Abstract
Context: Sport massage is often used to help prepare for exercise, expedite recovery from muscle soreness, and enhance athletic performance. However, the effect of sport massage on recovery from delayed-onset muscle soreness is unknown.
Objective: To determine the effect of a short sport massage treatment on intramuscular swelling and pain in response to eccentric exercise.
Design: We used a 2 × 8 (treatment × time) repeated-measures design to compare triceps surae muscle girth and pain ratings over the 72 hours after eccentric exercise.
Setting: University research laboratory.
Patients or Other Participants: Nineteen healthy, college-aged subjects.
Intervention(s): Delayed-onset muscle soreness was induced with several sets of eccentric triceps surae contractions at 90% of the estimated concentric, 1-repetition maximum weight. Subjects returned on 3 consecutive days after eccentric exercise with a cycle ergometer for active rest treatments. In addition, 1 leg received the sport massage.
Main Outcome Measure(s): Girth measurements were taken at 5.08 cm (2 in), 10.16 cm (4 in), 15.24 cm (6 in), and 20.32 cm (8 in) below the knee joint line, and pain was assessed with a visual analog scale before and after all 4 sessions.
Results: No interaction was noted between treatment and time for any girth or pain measurements, and no main effect was seen for treatment.
Conclusions: Sport massage did not reduce girth or pain in the lower leg after eccentric exercise within 72 hours.
Keywords: delayed-onset muscle soreness, active rest, rehabilitation, treatment
Clinicians continue to seek the most expedient treatment for relieving the signs and symptoms of delayed-onset muscle soreness (DOMS). Eccentric exercise has been reported to significantly contribute to the onset of DOMS.1–4 Delayed-onset muscle soreness is the feeling of soreness after large-force, eccentric exercise5–7 and usually peaks at 24 to 48 hours postexercise,2,6–8 with resolution at 5 to 7 days.6,7 It is associated with muscle pain, swelling, and decreased muscle endurance and force production, which can be detrimental to athletic performance.1 The findings from investigations evaluating treatments for DOMS have been inconclusive and conflicting.9–13
Active rest, which involves repetitive, low-intensity, concentric muscle contractions, appears to facilitate the clearance of postexercise intramuscular swelling, adhesions, and therefore soreness associated with DOMS.3 However, the external forces provided by sport massage may further accelerate muscle healing and return to activity. Sport massage is similar to conventional massage and is often used as an aid in preparing for exercise, expediting recovery from muscle soreness, and enhancing athletic performance.9–11,14 Protocols and techniques differ among those providing massage, but all share similar goals. Effleurage (stroking) and petrissage (kneading) are commonly used sport massage techniques.15 The goal of these massage techniques is to augment treatments for postexercise intramuscular edema resolution by facilitating fluid movement and increasing circulation.16
Conflicting research findings are noted regarding the efficacy of sport massage in the recovery from the signs and symptoms of DOMS. Although benefits of sport massage during the recovery from eccentric exercise have been reported, lengthy treatment sessions of 1510, 2011, or 309 minutes along with a complex sequence of techniques make these results difficult to effectively reproduce in some clinics and athletic training rooms. However, a simple, 5-minute sport massage treatment performed immediately after eccentric exercise and 24 hours later had no benefit on DOMS when compared with, and not supplemented by, other modalities.13
The most effective treatment for DOMS may vary among patients. If a short, easily reproducible sport massage treatment adds to the success of existing DOMS treatment programs, the recovery process for athletes and patients may be expedited. Our purpose was to determine the additional influence of a short sport massage treatment on intramuscular swelling and pain in response to bilateral eccentric exercise to the triceps surae muscles.
METHODS
Design
We used a 2 × 8 repeated-measures design. Treatment (sport massage + active rest, active rest alone) and time (baseline and postexercise on day 1, pretreatment and postexercise on days 1–4) were the independent variables. Girth measurements at 5.08 cm (2 in), 10.16 cm (4 in), 15.24 cm (6 in), and 20.32 cm (8 in) below the knee joint line and pain ratings assessed with a visual analog scale were the dependent variables. Each subject served as his or her own control.
