Abstract
Intermittent interleukin-2 (IL-2) therapy has been shown to increase the number of CD4+ T cells, preferentially cells with a naive phenotype, in patients with HIV infection. For this report we investigated the mechanism underlying this expansion by studying the relative roles of peripheral expansion and thymic output. In a cohort of six patients receiving IL-2 over a period of 1 year, the mean number of naive CD4+ T cells increased from 139 to 387 cells per μl while levels of T cell receptor rearrangement excision circles (TRECs) declined from 47,946 to 26,510 copies per 106 naive T cells, thus making it unlikely that the CD4+ T cell count increases were secondary to increase in thymic output. To examine directly the impact of IL-2 on peripheral expansion, peripheral blood mature, naive CD4+ T cells were labeled ex vivo with 5-bromodeoxyuridine as well as stained directly for Ki67. These studies revealed a 7-fold increase in the percentage of 5-bromodeoxyuridine-positive cells and a 20–40-fold increase in Ki67 staining in the naive CD4+ T cell pool in the setting of IL-2 administration. This degree of increase in mature CD4+ T cell turnover induced by IL-2 does not compromise the future replicative potential of these cells, because longitudinal measurements of telomere length went from 6,981 to 7,153 bp after 1 year of IL-2 therapy. These data strongly suggest that much of the increase in CD4+ cells associated with IL-2 treatment is caused by peripheral expansion of existing naive CD4+ T cells rather than increased thymic output and that these increases occur without compromising the potential of these cells for further cell division.
In healthy individuals, relatively constant numbers of peripheral T cells are maintained by the death of mature T cells, the proliferation of existing T cells, and generation of new T cells by thymopoiesis. HIV infection perturbs this homeostatic balance and over a period of years leads to decline in the number of CD4+ T cells. This process leads to immunodeficiency and increased susceptibility to opportunistic infections (1). The reason for the progressive loss of CD4+ T cells in HIV-infected patients is not understood fully, although many hypotheses have been proposed (2). The currently licensed antiretroviral reagents for treating HIV infection decrease viral load and lead to increases in CD4+ T cell number. One experimental approach that can increase the CD4+ T cell pool in HIV patients independent of significant effect on viral load is treatment with the T cell growth factor IL-2 (3, 4). Several studies have established that intermittent administration of IL-2 increases the number and percentage of CD4+ T cells in HIV-1-infected patients. The clinical benefit of this increase is unknown and is currently the focus of two phase-III studies, study of IL-2 in people with low CD4+ T cell counts on active anti-HIV therapy (SILCAAT) and the evaluation of subcutaneous proleukin in a randomized international trial (ESPRIT) (3, 5).
The underlying mechanism by which IL-2 treatment increases CD4+ T cells is not understood fully (4). Possibilities include increased production, by enhanced stem cell differentiation or increased peripheral expansion, and decreased destruction. Although the thymus may involute with age, recent data suggest that it can retain functional activity in adults (6). During maturation in the thymus, cells destined to become T cells undergo the rearrangement of their T cell receptor genes by site-specific somatic DNA-recombination events, leading to the formation of extrachromosomal circular excision products as a by-product. These episomes, T cell receptor rearrangement excision circles (TRECs), are stable and persist in the newly matured T cells. They do not replicate during cell division and thus are diluted during mitosis of these cells or lost when these cells die (7). Recently, Douek et al. (8) have developed a method to quantitate TRECs present in human T cells. Enumeration of the number of cells harboring TRECs is considered to be a direct measure of thymic emigrants. However, interpretation of such data also requires concomitant assessment of T cell division. In addition to thymopoiesis, T cells can be produced through the peripheral expansion of existing T cells. To assess the relative contribution of these two mechanisms of increased T cell production to the CD4+ T cell expansions seen in the setting of IL-2 therapy, we quantitated the changes in TREC levels and rates of turnover by Ki67 staining and BrdUrd incorporation in naive CD4+ lymphocytes during IL-2 therapy. Ki67 acts as a marker of activation and is increased from late G1 forward in the cell cycle, whereas BrdUrd incorporation is a direct measurement of the fraction of cells that have entered S phase during the time of labeling. The results suggest that much of the increase in CD4+ T cells associated with IL-2 treatment is caused by peripheral expansion of existing cells rather than increased thymic output.
Materials and Methods
Patients.
