Abstract
This paper describes a simple methodology for evaluating the bacterial binding of ciprofloxacin labelled with technetium Tc 99m. Using this methodology, the binding of 99mTc-ciprofloxacin by live Escherichia coli was compared with the binding of 99mTc-ciprofloxacin by killed E. coli and the binding of 99mTc-pertechnetate (99mTcO4 −) by live E. coli. The antimicrobial effect of 99mTc-ciprofloxacin on E. coli was evaluated. Four groups were defined: live E. coli with 99mTc-ciprofloxacin, live E. coli with 99mTcO4 −, killed E. coli with 99mTc-ciprofloxacin, and killed E. coli with 99mTcO4 −. After 0, 2, and 4 h of incubation of 1 × 108 colony-forming units of E. coli suspended in 5 mL of sterile distilled water with 1.85 MBq of 99mTc-ciprofloxacin or 99mTcO4 −, 1 mL from each sample was centrifuged. The radioactivity of the bacterial pellet and that of the supernatant were measured separately, and the percentage of sample radioactivity attributable to bacterial binding was calculated. Of the 99mTc-ciprofloxacin, 3.6% to 5.9% was bound to live or killed E. coli; only 0.1% to 0.2% of the 99mTcO4 − was bound to live E. coli (P < 0.0001). No significant difference in 99mTc-ciprofloxacin binding was found between live and killed E. coli (P = 0.887). An antimicrobial effect on E. coli was seen with 99mTc-ciprofloxacin: colony counts were reduced after 4 h. The small amount of 99mTc-ciprofloxacin binding and the lack of difference in binding between live and killed E. coli may limit the utility of this methodology in evaluating the presence of E. coli infection.
Résumé
Cette étude décrit une méthode simplifiée pour l’évaluation de la liaison bactérienne au 99mTc-ciprofloxacin. La liaison du 99mTc-ciprofloxacin à une suspension de E. coli viable a été comparée à la liaison du 99mTc-ciprofloxacin au E. coli tué et à celle du 99mTcO4− au E. coli viable. 99mTc-ciprofloxacin a aussi été évalué pour son effet antimicrobien sur E. coli. Quatre groupes ont été testés : E. coli viable avec 99mTc-ciprofloxacin (CL), E. coli viable avec 99mTcO4− (TL), E. coli tué avec 99mTc-ciprofloxacin (CK), et E. coli tué avec 99mTcO4− (TK). Une suspension de E. coli (1 × 108 unités formatrices de colonies) dans 5 mL d’eau distillée a été incubé avec 1,85 MBq 99mTc-ciprofloxacin ou 99mTcO4−. À 0, 2 et 4 heures, 1 mL de chaque échantillon a été centrifugé afin de séparer les bactéries du surnageant. La radioactivité du culot bactérien et celle du surnageant a été mesurée séparément et le pourcentage le la radioactivité totale de l’échantillon attribuable à la portion bactérienne a été calculé. De 3,6 à 5,9 % du 99mTc-ciprofloxacin se retrouvait lié au E. coli viable ou tué. Ceci était significativement plus élevé que le pourcentage de liaison de 99mTcO4− au E. coli (0,1–0,2 %). Aucune différence significative n’a été retrouvée dans le niveau de la liaison au 99mTc-ciprofloxacin par E. coli viable ou tué. La méthode employée a permis l’évaluation de la liaison bactérienne du 99mTc-ciprofloxacin au E. coli. Le faible pourcentage de liaison bactérienne de 99mTc-ciprofloxacin et l’absence de différence de la liaison entre E. coli viable ou tué pourraient limiter l’utilité du 99mTc-ciprofloxacin dans l’évaluation d’infections cliniques au E. coli.
(Traduit par les auteurs)
Introduction
Ciprofloxacin labelled with technetium Tc 99m was developed for scintigraphy in cases of clinical infection. Ciprofloxacin, a fluoroquinolone antibiotic, binds to and inhibits DNA gyrase of viable bacteria (1,2) and thus has a broad spectrum of antimicrobial activity. This feature makes 99mTc-ciprofloxacin potentially useful for detection of a variety of bacterial infections. However, false-positive and false-negative scans have invariably occurred in the many in vivo experimental studies in animals and clinical trials in humans that have investigated the accuracy of 99mTc-ciprofloxacin scintigraphy (3–17). Possible reasons for the inaccuracy include the presence of ciprofloxacin-resistant bacteria, insufficient intralesional bacteria, killed intralesional bacteria, and antibiotic therapy (3,4,7,10,14). Also, the exact mechanism of uptake of 99mTc-ciprofloxacin into sites of bacterial infection in vivo has not been proven, although it is speculated to be through binding of the radiopharmaceutical to bacterial DNA gyrase (4,13,16). In vitro studies of bacterial binding of 99mTc-ciprofloxacin could answer many of these questions in addition to reducing the number of in vivo experiments.
