Abstract
Context: Delayed-onset muscle soreness (DOMS) describes muscle pain and tenderness that typically develop several hours postexercise and consist of predominantly eccentric muscle actions, especially if the exercise is unfamiliar. Although DOMS is likely a symptom of eccentric-exercise–induced muscle damage, it does not necessarily reflect muscle damage. Some prophylactic or therapeutic modalities may be effective only for alleviating DOMS, whereas others may enhance recovery of muscle function without affecting DOMS.
Objective: To test the hypothesis that massage applied after eccentric exercise would effectively alleviate DOMS without affecting muscle function.
Design: We used an arm-to-arm comparison model with 2 independent variables (control and massage) and 6 dependent variables (maximal isometric and isokinetic voluntary strength, range of motion, upper arm circumference, plasma creatine kinase activity, and muscle soreness). A 2-way repeated-measures analysis of variance and paired t tests were used to examine differences in changes of the dependent variable over time (before, immediately and 30 minutes after exercise, and 1, 2, 3, 4, 7, 10, and 14 days postexercise) between control and massage conditions.
Setting: University laboratory.
Patients or Other Participants: Ten healthy subjects (5 men and 5 women) with no history of upper arm injury and no experience in resistance training.
Intervention(s): Subjects performed 10 sets of 6 maximal isokinetic (90°·s−1) eccentric actions of the elbow flexors with each arm on a dynamometer, separated by 2 weeks. One arm received 10 minutes of massage 3 hours after eccentric exercise; the contralateral arm received no treatment.
Main Outcome Measure(s): Maximal voluntary isometric and isokinetic elbow flexor strength, range of motion, upper arm circumference, plasma creatine kinase activity, and muscle soreness.
Results: Delayed-onset muscle soreness was significantly less for the massage condition for peak soreness in extending the elbow joint and palpating the brachioradialis muscle (P < .05). Soreness while flexing the elbow joint (P = .07) and palpating the brachialis muscle (P = .06) was also less with massage. Massage treatment had significant effects on plasma creatine kinase activity, with a significantly lower peak value at 4 days postexercise (P < .05), and upper arm circumference, with a significantly smaller increase than the control at 3 and 4 days postexercise (P < .05). However, no significant effects of massage on recovery of muscle strength and ROM were evident.
Conclusions: Massage was effective in alleviating DOMS by approximately 30% and reducing swelling, but it had no effects on muscle function.
Keywords: elbow flexors, muscle strength, range of motion, creatine kinase
Exercise consisting of predominantly eccentric muscle actions has the potential to cause greater injury to muscles than that involving largely isometric or concentric actions, especially if the exercise is unfamiliar.1–3 Muscle pain and tenderness generally develop 24 hours after such exercise and are usually described as delayed-onset muscle soreness (DOMS).2,4,5 Undoubtedly, DOMS is one of the symptoms of eccentric-exercise–induced muscle damage; however, DOMS does not necessarily indicate muscle damage.5 The level of DOMS does not reflect the extent of muscle damage, and the course of DOMS does not correspond to the course of changes in other indicators of muscle damage.5,6 In this context, it is necessary to separate DOMS from other symptoms of eccentric-exercise–induced muscle damage, especially when investigating prophylactic or therapeutic modalities. It may be that some interventions are effective only for alleviating DOMS, but others enhance recovery of muscle function without affecting DOMS. Thus, when a treatment is found to alleviate DOMS without any effects on recovery of muscle function, the treatment is still effective if DOMS is the main concern.
