Abstract
The objectives of this study were to establish optimal doses of 13C-glycocolic acid (GCA) for use in a GCA blood test as a marker for canine small intestinal bacterial metabolic activity. Four doses of GCA were administered orally to 8 healthy dogs. Blood samples were collected at various time points up to 480 min. The percent dose/min of 13C administered as GCA (PCD) and cumulative PCD (CUMPCD) were determined by fractional mass spectrometry. No dog showed any clinically obvious side effects. Doses of 1 and 2 mg/kg of bodyweight (BW) led to a significant increase in PCD and CUMPCD (P < 0.001). The mean CUMPCD was significantly higher for the 1 mg/kg BW dose compared with the 2 and 4 mg/kg BW doses (P < 0.05). Administration of 1 mg/kg BW of 13C-glycocholic acid led to an increase in CUMPCD over baseline in gas extracted from blood samples and appears to be the best parameter to evaluate for future clinical studies.
Résumé
Cette étude avait pour objectif d’établir les doses optimales d’acide glycolique-13C (GCA) pour utilisation dans un test sanguin à titre de marqueur de l’activité métabolique bactérienne dans le petit intestin de chien. Quatre doses de GCA ont été administrées par voie orale à 8 chiens en santé. Des échantillons sanguins ont été prélevés à différents temps pendant une période de 480 min. Le pourcentage dose/min de 13C administré comme GCA (PCD) et le PCD cumulatif (CUMPCD) ont été déterminés par spectrométrie de masse fractionnelle. Aucun chien n’a montré de signe clinique évident d’effets secondaires. Des doses de 1 et 2 mg/kg de poids corporel (BW) ont entraîné une augmentation significative du PCD et CUMPCD (P < 0,001). La CUMPCD moyenne était significativement plus élevée pour la dose de 1 mg/kg BW comparativement aux doses de 2 et 4 mg/kg BW (P < 0,05). L’administration de 1 mg/kg BW d’acide glycolique-13C a causé une augmentation du CUMPCD au-dessus de la valeur de base dans les gaz extraits d’échantillons sanguins et semble être le meilleur paramètre à évaluer lors d’études cliniques futures.
(Traduit par Docteur Serge Messier)
Some canine patients that present with chronic, relapsing diarrhea respond to antibiotic therapy. This condition has been referred to as “small intestinal bacterial overgrowth” by many authors (1,2), although this terminology remains controversial as there is little objective data regarding the normal bacterial flora of the canine small intestine. While quantitative aerobic and anaerobic bacterial culture of the duodenal juice is considered the gold-standard for diagnosis of small intestinal bacterial overgrowth, the technical difficulty of this procedure limits its routine use in clinical practice. Also, it has been recently recognized that the majority of microbial species present in biological samples escape identification through standard culture techniques alone (3,4). For example, it has been estimated that 60% to 80% of bacterial organisms present in the gastrointestinal tract of humans and pigs have not yet been identified (4,5). There is a significant need for a diagnostic test that will accurately reflect the bacterial content of the small intestine in dogs. Ideally, such a test would be sensitive and specific, non-invasive, readily available to general practitioners, and not require complicated sample handling.
14C- and 13C-glycocholic acid (GCA) breath tests have been used to diagnose small intestinal bacterial overgrowth in humans (6,7). The breath test is based on the deconjugation of the naturally occurring bile acid glycocholic acid by intestinal bacteria. 13C-glycocholic acid is the glycine conjugate of cholic acid. One carbon of the glycine residue is labeled with 13C, a stable carbon isotope, and thus can serve as a tracer. After oral administration, GCA reaches the small intestine where it enters the physiological bile acid pool and undergoes enterohepatic circulation. Bacteria present in the small intestine deconjugate the 13C-glycine portion from the core bile acid. The 13C-glycine is metabolized by intestinal bacteria and 13C is released. The 13C freely diffuses into the blood stream where it may be transported in 3 different forms: dissolved in the plasma, bound to hemoglobin, and as bicarbonate. Eventually 13CO2 is exhaled and can be quantified in breath (6). Alternatively, 13C can be measured in blood samples (8,9). Addition of 6 N HCl to a blood sample releases 13CO2, which can then be quantified by fractional mass spectrometry.
The presence of increased bacterial numbers in the upper small intestine may lead to an increase in the proportion of the orally administered dose of 13C-glycocholic acid, which undergoes deconjugation. Thus, as has been observed in humans, an increase in small intestinal bacterial numbers may lead to an increase in the fraction of the 13CO2 fraction of CO2 in the circulation in comparison to healthy individuals (6). The aim of this study was to establish an optimal dose of GCA for use in a GCA blood test as a potential marker for small intestinal bacterial biomass and metabolic activity in dogs.