A few studies have evaluated the in vitro binding of various formulations of 99mTc-ciprofloxacin to bacteria (18–21). The percentage of binding seemed to depend on the concentration of bacteria in solution, the species of bacteria, the presence of live versus killed bacteria, the concentration of 99mTc-ciprofloxacin in solution, the formulation of 99mTc-ciprofloxacin, and the duration of incubation of the bacteria with the radiopharmaceutical. However, the articles did not include specific information on the volume or activity of the radiopharmaceutical used, the phase of bacterial growth, or the stability of 99mTc-ciprofloxacin in the incubation solutions. Earlier studies had evaluated the binding of 99mTc-ciprofloxacin prepared with formamidine sulfinic acid as a reducing agent and then heated (18,19). This formulation has recently been modified: stannous chloride is used as a reducing agent, and heating is no longer required. Only 1 study has evaluated the bacterial binding and stability of a similar formulation of 99mTc-ciprofloxacin (21).
Binding of 99mTc-ciprofloxacin to various bacterial species varies greatly. In 1 study, 18.9% to 20.3% of the radiopharmaceutical was bound by 108 colony-forming units (cfu)/mL of live Escherichia coli at 4 h (18); in another study, 11.72% to 43.89% was bound to 109 cfu/mL of E. coli at 4 h (19). For Staphylococcus aureus (108 cfu/mL), 7.7% (18) and 15% to 40% (21) of the radiopharmaceutical was bound at 1 h. This variability may be due to differences in methodology or 99mTc-ciprofloxacin formulation. Despite the variability, binding of 99mTc-ciprofloxacin by live bacteria exceeded that of a control radiopharmaceutical (99mTc-methylene diphosphonate), of which less than 2.5% was bound (19,20).
Our primary objective was to develop and assess a simple methodology for evaluating the bacterial binding of 99mTc-ciprofloxacin. Using this methodology, we compared the binding by live and killed E. coli.
Materials and methods
Preliminary experimentation
A strain of E. coli isolated from a blood culture of a 4-d-old quarterhorse filly with septicemia was used. The Kirby Bauer antibiotic disc method demonstrated that the strain was susceptible to ciprofloxacin at a minimal inhibitory concentration (MIC) of 0.007 μg/mL, the same as for E. coli American Type Culture Collection (ATCC) 25922. The isolate was evaluated for overnight growth (approximately 18 h) that would consistently yield a concentration of approximately 1.5 × 109 cfu/mL. Bacterial concentration was confirmed as described in “Bacterial samples.” To confirm that the concentration would remain stable, the bacteria were maintained in distilled water for 6 h at room temperature. During this period the bacterial counts were unchanged.
Sample groups of bacteria
The 4 sample groups were as follows: live E. coli with 99mTc-ciprofloxacin, live E. coli with 99mTc-pertechnetate (99mTcO4 −), killed E. coli with 99mTc-ciprofloxacin, and killed E. coli with 99mTcO4 −. Each group contained 12 samples. Each sample was evaluated after 0, 2, and 4 h of incubation with 99mTc-ciprofloxacin or 99mTcO4 − at room temperature. The experiment was conducted on 3 separate days, so that 4 samples from each group were evaluated on a given day. A separate vial of 99mTc-ciprofloxacin was used for each sample. The radiopharmaceuticals were reconstituted each experimental day.
Labelling and quality control
Vials containing 185 MBq (5 mCi) of 99mTc-ciprofloxacin or 99mTcO4 − in a 2-mL volume were obtained from the Ohio State University Nuclear Pharmacy, Columbus, Ohio, USA. Labelled ciprofloxacin was reconstituted from kits provided by the manufacturer (Infecton; Draximage, Kirkland, Quebec). Each kit vial contained a lyophilized powder composed of 2 mg of ciprofloxacin and stannous chloride (SnCl2•2H2O) as a reducing agent. The kits were stored refrigerated and shielded from light. Before reconstitution, the vials were brought to room temperature. To produce 99mTc-ciprofloxacin, 185 MBq (5 mCi) of 99mTcO4 − was added to the kit vial. To assess radiopharmaceutical purity, instant thin-layer chromatography (ITLC) with acetone as the soluble phase was performed on all vials at time 0 and again at 4 h to verify that the amount of free pertechnetate was less than 5%.