A number of prophylactic or therapeutic measures have been examined for their efficacy in preventing or reducing DOMS and other outcomes of eccentric-exercise–induced muscle damage.6,7 Massage is widely used as a therapeutic modality for recovery from muscle fatigue and injury8–11 and is probably one of the most popular treatments after sports activities. Although physiologic theory to support how massage facilitates recovery from eccentric-exercise–induced muscle damage is obscure,8 a massage is often recommended by coaches and therapists to alleviate or prevent DOMS after a sporting activity.6,8–10
A number of authors have examined the effects of massage on DOMS and indirect markers of muscle damage such as impairment of muscle function, swelling, and changes in muscle proteins in the blood. Doubt has been cast on the effectiveness of massage therapy in aiding DOMS and recovery of muscle function.8,10 In fact, an 8-minute massage immediately postexercise has no effect on DOMS and recovery of muscle strength.12 Another author9 concluded that massage therapy might be a promising intervention for reducing DOMS if massage treatment enhances local blood and lymph flow and stated the necessity of further research. In line with the positive effect of massage on DOMS, a 30-minute massage, applied 2 hours after eccentric exercise of the elbow flexors and extensors, reduces DOMS.13 A recent group14 reported that a 30-minute therapeutic massage of one leg 2 hours after downhill running was effective in attenuating DOMS compared with the contralateral limb with no treatment, but it caused declines in muscle strength and power. Other researchers15,16 have also found that massage attenuates DOMS to varying degrees but does not affect muscle function. Furthermore, combining warm-up, stretching, and massage has been reported to have some effect on DOMS and muscle function; however, it is not clear how much of the effect was associated with massage.17 The findings about the effects of massage on DOMS and muscle function are inconclusive or contradictory in nature.8–10
One reason for the controversy seems to stem from the different eccentric exercise models used in the studies, which result in different magnitudes of damage to different muscles. A second possible confounding factor is the fact that individuals show wide variations in their responses to the same exercise protocol.2,5,18 The large variability in responses among individuals to the effects of eccentric exercise has made comparison with control conditions difficult. Most of the previous authors of massage studies12,13,15,17 have compared massage and control groups composed of different populations of subjects. The inconsistency among subjects in response to eccentric exercise is likely to act as a confounding factor, reducing the likelihood of exposing any positive effects the massage therapy may have provided. One solution to this problem is to use a “limb-to-limb” comparison model, in which a treatment limb is compared with responses from the contralateral (untreated) limb of the same subject. Two groups14,16 used a leg-to-leg comparison model by assigning one leg to massage and the contralateral leg to the control condition. No investigators have used an arm-to-arm comparison model to investigate the effects of massage on DOMS and other markers of muscle damage after eccentric exercise. Because of the subjective nature of pain sensation, comparing the massage and control conditions in the same subject would be preferable.
Therefore, our purpose was to examine the effects of massage on DOMS, muscle strength, range of motion (ROM), swelling, and a biochemical marker of muscle damage in the blood using the arm-to-arm comparison model. We expected that the arm-to-arm comparison model would offer a better indication of whether massage is effective in alleviating DOMS and enhancing recovery of muscle function after eccentric exercise.
METHODS
Subjects
Ten healthy subjects (5 men and 5 women) with no history of upper arm injury and no experience in resistance training were recruited after approval from the Institutional Ethics Committee. The number of subjects was determined by a power analysis with 80% power and a 1-tailed level of significance of P < .05 based on the data from our pilot study. The mean ± SEM age, height, and mass of the subjects were 23.0 ± 1.3 years, 163.2 ± 4.8 cm, and 63.7 ± 3.8 kg, respectively. During the experimental period, subjects were requested not to take any medication, change their diet, or perform any strenuous exercise.
Experimental Design
We used an arm-to-arm comparison model: one arm was the control, and the other arm was assigned to a treatment condition. Subjects performed an identical, maximal eccentric exercise of the elbow flexors with each arm, separated by 2 weeks. For the treatment condition, subjects received a 10-minute massage on the exercised arm 3 hours postexercise. Therefore, the independent variables were the 2 conditions: control and treatment (massage). Dependent variables consisted of maximal isometric and isokinetic voluntary strength, ROM, upper arm circumference, plasma creatine kinase (CK) activity, and muscle soreness. Subjects reported to the laboratory on 9 occasions, including one familiarization session before the baseline measurements. Measurements were taken before, immediately after and 30 minutes after the exercise, and on days 1, 2, 3, 4, 7, 10, and 14 postexercise. Changes in the measures over time were compared between the control and experimental arms.
Exercise
The exercise protocol consisted of 60 maximal voluntary eccentric contractions of the elbow flexors against the lever arm of the isokinetic dynamometer (Cybex 6000; Lumex Inc, Ronkonkoma, NY) moving at constant velocity of 90°·s−1. Subjects were seated on an arm-curl bench with the forearm in a supinated position and the elbow aligned with the axis of rotation of the dynamometer lever arm. The movement began at an elbow joint angle of 90° (the extended elbow is considered as 0°). The elbow joint was forcibly extended from the flexed position (90°) to the extended position (0°) in 1 second while the subject was asked to resist maximally against the motion. After each eccentric action, the lever arm of the isokinetic dynamometer returned to the starting point at the velocity of 9°·s−1 while the subject was relaxing the arm, so that a 10-second passive recovery period was allowed between eccentric repetitions. The 60 maximal eccentric actions were divided into 10 sets of 6 repetitions, with a 3-minute rest between sets. Torque output was recorded and displayed in real time for each eccentric action, and the data were saved in a desktop computer with AMLAB data-acquisition software (version II; AMLAB Technologies, Lewisham, Australia).
