Abstract
Context: Contralateral muscular imbalances have been suggested to increase the risk of lower extremity injury. Previous groups have assessed strength of the quadriceps and hamstring muscle groups; however, no previous authors have compared bilateral hip-abductor muscular performance.
Objective: To examine the strength and fatigability of the hip abductors in the dominant and nondominant legs.
Design: Single-group, repeated-measures design.
Setting: Musculoskeletal laboratory.
Patients or Other Participants: Forty-two healthy subjects (23 males, 19 females; age = 24.3 ± 2.7 years, height = 173.4 ± 9.8 cm, mass = 73.7 ± 11.6 kg).
Intervention(s): Subjects performed three 5-second maximal voluntary isometric contraction (MVIC) trials of the hip abductors with the dominant and nondominant legs. Following the maximal strength trials, subjects performed a submaximal (50% of MVIC) 30-second fatigue trial with each leg.
Main Outcome Measure(s): Peak torque (PT) was recorded from each MVIC trial. Surface electromyography was used to record muscle activity during the fatigue trials. Power spectral analysis was used to determine the median frequency of each 0.512-second portion of the fatigue trials. Median frequencies were plotted against time, and linear regression was used to determine the median frequency slope (MFslope). Data were analyzed using 2-tailed, paired t tests.
Results: Hip-abduction PT of the dominant leg (81.0 ± 23.7 Nm) was significantly larger than that of the nondominant leg (76.1 ± 9.9 Nm, P = 0.02). There was no difference in MFslope between the dominant (−0.37 ± 0.29) and nondominant limbs (−0.35 ± 0.34, P = 0.84). The PT and MFslope were not significantly correlated (r = −0.07, P = 0.53).
Conclusions: Hip-abduction strength differences exist between the dominant and nondominant legs. Measures of strength and fatigability were poorly related; therefore, clinicians may opt to assess hip strength and fatigability independent of each another.
Keywords: footedness, gluteus medius, electromyography, median frequency
INTRODUCTION
Limb dominance has been defined as one limb demonstrating increased dynamic control as a result of an imbalance in muscular strength and recruitment patterns.1–3 Both limbs can be negatively affected by this asymmetry. Dependence on the dominant limb can increase stress on the joints of that extremity. Overreliance on the dominant limb can also result in weakness in the contralateral limb, which can decrease the nondominant extremity's ability to absorb large forces associated with athletic activities.2
The concept of limb dominance in the upper extremity has long been accepted, yet researchers and clinicians often treat the 2 lower extremities as equal. Authors1–6 have addressed the idea of leg dominance as it relates to both strength and function. Several groups4,7 have reported no significant side-to-side differences in isokinetic strength of the flexors and extensors of the hip and knee. Conversely, epidemiologic studies have shown not only that strength imbalances exist but that they may result in increased injury rates for athletes with side-to-side strength differentials greater than 10%.3,8
Leg dominance differences at the hip and knee have been revealed during functional tasks, such as landing from a jump.1,9 The differences have been observed in both the frontal1 and transverse planes.9 Frontal-plane movement is of interest because of the potential for knee ligament injury from increased valgus motion. Dominance-related strength differentials of the hip abductors may be partly responsible for the reported functional differences.
Hip-abductor endurance also plays a role in valgus knee movement. Eggen et al10 reported that valgus movement of the knee increased after fatigue of the hip abductors. Hip-abductor strength and endurance have yet to be determined in relation to leg dominance. Therefore, our purpose was to examine the strength and fatigability of the hip abductors in the dominant and nondominant legs.
METHODS
Design and Setting
We used a repeated-measures design for this laboratory-based protocol. All testing procedures were approved by the institutional review board, and all subjects provided informed consent. Testing was performed at the University of Kentucky Musculoskeletal Laboratory. Before testing, the dominant leg was determined to be the leg used to kick a soccer ball.6 Testing consisted of 2 components for each limb: maximal voluntary isometric contraction (MVIC) trials and a fatigue trial. Leg order was counterbalanced for testing, and subjects were given at least 4 days between tests. The independent variables were the strength test, fatigue test, and test leg (dominant and nondominant). The dependent variables were peak torque (PT) and the slope of the electromyographic median frequency.