Bacterial samples and killing
Samples of approximately 1 × 108 cfu/mL of bacteria suspended in 5 mL of sterile distilled water were used. The E. coli was grown overnight on MacConkey medium (MacConkey II, BBL Stacker Plate; Becton Dickinson, Sparks, Maryland, USA). Several colonies were removed and suspended in 5 mL of brain heart infusion (BHI) (BBL; Becton Dickinson). A concentration of approximately 1 × 108 cfu/mL was achieved (comparison with a 0.5 McFarlane turbidity standard). Overnight growth of this suspension resulted in a concentration of approximately 1.5 × 109 cfu/mL. The suspension was diluted with 60 mL of sterile distilled water to obtain a stock solution, then divided into 5-mL samples in sterile glass test tubes. The bacterial concentration of the stock solution at time 0 was verified by plating serial dilutions on blood-agar plates (TSA II, BBL Stacker Plate; Becton Dickinson) and counting the bacterial colonies after incubation for 24 h at 37°C. As shown in our preliminary experimentation, bacteria were not multiplying during the study. The bacterial concentration of each sample was verified 4 h after incubation with 99mTc-ciprofloxacin to evaluate the antibiotic effect. Three randomly selected samples of bacteria incubated with 99mTcO4 − were similarly evaluated to test whether 99mTcO4 − killed bacteria.
Suspended bacteria grown overnight and counted as described earlier (approximately 1.5 × 109 cfu/mL in 5 mL BHI) were killed by means of heating to 66°C (± 1°C) in a water bath for 60 min. The killed suspensions were refrigerated until further use. On the morning of experimentation, 5 mL of each suspension was diluted in 60 mL of sterile distilled water, then 5-mL samples were placed in sterile glass test tubes. A sample was plated to ensure lack of viability and Gram’s staining performed to evaluate cell morphology. No viability was seen in any of these controls, and the morphology was similar to that of E. coli from fresh cultures.
Radiopharmaceutical incubation and measurement
Approximately 1.85 (1.7 to 2.0) MBq of 99mTc-ciprofloxacin or 99mTcO4 − in approximately 20 (19 to 25) μL was placed in each sample of live or killed bacteria, for a total radiopharmaceutical concentration of approximately 0.37 MBq/mL (10 μCi/mL) and a final ciprofloxacin concentration of 4 μg/mL.
The percentage of the total amount of sample radioactivity in the bacterial pellet was calculated. To obtain a bacterial pellet, 1 mL of each sample was withdrawn in a glass pipette (S/P; Baxter Healthcare, McGaw Park, Illinois, USA), placed in a polypropylene microcentrifugation tube (VWR International, West Chester, Pennsylvania, USA), and spun at 2000 × g (Mini-Spin; Eppendorf, Westbury, New York) for 10 min. The supernatant was transferred via pipette (Pipet-Plus; Rainin Instrument, Oakland, California, USA. Redi-Tips, Fisherbrand; Fisher Scientific, Pittsburgh, Pennsylvania, USA) to a new glass test tube. The pellet was resuspended with 1 mL of sterile distilled water and centrifugation repeated. The resuspension supernatant was added to the initial supernatant. The pellet was transferred via pipette into a new glass test tube. The pipette tip was saved, as the bacteria within it could not be completely evacuated; the radioactivity in the pipette tip was considered part of the bacterial pellet. Transfer of the pellet to a new glass test tube was necessary because of some binding of 99mTc-ciprofloxacin to the microcentrifuge tube, which was preserved and considered part of the supernatant after being washed with 1 mL of sterile distilled water. The water for washing was discarded. The radioactivity in the pellet, supernatant, and microcentrifugation tube was counted separately for 15 s in a sodium iodide well counter (Biodex Medical Systems, Shirley, New York) with dedicated nuclear medicine software (AtomLab 450, version 1.0.8; Biodex). The percentage of total activity in the pellet was calculated as follows: [pellet activity/(pellet + supernatant activity) × 100].
Statistical analysis
Linear multivariate analysis (repeated-measures analysis of variance) was used to evaluate differences between groups in the effect of time, treatment, and time on treatment. If a significant difference (P < 0.05) was found, it was further characterized by means of Tukey’s multiple comparisons. Friedman’s test was used to evaluate for an effect of time within treatments. If a significant difference over time (P < 0.05) was found, it was further characterized by means of the Wilcoxon signed-rank test. All analyses were conducted with the use of statistical software (SPSS 11.5; SPSS, Chicago, Illinois, USA).