The protocol was approved by the University Laboratory Animal Care Committee at Texas A&M University. Eight healthy, female hound dogs (median age: 3.0 y; range: 1 to 6.5 y) were enrolled in this study. Before the beginning of the study serum folate, cobalamin, unconjugated bile acids, and trypsin-like immunoreactivity concentrations were evaluated to screen for gastrointestinal disease.
Four doses of GCA (Glycocholic acid-13C [glycyl-1-13C]; CDN Isotopes, Point-Claire, Quebec), 0.5 mg/kg body weight (BW), 1 mg/kg BW, 2 mg/kg BW, and 4 mg/kg BW, were evaluated in a randomized study design. During each study period all dogs were given the same dose, with at least a 14-day rest period between individual study periods. Dogs were fed at 10 am on the day prior to each study period. At 3 pm of that day, all food dishes and unconsumed food were removed from the dog runs. Venous access was established in each dog by insertion of a 12-inch-long, 18.5-gauge, indwelling catheter (Venocath; Abbott Laboratories, Abbott Park, Illinois, USA) into a jugular vein. After collection of a 1 mL baseline blood sample, GCA dissolved in 50 mL of deionized H2O was administered using a gastric feeding tube. Additional 1 mL blood samples were collected at 15, 30, 45, 60, 75, 90, 105, 120, 135, 150, 165, 180, 210, 240, 270, 300, 330, 360, 390, 420, 450, and 480 min after GCA administration. Blood samples were collected every 15 min initially because bacterial metabolism of 13C-glycocholic acid was expected to occur early after administration. After the first 180 min, the sampling interval was changed to 30 min in order to reduce the total number of blood samples collected from the dogs. Blood samples were immediately transferred into evacuated tubes (Vacutainer Sodium Lithium 10 mL; Becton Dickinson, Franklin Lanes, New Jersey, USA) containing 2 mL of 6 N hydrochloric acid.
The 13CO2 released into the gas phase above the blood sample in each tube was determined by gas chromatograph isotope ratio mass spectrometry (Automated Breath 13Carbon Analyzer; Europa Scientific, Crewe, United Kingdom) and expressed as a relative isotope ratio 13CO2:12CO2 (percentage 13CO2 in total CO2 above background 13CO2 concentration in the environment) (10). This relative isotope ratio was then converted into an absolute ratio (RSample) by comparing the measured 13CO2:12CO2 of the sample with the absolute 13CO2:12CO2 ratio of the international calcium carbonate standard Pee Dee belemnite (PDB) (PDB is a limestone fossil of Belemnitella americana from the Cretaceous Pee Dee formation in South Carolina) and calculated from the following equation (11):
 |
where
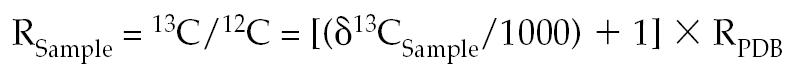 |
The δ13CSample is the relative difference of the sample to PDB. The PDB has, by international convention, an arbitrarily assigned δ13C value of 0%, its absolute 13C:12C (RPDB) has been reported as 0.0112372 (11). A negative or positive δ13C indicates that the sample contains relatively less or more 13C than the PDB, respectively.
The data was initially expressed as δ13CSample over baseline (DOB). The 13C expired (μmol/min) was calculated as DOB × 0.0112372 × CO2 produced. The CO2 production was estimated using the following calculation: (RER × RQ)/4.8 L, where RER is the resting energy requirement (BW0.75 × 70) and RQ the respiratory quotient (estimated RQ for canine diets was 0.8).
The percent dose/min of 13C administered as GCA (PCD) was calculated using the following formula:
 |
where MW is the molecular weight of the 13GCA.
The cumulative PCD (CUMPCD) is the cumulative % of the dose recovered, and was calculated as:
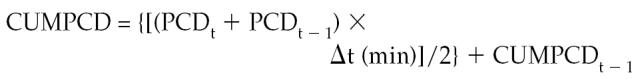 |
The PCDs and CUMPCDs were compared for each one of the 4 individual doses using a 1-way analysis of variance (ANOVA), followed by Tukey’s multiple comparison tests. The effect of the differing doses on PCDs and CUMPCDs over time was analyzed by using a 2-way ANOVA. Coefficients of variation (CV% = [standard deviation/mean] × 100) were calculated for both PCD and CUMPCD values for each time point and for each dose. The mean %CVs for the 1 mg/kg BW and 2 mg/kg BW doses were compared using a student’s t-test. Data was analyzed using a statistical software package (GraphPad Prism, 3.0; GraphPad Software, San Diego, California, USA).