Subjects
Forty-two subjects (23 males, 19 females; age = 24.3 ± 2.7 years, height = 173.4 ± 9.8 cm, mass = 73.7 ± 11.6 kg) volunteered to participate in the study.
Instruments
Strength was quantified by measuring PT with the Primus dynamometer (Baltimore Therapeutic Equipment, Baltimore, MD). The dynamometer was also used to quantify force production during the isometric fatigue protocol of the hip abductors. The dynamometer was calibrated weekly during testing.
Electromyographic (EMG) data were collected during the hip-abductor fatigue test. Before testing, the skin was shaved, abraded, and cleaned with an alcohol preparation pad. Bipolar, 5-mm diameter, Ag-AgCl surface electrodes (Ambu Inc, Linthicum, MD) were placed over the gluteus medius muscle. The edges of the surface electrodes were trimmed to reduce the interelectrode distance to 2.0 cm. The distance between the iliac crest and the greater trochanter was measured, and electrodes were placed at one third of this distance distal to the iliac crest.11 Electrodes were positioned parallel to the direction of the muscle fibers, and a ground electrode was placed over the clavicle.
A 16-channel EMG system (Run Technologies, Mission Viejo, CA) recorded all muscle activity during testing. Subjects wore a battery-operated amplifier (Run Technologies) that collected muscle activity from the surface electrodes. A Myopac transmitter belt unit (Run Technologies) transmitted raw EMG signal at 2000 Hz via a fiberoptic cable to its receiver unit. The unit specifications include an amplifier gain of 2000 and a common mode rejection ratio of 90 dB. After reaching the receiver unit, the signal was processed through a 16-bit A/D board into a personal computer. The EMG raw data from the fatigue trials were stored and later analyzed using Datapac Software (Run Technologies).
Procedures
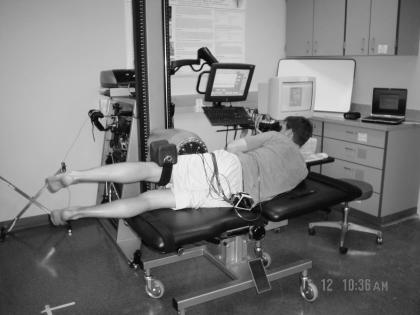
Subjects completed three 5-second MVICs of the gluteus medius muscle. Rest intervals of 30 seconds were given between trials. Subjects were positioned lying on their side for testing. The dynamometer's axis of rotation was aligned with a point on the subject representing the intersection of 2 straight lines. One line was directed inferiorly from the anterior superior iliac spine toward the patella, and the other line was medially directed from the greater trochanter of the femur toward the midline of the body. The lever arm of the dynamometer was set so that the resistance pad was located just proximal to the lateral femoral epicondyle. The hip was placed in a position that was neutrally aligned in all 3 planes (Figure 1). Subjects were instructed to push out into the resistance pad as hard as possible during each 5-second MVIC trial. They were also instructed to keep their toes pointed forward and not to bend their knees to help prevent alterations in muscle recruitment and compensation during testing. The PT measurements, recorded in Newton-meters, were obtained from each MVIC trial, with the overall largest value from the 3 trials representing each subject's PT. We determined a priori that if the coefficient of variation was greater than 10%, the subject's data would not be used for analysis; all subjects had a coefficient of variation less than this value. Also, we have demonstrated excellent intrasession reliability (intraclass correlation coefficient [2, 1] = 0.99) when measuring PT using this method.
Figure 1. Subject position during testing.