Results
Escherichia coli radioactivity
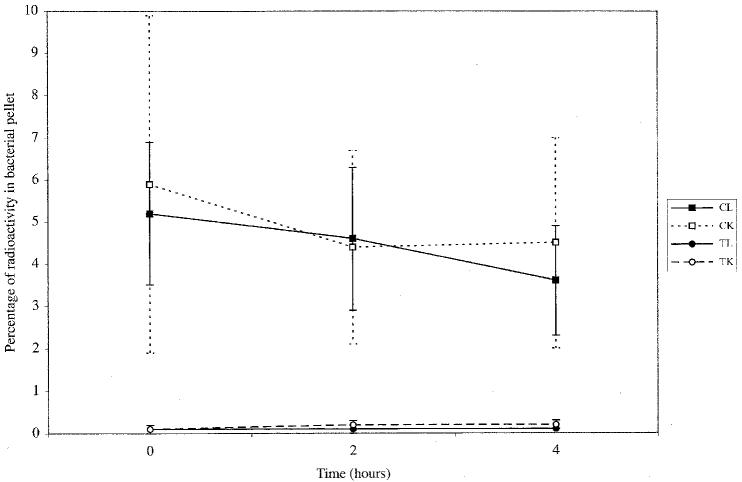
As Figure 1 shows, the samples treated with 99mTc-ciprofloxacin had a significantly higher percentage of radiopharmaceutical activity within the bacterial pellet than those treated with 99mTcO4 − (3.6% to 5.9% versus 0.1% to 0.2%; P < 0.0001). There was no significant difference in radioactivity between the live and killed bacterial pellets among those treated with 99mTc-ciprofloxacin (3.6% to 5.2% versus 4.4% to 5.9%; P = 0.887) or among those treated with 99mTcO4 − (P = 1).
Figure 1.
Mean percentage and standard deviation of bacterial pellet radioactivity over time after incubation of live or killed Escherichia coli with either ciprofloxacin labelled with technetium Tc 99m (CL and CK) or 99mTc-pertechnetate (TL and TK).
Bacterial binding of 99mTc-ciprofloxacin was almost immediate, as evidenced by the radioactivity in the bacterial pellet at time 0. No significant change in the percentage of activity within the bacterial pellet over time was found for the samples killed by 99mTc-ciprofloxacin (P = 0.758) or 99mTcO4 − (P = 0.651) (Figure 1). Among the live samples treated with 99mTc-ciprofloxacin, significant decreases in bacterial pellet activity were found between times 0 to 4 h (P = 0.029) and 2 to 4 h (P = 0.018).
Bacterial concentration over time
Incubation of live bacteria with 99mTc-ciprofloxacin caused a reduction in colony count over the 4-h incubation period, from 1.06 × 108 cfu/mL to 1.54 × 106 cfu/mL, on average. No change in concentration over time was seen in 3 randomly selected samples of bacteria incubated with 99mTcO4 − (average at 4 h 1.14 × 108 cfu/mL).
Discussion
Our primary objective was to develop a simple methodology for evaluating the in vitro binding of 99mTc-ciprofloxacin to bacteria. We selected E. coli because it multiplied to a predictable concentration overnight, and the concentration remained stable in water over the 6-h period needed to conduct the experiment. The original intent of this study was to evaluate the binding of 99mTc-ciprofloxacin to a variety of equine pathogens; for this reason, a strain of E. coli isolated from an infected horse was used, as opposed to an ATCC strain. The bacteria were killed with gentle heating in an attempt to preserve cell wall integrity as much as possible. The bacteria were separated from the supernatant by centrifugation. We initially attempted filtration with polyethersulfone and polyvinylidine fluoride membranes, as in other experiments (18,19), but 18% to 70% of the 99mTc-ciprofloxacin in solution bound to the filters. Flushing the filters with saline did not resolve the problem. Although there was some binding of 99mTc-ciprofloxacin to the polypropylene centrifugation tubes, this was accounted for by considering the tubes as part of the supernatant.
The concentration, activity, and volume of 99mTc-ciprofloxacin were not clearly defined in other studies. We used the lowest amount of radioactivity that could be measured with our dose calibrator (30 to 50 μCi). However, this resulted in a final concentration of ciprofloxacin in the bacterial suspensions of 4 μg/mL, much higher than therapeutic plasma levels (0.5 μg/mL) (22) and higher than the average MIC90 for 17 strains of E. coli isolated from horses (0.008 μg/mL) (23) and the MIC for the strain used in this study (0.007 μg/mL). Ideally, the sample concentration of ciprofloxacin would have mimicked the plasma concentration after injection of an imaging dose of 99mTc-ciprofloxacin: 0.04 μg/mL, based on injection of 2 mg of 99mTc-ciprofloxacin in a 500-kg horse with an approximate blood volume of 50 L.