Subjects
Nineteen healthy, college-aged individuals (10 men, 9 women) volunteered to participate in this study (age = 20.6 ± 1.2 years, height = 172.1 ± 8.5 cm, mass = 70.8 ± 13.7 kg). Subjects had no history of recent orthopaedic injury to either lower extremity or any other disability or medical condition that would prevent them from completing the exercise protocol or receiving clinical treatment. Subjects read and signed an informed consent agreement before participating. A university institutional review board approved this study.
Procedure
Baseline girth measurements of the lower leg were recorded at the 4 sites below the knee joint line. Subjects then performed an adequate warm-up and stretch for the lower extremity before exercising the triceps surae muscle group. To induce DOMS to both legs, subjects performed 20 submaximal, concentric, plantar-flexion contractions, followed by 4 or 5 sets of 35 eccentric triceps surae contractions at 90% of their estimated concentric, 1-repetition maximum weight.9 All plantar-flexion exercises were performed on a standing calf-raise machine (Cybex Eagle Fitness Systems, Medway, MA). Subjects did not pursue treatment for their calf muscle discomfort while participating in this study.
Treatments
After the exercise regimen intended to induce DOMS, subjects returned daily for 3 treatment sessions (at 24, 48, and 72 hours). During each of these sessions, girth and discomfort measurements were recorded first. Then all subjects rode a stationary bicycle for 5 minutes and immediately afterward received 5 minutes of sport massage to the experimental limb. The bicycle (Cybex cycle ergometer, Cybex Eagle Fitness Systems), set at a constant resistance of 90°/s, was used for active rest treatments. The seat was set at a comfortable height, and each subject pedaled at a self-selected moderate pace. The resistance was set so subjects could ride comfortably during active rest treatments. Sport massage was randomly assigned to 1 of each subject's legs by flipping a coin. All subjects and both lower extremities were treated with active rest, but only 1 leg received the sport massage, and this remained the experimental leg throughout data collection. Sport massage treatments consisted of repeating cycles of petrissage (kneading) and effleurage (broad stroking).15 Seventy-five seconds of petrissage was followed by 75 seconds of effleurage and repeated once for a total of 5 minutes of sport massage therapy. A certified athletic trainer (J.M.H.) performed all sport massage treatments. Each petrissage stroke and effleurage stroke was timed with the second hand of a clock to maintain a pace of about 1 stroke per second. Friction between the subject's skin and the researcher's hands was reduced with liberal amounts of hypoallergenic moisturizing lotion. This sport massage protocol is not an established protocol. It was designed to be easily reproduced by any certified athletic trainer in an athletic training room or clinical setting in which extended treatment durations may not be practical.
Data Collection
Girth measurements (centimeters) were assessed with a standard flexible tape measure. Subjects' lower legs were marked with permanent marker at the 4 sites below the knee joint line (Figure 1). The flexible tape measure was approximated in the coronal plane at each level on the lower leg and pulled with a force that was snug on the leg but did not cause the skin to deform. Measurements were assessed to the nearest 0.1 cm. All girth measurements were determined by the same researcher and with the same tape measure. Pain measurements were assessed with a visual analog scale with 2 extreme polar descriptors: no pain and worst possible pain. Subjects were asked to place a vertical mark intersecting with the 10-cm horizontal line at a distance between the 2 extreme descriptors that represented their current level of pain. Both girth measurements and pain level were assessed before and after all 4 sessions for a total of 8 levels of time. The investigator collecting data was not blinded to treatment leg or time of measurement (ie, pretreatment versus posttreatment).
Figure 1. Subjects' legs were marked at the joint line and at 5.08 cm (2 in), 10.16 cm (4 in), 15.24 cm (6 in), and 20.32 cm (8 in) below the joint line.