Cryopreserved or freshly isolated peripheral blood mononuclear cells (PBMCs) from HIV-infected participants enrolled in National Institute of Allergy and Infectious Diseases Institutional Review Board-approved protocols were used. The patients studied longitudinally were selected from a cohort of 31 patients enrolled in a randomized controlled trial of IL-2 in patients with HIV infection. They were selected based on the criteria of (i) adequate numbers of cryopreserved cells for study, (ii) a clear increase in CD4+ T cell count in response to IL-2, and (iii) viral loads <20,000 copies per ml at both time points for analysis. All participants in these analyses received IL-2 by continuous infusion for 5 days as described (9). IL-2 cycles were administered approximately every 2 months, and the total number of cycles ranged from four to seven over a 12-month period. Day 0 denotes the blood collected immediately before beginning of the first IL-2 infusion, day 5 refers to blood collected at the end of the first IL-2 infusion, and week 48 refers to the sample taken 48 weeks from the start of the IL-2 therapy. The base-line and follow-up characteristics of the patients are shown in Table 1. All patients were on a combination of two reverse-transcriptase inhibitors at the time of the study (1993–1994).
Table 1.
Base-line and follow-up characteristics of patients studied
| Patient no.
|
Age, years
|
Antiretovirals
|
No. of IL-2 cycles
|
CD4+ T cell count per mm3 | CD8+ T cell count per mm3 | Viral load, copies per ml | |||
|---|---|---|---|---|---|---|---|---|---|
| Start of study | End of study | Start of study | End of study | Start of study | End of study | ||||
| 1 | 31 | AZT and ddC | 5 | 643 | 907 | 1,141 | 400 | 354 | <50 |
| 2 | 28 | AZT and ddI | 6 | 395 | 1,796 | 1,066 | 1,840 | 19,482 | <50 |
| 3 | 38 | ddI and ddC | 4 | 476 | 1,544 | 502 | 746 | 807 | 101 |
| 4 | 28 | AZT and ddI | 6 | 456 | 845 | 1,076 | 1,367 | 13,474 | 16,420 |
| 5 | 38 | AZT and ddI | 7 | 991 | 2,549 | 1,133 | 2,096 | <10,000 | <10,000 |
| 6 | 34 | AZT and ddI | 6 | 466 | 1,221 | 612 | 922 | <50 | 2,373 |
| Mean | 32.8 | 6 | 571 | 1,449 | 922 | 1,229 | 8,529 | 6,298 | |
Antiretrovirals: AZT, zidovudine; ddI, didanosine; and ddC, zalcitabine.
, P = 0.004.
Immunophenotyping.
Cryopreserved PBMCs from the same time point as the TREC analysis were stained with monoclonal antibodies for CD45RO APC (clone UCHL-1), CD27 FITC (clone L128), and CD4 PerCP (clone SK3) or CD8 PerCP (clone SK1), all from Becton Dickinson Immunocytometry Systems, for 20 min at 4°C in the dark. Samples were subsequently fixed with fluorescence-activated cell sorter (FACS) lysis solution (Becton Dickinson Immunocytometry Systems) for 10 min in the dark at room temperature and washed once with PBS/0.5% BSA/0.1%NaN3 and analyzed on a four-color multiparameter flow cytometer (FACScalibur, Becton Dickinson Immunocytometry Systems). Intracellular staining for the nuclear antigen Ki67 was performed after surface staining. After fixation, cells were permeabilized for 10 min with FACS permeabilization solution (Becton Dickinson Immunocytometry Systems), subsequently stained with Ki67 PE antibody (clone B56, Becton Dickinson Immunocytometry Systems) for 30 min at 37°C in the dark, and washed twice with PBS/0.5% BSA/0.1%NaN3. Approximately 1.5–2 × 105 total events and a minimum of 10,000 events in the CD4+ or CD8+ gate were collected per sample. FLOW JO software was used for all flow-cytometric data analysis (Tree Star, San Carlos, CA).
TREC Measurements.
CD4+ and CD8+ T cells were obtained from frozen PBMCs by using magnetic beads (Dynabeads M-450 CD4, Dynabeads M-450 CD8, and detachaBead, Dynal, Great Neck, NY). TRECs in PBMCs and purified CD4+ and CD8+ cells were quantitated by real-time PCR using a cell-lysis method as described (10). The consistency of the DNA content of the cell lysate was checked by real-time PCR using a ribosomal protein gene and the Taqman assay kit from Applied Biosystems. The number of TRECs per naive T cell was calculated by measuring the number of naive cells present in PBMCs by flow-cytometric analysis as described above. Naive T cells were defined as the pool of cells with CD4+CD45RO−CD27+ and CD8+CD45RO−CD27+.
