Abstract
Background
Drug-resistant Streptococcus pneumoniae (SP) infections pose a significant global health threat, with a limited availability of effective treatments to prevent disease progression. Chlorogenic acid (CGA) exhibits potent antioxidant and antimicrobial properties, though its exact mechanisms in combating bacterial infections remain not yet fully elucidated. Pneumolysin (PLY), a pivotal virulence factor of SP, specifically contributes to the pathogenesis of these infections.
Purpose
This study aimed to investigate the role of SP-derived PLY in triggering macrophage ferroptosis and to elucidate the potential mechanism underlying CGA-mediated regulation of this process.
Methods
A macrophage-SP co-culture model was established to investigate PLY-induced ferroptosis and the mechanisms underlining CGA-mediated antimicrobial effects. Hemolysis assays were conducted to assess PLY activity, and macrophage apoptosis was evaluated using an Annexin V/PI apoptosis detection kit and CCK-8 assay. Key biomarkers, including intracellular ROS levels, mitochondrial membrane potential, MDA content, and total iron levels, were measured using commercial assay kits. Transmission electron microscopy (TEM) was utilized to analyze mitochondrial ultrastructural alterations, particularly morphological changes in the mitochondrial membrane and cristae. Additionally, the expression of key ferroptosis-related factors was analyzed via qRT-PCR, Western blotting, and immunofluorescence staining to delineate the underlying molecular pathways.
Results
CGA markedly suppressed SP proliferation and attenuated PLY activity. Co-culture with SP or PLY exposure significantly decreased macrophage viability and triggered apoptotic cell death, whereas CGA treatment markedly attenuated apoptosis. CGA upregulated the expression of Nrf2, SOD1, and HO-1 while substantially decreasing intracellular ROS accumulation. Additionally, CGA preserved mitochondrial membrane integrity and significantly lowered MDA content and total iron levels in macrophages. Furthermore, CGA significantly upregulated the expression of key ferroptosis-related factors, including GPX4 and SLC7A11.
Conclusions
CGA effectively attenuates SP-induced macrophage ferroptosis by activating the Nrf2/GPX4 signaling axis. Notably, PLY was identified as a critical mediator of SP-induced macrophage ferroptosis, and further investigations are warranted to elucidate the exact molecular mechanisms.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12865-025-00755-y.
Keywords: Streptococcus pneumonia, Pneumolysin, Chlorogenic acid, Macrophages, Ferroptosis
Introduction
Streptococcus pneumoniae (SP) is a Gram-positive bacterium responsible for a range of life-threatening infections, including pneumonia, meningitis, and bacteremia, resulting in over a million deaths annually [1]. SP’s ability to colonize the upper respiratory tract and invade the host is closely linked to its capacity to evade, suppress, and manipulate host immune responses [2]. A crucial virulence factor of SP is pneumolysin (PLY) [3], a multifunctional cytotoxin that induces necroptosis, apoptosis, and direct cytotoxicity in various cell types [4]. PLY is a major target for vaccine development aimed at combating SP infections [5]. However, the emergence of antibiotic-resistant SP strains, driven by excessive antibiotic use, has exacerbated the morbidity and mortality associated with these infections [6]. Even with antibiotics, bacterial toxins released following conventional treatment can continue to damage host cells, highlighting the need for novel therapeutic strategies that directly inhibit PLY activity [7]. In this context, alternative therapeutic approaches, alongside the development of novel antibiotics, are essential.
Ferroptosis is a form of iron-dependent regulated cell death triggered by excessive lipid peroxidation. It is characterized by increased reactive oxygen species (ROS) and the accumulation of lipid peroxides, distinguishing it from necrosis, apoptosis, and autophagy [8]. Morphologically, ferroptosis is marked by mitochondrial shrinkage, membrane rupture, and cristae loss [9]. Recent studies have shown that bacterial infections, including those caused by Staphylococcus aureus (SA), can trigger ferroptosis, and some reports suggest that modulating ferroptosis may serve as a potential therapeutic target [10]. Likewise, SP infection has been associated with ferroptosis. However, the underlying mechanisms, particularly those involving the Nrf2/GPX4 pathway, remain to be fully elucidated [11]. Ferroptosis is regulated by glutathione peroxidase 4 (GPX4), an enzyme that reduces lipid hydroperoxides and prevents ferroptosis [12]. Nuclear factor erythroid 2–related factor 2 (Nrf2) is a critical antioxidant transcription factor that maintains redox homeostasis and regulates genes involved in oxidative stress response [13]. Both SA and SP infections can impair Nrf2 activity, thereby promoting ferroptosis and exacerbating tissue injury.
CGA is a bioactive polyphenolic compound found in plants, widely recognized for its potent antioxidant and antimicrobial properties [14]. Beyond its antimicrobial effects, CGA has exhibited protective roles in various oxidative stress and inflammation models, including liver injury, primarily through activation of the Nrf2 signaling pathway [15]. It has also been reported to alleviate ferroptosis in chronic stress-induced duodenal injury [16]. Despite these findings, the impact of CGA on ferroptosis in the context of SP infection and its potential to mitigate PLY-induced cytotoxicity remain unexplored. Given CGA's capability to activate the Nrf2/GPX4 pathway, it presents a promising candidate for alleviating PLY-induced ferroptosis in macrophages. This study aims to determine whether CGA can reverse SP- or PLY-induced cytotoxicity by focusing on ferroptosis modulation and the involvement of the Nrf2/GPX4 axis.