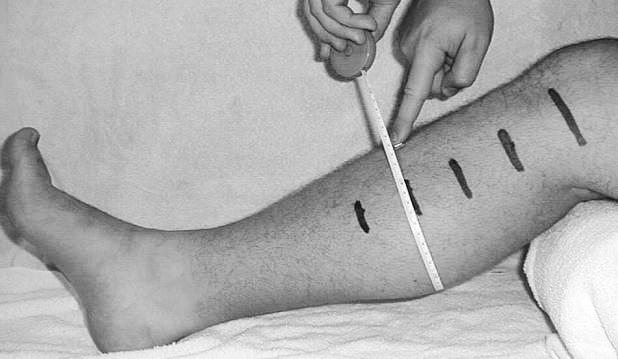
This girth measurement is at 15.24 cm (6 in) below the joint line. Marks were made with permanent marker so they would not wash off between days
Before data collection, we conducted a single-blinded test-retest reliability study to determine the intersession and intratester reliability of girth measurements taken by 1 investigator with the same tape measure. A university institutional review board approved this research, and all subjects read and signed an informed consent. Circumferential lower leg girth measurements were taken by the same investigator and at the same measurement sites used in the current study. Ten subjects (age = 23.7 ± 3.8 years, height = 171.5 ± 8.05 cm, mass = 68.4 ± 14.4 kg) lay supine on a plinth with their bodies concealed behind a curtain while 1 researcher performed initial girth measurements on both legs. A second researcher recorded all data for the first session and then rearranged the subjects for a second session of measurements. The variance between the 2 trials was 0.021, 0.0001, 0.443, and 0.491 cm for measures taken at 5.08 cm, 10.16 cm, 15.24 cm, and 20.32 cm below the medial joint line, respectively, and showed high reliability with repeated measurement (intraclass correlation coefficient [3,1] range = 0.993–0.997). We also calculated the SEM for the girth measurements taken during the sport massage study (range = 0.29–0.36 cm) for all measurement sites at all 8 levels of time (pretreatment and posttreatment × 4 days). Girth measurement sensitivity may have been affected because the values of the SEM were similar to the girth changes measured in subjects.
Statistical Analysis
We used a 2 × 8 (treatment × time) repeated-measures multiple analysis of variance to assess girth differences among treatments over time at the 4 measurement sites. Follow-up analysis of variance and t tests with Bonferroni correction were performed when appropriate. A 2 × 8 analysis of variance with repeated measures was calculated to assess subjective pain differences between treatments over time. The alpha level was set a priori at P ≤ .05. We used SPSS (version 11.0; SPSS Inc, Chicago, IL) for all analyses.
RESULTS
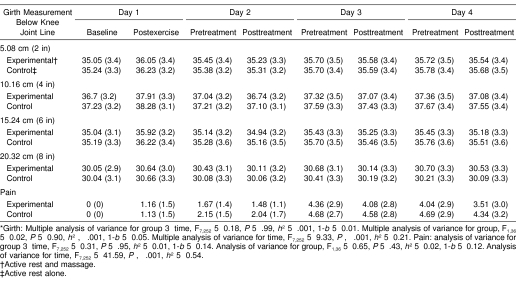
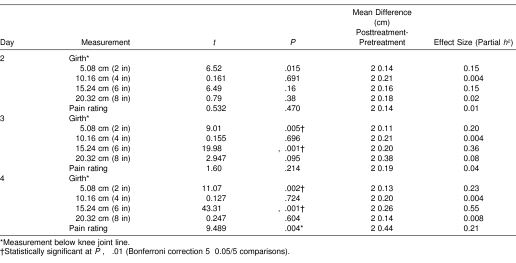
Means and SDs for girth and pain measurements over time are presented in Table 1. For girth, a significant main effect was noted only for time. The follow-up univariate analyses showed statistically significant girth differences over time at 5.08 cm (F7,252 = 20.12, P < .001, η2 = 0.36), 15.24 cm (F7,252 = 15.86, P < .001, η2 = 0.31), and 20.32 cm (F7,252 = 2.25, P = .016, η2 = 0.06) but not at 10.16 cm (F7,252 = 1.63, P = .126, η2 = 0.04, 1-β = 0.7). Post hoc t tests for girth measurements over time are presented in Table 2. Regarding pain, a significant main effect was noted only for time (see Table 1). Post hoc t test results for pain over time are also presented in Table 2.