The bacterial pellet activity of 99mTc-ciprofloxacin was measured at times 0, 2, and 4 h. Although imaging is routinely performed in vivo 24 h after injection, the 99mTc-ciprofloxacin, as evaluated in a preliminary experiment (29), was not stable enough to permit evaluation of bacterial binding at that time point.
We selected 99mTcO4 − as the control radiopharmaceutical because it is inexpensive, stable, and widely available. In addition, E. coli should not bind 99mTcO4 − in its unreduced form except under anaerobic conditions (24).
Binding of 99mTc-ciprofloxacin to live and killed E. coli was minimal and did not differ significantly between the 2 groups. Compared with previous findings, the binding to live E. coli was less in our study (3.6% to 5.2% versus 11.72% to 43.89%) (18,19), whereas the binding to killed E. coli was similar (4.4% to 5.9% versus 3%) (18). Overall, our results contrast with those of experiments that found higher binding of 99mTc-ciprofloxacin in live than in killed E. coli (18,20). There could be several explanations for the low binding by live bacteria in our study: lack of mediation of binding by DNA gyrase, decreased concentration of DNA gyrase because the E. coli were not multiplying, differences between the E. coli strains used, and in vitro dissociation of 99mTc-ciprofloxacin, with binding of free reduced pertechnetate to E. coli.
No studies exist proving that the mechanism of binding of 99mTc-ciprofloxacin to bacteria is via DNA gyrase. Binding may instead occur to nonspecific components present in both live and killed bacteria, such as the cell wall. One study indirectly supports this theory: in mice with soft tissue abscesses, pretreatment with ciprofloxacin did not change the 99mTc-ciprofloxacin scintigraphic imaging characteristics of the abscesses (25). If 99mTc-ciprofloxacin is specifically bound to bacterial DNA gyrase, pretreatment with ciprofloxacin should reduce the number of available binding sites and therefore the uptake of 99mTc-ciprofloxacin into infected sites. Further experimentation is needed to establish the exact binding mechanism.
DNA gyrase is necessary to prevent DNA-strand supercoiling during DNA replication (2,26); therefore, more enzyme should be present in actively multiplying (log-phase) bacterial populations. Bacterial replication may explain the greater overall percentage of 99mTc-ciprofloxacin binding in live bacteria and the significant difference in binding between live and killed bacteria seen in other studies. It would be interesting to repeat the experiment, comparing the effects of replicating versus static bacterial populations on the binding of 99mTc-ciprofloxacin. Being in a stationary phase of growth does not limit binding of unlabelled ciprofloxacin by certain strains of E. coli (27,28), but that may not be the case for 99mTc-ciprofloxacin or for the strain of E. coli used here. In other studies (18,21), it was not specified whether bacteria were actively multiplying, although in 1 study (18) the bacteria were suspended in IsoSensitest broth, which would be expected to promote multiplication. In the other study (21), the bacteria were suspended in chilled phosphate-buffered saline (PBS). Preliminary investigation showed significant dissociation of 99mTc-ciprofloxacin in PBS compared with distilled water when tested with ITLC (29).
Dissociation of 99mTc-ciprofloxacin in the bacterial suspension as an explanation for the lack of difference in binding between live and killed E. coli is unlikely, given that the least dissociation (7.5% at 6 h) occurred in distilled water in the preliminary investigation (29). In addition, in the vials of undiluted 99mTc-ciprofloxacin used in this experiment, ITLC showed less than 5% free pertechnetate from 99mTc-ciprofloxacin dissociation at 0 and 4 h. We therefore assume that all radiopharmaceutical activity in the bacterial pellet was from the binding of intact 99mTc-ciprofloxacin. The ITLC was not validated or performed to evaluate specifically for dissociation of the 99mTc-ciprofloxacin diluted in the bacterial samples. Therefore, we cannot completely eliminate the possibility that the presence of suspended bacteria caused dissociation, although it seems highly unlikely.