Measurement of Naive CD4+ T Cell Proliferation.
Ex vivo labeling of proliferating naive CD4+ T cells was carried out by incubating freshly isolated PBMCs with BrdUrd for 4 h at 37°C as described (11). Labeled cells were analyzed by FACS. Samples obtained from a separate cohort of patients who were receiving IL-2 were used in these experiments.
Measurement of Telomeric Terminal Restriction Fragment (TRF) Length.
The TRF was measured by using a modification of the procedure by Feng et al. (12). Genomic DNA was isolated from purified CD4+ T cells according to the manufacturer's recommended procedure (Puregene, Gentra Systems). Equal amounts of DNA were digested with AluI and HinFI, and 2 μg per well were loaded onto a 1% agarose gel. DNA molecular weight standards (DNA Marker Analysis System, Life Technologies, Rockville, MD) were loaded every 10–15 lanes to correct for uneven sample separation across the gel. DNA restriction fragments were resolved for 18 h at 15°C by field-inversion gel electrophoresis (FIGE Mapper Power Module, Bio-Rad) using a nonlinear switch-time ramp of 0.1–0.4 sec and a forward and reverse voltage gradient of 240 and 160 volts, respectively. The DNA was transferred to a nylon membrane (Hybond-NX, Amersham Pharmacia) and simultaneously hybridized with an alkaline phosphatase-conjugated telomeric probe (Life Technologies) and an alkaline phosphatase-conjugated DNA sizing probe (Lifecodes, Stamford, CT). Hybridized probes were detected after chemiluminescence development (Lumi-Phos Plus, Whatman) and exposure to Kodak Biomax MR 2 film (Sigma). The digitized lumigraph was analyzed by using LAB WORK GEL ANALYSIS software (Ultraviolet Products, San Gabriel, CA), and average TRF length was calculated by using weighted mean calculations that normalize the signal intensity relative to the size of each digestion product (13).
Results
Changes in CD4+ T Cells in IL-2-Treated Patients.
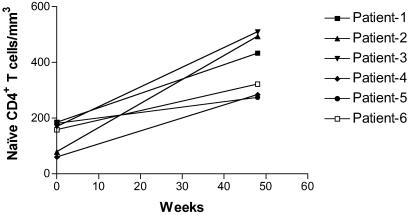
Multiple randomized, controlled studies have shown that intermittent IL-2 treatment increases CD4+ T cell count in HIV-infected individuals. The mechanism responsible for these increases remain unclear (14–19). The six patients studied longitudinally in this report were from a cohort of IL-2-treated subjects described earlier from whom stored PBMCs were available for analysis (9, 14). These patients received a total of 4–7 5-day IL-2 infusions during the study period of 12 months. During the IL-2 therapy, these patients were treated with a combination of two nucleoside reverse-transcriptase inhibitors, zidovudine and didanosine or zalcitabine (Table 1). On average CD4+ T cell numbers increased from a base-line count of 571 to 1,449 (P = 0.01) in the group of six patients selected for this study. In all patients there was an increase in total and naive CD4+ T cells over the time period examined (Fig. 1 and Table 1).
Fig 1.
Change in the absolute number of naive (CD45RO−CD27+) CD4+ T cells during intermittent IL-2 therapy. The patients received 4–7 IL-2 cycles over a 12-month period.
Decline in TREC Levels in T Cells of Patients Treated with Intermittent IL-2.
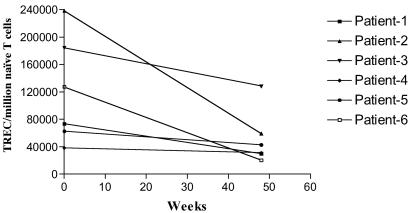
Before the analysis of PBMC samples from IL-2-treated patients, TREC levels in purified naive (CD45RA+) and memory (CD45RO+) cells from four HIV-1-positive patients were quantitated by real-time PCR method. In all these samples, greater than 97% of the total TRECs were present in naive cells (data not shown). These results confirmed earlier observations that the vast majority of the TRECs are present within naive T cells (8). Based on this observation, TRECs present in PBMC samples from IL-2-treated patients were quantitated, and the data were expressed as the number of TRECs per million naive T cells (Fig. 2). Compared with base-line, the concentration of TRECs in the total naive T cell pool (CD4 and CD8) decreased (P = 0.02) by an average of 57% over 12 months.