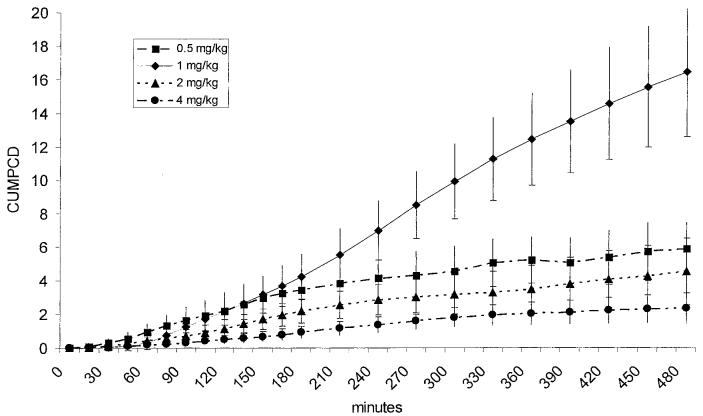
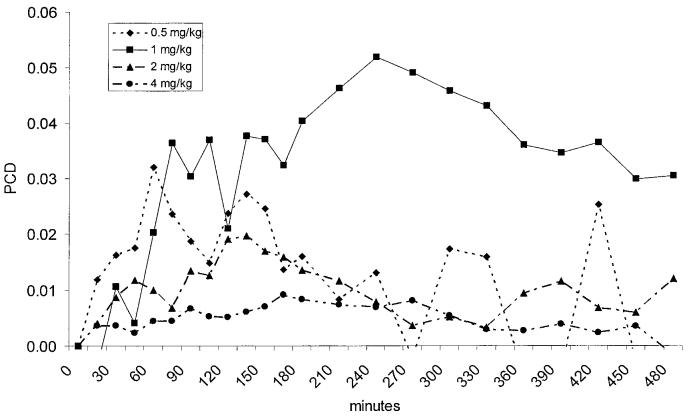
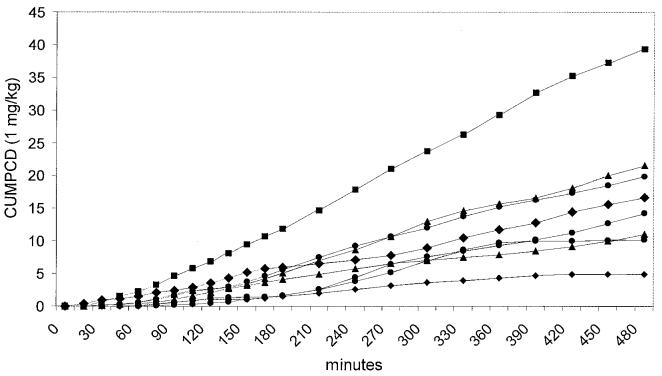
Prior to being enrolled into the study none of the dogs had any history of clinical signs or any changes in evaluated blood parameters that would be consistent with gastrointestinal disease. None of the dogs showed any clinically obvious side effects after oral administration of GCA. Neither the 0.5 mg/kg BW nor the 4 mg/kg BW doses lead to a significant increase in PCD over time (P = 0.083 and P = 0.107, respectively), but both doses led to a significant increase in CUMPCD over time (P < 0.0001 for both doses). Over time, both the 1 and 2 mg/kg BW doses led to significant increases in PCD (P < 0.0001 and P = 0.024, respectively) and CUMPCD (P < 0.0001 for both doses; Figure 1). The time-points for peak PCDs showed a high degree of variation between dogs and doses (Figure 2). The mean CUMPCD was significantly higher for the 1 mg/kg BW dose than the other doses (P < 0.05; Figure 1). Administration of GCA in the amount of 1 mg/kg BW led to an increase in CUMPCD over baseline in gas extracted from blood samples in all 8 healthy dogs (Figure 3), whereas 7/8 dogs had an increase in CUMPCD over baseline after administration of GCA in the amount of 2 mg/kg BW. There was no significant difference between the mean %CVs for CUMPCD between the 1 mg/kg BW and 2 mg/kg BW doses (P = 0.535).
Figure 1.