Materials and methods
Bacterial culture, determination of the Minimum Inhibitory Concentration (MIC), and growth measurements
The clinical isolates of drug-resistant Streptococcus pneumoniae (SP) were obtained from the First People's Hospital of Hangzhou, China. The isolates were incubated in Luria Bertani (LB) broth at 37 °C with shaking at 200 × g. Upon reaching the logarithmic growth phase, the bacterial culture was diluted with PBS to a final density of 105 CFU/mL. Based on clinical use, penicillin G (PG), a resistant antibiotic, and vancomycin (VA), a sensitive antibiotic, were selected. According to the Clinical and Laboratory Standards Institute (CLSI) 2021-M100 guidelines, the MICs of PG, VA, and chlorogenic acid (prepared in sterile water) against the isolates were determined using the microdilution method. Test bacteria were exposed to serial dilutions of the drugs in 96-well plates, and absorbance at OD600 was measured after 18 h of incubation 7A.
Cell culture and determination of non-toxic drug concentrations
Human macrophages were employed to assess the cytotoxicity of CGA. Macrophages were cultured in RPMI-1640 medium (Servicebio, China) at 37 °C in a 5% CO₂ atmosphere. Once cells reached the logarithmic growth phase, they were seeded into 96-well plates at a density of 5 × 104 cells/well and into 6-well plates at a density of 5 × 10⁶ cells/well. To induce THP-1 cell differentiation into macrophages, 100 ng/mL phorbol 12-myristate 13-acetate (PMA) was added and incubated for 48 h. Following differentiation, the drugs were added to the culture medium, and cells were incubated for an additional 24 h. Cell viability was evaluated using the CCK-8 assay.
Coculture of macrophages and PLY
Following macrophage differentiation as described in Sect."Cell Culture and Determination of Non-Toxic Drug Concentrations", the culture medium was replaced with antibiotic-free RPMI-1640 medium. Cells were then infected with either purified PLY protein (purified in this laboratory) or antibiotic-resistant Streptococcus pneumoniae (SP) at a multiplicity of infection (MOI) of 10 for 18 h at 37 °C in a 5% CO₂ atmosphere. Drugs were added to the culture medium as indicated, and co-incubation was continued for 18 h prior to downstream experiments.
Hemolytic activity assay of the PLY protein
PLY protein at various concentrations (0, 4, 8, 16, 32, or 64 μg/mL) was used either alone or in combination with CGA and antibiotics in 195 μL of PBS. Five microliters of goat red blood cells (Servicebio, Hubei, China) were added to the mixture and incubated at 37 °C for 10–30 min. After incubation, samples were centrifuged at 2000 rpm for 5 min. PBS and 1% Triton X-100 were used as negative and positive controls, respectively. Hemolysis was quantified by measuring absorbance at 543 nm. The hemolysis rate for various concentrations of chlorogenic acid was calculated by comparison with control groups using the following formula: Haemolysis rate (%) = (OD₅₄₃ nm of experimental group – OD₅₄₃ nm of negative control)/(OD₅₄₃ nm of positive control – OD₅₄₃ nm of negative control) × 100%.
Real-Time PCR (qRT-PCR)
Total RNA was extracted from SP strains 18 h post-drug intervention and from macrophages following 18 h infection with SP or PLY, using TRIzol reagent (Servicebio). The extracted RNA was used to evaluate the expression of the PLY gene in the SP strain, as well as the transcription levels of iron-dependent genes (GPX4, SLC7A11) and genes involved in endogenous antioxidant pathways (NRF2, SOD1, HO-1) in macrophages. Complementary DNA (cDNA) was synthesized using the ReverTra Ace qPCR kit (Biosharp, China), followed by quantitative PCR performed with a SYBR Green real-time PCR kit according to the manufacturer’s protocol. Calculate relative gene expression using the 2 ^—Δ Δ Ct method.
Table 1.
The primer sequences for qRT-PCR
| Gene | Forward (5′−3′) | Reverse (5′−3′) |
|---|---|---|
| GAPDH | GGAAGCTTGTCATCAATGGAAATC | TGATGACCCTTTTGGCTCCC |
| NRF2 | GGATTTGATTGACATACTTTGGAGG | TTTCTGACTGGATGTGCTGGG |
| SOD1 | CGAGCAGAAGGAAAGTAATGGA | CCACACCATCTTTGTCAGCAGT |
| HO-1 | ATGCCCCAGGATTTGTCAGAG | GGAAGTAGACAGGGGCGAAGAC |
| PLY | AACAGGCAAGGTGGATATGGTAGAG | TGAAAGGTCGCAACTACATTGTCAC |
| 16sDNA | CAGGTGCGAATGATGTGTGG | ACAATCCTTCCATTGGCGTG |
Determination of cell Viability Via Annexin V/PI and CCK-8 assays
The CCK-8 assay was used to evaluate cytotoxicity. As described in Sect."Cell Culture and Determination of Non-Toxic Drug Concentrations", macrophages differentiated in 96-well plates were cultured in the presence of various concentrations of CGA (0, 16, 32, 64, or 128 μg/mL) for 24 h at 37 °C in a 5% CO₂ incubator. After adding 10% CCK-8 reagent to each well, cells were incubated for 50–60 min, and absorbance at OD450 nm was measured to assess cell viability.
Cell apoptosis was evaluated using the Annexin-V-FITC apoptosis detection kit (Beyotime, Shanghai, China). The culture supernatant was first collected. Cells were digested with EDTA-free trypsin, combined with the collected supernatant, and centrifuged at 500 g and 4 °C for 5 min. The cells were washed twice with precooled PBS (500 g, 4 °C, 5 min) and gently resuspended in precooled 1 × Binding Buffer to a concentration of 5 × 10⁶ cells/mL. A total of 100 μL of cell suspension was used, to which 5 μL of Annexin V-FITC, 5 μL of PI, and 10 μL Hoechst were added. The mixture was gently mixed and incubated in the dark at room temperature for 8–10 min. Finally, 400 μL of precooled 1 × Binding Buffer was added and gently mixed. Apoptosis was analyzed using a flow cytometer or fluorescence microscope within 1 h.
Measurement of intracellular ROS, mitochondrial membrane potential, MDA, and total iron levels
As described in Sect."Coculture of Macrophages and PLY", after co-culturing macrophages with drugs, PLY, or SP in six-well plates for 18 h, the following assays were performed.