Two minutes after the completion of the MVICs, subjects performed a hip fatigue trial. They were again tested lying on their side, with the hip in a position of neutral alignment. Participants were required to maintain a submaximal (50% MVIC) contraction for the 30-second fatigue trial. This value was chosen because of previous reports that EMG markers of fatigue increased with force up to 60% MVIC,12 with no benefit resulting from testing above this level. Before the fatigue trial, subjects were given a 5- to 10-second practice trial to familiarize themselves with the procedures of the fatigue trial and to experience the effort required to achieve the target load. Approximately 30 seconds' rest was given between the practice and test trials. Visual and verbal feedback was provided to help ensure consistent effort. During both the practice and fatigue trials, subjects could view an image representing their force production on a line graph on the monitor located directly in their line of sight. A dotted horizontal line represented the target load, and as the trial proceeded, a red line tracked across the screen in real time, giving the subject a clear visual representation of force production.
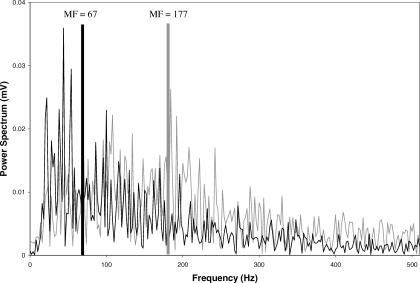
The EMG data from the isometric fatigue trials were arranged in 0.512-second time epochs and transformed using fast Fourier transformation (Figure 2). The signal was also treated by using the data demeaning and Hanning window functions available with the Datapac system. The median frequency (MF) values for each 0.512-second epoch were used to quantify the resulting power spectrum. The MF of the initial epoch was recorded, and the MF data were then plotted against time using Microsoft Excel (version 2003; Microsoft Corp, Redmond, WA).5 The resulting slope (MFslope) was calculated and recorded for each subject's dominant and nondominant legs (Figure 3). The premise of this technique is that slow-twitch muscle fibers have low-frequency signals, whereas fast-twitch fibers have higher-frequency signals. During fatiguing efforts, a shift occurs from higher- to lower-frequency motor units as fast-twitch fibers become fatigued and more emphasis is placed on slow-twitch fibers.13,14 The MFslope values are generally negative, representing this shift. If a muscle demonstrates a steeper negative MFslope, it is fatiguing at a faster rate.
Figure 2. Representative sample power spectrum of one 0.512-second window of the gluteus medius muscle at the start of the fatigue trial (gray trace: median frequency [MF] = 177 Hz) and at the end of the fatigue trial (black trace: MF = 67 Hz).
Figure 3. Median frequency of the gluteus medius plotted as a function of time for one subject.
Linear regression slopes were calculated for each subject's dominant and nondominant hips
Statistical Analysis
We performed 2-tailed, paired t tests to assess potential differences in PT and MFslope between the dominant and nondominant hip abductors. Also, a Pearson product moment correlation was performed to evaluate the relationship between the measures of strength and fatigability. An α level of P ≤ 0.05 was considered significant for all analyses.
RESULTS
Significant differences in PT were noted between the dominant and nondominant hip abductors (P = 0.02, effect size = 0.22). Mean PT values were 81.0 ± 23.7 Nm for the dominant hip and 76.1 ± 19.9 Nm for the nondominant hip. The mean coefficient of variation during strength testing was 3.45%. Regardless of leg dominance, the average within-subject bilateral strength differential was 11.6 ± 8.31%. The average MF of the initial 0.512-second epoch was 146.18 ± 24.19 Hz. No dominance-related differences in fatigability were present between limbs (P = 0.84, observed power = 0.17). Mean MFslope for the dominant hip was −0.37 ± 0.29, whereas the nondominant hip had a mean MFslope of −0.35 ± 0.34. The correlation between PT and MFslope (r = −0.07) was not significant (P = 0.53).
DISCUSSION
Our purpose was to evaluate the strength and fatigability of the hip abductors of the dominant and nondominant legs. The hip abductors of the dominant limb were stronger than those of the nondominant limb. However, statistical significance may have been a result of the large sample size, and the small effect size of 0.22 indicates that although the differences were statistically significant, they may not be clinically meaningful. This being said, it is still of interest that regardless of leg dominance, the average side-to-side strength differential was approximately 11%. Clinically, this may be of more importance, as it indicates that imbalances in hip-abductor strength are prevalent and may not be strictly related to limb dominance. Athletes are commonly allowed to return to full participation with a remaining strength deficit of 15%.15 However, side-to-side strength imbalances in the range of 10% to 15% in other muscle groups have been associated with increased injury rates. Knapik et al3 reported increased lower extremity injury rates in female athletes demonstrating 15% strength deficits of either the left quadriceps or hamstring muscles. Burkett8 reported that a 10% side-to-side strength differential in the hamstrings was associated with an increased incidence of hamstring strains.