Table 1. Mean (SD) for Girth (Centimeters) and Pain Measurements (12-mm Visual Analog Scale) Across Days*.
Table 2. Post Hoc t Tests for Within-Day Repeated Comparisons at 4 Girth Measurement Sites and Pain Across 3 Treatment Days for Combined Groups.
DISCUSSION
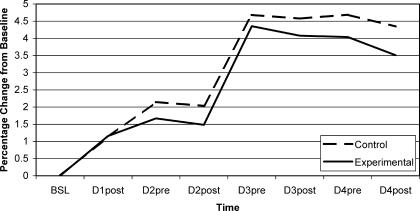
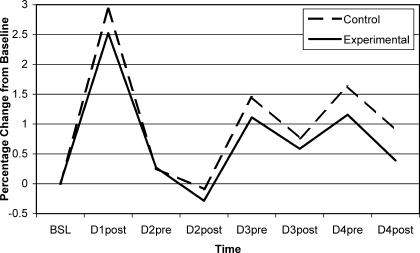
The eccentric exercise protocol induced DOMS effectively because both pain and girth measures significantly increased at 24 hours over baseline (see Table 1). Pain measurements remained elevated during the 3 days after DOMS induction (Figure 2), but the girth measurements did not (Figure 3). Table 1 compares the 7 postbaseline girth measures over 4 days. Girth was significantly different from baseline for pretreatment measures but not posttreatment measures (see Table 1). Furthermore, within-session reduction of pain and girth was evident in all sessions, but not all values achieved statistical significance. The treatment protocol used in this experiment mostly affected girth measures at 15.24 cm below the knee joint line (see Table 2), which is approximately the level of the muscle bellies of the triceps surae. Because the treatment groups did not differ according to whether they had sport massage or not, active rest may have contributed more to the observed within-session reduction in girth and pain. However, we cannot discount the possibility that the girth and pain reductions were a natural result of the passage of time or another factor that was not controlled in the design of this study. In addition, the changes may also be attributed to measurement error because the SEM values were similar to the observed girth changes. Still, our results provide no scientific evidence that a short sport massage treatment including effleurage and petrissage expedites recovery from DOMS over the effects of active rest alone.
Figure 2. Pain ratings for control and experimental groups.
Pain data were collected 7 times after baseline and were significantly higher than baseline (0) throughout the experiment. BSL indicates baseline; D1post, day 1, postexercise; D2pre, day 2, pre-exercise, etc
Figure 3. Girth measurements at 15.24 cm (6 in) below the knee joint line for control (active rest only) and experimental (active rest and sport massage) sides are presented as percentage change from baseline.
The first intervention (active rest and randomly assigned sport massage) took place once pretreatment measurements were taken at 24 hours after eccentric exercise (D2pre). The same treatments were applied to the control and experimental legs at the same time at 48 hours (D3) and 72 hours (D4). Treatments were all followed by posttest girth and pain measurements. BSL indicates baseline; D1post, day 1, postexercise; D2pre, day 2, pre-exercise, etc
Previous authors9–11,13 have used massage protocols of various durations and timings. Weber et al13 reported no differences in pain or force production of the elbow flexors between sport massage and control groups when sport massage was applied immediately after and at 24 hours after eccentric exercise. Sport massage has also provided pain relief when applied 2 hours after9,11 and immediately after10 eccentric exercise. We chose the timing of the initial sport massage treatment (ie, 24 hours postexercise) to resemble the timing of an athlete's initial complaint of DOMS after intense exercise. Furthermore, the duration of the sport massage treatment we used is reasonable for an athletic training room accommodating several athletes and several sports teams. Unfortunately, the brevity of the intervention may account for the observed effect, as is illustrated by small effect sizes (see Table 2). It is difficult to determine the meaningfulness of the small treatment effects on daily function because we did not measure any functional outcomes other than girth and pain.