Fig 2.
Decrease (P = 0.02) in the concentration of TRECs in naive T cells after 1 year of intermittent IL-2 treatment in HIV-infected individuals. TREC levels in PBMCs were quantitated and the data expressed as the number of TRECs per million naive (CD45RO−CD27+) T cells.
IL-2 treatment preferentially increases the number of naive CD4+ T cells and has a minimal effect on CD8+ T cell number (Fig. 1 and Table 2). To understand better the potential role of the thymus in this preferential effect of IL-2 on CD4+ T cell expansion, the levels of TRECs were measured in purified CD4+ and CD8+ cells from all three patients from whom enough frozen cells were available. The drop in the level of TRECs was caused predominantly by changes in the concentration of TRECs in CD4+ cells. Minimal changes were seen within the CD8+ T cells. TREC concentrations declined by an average of 76 ± 13% in CD4+ T cells and 1 ± 18% in CD8+ T cells (Table 2). The decline in the concentration of TRECs in CD4+ T cells along with the increase in the total number of naive CD4+ T cells suggest that the naive CD4+ T cell expansions in IL-2-treated patients are caused, in large part, by an increased proliferation of mature naive T cells.
Table 2.
Decline of TRECs in CD4+ T cells of IL-2-treated patients
| Patient no.
|
Cell type
|
Naive cell no. per mm3 | TRECs per million cells | TRECs per million naive cells | |||
|---|---|---|---|---|---|---|---|
| Pre-IL-2 | Post-IL-2 | Pre-IL-2 | Post-IL-2 | Pre-IL-2 | Post-IL-2 | ||
| 1 | CD4+ | 185 | 433 | 45,931 | 11,031 | 159,641 | 23,107 |
| CD8+ | 274 | 99 | 27,017 | 18,392 | 112,505 | 74,311 | |
| 2 | CD4+ | 79 | 494 | 151,109 | 17,655 | 755,545 | 64,187 |
| CD8+ | 228 | 206 | 27,708 | 18,731 | 129,547 | 167,306 | |
| 3 | CD4+ | 170 | 510 | 36,864 | 16,939 | 103,219 | 51,282 |
| CD8+ | 184 | 249 | 44,344 | 40,907 | 120,982 | 122,557 | |
CD4+ and CD8+ T cells were purified as described in Materials and Methods, and the amount of TRECs in these cells was quantitated. The number of naive cells present in CD4+ and CD8+ cells was estimated by immunostaining. Naive cells were defined as CD45RO−/CD27+. Pre- and post-IL-2 refers to day-0 and week-48 samples, respectively.
Increased Proliferation of Naive CD4+ T Cells in IL-2-Treated Patients.
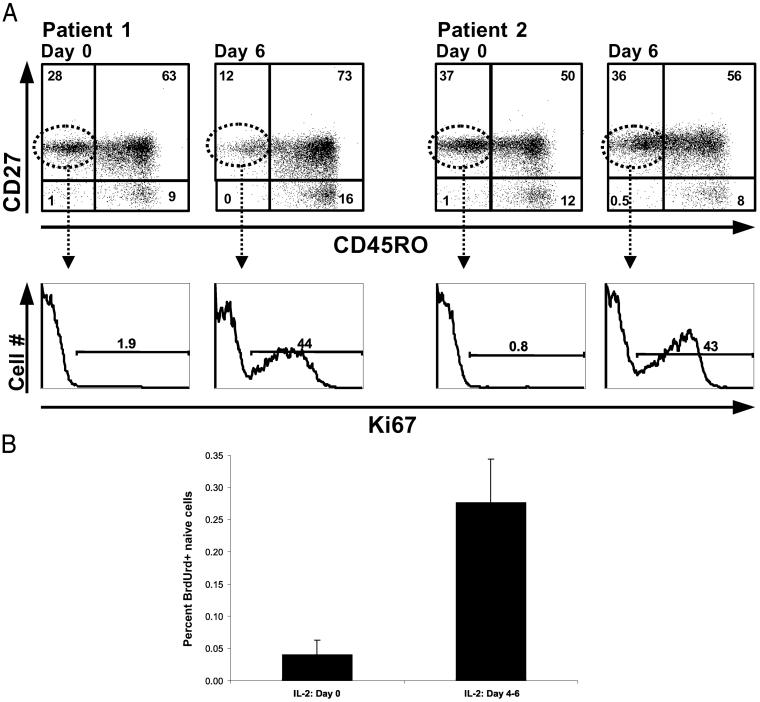
To evaluate directly the proliferation of mature, naive CD4+ T cells in the setting of IL-2 therapy, the percentage of Ki67+ cells was measured immediately after a course of IL-2 treatment (Fig. 3A). The percentage of naive (CD45RO−/CD27+) CD4+ T cells positive for Ki67 increased dramatically from values of 1–2% at day 0 to more than 40% on day 6 of IL-2 treatment. Interestingly, 2 months after an IL-2 cycle the percentage of Ki67+ CD4+ naive cells declined after this initial increase to levels below base-line values. Similar increases and declines in the percentage of CD4+Ki67+ cells were seen in all six patients studied. Overall the percentage of naive CD4+ T cells that was Ki67-positive increased from 1.3 ± 0.4% at day 0 to 46 ± 5% at day 6 of an IL-2 cycle and declined to 0.5 ± 0.1% at 1 year.