Binding of 99mTc-ciprofloxacin was seen immediately with both live and killed E. coli, as is also true for unlabelled ciprofloxacin, which reaches concentration equilibrium in bacteria within 1 to 2 min of antibiotic exposure (30). Although the binding was unchanged over time in killed E. coli, it decreased between 0 to 4 and 2 to 4 h in live E. coli, perhaps in relation to the decrease in live bacterial concentration of the suspension between 0 and 4 h due to the antimicrobial effect of 99mTc-ciprofloxacin or residual free ciprofloxacin in solution. In comparison, Hall (18) found significantly greater binding of 99mTc-ciprofloxacin by 108 cfu/mL of E. coli at 2 h (20.8%) than at 1 (16.1%) or 4 h (18.9%). However, in that experiment, the bacterial concentration in solution and the antibacterial effect were not re-evaluated at each time point.
The percentage of bacterial pellet activity differed significantly for live E. coli between those exposed to 99mTc-ciprofloxacin and those exposed to 99mTcO4 −. As our control radiopharmaceutical, 99mTcO4 − was not expected to bind with E. coli. The apparent activity of 99mTcO4 − in live E. coli in our study (0.1%) is less than that reported for live E. coli after 21 h of incubation with 99mTc-methylene diphosphonate (1.02%) (19).
The reduction in the concentration of live bacteria after 4 h of incubation with 99mTc-ciprofloxacin may indicate preservation of the ability of the molecule to bind to bacterial DNA gyrase. Alternatively, the antimicrobial activity may have resulted from residual unlabelled ciprofloxacin or from dissociated ciprofloxacin, although the latter is less likely. Further experimentation to establish the cause of the apparent preservation of antimicrobial effect is warranted, with a goal of elucidating the exact mechanism of 99mTc-ciprofloxacin binding. Also, concerns have been raised that the clinical use of 99mTc-ciprofloxacin could select for fluoroquinolone-resistant bacteria (31). If antimicrobial activity is preserved, resistance could occur; this concern is minor relative to resistance caused by other uses of fluoroquinolones. Also, at the much lower plasma concentration of radiopharmaceutical that would be achieved in vivo, an antimicrobial effect of the magnitude that we observed would not be expected.
The antimicrobial effect of 99mTc-ciprofloxacin had a negative effect on our experimental methodology. Our goal was to maintain a constant concentration of bacteria in solution in order to accurately compare bacterial binding between time points, between live and killed bacteria, and between radiopharmaceuticals. Because the concentration of live bacteria in solution decreased significantly after incubation with 99mTc-ciprofloxacin, exact comparisons could not be made. This effect may explain the significant decrease in 99mTc-ciprofloxacin binding over time that we observed in live E. coli.
No evidence of an antimicrobial effect of 99mTcO4 − was seen. Although radiation can kill bacteria, concentrations of 460 MBq/mL (12.5 mCi/mL) and higher of 99mTcO4 − were required for an antimicrobial effect on S. epidermidis (32). Our model used a concentration of 0.37 MBq/mL (10 μCi/mL).
To conclude, using the methods described herein, a small amount (< 6%) of 99mTc-ciprofloxacin binding may be seen with both live and killed E. coli. This may limit the usefulness of this radiopharmaceutical in evaluating the presence of clinical E. coli infection for 2 reasons. First, because the degree of binding in killed bacteria was similar to that in live bacteria, false-positive detection of “sterile” or resolved sites of infection might occur in vivo. Second, the small degree of binding seen in vitro might be insufficient to be clinically detected in vivo. Further in vitro evaluation of the binding of 99mTc-ciprofloxacin to different bacterial species is warranted, and clinical evaluation of 99mTc-ciprofloxacin is necessary to expand on our preliminary data.
Acknowledgments
This study was funded by the Ohio State University College of Veterinary Medicine Equine Research Funds. The authors also acknowledge Draximage (Kirkland, Quebec) for supplying the ciprofloxacin radiolabelling kits, the Ohio State University Nuclear Pharmacy for the radiopharmaceutical reconstitution, and the Comparative Orthopedic Research Laboratory for use of its facilities.
Footnotes
This project was reported as a portion of Dr. Alexander’s master’s thesis.
Dr. Alexander’s current address is Faculté de médecine vétérinaire, Université de Montréal, 3200, rue Sicotte, St-Hyacinthe, Québec J2S 7C6.
References
- 1.Hertzberg RP. Agents interfering with nucleic acids. In: Hansch C, Sammes PG, Taylor JB, eds. Comprehensive Medicinal Chemistry: the Rational Design, Mechanistic Study & Therapeutic Application of Chemical Compounds. New York: Pergamon Press, 1990:753–791.