Few researchers have addressed the relationship between side-to-side hip-abductor strength imbalances and lower extremity injury. Fredericson et al16 reported that distance runners with iliotibial band syndrome demonstrated hip-abduction side-to-side imbalances of approximately 20%. Of the 42 healthy subjects we tested, 12 had side-to-side imbalances greater than 15%, and 6 had imbalances greater than 20%. The fact that our subjects were asymptomatic does not contradict the conclusions of Fredericson et al16 but merely highlights differences in activity level between the sample populations. Obtaining strength imbalance information such as this may assist athletic trainers in implementing preventive strengthening programs.
Our results indicated no difference between dominant and nondominant hip-abductor fatigability. Changes in MF have been widely used to quantify fatigability in research addressing the paraspinal muscle group, but to our knowledge, they have not been evaluated in the gluteus medius muscle. Because of the large variability of the current results and the lack of comparable previous literature, we must question the validity and reliability of this measure of fatigability in the gluteus medius muscle. Future research evaluating the validity and reliability of MFslope is necessary.
Despite the questionable validity of MFslope, it is of interest that the measures of strength and fatigability employed in the current investigation were poorly correlated. The lack of a significant relationship between PT and MFslope may reinforce the idea that we, as clinicians, must evaluate strength and fatigability independently.
The average MF of the initial epoch when we tested the gluteus medius (146.2 ± 24.2 Hz) was much higher than previously reported values associated with the paraspinals (53.2 Hz).17 As MF has been previously used to describe fiber type composition,18 we surmise that the gluteus medius muscle has a larger proportion of fast-twitch fibers than the primarily tonic paraspinals. Also, the initial MF values reported in this investigation are more similar to those reported for the tibialis anterior muscle (116 ± 20 Hz),19 which contracts more dynamically during normal gait, similar to the gluteus medius muscle.
CONCLUSIONS
Side-to-side strength imbalances exist between the hip abductors of asymptomatic healthy individuals. Hip-abductor imbalances have been previously reported in injured athletes. To reduce the potential risk of injury, lower extremity strength imbalances should be identified and corrected. The role of the hip abductors should not be overlooked, and lower extremity strengthening programs should include exercises targeting these muscles. Future investigations are needed to evaluate the relationship between hip-abductor strength and fatigability and the incidence of noncontact injuries to the lower extremity.
REFERENCES
- Ford KR, Myer GD, Hewett TE. Valgus knee motion during landing in high school female and male basketball players. Med Sci Sports Exerc. 2003;35:1745–1750. doi: 10.1249/01.MSS.0000089346.85744.D9. [DOI] [PubMed] [Google Scholar]
- Hewett TE, Myer GD, Ford KR. Prevention of anterior cruciate ligament injuries. Curr Women's Health Rep. 2001;1:218–224. [PubMed] [Google Scholar]
- Knapik JJ, Bauman CL, Jones BH, Harris JM, Vaughan L. Preseason strength and flexibility imbalances associated with athletic injuries in female collegiate athletes. Am J Sports Med. 1991;19:76–81. doi: 10.1177/036354659101900113. [DOI] [PubMed] [Google Scholar]