Fig 3.
(A) Increases in naive CD4+ T cell proliferation during an IL-2 cycle. Proliferation of naive CD4+ cells was measured by Ki67 intracellular staining on days 0 and 6 of an IL-2 cycle in two patients. (Upper) The gating to identify naive CD4+ T cells (CD45RO−CD27+). (Lower) The proportion of naive CD4+ T cells that are Ki67-positive. (B) Increases in ex vivo proliferation of CD4+ CD45RO− cells from IL-2-treated patients (n = 18, P = 0.002). PBMCs were labeled with BrdUrd and stained with anti-BrdUrd, anti-CD45RO, and anti-CD4 antibodies and measured by flow cytometry. CD4+ CD45RO− cells from this cohort of patients were also CD27+ as shown by staining of parallel tubes of cells.
Another way to examine directly naive CD4+ T cell proliferation is to determine the percentage of cells entering S phase through use of a marker of DNA synthesis such as BrdUrd. Direct examination of the ex vivo proliferation of the CD4+/CD45RO− T cell subset by labeling with BrdUrd for 4 h immediately after isolation revealed a 6–7-fold increase (P = 0.002) in the percentage of cells in S phase at the end of an IL-2 cycle (Fig. 3B). Thus, in IL-2-treated patients the declines in TREC values are accompanied by short-term increases in the proliferation of naive CD4+ cells as indicated by Ki67 staining and BrdUrd labeling. These data indicate that IL-2-induced proliferation of naive T cells is likely a major mechanism leading to the increases in naive CD4+ T cells seen with IL-2 therapy.
IL-2-Induced Proliferation of Naive CD4+ T Cells Does Not Lead to Shortening of Telomeres.
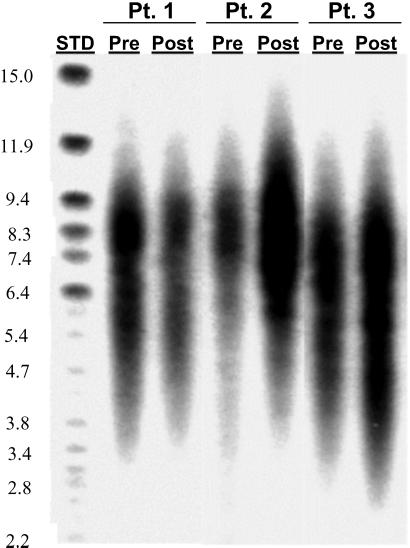
A major concern about the renewal of CD4+ T cells by continued proliferation of existing cells in HIV-1-infected patients is that these cells might reach a stage of replicative senescence and not be able to divide further after a certain length of time and number of cycles. Lengths of telomeres present at the end of a chromosome can be used as a marker of the proliferative potential of eukaryotic cells (20, 21). The average telomere lengths of CD4+ cells from IL-2-treated patients were estimated by TRF-length analysis (Fig. 4). There was no difference in the average TRF length between CD4+ T cells obtained before IL-2 infusion and cells obtained 1 year after initiation of IL-2 treatment. The average TRF length in CD4+ T cells went from 6,981 to 7,153 bp after 1 year of IL-2 therapy. These data indicate that CD4+ T cells after IL-2 retain the same potential to divide as that of the CD4+ T cells present before IL-2.
