] [Google Scholar]
- 25.Penichet M. L., Kang, Y. S., Pardridge, W. M., Morrison, S. L. & Shin, S. U. (1999) J. Immunol. 163, 4421-4426. [PubMed] [Google Scholar]
- 26.Shin S. U., Wu, D., Ramanathan, R., Pardridge, W. M. & Morrison, S. L. (1997) J. Immunol. 158, 4797-4804. [PubMed] [Google Scholar]
- 27.White S., Taetle, R., Seligman, P. A., Rutherford, M. & Trowbridge, I. S. (1990) Cancer Res. 50, 6295-6301. [PubMed] [Google Scholar]
- 28.Coloma M. J., (1997) Microbiology and Molecular Genetics (Univ. of California, Los Angeles), pp. 263.
- 29.Morrison S. L., Wims, L., Wallick, S., Tan, L. & Oi, V. T. (1987) Ann. N.Y. Acad. Sci. 507, 187-198. [DOI] [PubMed] [Google Scholar]
- 30.Galfr G., Milstein, C. & Wright, B. (1979) Nature (London) 277, 131-133. [DOI] [PubMed] [Google Scholar]
- 31.Geering G., Old, L. J. & Boyse, E. A. (1966) J. Exp. Med. 124, 753-772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tanoguchi H., Tachibana, M. & Murai, M. (1997) Br. J. Cancer 76, 1262-1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Book A. A., Fielding, K. E., Kundu, N., Wilson, M. A., Fulton, A. M. & Laterra, J. (1998) J. Neuroimmunol. 92, 50-59. [DOI] [PubMed] [Google Scholar]
- 34.Book A. A., Ranganathan, S., Abounader, R., Rosen, E. & Laterra, J. (1999) Brain Res. 833, 173-180. [DOI] [PubMed] [Google Scholar]
- 35.Lozzio C. B. & Lozzio, B. B. (1975) Blood 45, 321-334. [PubMed] [Google Scholar]
- 36.Haugland R. P. (1996) in Handbook of Fluorescent Probes and Research Chemicals, ed. Spence, M. T. Z. (Molecular Probes, Eugene, OR), Vol. 1, pp. 679. [Google Scholar]
- 37.Koopman G., Reutelingsperger, C. P., Kuijten, G. A., Keehnen, R. M., Pals, S. T. & van Oers, M. H. (1994) Blood 84, 1415-1420. [PubMed] [Google Scholar]
- 38.Chignola R., Foroni, R., Candiani, C., Franceschi, A., Pasti, M., Stevanoni, G., Anselmi, C., Tridente, G. & Colombatti, M. (1994) Int. J. Cancer 57, 268-274. [DOI] [PubMed] [Google Scholar]
- 39.Muraszko K., Sung, C., Walbridge, S., Greenfield, L., Dedrick, R. L., Oldfield, E. H. & Youle, R. J. (1993) Cancer Res. 53, 3752-3757. [PubMed] [Google Scholar]
- 40.Gow A. J., Branco, F., Christofidou-Solomidou, M., Black-Schultz, L., Albelda, S. M. & Muzykantov, V. R. (1999) Am. J. Physiol. 277, L271-L281. [DOI] [PubMed] [Google Scholar]
- 41.Lesley J., Schulte, R. & Woods, J. (1989) Exp. Cell Res. 182, 215-233. [DOI] [PubMed] [Google Scholar]
- 42.Kovar J., Stunz, L. L., Stewart, B. C., Kriegerbeckova, K., Ashman, R. F. & Kemp, J. D. (1997) Pathobiology 65, 61-68. [DOI] [PubMed] [Google Scholar]
- 43.Trowbridge I. S. (1988) Prog. Allergy 45, 121-146. [DOI] [PubMed] [Google Scholar]
- 44.Basset P., Quesneau, Y. & Zwiller, J. (1986) Cancer Res. 46, 1644-1647. [PubMed] [Google Scholar]
- 45.Sauvage C. A., Mendelsohn, J. C., Lesley, J. F. & Trowbridge, I. S. (1987) Cancer Res. 47, 747-753. [PubMed] [Google Scholar]
- 46.Miller N. & Whelan, J. (1997) Hum. Gene Ther. 8, 803-815. [DOI] [PubMed] [Google Scholar]
- 47.Weiner H. L. (1994) Proc. Natl. Acad. Sci. USA 91, 10762-10765. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.