Massage
A standard 10-minute sports massage was applied to the exercised arm by a qualified massage therapist 3 hours postexercise for the massage condition. The therapist was a professional masseuse who had been working for an Australian football club for several years. The 3-hour time point was chosen based on a previous study.13 The massage protocol used deeply applied clearing techniques with palmar and finger stroking to the muscles. Massage was applied as the subject lay on his or her back on a massage table. The 10-minute massage consisted of effleurage (stroking) of the hand (30 seconds), wrist to elbow (1 minute), and elbow to shoulder (1 minute); petrissage (kneading) of the wrist to the elbow (30 seconds) and elbow to shoulder (30 seconds); frictions to the forearm (1 minute), biceps, triceps, and deltoids (1 minute); thumb petrissage of the wrist to the elbow (1 minute) and elbow to shoulder (1 minute); and repeat effleurage of the hand (30 seconds), wrist to elbow (1 minute), and elbow to shoulder (1 minute). Under verbal instruction recorded on an audiocassette, the same therapist performed the massage protocol throughout. The therapist was requested to keep the depth and rate of massage as consistent as possible.
Criterion Variables
Maximal voluntary isometric and isokinetic elbow flexor strength, elbow joint angles and ROM, upper arm circumference, plasma CK activity, and muscle soreness were measured for the exercised arm. All measurements were taken twice during the familiarization session. Measurements were taken before, immediately and 30 minutes after, and 1, 2, 3, 4, 7, 10, and 14 days postexercise. Plasma CK activity and muscle soreness were measured at the same time points as those described previously except for immediately and 30 minutes postexercise.
Muscular Strength
We used an isokinetic dynamometer to record isometric and isokinetic concentric torque during maximal voluntary contractions of the elbow flexors. Verbal encouragements were given during the measurements. For the isometric contractions, subjects were asked to sustain maximal effort for 3 seconds at fixed elbow joint angles of 90° and 30°, where 0° was referred to as a full extension angle. The 90° position has been used in previous studies2,5,17 to measure isometric strength of the elbow flexors, and the 30° position was added to examine a possible effect of an optimal angle shift. Two measurements were performed for each angle, and the highest peak torque value was used for subsequent analysis. The rest between maximal isometric contractions was 30 seconds, and a 1-minute recovery period was allowed between tests at different joint angles.
We assessed concentric maximal voluntary torque of the elbow flexors isokinetically at 5 velocities (30, 90, 150, 210, and 300°·s−1) with the same subject positioning as in the isometric assessment, with 90° ROM identified as extension (0°) to flexion (90°). The isokinetic strength testing was performed in order of increasing velocity from 30°·s−1 to 300°·s−1, with the highest peak torque from 2 trials being accepted. A 5-second period was provided between attempts at a given velocity and a 1-minute recovery period between different velocities.
Range of Motion
A plastic goniometer (Sammon Preston Rolyan, Bolingbrook, IL) was used to measure the total active ROM for the elbow joint. The ROM was determined as the difference between the actively flexed and extended elbow joint angles. The active flexion angle was defined as the angle at the elbow when attempting to fully flex the elbow joint to touch the shoulder with the palm, and the active extended angle was the angle when attempting to extend the elbow joint as much as possible. To measure the elbow joint angles, we used a semipermanent ink pen to create landmarks on the skin and obtain consistent measurements. These measurements consisted of the lateral epicondyle of the humerus, the acromion process, and the midpoint of the styloid process of the ulna and radius. Two measurements were taken for each angle, and the mean value of the 2 measurements was used for analysis.
Upper Arm Circumference
A constant tension tape measure was used to measure the upper arm circumference of 5 marked sites: 3, 5, 7, 9, and 11 cm from the elbow crease. The marks were maintained using a semipermanent ink marker during the experimental period. Measurements were taken while the subject's relaxed arm was hanging by the side. Two measurements were taken from each marked site and averaged. The mean value of the 5 sites was calculated and used for further analysis.
Plasma Creatine Kinase Activity
Approximately 50 μL of blood was collected from a finger of the exercised arm in a heparinized tube from a finger prick made with a spring-loaded lancet. The blood sample was immediately analyzed using a Reflotron spectrophotometer (Boehringer-Manheim, Pode, Czech Republic) for plasma CK activity. The normal reference range for CK using this method is 50 to 220 IU·L−1, and the assay can accurately detect values between 20 and 2000 IU·L−1, according to the manufacturer's manual. When the value exceeded 2000 IU·L−1, another blood sample was taken and diluted to obtain a value within the range, and the actual value was calculated.