Although several theories on the underlying mechanism of DOMS after eccentric exercise have been suggested,1–3,6 the development of DOMS may resemble the sequence of events observed in the acute inflammatory cycle.2,17,18 Within a few hours after muscle damage, biochemical infiltrates begin to collect in the area of muscle injury.2,3 Sport massage may mitigate the accumulation of white blood cells along vessel walls after muscle damage.9 Macrophage accumulation, which depends on this initial white blood cell migration to the injury site, contributes considerably to activation of nociceptors through prostaglandin production and edema accumulation.3 Increased blood flow resulting from the mechanical action of sport massage may interfere with this early event in the acute inflammatory cascade by possibly shearing marginated white blood cells from vessel walls. White blood cells may be prevented from migrating to the injury site, macrophage accumulation is reduced, and the intensity of DOMS is lessened.9 If this is the case, the timing of the sport massage intervention used in this study (24 hours after eccentric exercise) may have been too late to provide relief of signs or symptoms. The induction of DOMS was used as a model to study a method of reducing muscular discomfort and edema after eccentric exercise that may involve a series of events resembling those occurring during the acute phase of inflammation.
Massage may have systemic psychological19 and biochemical9 effects on the signs and symptoms of DOMS. MacDonald19 reported that massage reduced self-reported perceived ratings of emotional and physical stress and physical pain in primary caregivers. We did not assess subjects' psychological and biochemical influences, but these factors may have affected both legs equally. In addition, the external forces provided by massage may be interpreted as a stressor by the body, resulting in the release of the anti-inflammatory hormone cortisol.9 This release could also produce effects that would equally influence each leg. Further controlled laboratory research is needed to develop this theory.
CONCLUSIONS
The eccentric exercise protocol we used effectively induced pain and swelling over time. A 5-minute sport massage protocol did not provide relief from eccentric exercise-induced DOMS, and the sport massage provided did not reduce the within-session discomfort or swelling after eccentric exercise. Five minutes of sport massage performed at 24, 48, and 72 hours after exercise did not alter the time course of DOMS over the time frame we studied. Although many clinicians may have anecdotal success with sport massage treatments, the nature of their effectiveness should be investigated with further controlled scientific research aimed at practical application in sports medicine clinics and athletic training rooms.
Acknowledgments
We thank John Spiker, President of Healthworks Rehabilitation and Fitness, Morgantown, WV, for letting us use his facility for data collection. We also thank Mia Ericson for her help with this project.