- 2.Petri WA. Antimicrobial agents: sulfonamides, trimethoprim–sulfamethoxazole, quinolones. In: Hardman JG, Limbird LE, Gilman AG, eds. Goodman and Gilman’s the Pharmacological Basis of Therapeutics. Chicago: McGraw-Hill, 2001.
- 3.Yapar Z, Kibar M, Yapar AF, Togrul E, Kayaselcuk U, Sarpel Y. The efficacy of technetium-99m ciprofloxacin (Infecton) imaging in suspected orthopaedic infection: a comparison with sequential bone/gallium imaging. Eur J Nucl Med. 2001;28:822–830. doi: 10.1007/s002590100555. [DOI] [PubMed] [Google Scholar]
- 4.Vinjamuri S, Hall AV, Solanki KK, et al. Comparison of 99mTc Infecton imaging with radiolabelled white-cell imaging in the evaluation of bacterial infection. Lancet. 1996;347:233–235. doi: 10.1016/s0140-6736(96)90407-9. [DOI] [PubMed] [Google Scholar]
- 5.Sundram FX, Wong WY, Ang ES, Goh AS, Ng DC, Yu S. Evaluation of technetium-99m ciprofloxacin (Infecton) in the imaging of infection. Ann Acad Med Singapore. 2000;29:699–703. [PubMed] [Google Scholar]
- 6.Southwood LL, Kawcak CE, McIlwraith CW, Frisbie DD, Steyn PE. Use of scintigraphy for assessment of fracture healing and early diagnosis of osteomyelitis following fracture repair in rabbits. Am J Vet Res. 2003;64:736–745. doi: 10.2460/ajvr.2003.64.736. [DOI] [PubMed] [Google Scholar]
- 7.Sonmezoglu K, Sonmezoglu M, Halac M, et al. Usefulness of 99mTc-ciprofloxacin (Infecton) scan in diagnosis of chronic orthopedic infections: comparative study with 99mTc-HMPAO leukocyte scintigraphy. J Nucl Med. 2001;42:567–574. [PubMed] [Google Scholar]
- 8.Sarda L, Saleh-Mghir A, Peker C, Meulemans A, Cremieux AC, Le Guludec D. Evaluation of (99m)Tc-ciprofloxacin scintigraphy in a rabbit model of Staphylococcus aureus prosthetic joint infection. J Nucl Med. 2002;43:239–245. [PubMed] [Google Scholar]
- 9.Sarda L, Cremieux AC, Lebellec Y, et al. Inability of 99mTc-ciprofloxacin scintigraphy to discriminate between septic and sterile osteoarticular diseases. J Nucl Med. 2003;44:920–926. [PubMed] [Google Scholar]
- 10.Malamitsi J, Giamarellou H, Kanellakopoulou K, et al. Infecton: a 99mTc-ciprofloxacin radiopharmaceutical for the detection of bone infection. Clin Microbiol Infect. 2003;9:101–109. doi: 10.1046/j.1469-0691.2003.00506.x. [DOI] [PubMed] [Google Scholar]
- 11.Larikka MJ, Ahonen AK, Niemela O, et al. 99m Tc-ciprofloxacin (Infecton) imaging in the diagnosis of knee prosthesis infections. Nucl Med Commun. 2002;23:167–170. doi: 10.1097/00006231-200202000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Larikka MJ, Ahonen AK, Niemela O, et al. Comparison of 99mTc ciprofloxacin, 99mTc white blood cell and three-phase bone imaging in the diagnosis of hip prosthesis infections: improved diagnostic accuracy with extended imaging time. Nucl Med Commun. 2002;23:655–661. doi: 10.1097/00006231-200207000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Hall AV, Solanki KK, Vinjamuri S, Britton KE, Das SS. Evaluation of the efficacy of 99mTc-Infecton, a novel agent for detecting sites of infection. J Clin Pathol. 1998;51:215–219. doi: 10.1136/jcp.51.3.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dumarey N, Blocklet D, Appelboom T, Tant L, Schoutens A. Infection is not specific for bacterial osteo-articular infective pathology. Eur J Nucl Med Mol Imaging. 2002;29:530–535. doi: 10.1007/s00259-001-0749-2. [DOI] [PubMed] [Google Scholar]
- 15.De Winter F, Gemmel F, Van Laere K, et al. 99mTc-ciprofloxacin planar and tomographic imaging for the diagnosis of infection in the postoperative spine: experience in 48 patients. Eur J Nucl Med Mol Imaging. 2004;31:233–239. doi: 10.1007/s00259-003-1349-0. [DOI] [PubMed] [Google Scholar]
- 16.Britton KE, Vinjamuri S, Hall AV, et al. Clinical evaluation of technetium-99m Infecton for the localisation of bacterial infection. Eur J Nucl Med. 1997;24:553–556. doi: 10.1007/BF01267688. [DOI] [PubMed] [Google Scholar]
- 17.Britton KE, Wareham DW, Das SS, et al. Imaging bacterial infection with (99m)Tc-ciprofloxacin (Infecton) J Clin Pathol. 2002;55:817–823. doi: 10.1136/jcp.55.11.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hall AV. Investigation of a Novel Radiopharmaceutical, 99m Technetium Labelled Ciprofloxacin-99mTc-Infecton; Its In-Vitro Binding to Bacteria and Its Clinical Efficacy as an Imaging Agent in Infection [MRC Path dissertation]. London: St-Bartholomew’s Hospital, 1996.