Muscle Soreness
Muscle soreness was rated with a visual analog scale that incorporated a 100-mm line, with 0 indicating no pain and 100 representing extremely painful. Subjects were asked to mark their perceived soreness on the 100-mm line when the elbow joint was forcibly flexed and extended by an investigator and when an investigator palpated the brachialis and brachioradialis. The pressure applied to the muscles during the palpation was kept as similar as possible between days by consistently matching the indentation of the palpated sites. Distance from the left edge of the line (0) to the marked point was measured in millimeters, and this value was used for the analysis.
Reliability of the Measurements
The same investigator took all the measurements. We used the intraclass correlation coefficient to analyze the reliability of the measurements with data from the 10 subjects for the 2 pre-exercise measurements taken during the familiarization session and before exercise. The formula for the intraclass correlation coefficient was R = (MSS − MSE)/MSS, where MSS was the mean square for subjects and MSE was the mean square for error, which is computed as follows: (sums of squares for trials + sums of squares for interaction)/(df for trials + df for interaction). The R values for isometric and isokinetic strength, ROM, upper arm circumference, plasma CK activity, and muscle soreness were 0.91, 0.90, 0.89, 0.98, 0.94, and 0.95, respectively.
Data Analysis
Changes in muscle strength, ROM, circumference, plasma CK activity, and muscle soreness over time were compared between the massage and control conditions using a 2-way repeated-measures analysis of variance. When the analysis of variance showed a significant difference between conditions, we applied a Tukey post hoc test to find the location of the significance. Peak soreness (extension, flexion, and palpation) was compared between conditions by a paired t test. Paired t tests were also used to examine differences between conditions for peak plasma CK activity and change in arm circumference. Data analysis was performed using a statistical software package (SPSS version 11.0; SPSS Inc, Chicago, IL). Statistical significance was set at P < .05 for all analyses. Data are presented as mean ± SEM unless otherwise stated.
RESULTS
Exercise
All subjects performed 2 bouts of maximal eccentric exercise. Baseline values for the maximal isometric and isokinetic strength showed no significant differences (P = .93 and .95, respectively) between the massage and control arms. Also, peak torque and total work values recorded during the eccentric exercise protocol were similar for the 2 conditions, and no significant differences between the arms were evident.
Muscular Strength
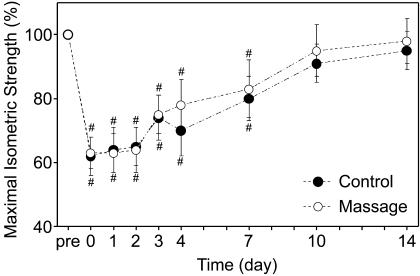
Maximal isometric torque was significantly larger at an elbow angle of 90° (37.2 ± 6.6 Nm) than at 30° (27.3 ± 4.6 Nm) before exercise and throughout the measurements; however, the magnitude of decrease in torque postexercise was similar between the 2 angles. No significant differences (P = .74) in maximal isometric torque at 2 different angles were observed between the massage and control arms. As shown in Figure 1, isometric torque decreased to approximately 60% of pre-exercise values immediately postexercise and remained at this level for 2 days, after which the torque returned to the pre-exercise level by 10 days postexercise. The treatment and control arms displayed a similar degree of strength loss postexercise, and no significant difference (P = .64) between arms was evident for the changes in isometric torque over time.
Figure 1. Changes in maximal voluntary isometric torque from baseline (pre), immediately after (0), and 1 to 14 days postexercise for the massage and control arms expressed as a percentage of baseline.
# Indicates a significant difference from baseline
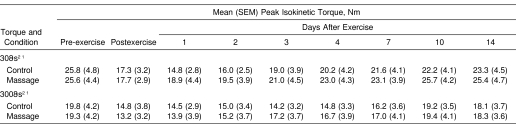
The isokinetic torque at 5 velocities showed similar changes postexercise, although some differences among the velocities were evident for the absolute values. The largest difference among the velocities was observed between 30°·s−1 and 300°·s−1 (Table 1). Changes in maximal voluntary isokinetic torque were similar to those in the isometric torque during the postexercise period. Furthermore, no significant difference (P = .82) between the treatment and the control arms for any of the velocities tested was evident. The isokinetic torque recovered to the pre-exercise level by 10 days postexercise for both conditions.