Intracellular ROS levels were detected using the ROS assay kit (Beyotime, Shanghai, China). The DCFH-DA probe was diluted 1:1000 in serum-free medium. The cell culture medium was discarded, and cells were washed 1–2 times with PBS. After removing the PBS, 1000 μL of DCFH-DA working solution was added to each well along with 10 μL Hoechst stain. Cells were incubated at 37 °C in the dark for 30 min. Following incubation, the DCFH-DA solution was removed, and the cells were washed 2–3 times with PBS before fluorescence detection by microscopy or flow cytometry (488 nm excitation, 525 nm emission).
Mitochondrial membrane potential was measured using the JC-1 staining kit (Beyotime, Shanghai, China). After removing the culture medium and washing the cells with PBS, cells were scraped and resuspended in PBS. Subsequently, 1 mL of cell suspension and 1 mL of JC-1 staining solution were mixed and incubated at 37 °C for 20 min. The supernatant was removed, and the cells were washed twice with 1 × JC-1 buffer. Cells were then resuspended in 2 mL of medium and analyzed for red/green fluorescence using flow cytometry or fluorescence microscopy.
Cells were lysed using the lysis buffer provided in the kit. Lipid peroxidation was quantified by measuring malondialdehyde (MDA) concentrations using the MDA assay kit (Solarbio, Shanghai, China) at 532 nm. The values were normalized to total protein content. Total iron levels were measured using a colorimetric assay kit (Solarbio, Shanghai, China) at 593 nm and normalized to cell number.
Immunofluorescence
For immunofluorescence analysis, cells were fixed with 4% paraformaldehyde at room temperature for 15 min, followed by 2–3 washes with PBS. Cells were then permeabilized using 0.5% Triton X-100 (Beyotime) for 15 min. After blocking with 5% BSA for 30 min, cells were incubated overnight at 4 °C with anti-GPX4 primary antibody (1:100). Subsequently, cells were incubated with a fluorescent secondary antibody (1:200) at room temperature and counterstained with DAPI (Beyotime). Fluorescent images were captured using an inverted fluorescence microscope.
Transmission Electron Microscopy (TEM)
For ultrastructural examination, cells were fixed with glutaraldehyde followed by osmium tetroxide. After fixation, cells were dehydrated using a graded ethanol series, embedded in resin, sectioned using an ultramicrotome, stained, and examined under a transmission electron microscope.
Western blotting analysis
Cells were lysed in RIPA buffer, and protein concentrations were determined using the BCA assay. Equal amounts of protein were loaded onto SDS-PAGE gels (1.5 mm thick) and subsequently transferred to PVDF membranes. Membranes were blocked with 5% skim milk for 1.5 h at room temperature, followed by overnight incubation at 4 °C with primary antibodies: anti-GPX4, anti-SLC7A11, anti-Nrf2, anti-HO-1, and anti-SOD1 (Servicebio, Wuhan, China). After incubation with secondary antibodies for 1 h at room temperature, protein bands were visualized using enhanced chemiluminescence (ECL) and captured with a gel imaging system (Bio-Rad, Shanghai, China). Protein band intensities were quantified using ImageJ software, with GAPDH used as the loading control.
Statistical analysis
All data were expressed as mean ± standard deviation (SD). Differences between groups were analyzed using one-way analysis of variance (ANOVA), followed by multiple comparison tests. Statistical significance was defined as P < 0.05 (significant) and P < 0.01 (highly significant).
Supplementary information
Supplementary information Supplementary information.docx.
Results
Inhibition of SP growth by CGA
The broth microdilution assay revealed that the MIC values of penicillin G (PG) and vancomycin (VA) against Streptococcus pneumoniae (SP) were 1024 μg/mL (Fig. 1A) and 128 μg/mL (Fig. 1B), respectively. For chlorogenic acid (CGA), the MIC exceeded 256 μg/mL, though antibacterial activity against SP was observed at 256 μg/mL (Fig. 1C). Cytotoxicity assessments via the CCK-8 assay indicated that the highest non-toxic concentrations of PG and CGA toward macrophages were 256 μg/mL (1/4 MIC; Fig. 1D) and 64 μg/mL (Fig. 1E), respectively; these concentrations were selected for subsequent cellular experiments. Bacterial growth curves demonstrated that 64 μg/mL CGA significantly inhibited SP proliferation and exhibited concentration-dependent antibacterial activity (Fig. 1F). Notably, compared to monotherapy, the combination of PG (256 μg/mL) and CGA (64 μg/mL) synergistically enhanced antibacterial efficacy (Fig. 1G).
Fig. 1.
Detect the effect of CGA on SP growth. Co culture Streptococcus pneumoniae with different concentrations of drugs for 18 h to test its antibacterial effect and determine the minimum inhibitory concentration (A, B, C) of the drugs. The CCK-8 method was used to determine the cytotoxicity of PG and CGA on macrophages (D, E). Treat the time killing curve of SP with CGA (0, 16, 32, 64, 128 μ g/ml) alone (F) or CGA (64μg/ml) in combination with PG (256 μ g/ml) (G). The molecular structure of CGA (H). Compared with the SP control group alone, * * P < 0.01 indicates a significant difference. Compared with the specified group * P < 0.05 indicates a significant difference
Effects of CGA on the hemolytic activity of the PLY protein
A Hemolysis assay was conducted to evaluate the effect of CGA on PLY-induced Hemolytic activity. The results indicated that at a concentration of 8μg/mL, PLY caused approximately 50% hemolysis, which was used as the reference dose for subsequent experiments (Fig. 2A). CGA significantly reduced hemolysis in a concentration-dependent manner (Fig. 2B). Furthermore, the combination of CGA (64 μg/mL) and PG (256 μg/mL) further suppressed PLY-induced hemolysis (Fig. 2C). PCR analysis also revealed that CGA reduced ply gene expression in SP, and this inhibitory effect was more pronounced when CGA was combined with PG (Fig. 2D).