Cumulative percent dose/min of 13C administered as 13C-glycocholic acid (CUMPCD). All 4 doses led to a significant increase of CUMPCD over time (P < 0.001). The lower CUMPCD of the 0.5 mg/kg BW dose compared to the 1 mg/kg BW dose suggesting a lack of substrate for intestinal bacteria, while the lower CUMPCD of the 2 and 4 mg/kg BW doses suggests saturation of the deconjugation capacity of the small intestinal microflora.
Figure 2.
The percent dose/min of 13C (PCD) administered as 13C-glycocholic acid (GCA). The 1 and 2 mg/kg BW doses led to a significant increase in PCD. However, the time-point for the peak PCD showed a high degree of variation between dogs and doses. Therefore, determination of CUMPCD (Figure 2) is suggested for future clinical studies.
Figure 3.
Individual cumulative percent dose/min of 13C administered as 13C-glycocholic acid (CUMPCD) in 8 healthy dogs after administration of 1 mg/kg BW dose of 13C-glycocholic acid.
Previous studies have shown that bacterial deconjugation (the ability of bacteria to remove a glycine or taurine moiety) of bile acids, and the subsequent appearance of unconjugated bile acids in serum, can serve as an non-invasive marker for small intestinal bacterial metabolic activity in dogs (12). Sample preparation for measurement of serum unconjugated bile acids is technically challenging, timeconsuming and, therefore, relatively expensive. Also, it has been speculated that spurious elevations of serum unconjugated bile acid concentrations and hence a false positive diagnosis of small intestinal bacterial overgrowth may be obtained through small amounts of bile entering the small intestine during the fasted state as a result of migrating motor complexes and spontaneous gall bladder contraction (13). The GCA blood test potentially combines the benefits of the measurement of a bacteria-specific metabolic activity with the minimally invasive nature of a blood test, and has a considerably lower technical difficulty in sample preparation than the unconjugated bile acids test. As the compound being administered is not present in the normal bile acid pool, this test is potentially less dependant upon strict fasting and less susceptible to interference from migrating motor complexes and spontaneous gall bladder contraction. Carbon dioxide is easily extracted from a blood sample by adding hydrochloric acid, and no subsequent processing is necessary before measurement of the 13C-labeled CO2 via fractional mass spectrometry. While the measurement of 13C in clinical samples necessitates the use of expensive equipment (an automated breath 13C analyzer), recent studies have shown that samples can be obtained easily in clinical settings and shipped to an appropriate laboratory that has the necessary equipment for analysis of samples (14). Also, a recent study has demonstrated that samples are stable for up to 3 wk at room temperature, thus allowing storage and shipment to the laboratory (15).
In this study we evaluated 4 different doses of GCA. After oral administration of GCA of amounts up to 4 mg/kg BW, no gross clinical evidence of adverse effects were observed during the course of the study, suggesting that the GCA test is safe in healthy dogs. Safety of GCA needs to be further evaluated in dogs with gastrointestinal disease.
In this study, only the 1 mg/kg BW and 2 mg/kg BW doses led to a significant increase in both PCD and CUMPCD over time. The lower CUMPCD of the 0.5 mg/kg BW dose compared to the 1 mg/kg BW dose, suggesting a lack of substrate for intestinal bacteria. Increasing the GCA dose to 2 and 4 mg/kg BW led to a decrease in CUMPCD, suggesting saturation of the capacity of the intestinal microflora to deconjugate administered GCA. Determination of PCD and their summation (cumulative PCD or CUMPCD) have been traditionally used in humans to measure bacterial metabolic mass (6). Determination of a peak PCD would be preferable compared to CUMPCD as fewer samples, such as a baseline sample and a sample taken at the time of the PCD peak, would be required. The peak PCD for the 1 mg/kg BW and 2 mg/kg BW doses showed a high degree of variation between individual dogs, preventing the selection of an optimal sampling time point for the determination of PCD. This variation might be due to differences in intestinal motility or differences in composition of the intestinal microflora leading to different deconjugation kinetics, between individual dogs. The CUMPCD showed a lower degree of variation between dogs, suggesting that the CUMPCD would be a more reliable parameter than the PCD. The minimal number of blood samples required for accurate assessment of the CUMPCD remains to be determined in dogs with suspected small intestinal bacterial overgrowth. Administration of a 1 mg/kg BW dose of 13C-glycocholic acid led to an increase in CUMPCD over baseline in gas extracted from blood samples from all 8 healthy dogs. While a 1 mg/kg BW dose appears to be the best dose to use in healthy dogs, it may be necessary to evaluate other doses in clinically ill dogs with suspected small intestinal bacterial overgrowth in order to avoid a possible substrate effect as noted in the current study at the 0.5 mg/kg BW dose. Additional studies that evaluate the clinical utility of the GCA test in dogs with suspected small intestinal bacterial overgrowth are warranted.