Table 1. Changes in Peak Isokinetic Torque Before, Immediately After, and 1 to 14 Days After Exercise for the Control and Massage Conditions (N = 10).
Range of Motion
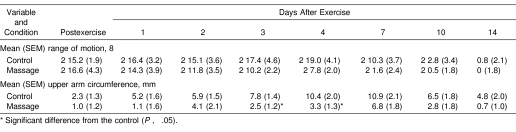
No significant difference in the pre-exercise ROM values was evident between the control and massage arms (P = .70). The ROM decreased significantly (P = .04) immediately postexercise by approximately 30% from baseline and did not recover for the next 4 days. Changes in ROM postexercise were similar between conditions (Table 2).
Table 2. Changes in Range of Motion and Upper Arm Circumference From the Pre-exercise Level to Immediately After and 1 to 14 Days After Exercise for the Control and Massage Conditions (N = 10).
Upper Arm Circumference
The baseline upper arm circumference was not significantly different between the arms (P = .74). Upper arm circumference increased significantly (P = .04) postexercise in both conditions, and the massaged arm showed a significantly smaller increase than the control arm (P = .04) (see Table 2). Significant differences in circumference between the massage and control arms were recorded at 3 (P = .04) and 4 days (P = .03) postexercise.
Plasma CK Activity
No significant difference in plasma CK activity between arms was evident before exercise (P = .90). Plasma CK activity increased significantly postexercise for both conditions (P = .01); however, significantly smaller CK increases occurred for the massaged arm than for the control (P = .02) (Figure 2). The CK peak value for the massage condition (982 ± 356 IU·L−1) was 36% lower than that for the control condition (2704 ± 637 IU·L−1).
Figure 2. Changes in plasma creatine kinase (CK) activity before (pre) and 1 to 14 days postexercise for the massage and control arms.
* Indicates a significant difference between arms; #, a significant difference from baseline
Muscle Soreness
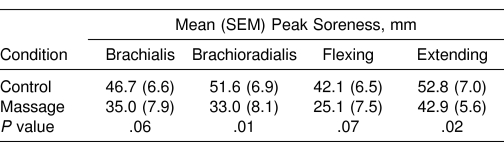
Muscle soreness developed after both exercise bouts. The course of development of soreness differed, depending on the type of measurement. Peak soreness for palpation of the brachioradialis and brachialis and elbow joint flexion was reported 1 to 3 days postexercise, whereas peak soreness on elbow joint extension occurred 4 days postexercise. All reports of soreness resolved by 7 days postexercise. As shown in Table 3, the highest peak soreness score was observed for extension, followed by palpation of the brachioradialis. Significant differences between the massage and the control conditions were found for peak soreness with palpation of the brachioradialis and extending the elbow joint (P = .01 to .02), with peak values for the other 2 soreness variables showing borderline significance (P = .06 to .07). The massage resulted in a 20% to 40% decrease in the severity of soreness compared with no treatment in the same individuals.
Table 3. Peak Muscle Soreness With Palpating the Brachialis and Brachioradialis Muscles and Flexing and Extending the Elbow Joint After Exercise for the Control and Massage Conditions (N = 10).
DISCUSSION
We investigated the effects of a 10-minute massage performed 3 hours after an eccentric exercise on DOMS and other indicators of eccentric-exercise–induced muscle damage. We used a self-report visual analog scale to quantify the magnitude of muscle soreness for palpation, extension, and flexion of the elbow flexors; this scale has been reported to be the most satisfactory means of assessing pain sensation.19 Because the perception of pain is highly subjective and varies widely among individuals, the use of soreness as a quantifier of muscle injury is problematic.5 Yet it is the most widely experienced negative consequence of eccentric exercise, making it an important variable to consider. To minimize the confounding effects associated with difference in individual responses, we used the arm-to-arm comparison model to compare massage and control conditions.