Fig. 2.
Use hemolysis assay to determine the effect of CGA on the hemolytic activity of PLY protein. The hemolytic activity of PLY protein (4、8、16、32、64 μ g/ml) at different concentrations was evaluated by co culturing with goat red blood cells for 10 min, confirming that PLY protein has hemolytic activity (A). The effects of different concentrations of CGA (0, 16, 32, 64, 128 μ g/ml) alone or in combination with PG on the hemolytic activity of PLY protein (8 μ g/ml) were shown (B and C). Effect of CGA (64 μg/mL) and PG (256 μg/mL) combination on ply gene expression in SP strain. RT-qPCR analysis of ply mRNA levels normalized to 16S rRNA (D). Compared with the control group, # P < 0.05 and # # P < 0.01; Compared with the SP group, * P < 0.05 and * * P < 0.01; Compared with the specified group, * P < 0.05 and * * P < 0.01
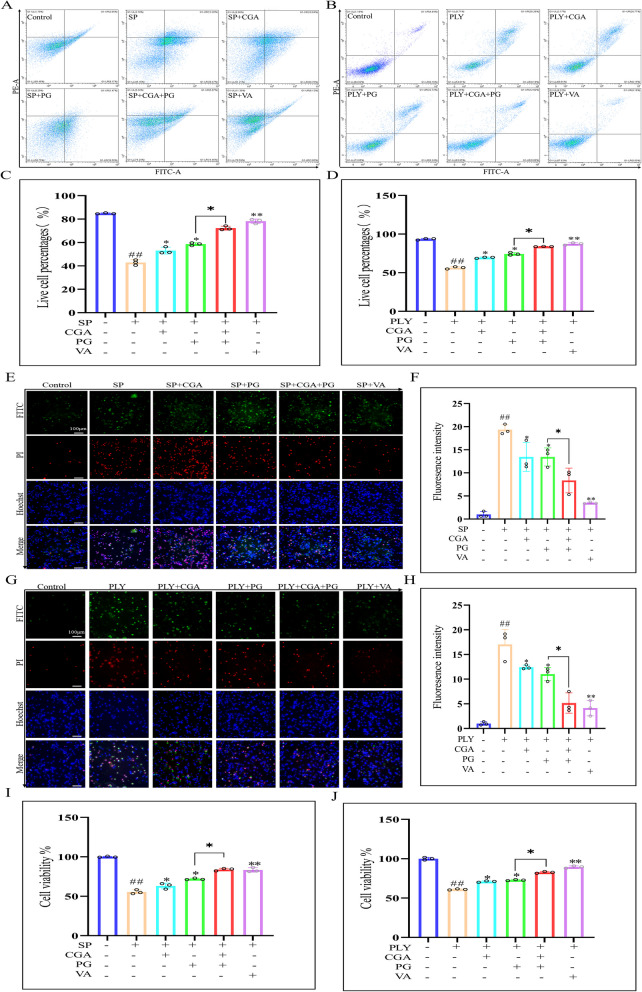
CGA reduces cell damage induced by PLY or SP
Apoptosis was assessed using Annexin V/PI staining to evaluate the protective effect of CGA on macrophages. Flow cytometry results indicated that the proportion of apoptotic cells significantly decreased after 18 h of CGA treatment compared to the SP or PLY groups, suggesting that CGA alleviated macrophage damage induced by SP or PLY. The combination of CGA and PG further enhanced cell viability (Fig. 3A and B). Post-apoptotic fluorescence analysis showed reduced green and red fluorescence intensity, indicating a decrease in apoptotic cells, consistent with flow cytometry results (Fig. 3E and G). CCK-8 assay results also demonstrated a significant increase in cell viability following CGA treatment, further confirming its protective effect (Fig. 3I and J).
Fig. 3.
The protective effect of CGA on macrophages. After co culturing the drug with SP for 18 h, the Annexin V/PI apoptosis detection kit was used to perform flow cytometry analysis on PLY or SP induced cell damage (A and B), and corresponding statistical graphs (C and D) were attached. The fluorescence results are used to enhance validation (E and G) and generate fluorescence intensity statistical graphs (F and H). Measure cell survival rate using CCK-8 assay (I, J). Compared with the control group, # P < 0.05 and # # P < 0.01; Compared with the PLY or SP group, * P < 0.05 and * * P < 0.01; Compared with the specified group, * P < 0.05 and * * P < 0.01
PLY or SP induces ferroptosis in macrophages
The accumulation of reactive oxygen species (ROS) resulting from metabolic activity can trigger ferroptosis. To explore whether PLY and SP infection promoted intracellular ROS accumulation, the ferroptosis inhibitor ferrostatin-1 (Fer-1, 5 μM) was applied. Flow cytometry analysis revealed significantly elevated ROS levels in the PLY and SP groups compared to the control, which were reversed by Fer-1 treatment (Fig. 4A). Fluorescence microscopy confirmed these results, showing increased green fluorescence following PLY or SP exposure, which was reduced upon Fer-1 intervention (Fig. 4B).
Fig. 4.