- 19.Solanki K. Imaging of Infections [US patent application]. London: British Technology Group, 1995.
- 20.Solanki KK, Bomanji J, Siraj Q, Small M, Britton KE. Tc-99m “Infecton” — a new class of radiopharmaceutical for imaging infection [abstract] J Nucl Med. 1993;34:119. [Google Scholar]
- 21.Oh SJ, Ryu JS, Shin JW, et al. Synthesis of 99mTc-ciprofloxacin by different methods and its biodistribution. Appl Radiat Isot. 2002;57:193–200. doi: 10.1016/s0969-8043(02)00107-0. [DOI] [PubMed] [Google Scholar]
- 22.Dowling PM, Wilson RC, Tyler JW, Duran SH. Pharmacokinetics of ciprofloxacin in ponies. J Vet Pharmacol Ther. 1995;18:7–12. doi: 10.1111/j.1365-2885.1995.tb00543.x. [DOI] [PubMed] [Google Scholar]
- 23.Ensink JM, van Klingeren B, Houwers DJ, Klein WR, Vulto AG. In-vitro susceptibility to antimicrobial drugs of bacterial isolates from horses in The Netherlands. Equine Vet J. 1993;25:309–313. doi: 10.1111/j.2042-3306.1993.tb02969.x. [DOI] [PubMed] [Google Scholar]
- 24.Lloyd JR, Cole JA, Macaskie LE. Reduction and removal of heptavalent technetium from solution by Escherichia coli. J Bacteriol. 1997;179:2014–2021. doi: 10.1128/jb.179.6.2014-2021.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ryu JK, Shin JW, Oh SJ, Cheon JH, Moon DH, Lee HK. Effect of ciprofloxacin pretreatment on 99mTc-ciprofloxacin (CIP) imaging of experimental bacterial infection [abstract] J Nucl Med. 2000;41:322. [Google Scholar]
- 26.Hooper DC, Wolfson JS, Ng EY, Swartz MN. Mechanisms of action of and resistance to ciprofloxacin. Am J Med. 1987;82:12–20. [PubMed] [Google Scholar]
- 27.Zeiler HJ. Evaluation of the in vitro bactericidal action of ciprofloxacin on cells of Escherichia coli in the logarithmic and stationary phases of growth. Antimicrob Agents Chemother. 1985;28:524–527. doi: 10.1128/aac.28.4.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zeiler HJ, Voigt WH. Efficacy of ciprofloxacin in stationary-phase bacteria in vivo. Am J Med. 1987;82:87–90. [PubMed] [Google Scholar]
- 29.Alexander K. In Vitro Experimentation and Detection of Clinical Infections Using 99m-Tc-Ciprofloxacin [Master’s thesis]. Columbus, Ohio: Ohio State University, 2004.
- 30.Piddock LJ. Mechanisms of fluoroquinolone resistance: an update, 1994–1998. Drugs. 1999;58:11–18. doi: 10.2165/00003495-199958002-00003. [DOI] [PubMed] [Google Scholar]
- 31.Welling MM, Paulusma-Annema A, Balter HS, Pauwels EK, Nibbering PH. Technetium-99m labelled antimicrobial peptides discriminate between bacterial infections and sterile inflammations. Eur J Nucl Med. 2000;27:292–301. doi: 10.1007/s002590050036. [DOI] [PubMed] [Google Scholar]
- 32.Stathis VJ, Miller CM, Doerr GF, Coffey JL, Hladik WB. Effect of technetium Tc 99m pertechnetate on bacterial survival in solution. Am J Hosp Pharm. 1983;40:634–637. [PubMed] [Google Scholar]