Fig 4.
TRF length does not decline in CD4+ T cells of patients treated with IL-2 for 1 year. Mean CD4+ T cell TRF lengths were determined for three patients before (Pre) and 12 months after (Post) receiving IL-2. STD, DNA analysis marker (Life Technologies). The sizes of the DNA markers are shown (in kb) on the left.
Discussion
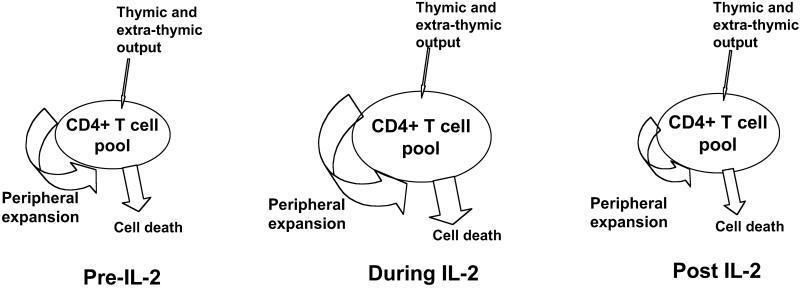
In addition to viral suppression, a major objective of the treatment of patients with HIV infection is to restore and preserve immune function. IL-2, a T cell growth factor that plays a major role in T cell activation and proliferation, is being administered as an experimental treatment of HIV-1-infected individuals with this objective (3–5). The group of patients analyzed in this study experienced a 2-fold increase in their CD4+ T cell counts after 1 year of IL-2 treatment. In prior studies it has been demonstrated that IL-2 treatment leads to preferential expansion of naive CD4+ cells in HIV-1-infected patients (18). However, the underlining mechanisms by which IL-2 increases CD4+ T cells are not understood (3, 19, 22). The increase could be caused by the proliferation of existing cells, enhanced survival of existing cells, de novo production of naive cells by the thymus, or a combination of these factors. The present study demonstrates that peripheral expansion of mature naive CD4+ T cells is a major in vivo activity of IL-2 (Fig. 5).
Fig 5.
IL-2 exerts multiple effects on the CD4+ T cell pool. New cells may enter the pool via homeostatic-driven proliferation or via stem cell differentiation through a thymic environment. During the time of an IL-2 cycle there is a pronounced increase in the size of the pool caused by peripheral expansion as indicated by decreases in TREC levels and increases in Ki67 expression and spontaneous BrdUrd incorporation. After IL-2 an increase in the CD4+ T cell pool is maintained. We postulate that persistent increases in the CD4+ T cell pool result from a state of decreased turnover and increased cell survival.
During maturation in the thymus, T cells undergo the rearrangement of their T cell receptor genes, resulting in the formation of stable episomal TRECs that persist in the newly matured T cells and are diluted out during mitosis of these cells or lost when these cells die. A number of studies have shown that TREC levels decline with HIV infection and increase after treatment with highly active antiretroviral therapy, suggesting that changes in viral replication can lead also to changes in levels of TRECs (8, 9, 23–25). Recent data suggest that the decline in TREC levels in HIV patients may be caused by increased cell division associated with immune activation by HIV rather than a decrease in thymic function (11, 26). Similarly the increased level of TRECs seen in patients treated with the highly active antiretroviral therapy may be caused by reduced immune activation and proliferation rather than an increase in the number of T cells with TRECs entering the periphery from the thymus (11, 24). In this regard it may be more accurate to refer to TREC-containing cells as “primary” rather than “recent” thymic emigrants.
In the IL-2-treated patients studied here, increases in the total numbers of naive CD4+ T cells were associated with decreases in the concentration of TRECs. We believe the most likely explanation for this observation is a dilution of TRECs caused by increased proliferation. This conclusion is supported by the short-term increases in in vivo and ex vivo proliferation of naive CD4+ cells from IL-2-treated patients as indicated by independent examinations of Ki67 and BrdUrd staining. Interestingly, IL-2 administration is accompanied by an increase in the levels of tumor necrosis factor α (TNFα) and IL-6, a cytokine milieu that, along with IL-2, has been shown to promote the growth of naive CD4+ T cells in vitro (27, 28). Decreased death and increased half-life also may contribute to the long-term raise in naive CD4+ T cells seen in IL-2-treated patients. This is suggested by the lower levels of Ki67 staining seen in these cells after 1 year of IL-2 therapy.