The effect of PLY or SP on macrophage ferroptosis. One hour before infection, macrophages were pretreated with Fer-1 (5 μ M), and cells co cultured with PLY or SP for 18 h were collected. The ROS levels of cells exposed to SP or PLY macrophages were evaluated using DCFH-DA probe. Representative flow cytometry results (A) and fluorescence staining results (B) of ROS expression in each group were analyzed, and statistical graphs were constructed (S1 A、B). Use JC-1 to check mitochondrial membrane potential (C) and generate a statistical graph (S1 C). Immunofluorescence staining was used to validate changes in GPX4 expression (D) and generate corresponding statistical graphs (S1 D).Representative results (E) of GPX4 and SLC7A11 protein expression in each group were analyzed by western blot, and statistical results (F and G) were calculated. Quantify MDA and total iron levels (H and I) using detection kits, and evaluate cell survival rate (J) using CCK8 kit. Observe mitochondrial morphology (K) through electron microscopy. Compared with the control group, # P < 0.05 and # # P < 0.01; Compared with the PLY or SP group, * P < 0.05 and * * P < 0.01; Compared with the specified group, * P < 0.05 and * * P < 0.01
Mitochondrial membrane potential (MMP) was also assessed. PLY or SP infection led to a marked reduction in MMP, which was alleviated by pretreatment with Fer-1 (Fig. 4C). Immunofluorescence staining revealed enhanced red fluorescence following Fer-1 treatment, indicating upregulation of GPX4 protein expression (Fig. 4D). Western blot analysis demonstrated that compared to the control group, stimulation with PLY or SP significantly reduced GPX4 and SLC7A11 protein expression, whereas Fer-1 treatment significantly restored the expression of both proteins (Fig. 4E).
Additionally, levels of malondialdehyde (MDA) and intracellular total iron, markers of lipid peroxidation and ferroptosis, were significantly elevated in PLY or SP-infected cells and reversed following Fer-1 treatment (Fig. 4H and I). Notably, Fer-1 also improved cell viability compared to the reduction observed after PLY or SP exposure (Fig. 4J). Finally, transmission electron microscopy revealed classic ferroptosis-associated mitochondrial alterations in infected macrophages, including membrane blurring and loss of cristae (Fig. 4k). Collectively, these results indicate that PLY and SP can induce ferroptosis in macrophages.
Chlorogenic acid reverses ferroptosis induced by PLY or SP in macrophages
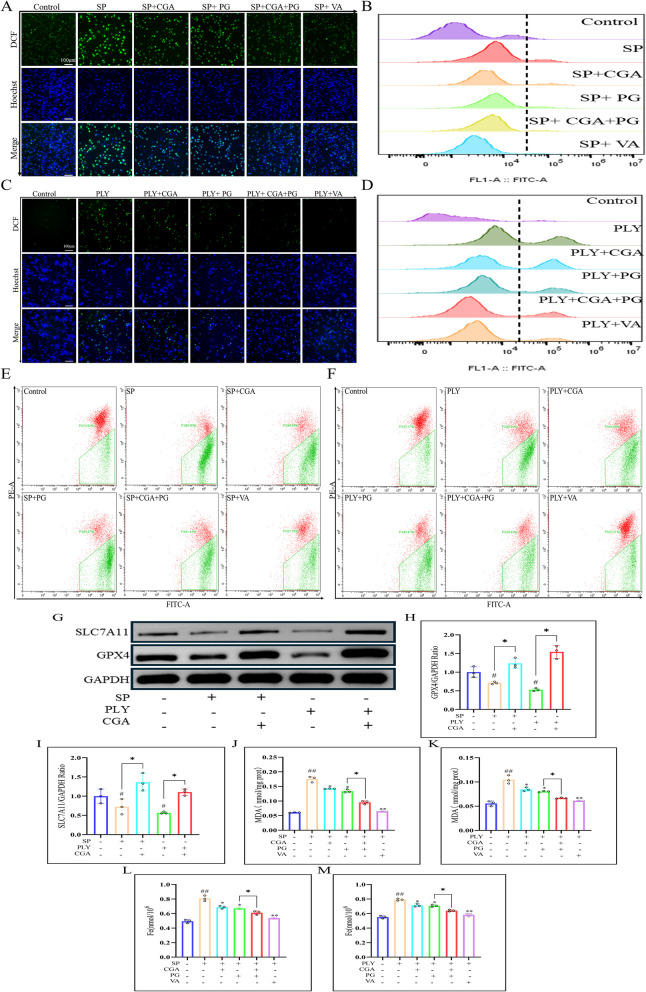
To determine whether CGA reverses PLY- or SP-induced ferroptosis, ROS levels in macrophages were evaluated using fluorescence staining. Compared with the PLY or SP model group, green fluorescence intensity was reduced after CGA (64 μg/mL) treatment, indicating a significant decrease in intracellular ROS. The effect was more pronounced when CGA was combined with PG (256 μg/mL) (Fig. 5A and C). Flow cytometry analysis confirmed this trend, demonstrating a notable reduction in ROS following CGA treatment (Fig. 5B and D).
Fig. 5.
The effect of CGA on PLY or SP induced macrophage ferroptosis. While co culturing PLY or SP with cells, CGA (64 μ g/ml), PG (256 μ g/ml), and VA (64 μ g/ml) were added. After 18 h (37° C, 5% CO2), the effect of CGA on intracellular ROS levels was evaluated using fluorescence staining (A and C), and fluorescence intensity was measured (S2A and B). In addition, ROS levels in macrophages exposed to SP or PLY were measured using flow cytometry (B and D), and statistical graphs (S2C and D) were generated. Use JC-1 staining to detect the effect of CGA on MMP after PLY or SP stimulation (E and F), and calculate the fluorescence intensity to construct statistical graphs (S2E and F). Perform Western blot analysis to detect the effect of PGA on intracellular GPX4 and SLC7A11 protein levels (G), and perform statistical analysis (H and I). Use MDA and total iron assay kits to evaluate changes in MDA (J and K) and total iron (L, M) in macrophages. Compared with the control group, # P < 0.05 and # # P < 0.01; Compared with the PLY or SP group, * P < 0.05 and * * P < 0.01; Compared with the specified group, * P < 0.05 and * * P < 0.01
Flow cytometry results showed that CGA (64 μg/mL) treatment significantly increased mitochondrial membrane potential (MMP) compared to the PLY and SP model groups. The combination of CGA and PG further enhanced MMP levels and had a more substantial impact than either treatment alone (Fig. 5E and F).