The arm-to-arm comparison model is advantageous when comparing 2 conditions in a relatively small number of subjects; however, it may produce a carryover effect, especially for the blood markers of muscle damage, if the time between the bouts is short. We avoided this potential problem by providing an adequate interval between the bouts based on previous studies, which was more than 2 weeks.2,18 Yet a possible placebo effect should also be considered, because it is difficult to eliminate a possible placebo effect in the arm-to-arm comparison model. Practically, people expect to have some effects of massage when they receive it, and psychological effects may always exist to some degree. We did not include a placebo treatment such as touching, because subjects might have noticed a difference if they had received a placebo treatment for one arm and actual treatment for the other arm. However, subjects were randomly grouped by test order (control-treatment or treatment-control), and dominant and nondominant arms were equally balanced over the 2 conditions. Moreover, the changes in muscle strength (see Table 1 and Figure 1), ROM, and upper arm circumference (see Table 2) immediately postexercise were not significantly different between the control and massage arms, and the massage was performed 3 hours postexercise and before DOMS developed. It seems unlikely that the changes in the criterion measures were altered by the psychological effects of massage, because the placebo effect would not account for the differences in upper arm circumference or CK values. This suggests that the reduction in DOMS for the massage condition was a real and not a placebo response. It seems reasonable to assume that differences between arms, if any, were due to the effects of massage. Massage was effective in reducing the magnitude of DOMS (see Table 3), swelling (see Table 2), and plasma CK activity (see Figure 2). In contrast, no positive effects of massage were found for muscle strength (see Figure 1 and Table 1) and ROM (see Table 2).
In this study, the subjects included both sexes to generalize the findings. Although there may be sex-based differences in responses to eccentric-exercise–induced muscle damage,20 controversies exist concerning the effects of sex on the magnitude of muscle damage, inflammatory response, and change in plasma CK activity after eccentric exercise.18,22 Even if there is a sex effect, the arm-to-arm comparison model could minimize the effect, because the comparisons between the control and treatment conditions are made within the same subject. Because the influence of the menstrual cycle on eccentric-exercise–induced muscle damage is small,23 the menstrual cycle was not considered in this study. Therefore, it seems unlikely that the choice of subjects affected the findings.
Delayed-onset muscle soreness is a symptom of eccentric-exercise–induced muscle damage and occurs 8 to 12 hours postexercise, when the affected muscle contracts or stretches or is palpated; it peaks at 2 to 3 days and slowly dissipates by 8 to 10 days postexercise.1,6,7 The course of muscle soreness development is different from changes in muscle strength and ROM, upper arm circumference, and plasma CK activity.5 Although the underlying mechanism of DOMS remains uncertain, it is generally accepted that DOMS is caused by inflammation of the damaged muscle and/or connective tissue and the efflux of substances from the damaged tissue to the extracellular space that sensitize the free nerve endings.1,6,24 Delayed-onset muscle soreness is thought to be the result of activation of the group IV pain receptors, which are responsible for the transmission of dull, aching pain signals.1 These receptors can respond to pressure and shear stress and/or chemical substances, such as bradykinin, serotonin, and histamine, that accumulate in the interstitium.1 The responses of group IV receptors to any one stimulus may be sensitized and potentiated if the chemical environment of the interstitium is altered. This is a possible mechanism for the development of DOMS after eccentric exercise.1,6,24
Our findings support previous results regarding the positive effects of massage on DOMS. In addition, we found significant effects of massage on muscle swelling and CK response. The massage protocols used in previous studies have varied widely in terms of the timing, duration, and frequency. Most have consisted of one session of massage at 2 to 4 hours postexercise.12–15 Only Tiidus and Shoemaker16 repeated the 10-minute massage 2 and 4 days postexercise. Massage duration has been between 8 and 30 minutes in previous studies.12–17 All groups except Weber et al12 reported that massage had a positive effect on DOMS. We also found that massage intervention reduced soreness more than 30% compared with the control (see Table 3). This suggests that a massage performed postexercise but before DOMS develops can alleviate soreness, no matter how the massage is performed.
It is difficult to explain how massage reduces DOMS, because no authors have yet described the effects of massage on cellular events or pathophysiologic changes in the muscle or connective tissue after eccentric exercise. Increasing blood flow appears to be a major consequence of massage.8,10 Increases in blood and lymph flow may enhance removal of pain substrates that start to accumulate in the injured area, reducing edema. We found smaller increases in upper arm circumference 3 and 4 days postexercise for the massage condition compared with the control (see Table 2). This may explain why DOMS was attenuated by massage, if indeed edema is associated with muscle soreness. Smith et al13 showed that circulating neutrophil levels were elevated from baseline for several hours after massage compared with the control condition and speculated that this was due to a reduced emigration of neutrophils into tissue spaces. However, no authors have yet shown that massage can decrease the migration of neutrophils or other leukocytes (ie, macrophages) to the injured sites. Massage to sore muscles could increase discharge from other low-threshold sensory fibers and block pain sensation temporarily1; however, the massage in our study was performed before soreness occurred.