Telomeric DNA sequences that are present at the extreme ends of chromosomes shorten progressively during each division of the eukaryotic cells. The telomere length not only serves as an indicator of the replicative history of a cell but also as a determining factor for the residual replicative capacity of the cell. It has been shown earlier that there is no change or a slight increase in telomere length of CD4+ T cells in HIV patients (29, 30). Despite that observation, a major concern about the IL-2-mediated increase in the proliferation of naive CD4+ cells is that it may result in the exhaustion of renewal capacity of these cells, leading to premature senescence. In the present study, the telomere lengths in IL-2-treated patients showed no significant changes during the course of 1 year, during which time the CD4+ T cell number doubled. Given that peripheral expansion plays a role in that doubling, it seems possible that IL-2-induced expansions may be associated with the induction of telomerase. These data indicate that the CD4+ T cells induced by IL-2 treatment retain their full replicative potential, suggesting that IL-2 along with highly active antiretroviral therapy can be used successfully to increase and maintain normal levels of CD4+ T cells in HIV-infected subjects.
Acknowledgments
We thank Dr. A. S. Fauci for his ongoing guidance and support, Dr. D. C. Douek for help in the TREC assay, and Ms. Mary Rust for her editorial assistance. This project has been funded with federal funds from the Department of Health and Human Services under contract number NO1-CO-56000. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Abbreviations
TREC, T cell receptor rearrangement excision circle
PBMC, peripheral blood mononuclear cell
TRF, terminal restriction fragment
References
- 1.Haynes B. F., Markert, M. L., Sempowski, G. D., Patel, D. D. & Hale, L. P. (2000) Annu. Rev. Immunol. 18, 529-560. [DOI] [PubMed] [Google Scholar]
- 2.Grossman Z. & Paul, W. E. (2000) Nat. Med. 6, 976-977. [DOI] [PubMed] [Google Scholar]
- 3.Sereti I. & Lane, H. C. (2001) Clin. Infect. Dis. 32, 1738-1755. [DOI] [PubMed] [Google Scholar]
- 4.Emery S. & Lane, H. C. (1997) Curr. Opin. Immunol. 9, 568-572. [DOI] [PubMed] [Google Scholar]
- 5.Emery S., Abrams, D. I., Cooper, D. A., Darbyshire, J. H., Lane, H. C., Lundgren, J. D. & Neaton, J. D. (2002) Control Clin. Trials 23, 198-220. [DOI] [PubMed] [Google Scholar]
- 6.Douek D. C. & Koup, R. A. (2000) Vaccine 18, 1638-1641. [DOI] [PubMed] [Google Scholar]
- 7.Steffens C. M., Smith, K. Y., Landay, A., Shott, S., Truckenbrod, A., Russert, M. & Al-Harthi, L. (2001) AIDS 15, 1757-1764. [DOI] [PubMed] [Google Scholar]
- 8.Douek D. C., McFarland, R. D., Keiser, P. H., Gage, E. A., Massey, J. M., Haynes, B. F., Polis, M. A., Haase, A. T., Feinberg, M. B., Sullivan, J. L., et al. (1998) Nature (London) 396, 690-695. [DOI] [PubMed] [Google Scholar]
- 9.Kovacs J. A., Baseler, M., Dewar, R. J., Vogel, S., Davey, R. T., Falloon, J., Polis, M. A., Walker, R. E., Stevens, R., Salzman, N. P., et al. (1995) N. Engl. J. Med. 332, 567-575. [DOI] [PubMed] [Google Scholar]
- 10.McFarland R. D., Douek, D. C., Koup, R. A. & Picker, L. J. (2000) Proc. Natl. Acad. Sci. USA 97, 4215-4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lempicki R. A., Kovacs, J. A., Baseler, M. W., Adelsberger, J. W., Dewar, R. L., Natarajan, V., Bosche, M. C., Metcalf, J. A., Stevens, R. A., Lambert, L. A., et al. (2000) Proc. Natl. Acad. Sci. USA 97, 13778-13883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng Y. R., Norwood, D., Shibata, R., Gee, D., Xiao, X., Martin, M., Zeichner, S. L. & Dimitrov, D. S. (1998) J. Med. Primatol. 27, 258-265. [DOI] [PubMed] [Google Scholar]