REFERENCES
- Smith LL. Causes of delayed onset muscle soreness and the impact on athletic performance: a review. J Appl Sport Sci Res. 1992;6:135–141. [Google Scholar]
- Smith LL. Acute inflammation: the underlying mechanism in delayed onset muscle soreness? Med Sci Sports Exerc. 1991;23:542–551. [PubMed] [Google Scholar]
- Cheung K, Hume P, Maxwell L. Delayed onset muscle soreness: treatment strategies and performance factors. Sports Med. 2003;33:145–164. doi: 10.2165/00007256-200333020-00005. [DOI] [PubMed] [Google Scholar]
- Nosaka K, Newton M, Sacco P. Muscle damage and soreness after endurance exercise of the elbow flexors. Med Sci Sports Exerc. 2002;34:920–927. doi: 10.1097/00005768-200206000-00003. [DOI] [PubMed] [Google Scholar]
- Gulick DT, Kimura IF, Sitler M, Paolone AM, Kelly JD., IV. Various treatment techniques on signs and symptoms of delayed onset muscle soreness. J Athl Train. 1996;31:145–152. [PMC free article] [PubMed] [Google Scholar]
- Cleak MJ, Eston RG. Delayed onset muscle soreness: mechanisms and management. J Sports Sci. 1992;10:325–341. doi: 10.1080/02640419208729932. [DOI] [PubMed] [Google Scholar]
- Gulick DT, Kimura IF. Delayed onset muscle soreness: what is it and how do we treat it? J Sport Rehabil. 1996;5:234–243. [Google Scholar]
- Isabell WK, Durrant E, Myrer W, Anderson S. The effects of ice massage, ice massage with exercise, and exercise on the prevention and treatment of delayed onset muscle soreness. J Athl Train. 1992;27:208–217. [PMC free article] [PubMed] [Google Scholar]
- Smith LL, Keating MN, Holbert D. The effects of athletic massage on delayed onset muscle soreness, creatine kinase, and neutrophil count: a preliminary report. J Orthop Sports Phys Ther. 1994;19:93–99. doi: 10.2519/jospt.1994.19.2.93. et al. [DOI] [PubMed] [Google Scholar]
- Rodenburg JB, Steenbeek D, Schiereck P, Bar PR. Warm-up, stretching and massage diminish harmful effects of eccentric exercise. Int J Sports Med. 1994;15:414–419. doi: 10.1055/s-2007-1021080. [DOI] [PubMed] [Google Scholar]
- Hilbert JE, Sforzo GA, Swensen T. The effects of massage on delayed onset muscle soreness. Br J Sports Med. 2003;37:72–75. doi: 10.1136/bjsm.37.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert MI, Marcus P, Burgess T, Noakes TD. Electro-membrane microcurrent therapy reduces signs and symptoms of muscle damage. Med Sci Sports Exerc. 2002;34:602–607. doi: 10.1097/00005768-200204000-00007. [DOI] [PubMed] [Google Scholar]
- Weber MD, Servedio FJ, Woodall WR. The effects of three modalities on delayed onset muscle soreness. J Orthop Sports Phys Ther. 1994;20:236–242. doi: 10.2519/jospt.1994.20.5.236. [DOI] [PubMed] [Google Scholar]
- Martin NA, Zoeller RF, Robertson RJ, Lephart SM. The comparative effects of sports massage, active recovery, and rest in promoting blood lactate clearance after supramaximal leg exercise. J Athl Train. 1998;33:30–35. [PMC free article] [PubMed] [Google Scholar]
- Tiidus PM. Manual massage and recovery of muscle function following exercise: a literature review. J Orthop Sports Phys Ther. 1997;25:107–112. doi: 10.2519/jospt.1997.25.2.107. [DOI] [PubMed] [Google Scholar]
- Callaghan MJ. The role of massage in the management of the athlete: a review. Br J Sports Med. 1993;27:28–33. doi: 10.1136/bjsm.27.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacIntyre DL, Reid WD, McKenzie DC. Delayed muscle soreness: the inflammatory response to muscle injury and its clinical implications. Sports Med. 1995;20:24–40. doi: 10.2165/00007256-199520010-00003. [DOI] [PubMed] [Google Scholar]
- MacIntyre DL, Reid WD, Lyster DM, Szasz IJ, McKenzie DC. Presence of WBC, decreased strength, and delayed soreness in muscle after eccentric exercise. J Appl Physiol. 1996;80:1006–1013. doi: 10.1152/jappl.1996.80.3.1006. [DOI] [PubMed] [Google Scholar]
- MacDonald G. Massage as a respite intervention for primary caregivers. Am J Hosp Palliat Care. 1998;15:43–47. doi: 10.1177/104990919801500109. [DOI] [PubMed] [Google Scholar]