Cardinal signs of acute inflammation include redness, heat, swelling, pain, and impairment of function.25 Among these signs, swelling, pain, and impairment of muscle function appear in eccentric-exercise–induced muscle damage.2,25 Our findings of reduced muscle swelling in the massage condition may support the concept of an ameliorated inflammatory response after treatment, as does the smaller CK efflux observed. Because we did not measure direct indicators of inflammation, it is not possible to state that the severity of DOMS is linked to the processes of inflammation and/or subsequent muscle edema. Further study is necessary to investigate how massage affects the inflammatory responses induced by eccentric exercise.
It is interesting that increases in plasma CK activity were significantly smaller for the massage condition than the control (see Figure 2). The blunted CK response for the massaged arm could be explained either by smaller CK efflux from the damaged muscle or increased clearance of CK from the circulation. It may be that massage enhanced the transport of CK from the damaged muscle to the circulation via the lymph fluid and increased CK clearance from the blood by increasing blood and lymph flow.13 It is also possible to assume that massage assists in flushing neutrophils and macrophages from the injured area, thus avoiding fiber necrosis and CK efflux.26 However, no concrete evidence to support these speculations is available at this time.
Although massage had positive effects on DOMS, swelling, and plasma CK activity, no significant protective effects occurred against losses in muscle strength and ROM. These findings are consistent with those of previous authors,12,13,15,16 who did not note beneficial effects of massage on either loss or recovery of muscle function. It might be more important for athletes and coaches to enhance recovery of muscle function after eccentric exercise than reduce DOMS and swelling. If this is the case, massage will not fulfill that purpose. Increasing blood flow by massage to deliver oxygen and other substances necessary for the regeneration of the damaged tissue is apparently not effective enough. The actual physiologic mechanisms by which massage could influence the regeneration process are obscure.10 Our findings thus support the idea that DOMS should be treated with caution as an indicator of muscle damage and may be more associated with individual responses to the sensations eliciting pain than the mechanisms responsible for muscle injury per se. This possibility makes it all the more important to consider such variations in the design and interpretation of studies such as this one.
In summary, using an arm-to-arm comparison model to quantify the effects of a therapeutic massage after high-intensity eccentric exercise, we found reductions in muscle soreness and muscle swelling and a lowered CK efflux compared with responses in the contralateral arm. However, massage had no protective effect on muscle strength and ROM. Our findings suggest that massage, used appropriately, is beneficial in reducing DOMS and swelling associated with high-intensity eccentric exercise, but recreational athletes and sports professionals who use massage should be cognizant of the fact that no positive effects of massage on recovery of muscle function can be expected.
REFERENCES
- Armstrong RB. Mechanisms of exercise-induced delayed onset muscular soreness: a brief review. Med Sci Sports Exerc. 1984;16:529–538. [PubMed] [Google Scholar]
- Clarkson PM, Nosaka K, Braun B. Muscle function postexercise-induced muscle damage and rapid adaptation. Med Sci Sports Exerc. 1992;24:512–520. [PubMed] [Google Scholar]
- Keskula DR. Clinical implications of eccentric exercise in sports medicine. J Sport Rehabil. 1996;5:321–329. [Google Scholar]
- Gulick DT, Kimura IF. Delayed-onset muscle soreness: what is it and how do we treat it? J Sport Rehabil. 1996;5:234–243. [Google Scholar]
- Nosaka K, Newton M, Sacco P. Delayed-onset muscle soreness does not reflect the magnitude of eccentric-exercise–induced muscle damage. Scand J Med Sci Sports. 2002;12:337–346. doi: 10.1034/j.1600-0838.2002.10178.x. [DOI] [PubMed] [Google Scholar]
- Cheung K, Hume P, Maxwell L. Delayed-onset muscle soreness: treatment strategies and performance factors. Sports Med. 2003;33:145–164. doi: 10.2165/00007256-200333020-00005. [DOI] [PubMed] [Google Scholar]