Western blot analysis confirmed that CGA significantly upregulated the protein expression of GPX4 and SLC7A11, suggesting that CGA exerts its protective effects on macrophages by inhibiting ferroptosis (Fig. 5G). In addition, CGA treatment effectively reduced intracellular levels of MDA (Fig. 5J and K) and total iron (Fig. 5L and M), with the combination of CGA and PG showing the strongest effect.
Chlorogenic acid activates the NRF2 pathway, which is inhibited by PLY or SP in macrophages
Given the role of NRF2 in regulating oxidative stress and ferroptosis, we next investigated whether the protective effect of CGA on macrophages is associated with NRF2 pathway activation. Immunoblotting revealed that CGA (64 μg/mL) treatment for 18 h significantly upregulated NRF2, HO-1, and SOD1 protein levels in macrophages infected with PLY or SP (Fig. 6A). These results were further confirmed by qPCR analysis, which showed similar upregulation at the mRNA level (Fig. 6E–G), indicating that CGA activates the NRF2 pathway in macrophages.
Fig. 6.
The impact of CGA on the NRF2 pathway. After infecting macrophages with SP or PLY for 18 h, Western blot analysis was performed on the expression of NRF2 pathway related proteins NRF2, Ho-1, and SOD1 (A), and statistical analysis results (B-D) were obtained. After 18 h of cell incubation, the expression of Nrf2, Ho-1, and SOD1 genes (E–G) in PLY or SP infected cells was evaluated by RT qPCR with or without CGA (64 μ G/ml). #P < 0.05 and # P < 0.01; Compared with the PLY or SP group, * P < 0.05 and * * P < 0.01; Compared with the specified group, * P < 0.05 and * * P < 0.01
Chlorogenic acid activates the NRF2 pathway to alleviate ferroptosis in macrophages induced by PLY or SP
To confirm the role of NRF2 activation in ferroptosis, the effect of CGA on the key ferroptosis regulatory protein GPX4 was evaluated. Macrophages were pretreated with ML385 (10 μM), a specific NRF2 inhibitor. Compared with cells treated with PLY or SP alone, ML385 pretreatment significantly reduced GPX4 protein expression, while CGA administration restored its levels (Fig. 7A and C). Additionally, ML385 pretreatment increased MDA levels and promoted ferroptosis, whereas CGA treatment reduced both MDA and total intracellular iron levels (Fig. 7E–H). Notably, CGA significantly improved cell viability compared to the marked reduction observed in the ML385 group (Fig. 7I and J). These findings suggest that CGA may upregulate GPX4 expression via NRF2 pathway activation, thereby inhibiting ferroptosis in macrophages.
Fig. 7.
The effect of CGA activation of NRF2 pathway on macrophage ferroptosis. Pre treat cells with ML385 (10 μ M) for 1 h. After co incubation with PLY or SP for 18 h, use immunofluorescence to detect the effect of CGA (64 μ g/ml) activation of NRF2 pathway on GPX4 protein expression after infection (A and C), and calculate the fluorescence intensity (B and D). Measure MDA (E and F) and total iron levels (G and H) using a detection kit. Evaluate the effect of CGA on macrophage survival rate through CCK8 assay (I, J). Compared with the control group, # P < 0.05 and # # P < 0.01; Compared with the PLY or SP group, * P < 0.05 and * * P < 0.01; Compared with the specified group, * P < 0.05 and * * P < 0.01
Discussion
Previous studies have demonstrated that PLY mediates SP-induced ferroptosis in macrophages, and that CGA mitigates this process by activating the NRF2/GPX4 pathway. This protective effect is likely attributable to CGA-mediated inhibition of PLY hemolytic activity. Additionally, CGA reduces the expression of PLY in SP, and its combination with PG further amplifies this inhibitory effect.
Ferroptosis, an iron-dependent form of programmed cell death, is characterized by the accumulation of lipid peroxides, often exacerbated by excessive ROS and iron overload [17]. Bacterial infections have been shown to trigger ferroptosis as part of the host cellular response to injury [18]. The present findings indicate that PLY-mediated SP infection activates ferroptosis in macrophages, as evidenced by increased levels of ROS, MDA, and intracellular iron. However, treatment with the ferroptosis inhibitor ferrostatin-1 (Fer-1) reversed these effects and partially restored cell viability. Previous studies have demonstrated that the combination of CGA and levofloxacin exhibits significant synergistic effects against Klebsiella pneumoniae biofilm formation, markedly reducing mortality and inflammatory responses in mice [19]. Additionally, berberine and CGA synergistically restore the antibacterial activity of ampicillin against methicillin-resistant Staphylococcus aureus [20]. CGA-antibiotic combinations generally exhibit additive or synergistic effects, as CGA directly targets bacterial cell walls and membranes to combat superbugs [21]. Consistent with these findings, CGA alone inhibits SP growth, while its combination with PG significantly enhances antibacterial efficacy. The underlying mechanisms may involve potentiation of PG’s antimicrobial activity or targeted disruption of SP biofilms.
PLY, a critical virulence factor of SP, is essential for host colonization and pathogenicity [22]. It is recognized as a promising target for vaccine development and therapeutic interventions against severe pneumococcal diseases [23]. Previous studies reported that downregulation of PLY alleviated lung injury in pneumonia models, while wogonin inhibited PLY’s hemolytic activity and reduced SP virulence [24]. Additionally, SP mutants lacking PLY failed to induce hemolysis and protected mice from lethal infection [25]. In this study, PLY-induced ferroptosis in macrophages was observed in vitro. CGA effectively suppressed the hemolytic activity of PLY and downregulated its expression in SP. Furthermore, CGA treatment upregulated the ferroptosis-suppressing proteins GPX4 and SLC7A11, with combination therapy alongside PG yielding even greater protective effects on macrophage viability. These findings suggest that targeting ferroptosis may represent a promising therapeutic approach for SP-induced macrophage injury.