- Calmels PM, Mellen MN, van der Borne I, Jourdin P, Minaire P. Concentric and eccentric isokinetic assessment of flexor-extensor torque ratios at the hip, knee, and ankle in a sample population of healthy subjects. Arch Phys Med Rehabil. 1997;78:1224–1230. doi: 10.1016/s0003-9993(97)90336-1. [DOI] [PubMed] [Google Scholar]
- Clark BC, Manini TM, The DJ, Doldo NA, Ploutz-Snyder LL. Gender differences in skeletal muscle fatigability are related to contraction type and EMG spectral compression. J Appl Physiol. 2003;94:2263–2272. doi: 10.1152/japplphysiol.00926.2002. [DOI] [PubMed] [Google Scholar]
- Hoffman M, Schrader J, Applegate T, Koceja D. Unilateral postural control of the functionally dominant and nondominant extremities of healthy subjects. J Athl Train. 1998;33:319–322. [PMC free article] [PubMed] [Google Scholar]
- Nadler SF, Malanga GA, Bartoli LA, Feinberg JH, Prybicien M, Deprince M. Hip muscle imbalance and low back pain in athletes: influence of core strengthening. Med Sci Sports Exerc. 2002;34:9–16. doi: 10.1097/00005768-200201000-00003. [DOI] [PubMed] [Google Scholar]
- Burkett LN. Causative factors in hamstring strains. Med Sci Sports. 1970;2:39–42. [PubMed] [Google Scholar]
- Jacobs C, Mattacola CG. Eccentric hip strength and kinematic differences between the dominant and non-dominant legs of men and women during a hopping task. J Athl Train. 2004;39:S-34–S-35. (suppl) [Google Scholar]
- Eggen J, Carcia C, Gansneder B, Shultz S. Hip abductor fatigue affects knee motion during the landing phase of a drop jump. J Athl Train. 2003;38:S-22. (suppl) [Google Scholar]
- Cram JR, Kasman GS. Electrode placements. In: Introduction to Surface Electromyography. Gaithersburg, MD: Aspen, 1998: 237–384.
- Bilodeau M, Schindler-Ivens S, Williams DM, Chandran R, Sharma SS. EMG frequency content changes with increasing force and during fatigue in the quadriceps femoris muscle of men and women. J Electromyogr Kinesiol. 2003;13:83–92. doi: 10.1016/s1050-6411(02)00050-0. [DOI] [PubMed] [Google Scholar]
- Basmajian JV, De Luca CJ. Muscles Alive: Their Functions Revealed by Electromyography. 5th ed. Baltimore, MD: Williams & Wilkins; 1985.
- Bigland-Ritchie B, Cafarelli E, Vollestad NK. Fatigue of submaximal static contractions. Acta Physiol Scand Suppl. 1986;556:137–148. [PubMed] [Google Scholar]
- Wilk KE, Reinold MM, Hooks TR. Recent advances in the rehabilitation of isolated and combined anterior cruciate ligament injuries. Orthop Clin North Am. 2003;34:107–137. doi: 10.1016/s0030-5898(02)00064-0. [DOI] [PubMed] [Google Scholar]
- Fredericson M, Cookingham CL, Chaudhari AM, Dowdell BC, Oestreicher N, Sahrmann SA. Hip abductor weakness in distance runners with iliotibial band syndrome. Clin J Sport Med. 2000;10:169–175. doi: 10.1097/00042752-200007000-00004. [DOI] [PubMed] [Google Scholar]
- Elfving B, Nemeth G, Arvidsson I, Lamontagne M. Reliability of EMG spectral parameters in repeated measurements of back muscle fatigue. J Electromyogr Kinesiol. 1999;9:235–243. doi: 10.1016/s1050-6411(98)00049-2. [DOI] [PubMed] [Google Scholar]
- Kupa EJ, Roy SH, Kandarian SC, De Luca CJ. Effects of muscle fiber type and size on EMG median frequency and conduction velocity. J Appl Physiol. 1995;79:23–32. doi: 10.1152/jappl.1995.79.1.23. [DOI] [PubMed] [Google Scholar]
- Krivickas LS, Taylor A, Maniar RM, Mascha E, Reisman SS. Is spectral analysis of the surface electromyographic signal a clinically useful tool for evaluation of skeletal muscle fatigue? J Clin Neurophysiol. 1998;15:138–145. doi: 10.1097/00004691-199803000-00006. [DOI] [PubMed] [Google Scholar]