- Connolly DA, Sayers SP, McHugh MP. Treatment and prevention of delayed onset muscle soreness. J Strength Cond Res. 2003;17:197–208. doi: 10.1519/1533-4287(2003)017<0197:tapodo>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Tiidus PM. Manual massage and recovery of muscle function following exercise: a literature review. J Orthop Sports Phys Ther. 1997;25:107–112. doi: 10.2519/jospt.1997.25.2.107. [DOI] [PubMed] [Google Scholar]
- Ernst E. Does post-exercise massage treatment reduce delayed-onset muscle soreness? A systematic review. Br J Sports Med. 1998;32:212–214. doi: 10.1136/bjsm.32.3.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiidus PM. Massage and ultrasound as therapeutic modalities in exercise-induced muscle damage. Can J Appl Physiol. 1999;24:267–278. doi: 10.1139/h99-022. [DOI] [PubMed] [Google Scholar]
- Robertson A, Watt JM, Galloway SD. Effects of leg massage on recovery from high intensity cycling exercise. Br J Sports Med. 2004;38:173–176. doi: 10.1136/bjsm.2002.003186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber MD, Servedio FJ, Woodall WR. The effects of three modalities on delayed onset muscle soreness. J Orthop Sports Phys Ther. 1994;20:236–242. doi: 10.2519/jospt.1994.20.5.236. [DOI] [PubMed] [Google Scholar]
- Smith LL, Keating MN, Holbert D. The effects of athletic massage on delayed onset muscle soreness, creatine kinase, and neutrophil count: a preliminary report. J Orthop Sports Phys Ther. 1994;19:93–99. doi: 10.2519/jospt.1994.19.2.93. et al. [DOI] [PubMed] [Google Scholar]
- Farr T, Nottle C, Nosaka K, Sacco P. The effects of therapeutic massage on delayed onset muscle soreness and muscle function following downhill walking. J Sci Med Sport. 2002;5:297–306. doi: 10.1016/s1440-2440(02)80018-4. [DOI] [PubMed] [Google Scholar]
- Hilbert JE, Sforzo GA, Swensen T. The effects of massage on delayed onset muscle soreness. Br J Sports Med. 2003;37:72–75. doi: 10.1136/bjsm.37.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiidus PM, Shoemaker JK. Effleurage massage, muscle blood flow and long-term post-exercise strength recovery. Int J Sports Med. 1995;16:478–483. doi: 10.1055/s-2007-973041. [DOI] [PubMed] [Google Scholar]
- Rodenburg JB, Steenbeek D, Schiereck P, Bar PR. Warm-up, stretching and massage diminish harmful effects of eccentric exercise. Int J Sports Med. 1994;15:414–419. doi: 10.1055/s-2007-1021080. [DOI] [PubMed] [Google Scholar]
- Nosaka K, Clarkson PM. Variability in serum creatine kinase response after eccentric exercise of the elbow flexors. Int J Sports Med. 1996;17:120–127. doi: 10.1055/s-2007-972819. [DOI] [PubMed] [Google Scholar]
- Ohnhaus EE, Adler R. Methodological problems in the measurements of pain: a comparison between the verbal rating scale and the visual analogue scale. Pain. 1975;1:379–384. doi: 10.1016/0304-3959(75)90075-5. [DOI] [PubMed] [Google Scholar]
- Tiidus PM. Estrogen and gender effects on muscle damage, inflammation, and oxidative stress. Can J Appl Physiol. 2000;25:274–287. doi: 10.1139/h00-022. [DOI] [PubMed] [Google Scholar]
- Stupka N, Tarnopolsky MA, Yardley NJ, Phillips SM. Cellular adaptation to repeated eccentric-exercise–induced muscle damage. J Appl Physiol. 2001;91:1669–1678. doi: 10.1152/jappl.2001.91.4.1669. [DOI] [PubMed] [Google Scholar]
- Stupka N, Lowther S, Chorneyko K, Bourgeois JM, Hogben C, Tarnopolsky MA. Gender differences in muscle inflammation after eccentric exercise. J Appl Physiol. 2000;89:2325–2332. doi: 10.1152/jappl.2000.89.6.2325. [DOI] [PubMed] [Google Scholar]
- Clarkson PM, Hubal MJ. Are women less susceptible to exercise-induced muscle damage? Curr Opin Clin Nutr Metab Care. 2001;4:527–531. doi: 10.1097/00075197-200111000-00011. [DOI] [PubMed] [Google Scholar]
- Stauber WT, Clarkson PM, Fritz VK, Evans WJ. Extracellular matrix disruption and pain after eccentric muscle action. J Appl Physiol. 1990;69:868–874. doi: 10.1152/jappl.1990.69.3.868. [DOI] [PubMed] [Google Scholar]
- Smith LL. Acute inflammation: the underlying mechanism in delayed-onset muscle soreness. Med Sci Sports Exerc. 1991;23:542–51. [PubMed] [Google Scholar]
- Cannon JG, Orencole SF, Fielding RA. Acute phase response in exercise: interaction of age and vitamin E on neutrophils and muscle enzymes release. Am J Physiol. 1990;259:R1214–1219. doi: 10.1152/ajpregu.1990.259.6.R1214. et al. (6 pt 2) [DOI] [PubMed] [Google Scholar]