NRF2 is a central transcription factor regulating cellular antioxidant defense, maintaining redox homeostasis by inducing the expression of antioxidant proteins [26]. NRF2 also regulates genes associated with iron metabolism, including those involved in storage, export, and degradation [27]. Downregulation of NRF2 has been shown to exacerbate ferroptosis in multiple cell types [28]. In the current study, CGA activated the NRF2 pathway in macrophages, resulting in upregulation of GPX4 and attenuation of ferroptosis-related oxidative damage. Moreover, inhibition of NRF2 with ML385 reduced GPX4 expression and intensified cellular damage. These results suggest that CGA prevents ferroptosis by upregulating GPX4 via NRF2 activation, thereby reducing ROS accumulation, MDA production, and intracellular iron levels.
Heme oxygenase-1 (HO-1), a downstream target of NRF2, plays a vital role in antioxidant defense [29], while superoxide dismutase (SOD1) scavenges superoxide anions to reduce oxidative burden [30]. Studies have demonstrated that NRF2 inhibition downregulates HO-1 and SOD1 expression, weakening cellular defense mechanisms [31]. Disruption of NRF2 signaling and its downstream effectors may enhance susceptibility to ferroptosis, autophagy, and pyroptosis. Although this study mainly focused on the role of NRF2 in regulating PLY-mediated ferroptosis, future investigations are warranted to explore the potential involvement of other programmed cell death pathways.
Although the present in vitro findings indicate that CGA effectively inhibits PLY-induced hemolysis and protects macrophages, further experiments are required to validate these results. Moreover, since the current findings are based primarily on macrophage models, additional studies utilizing other cell types are necessary. Future in vivo and in vitro studies are needed to fully elucidate the therapeutic potential of CGA in SP infection. In particular, while CGA has been shown to inhibit PLY-induced hemolysis, the extent to which CGA modulates PLY production by SP in vivo remains unclear. Further research is needed to determine whether CGA intervention can serve as a viable strategy against drug-resistant SP infections by targeting PLY production and activity.
Supplementary Information
Acknowledgements
Not applicable.
Clinical trial number
Not applicable.
Abbreviations
- SP
Streptococcus pneumoniae
- SA
Staphylococcus aureus
- CGA
Chlorogenic acid
- PLY
Pneumolysin
- ROS
Reactive oxygen species
- MDA
Malondialdehyde
- TEM
Transmission electron microscopy
- GPX4
Glutathione peroxidase 4
- NRF2
Nuclear factor erythroid 2-related factor 2
- HO-1
Heme oxygenase-1
- SOD1
Superoxide dismutase
- Fer-1
Ferrostatin-1
Authors’ contributions
L.Y. and D.Y. conceived and designed the experiment. L.Y. performed the experiments, analyzed and interpreted the data, and drafted the manuscript. D.Y.、H.Z.、 WB.W.and H.W. substantively revised the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (grant number 81930111) and also received support from the biosafety laboratory of integrated Chinese and Western medicine at Zhejiang Chinese Medicine University (BSL20205713156). Additionally, it was backed by the Zhejiang Province Traditional Chinese Medicine Science and Technology project (2023ZF157) and the Research Project of Zhejiang Chinese Medical University (2021RCZXZK23, 2022GJYY025). The funding organizations contributed to the study design, data collection and analysis, decision-making regarding publication, and the manuscript preparation.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lan Yang and Daojun Yu contributed equally to this work.
References
- 1.Klabunde B, Wesener A, Bertrams W, Ringshandl S, Halder LD, Vollmeister E, et al. Streptococcus pneumoniae disrupts the structure of the Golgi apparatus and subsequent epithelial cytokine response in an H2O2-dependent manner. Cell Commun Signal. 2023;21:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weiser JN, Ferreira DM, Paton JC. Streptococcus pneumoniae: transmission, colonization and invasion. Nat Rev Microbiol. 2018;16:355–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shizukuishi S, Ogawa M, Kuroda E, Hamaguchi S, Sakuma C, Kakuta S, et al. Pneumococcal sialidase promotes bacterial survival by fine-tuning of pneumolysin-mediated membrane disruption. Cell Rep. 2024;43:113962. [DOI] [PubMed] [Google Scholar]
- 4.Nerlich A, von Wunsch Teruel I, Mieth M, Hönzke K, Rückert JC, Mitchell TJ, et al. Reversion of pneumolysin-induced executioner caspase activation redirects cells to survival. J Infect Dis. 2021;223:1973–83. [DOI] [PubMed] [Google Scholar]
- 5.Yuan Y, Xu W, Li L, Guo T, Liu B, Xiao J, et al. A Streptococcus pneumoniae endolysin mutant protein ΔA146Ply elicits rapid broad-spectrum mucosal protection in mice via upregulation of GPX4 through TLR4/IRG1/NRF2 to alleviate macrophage ferroptosis. Free Radic Biol Med. 2024;222:344–60. [DOI] [PubMed] [Google Scholar]
- 6.Shang Z, Sharma V, Pai L, Kumar T, Patil S. Optimizing the production and efficacy of antimicrobial bioactive compounds from Streptomyces kanamyceticus in combating multi-drug-resistant pathogens. Front Cell Infect Microbiol. 2024;14:1500440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dao TH, Echlin H, McKnight A, Marr ES, Junker J, Jia Q, et al. Streptococcus pneumoniae favors tolerance via metabolic adaptation over resistance to circumvent fluoroquinolones. mBio. 2024;15:e0282823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen QM, Maltagliati AJ. Nrf2 at the heart of oxidative stress and cardiac protection. Physiol Genomics. 2018;50:77–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu L, Zhang L, Xiang Y, Zhang X. Knockdown of lncRNA NEAT1 suppresses streptococcus pneumoniae-induced ferroptosis in alveolar epithelial cells by regulating the Nrf2-GPX4 pathway. Toxicon. 2024;243:107705. [DOI] [PubMed] [Google Scholar]