- 13.Harley C., Bfutcher, A. B. & Greider, C. W. (1990) Nature (London) 345, 458-460. [DOI] [PubMed] [Google Scholar]
- 14.Kovacs J. A., Vogel, S., Albert, J. M., Falloon, J., Davey, R. T., Jr., Walker, R. E., Polis, M. A., Spooner, K., Metcalf, J. A., Baseler, M., Fyfe, G. & Lane, H. C. (1996) N. Engl. J. Med. 335, 1350-1356. [DOI] [PubMed] [Google Scholar]
- 15.Davey R. T., Chaitt, D. G., Piscitelli, S. C., Wells, M., Kovacs, J. A., Walker, R. E., Falloon, J., Polis, M. A., Metcalf, J. A., Masur, H., et al. (1997) J. Infect. Dis. 175, 781-789. [DOI] [PubMed] [Google Scholar]
- 16.Carr A., Emery, S., Lloyd, A., Hoy, J., Garsia, R., French, M., Stewart, G., Fyfe, G. & Cooper, D. A. (1998) J. Infect. Dis. 178, 992-999. [DOI] [PubMed] [Google Scholar]
- 17.Gougeon M. L., Rouzioux, C., Liberman, I., Burgard, M., Taoufik, Y., Viard, J. P., Bouchenafa, K., Capitant, C., Delfraissy, J. F. & Levy, Y. (2001) AIDS 15, 1729-1731. [DOI] [PubMed] [Google Scholar]
- 18.Kovacs J. A., Vogel, S., Metcalf, J. A., Baseler, M., Stevens, R., Adelsberger, J., Lempicki, R., Hengel, R. L., Sereti, I., Lambert, L., et al. (2001) Eur. J. Immunol. 31, 1351-1360. [DOI] [PubMed] [Google Scholar]
- 19.De Paoli P., Bortolin, M. T., Zanussi, S., Monzoni, A., Pratesi, C. & Giacca, M. (2001) Clin. Exp. Immunol. 125, 440-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.von Zglinicki T. (2001) Cancer Lett. 168, 111-116. [DOI] [PubMed] [Google Scholar]
- 21.Urquidi V., Tarin, D. & Goodison, S. (2000) Annu. Rev. Med. 51, 65-79. [DOI] [PubMed] [Google Scholar]
- 22.Sereti I., Herpin, B., Metcalf, J. A., Stevens, R., Baseler, M. W., Hallahan, C. W., Kovacs, J. A., Davey, R. T. & Lane, H. C. (2001) AIDS 15, 1765-1775. [DOI] [PubMed] [Google Scholar]
- 23.Hatzakis A., Touloumi, G., Karanicolas, R., Karafoulidou, A., Mandalaki, T., Anastassopoulou, C., Zhang, L., Goedert, J. J., Ho, D. D. & Kostrikis, L. G. (2000) Lancet 355, 599-604. [DOI] [PubMed] [Google Scholar]
- 24.Zhang L., Lewin, S. R., Markowitz, M., Lin, H. H., Skulsky, E., Karanicolas, R., He, Y., Jin, X., Tuttleton, S., Vesanen, M., et al. (1999) J. Exp. Med. 190, 725-732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chavan S., Bennuri, B., Kharbanda, M., Chandrasekaran, A., Bakshi, S. & Pahwa, S. (2001) J. Infect. Dis. 183, 1445-1454. [DOI] [PubMed] [Google Scholar]
- 26.Hazenberg M. D., Otto, S. A., Cohen Stuart, J. W., Verschuren, M. C., Borleffs, J. C., Boucher, C. A., Coutinho, R. A., Lange, J. M., Rinke de Wit, T. F., Tsegaye, A., et al. (2000) Nat. Med. 6, 1036-1042. [DOI] [PubMed] [Google Scholar]
- 27.Brinkmann V., Kinzel, B. & Kristofic, C. (1996) J. Immunol. 156, 4100-4106. [PubMed] [Google Scholar]
- 28.Unutmaz D., Pileri, P. & Abrignani, S. (1994) J. Exp. Med. 180, 1159-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Palmer L. D., Weng, N., Levine, B. L., June, C. H., Lane, H. C. & Hodes, R. J. (1997) J. Exp. Med. 185, 1381-1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wolthers K. C., Bea, G., Wisman, A., Otto, S. A., de Roda Husman, A. M., Schaft, N., de Wolf, F., Goudsmit, J., Coutinho, R. A., van der Zee, A. G., et al. (1996) Science 274, 1543-1547. [DOI] [PubMed] [Google Scholar]