Footnotes
Parts of this manuscript have been presented as a research abstract at the 2004 ACVIM Forum in Minneapolis, Minnesota, USA.
References
- 1.Ludlow CL, Davenport DJ. Small intestinal bacterial overgrowth. In: Bonagura JD, ed. Kirk’s Current Veterinary Therapy. WB Saunders, 2000:637–641.
- 2.Morris TH, Sorensen SH, Turkington J, Batt RM. Diarrhoea and increased intestinal permeability in laboratory beagles associated with proximal small intestinal bacterial overgrowth. Lab Anim. 1994;28:313–319. doi: 10.1258/002367794780745047. [DOI] [PubMed] [Google Scholar]
- 3.Suau A, Bonnet R, Sutren M, et al. Direct analysis of genes encoding 16S rRNA from complex communities reveals many novel molecular species within the human gut. Appl Environ Microbiol. 1999;65:4799–4807. doi: 10.1128/aem.65.11.4799-4807.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leser TD, Amenuvor JZ, Jensen TK, Lindecrona RH, Boye M, Moller K. Culture-independent analysis of gut bacteria: the pig gastrointestinal tract microbiota revisited. Appl Environ Microbiol. 2002;68:673–690. doi: 10.1128/AEM.68.2.673-690.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Langendijk PS, Schut F, Jansen GJ, et al. Quantitative fluorescence in situ hybridization of Bifidobacterium spp. with genus-specific 16S rRNA-targeted probes and its application in fecal samples. Appl Environ Microbiol. 1995;61:3069–3075. doi: 10.1128/aem.61.8.3069-3075.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peled Y, Levy-Gigi C, Ayalon D, Gilat T. The cholyl glycine-1-14C breath test in various gastrointestinal disorders. Digestion. 1979;19:267–276. doi: 10.1159/000198356. [DOI] [PubMed] [Google Scholar]
- 7.Solomons NW, Schoeller DA, Wagonfeld JB, Ott D, Rosenberg IH, Klein PD. Application of a stable isotope (13C)-labeled glycocholate breath test to diagnosis of bacterial overgrowth and ileal dysfunction. J Lab Clin Med. 1977;90:431–439. [PubMed] [Google Scholar]
- 8.Moeller EM, Steiner JM, Williams DA, Klein PD. Preliminary studies of a canine 13C-aminopyrine demethylation blood test. Can J Vet Res. 2001;65:45–49. [PMC free article] [PubMed] [Google Scholar]
- 9.Cornetta AM, Simpson KW, Strauss-Ayali D, McDonough PL, Gleed RD. Use of a [13C]urea breath test for detection of gastric infection with Helicobacter spp in dogs. Am J Vet Res. 1998;59:1364–1369. [PubMed] [Google Scholar]
- 10.Choi MG, Camilleri M, Burton DD, Zinsmeister AR, Forstrom LA, Nair KS. Reproducibility and simplification of 13C-octanoic acid breath test for gastric emptying of solids. Am J Gastroenterol. 1998;93:92–98. doi: 10.1111/j.1572-0241.1998.092_c.x. [DOI] [PubMed] [Google Scholar]
- 11.Boutton T. Stable carbon isotope ratios of natural materials: I. sample preparation and mass spectrometric analysis. In: Coleman DC, Fry B, eds. Carbon Isotope Techniques. Academic Press, 1991:155–171.
- 12.Melgarejo T, Williams DA, O’Connell NC, Setchell KD. Serum unconjugated bile acids as a test for intestinal bacterial overgrowth in dogs. Dig Dis Sci. 2000;45:407–414. doi: 10.1023/a:1005493416946. [DOI] [PubMed] [Google Scholar]
- 13.Ruaux CG, Steiner JM, Williams DA. Postprandial changes in serum unconjugated bile acid concentrations in healthy beagles. Am J Vet Res. 2002;63:789–793. doi: 10.2460/ajvr.2002.63.789. [DOI] [PubMed] [Google Scholar]
- 14.Chiaramonte D, Steiner JM, Broussard JD, et al. Use of a 13C-aminopyrine blood test: first clinical impressions. Can J Vet Res. 2003;67:183–188. [PMC free article] [PubMed] [Google Scholar]
- 15.Moeller EM, Steiner JM, Teague SR, Ruaux CG, Suchodolski JS, Williams DA. Evaluation of sample stability for the 13C-aminopyrine blood test[Abstract] . J Vet Intern Med. 2003;17:448. [Google Scholar]