- 10.Hu H, Hua SY, Lin X, Lu F, Zhang W, Zhou L, et al. Hybrid biomimetic membrane coated particles-mediated bacterial ferroptosis for acute MRSA pneumonia. ACS Nano. 2023;17:11692–712. [DOI] [PubMed] [Google Scholar]
- 11.Alrouji M, Anwar S, Venkatesan K, Shahwan M, Hassan MI, Islam A, et al. Iron homeostasis and neurodegeneration in the ageing brain: insight into ferroptosis pathways. Ageing Res Rev. 2024;102:102575. [DOI] [PubMed] [Google Scholar]
- 12.Lei G, Mao C, Horbath AD, Yan Y, Cai S, Yao J, et al. BRCA1-mediated dual regulation of ferroptosis exposes a vulnerability to GPX4 and PARP co-inhibition in BRCA1-deficient cancers. Cancer Discov. 2024;14:1476–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dai M, Ouyang W, Yu Y, Wang T, Wang Y, Cen M, et al. IFP35 aggravates Staphylococcus aureus infection by promoting Nrf2-regulated ferroptosis. J Adv Res. 2024;62:143–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang J, Xie M, He L, Song X, Cao T. Chlorogenic acid: a review on its mechanisms of anti-inflammation, disease treatment, and related delivery systems. Front Pharmacol. 2023;14:1218015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi A, Shi H, Wang Y, Liu X, Cheng Y, Li H, et al. Activation of Nrf2 pathway and inhibition of NLRP3 inflammasome activation contribute to the protective effect of chlorogenic acid on acute liver injury. Int Immunopharmacol. 2018;54:125–30. [DOI] [PubMed] [Google Scholar]
- 16.Zhao Y, Wang C, Yang T, Wang H, Zhao S, Sun N, et al. Chlorogenic acid alleviates chronic stress-induced duodenal ferroptosis via the inhibition of the IL-6/JAK2/STAT3 signaling pathway in rats. J Agric Food Chem. 2022;70:4353–61. [DOI] [PubMed] [Google Scholar]
- 17.Jiang X, Stockwell BR, Conrad M. Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol. 2021;22:266–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao J, Wang Q, Tang Y-D, Zhai J, Hu W, Zheng C. When ferroptosis meets pathogenic infections. Trends Microbiol. 2023;31:468–79. [DOI] [PubMed] [Google Scholar]
- 19.Tan S, Gao J, Li Q, Guo T, Dong X, Bai X, et al. Synergistic effect of chlorogenic acid and levofloxacin against Klebsiella pneumonia infection in vitro and in vivo. Sci Rep. 2020;10:20013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fu S, Yi X, Li Y, Li Y, Qu X, Miao P, et al. Berberine and chlorogenic acid-assembled nanoparticles for highly efficient inhibition of multidrug-resistant Staphylococcus aureus. J Hazard Mater. 2024;473:134680. [DOI] [PubMed] [Google Scholar]
- 21.Feng S, Zhang Y, Fu S, Li Z, Zhang J, Xu Y, et al. Application of chlorogenic acid as a substitute for antibiotics in multidrug-resistant Escherichia coli-induced mastitis. Int Immunopharmacol. 2023;114:109536. [DOI] [PubMed] [Google Scholar]
- 22.Cockeran R, Anderson R, Feldman C. Pneumolysin as a vaccine and drug target in the prevention and treatment of invasive pneumococcal disease. Arch Immunol Ther Exp (Warsz). 2005;53:189–98. [PubMed] [Google Scholar]
- 23.Anderson R, Feldman C. Pneumolysin as a potential therapeutic target in severe pneumococcal disease. J Infect. 2017;74:527–44. [DOI] [PubMed] [Google Scholar]
- 24.Domon H, Isono T, Hiyoshi T, Tamura H, Sasagawa K, Maekawa T, et al. Clarithromycin inhibits pneumolysin production via downregulation of ply gene transcription despite autolysis activation. Microbiol Spectr. 2021;9:e0031821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gu K, Ding L, Wang Z, Sun Y, Sun X, Yang W, et al. Wogonin attenuates the pathogenicity of Streptococcus pneumoniae by double-target inhibition of Pneumolysin and Sortase A. J Cell Mol Med. 2023;27:563–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He F, Antonucci L, Karin M. NRF2 as a regulator of cell metabolism and inflammation in cancer. Carcinogenesis. 2020;41:405–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xue D, Zhou X, Qiu J. Emerging role of NRF2 in ROS-mediated tumor chemoresistance. Biomed Pharmacother. 2020;131:110676. [DOI] [PubMed] [Google Scholar]
- 28.Kerins MJ, Ooi A. The roles of NRF2 in modulating cellular iron homeostasis. Antioxid Redox Signal. 2018;29:1756–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun Y-Y, Zhu H-J, Zhao R-Y, Zhou S-Y, Wang M-Q, Yang Y, et al. Remote ischemic conditioning attenuates oxidative stress and inflammation via the Nrf2/HO-1 pathway in MCAO mice. Redox Biol. 2023;66:102852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang B, Pan J, Zhang X-N, Wang H, He L, Rong X, et al. Nrf2 activation suppresses motor neuron ferroptosis induced by the SOD1G93A mutation and exerts neuroprotection in amyotrophic lateral sclerosis. Neurobiol Dis. 2023;184:106210. [DOI] [PubMed] [Google Scholar]
- 31.Huang Q-L, Huang L-N, Zhao G-Y, Liu C, Pan X-Y, Li Z-R, et al. Naringin attenuates Actinobacillus pleuropneumoniae-induced acute lung injury via MAPK/NF-κB and Keap1/Nrf2/HO-1 pathway. BMC Vet Res. 2024;20:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.