Abstract
Objective: To present guidelines for the recognition, prophylaxis, and management of asthma that lead to improvement in the quality of care certified athletic trainers and other heath care providers can offer to athletes with asthma, especially exercise-induced asthma.
Background: Many athletes have difficulty breathing during or after athletic events and practices. Although a wide variety of conditions can predispose an athlete to breathing difficulties, the most common cause is undiagnosed or uncontrolled asthma. At least 15% to 25% of athletes may have signs and symptoms suggestive of asthma, including exercise-induced asthma. Athletic trainers are in a unique position to recognize breathing difficulties caused by undiagnosed or uncontrolled asthma, particularly when asthma follows exercise. Once the diagnosis of asthma is made, the athletic trainer should play a pivotal role in supervising therapies to prevent and control asthma symptoms. It is also important for the athletic trainer to recognize when asthma is not the underlying cause for respiratory difficulties, so that the athlete can be evaluated and treated properly.
Recommendations: The recommendations contained in this position statement describe a structured approach for the diagnosis and management of asthma in an exercising population. Athletic trainers should be educated to recognize asthma symptoms in order to identify patients who might benefit from better management and should understand the management of asthma, especially exercise-induced asthma, to participate as active members of the asthma care team.
Keywords: airway hyperresponsiveness, airway obstruction, exercise-induced asthma, exercise-induced bronchospasm, pulmonary function tests, certified athletic trainer
INTRODUCTION
Asthma is defined as a chronic inflammatory disorder of the airways characterized by variable airway obstruction and bronchial hyperresponsiveness.1 Airway obstruction can lead to recurrent episodes of wheezing, breathlessness, chest tightness, and coughing, particularly at night or in the early morning.1 Asthma can be triggered by many stimuli, including allergens (eg, pollen, dust mites, animal dander), pollutants (eg, carbon dioxide, smoke, ozone), respiratory infections, aspirin, nonsteroidal anti-inflammatory drugs (NSAIDs), inhaled irritants (eg, cigarette smoke, household cleaning fumes, chlorine in a swimming pool), particulate exposure (eg, ambient air pollutants, ice rink pollution), and exposure to cold and exercise.1–5 Airflow limitation is often reversible, but as asthma symptoms continue, patients may develop “airway remodeling” that leads to chronic irreversible airway obstruction.6,7 Severe attacks of asthma can also cause irreversible airflow obstruction that can lead to death.4,8
The National Heart, Lung, and Blood Institute of the National Institutes of Health launched the National Asthma Education and Prevention Program (NAEPP) in March 1989 to address the increasing prevalence of asthma in the United States and its economic costs to the society; the program was updated in 1997 (as NAEPPII).1 An updated expert panel report from the NAEPP is expected to be released in 2006. The Global Initiative for Asthma (GINA) was also developed to provide worldwide guidelines for asthma awareness and management.2 These guidelines are extremely comprehensive and have been regularly updated to reflect advances in the diagnosis and management of asthma. Nevertheless, the guidelines do not describe the role of certified athletic trainers or other allied health care professionals in recognizing and managing asthma in an athletic population.
PURPOSE
The purpose of this position statement is to provide athletic trainers and other allied health care professionals who care for athletes with information to:
Identify the characteristics and diagnostic criteria of asthma, especially exercise-induced asthma (EIA) or exercise-induced bronchospasm (EIB).
Provide guidelines for referral so that patients with asthma and those in whom asthma is suspected can receive a thorough evaluation.
Describe management plans to prevent attacks and to control asthma exacerbations when they occur.
Educate certified athletic trainers and athletes about pharmacologic and nonpharmacologic therapies and techniques to help control asthma.
RECOMMENDATIONS
Based on current research and literature, the National Athletic Trainers' Association provides the following guidelines for the identification, examination, management, and prophylaxis of asthma, including EIA, and the education of athletes, parents, coaches, and health care personnel about asthma. Not all individuals who suffer from asthma present in the same manner, nor do they all respond to the same management or treatment plan. Therefore, these recommendations are intended to provide the certified athletic trainer and other health care professionals with an overall guide for a better understanding of the asthmatic condition.
Asthma Identification and Diagnosis
1. All athletes must receive preparticipation screening evaluations sufficient to identify the possible presence of asthma.9–12 In most situations, this evaluation includes only obtaining a thorough history from the athlete. However, in special circumstances, additional screening evaluations (eg, spirometry testing or the challenge testing described below) should also be performed because the history alone is not reliable.10
- 2. Athletic trainers should be aware of the major signs and symptoms suggesting asthma, as well as the following associated conditions5,13,14:
- a. Chest tightness (or chest pain in children)
- b. Coughing (especially at night)
- c. Prolonged shortness of breath (dyspnea)
- d. Difficulty sleeping
- e. Wheezing (especially after exercise)
- f. Inability to catch one's breath
- g. Physical activities affected by breathing difficulty
- h. Use of accessory muscles to breathe
- i. Breathing difficulty upon awakening in the morning
- j. Breathing difficulty when exposed to certain allergens or irritants
- k. Exercise-induced symptoms, such as coughing or wheezing
- l. An athlete who is well conditioned but does not seem to be able to perform at a level comparable with other athletes who do not have asthma
- m. Family history of asthma
-
n. Personal history of atopy, including atopic dermatitis/ eczema or hay fever (allergic rhinitis)Note: Although there is a correlation between the presence of symptoms and EIA, the diagnosis should not be based on history alone.5 Rather, these symptoms should serve to suggest that an athlete may have asthma.
- 3. The following types of screening questions can be asked to seek evidence of asthma13:
- a. Does the patient have breathing attacks consisting of coughing, wheezing, chest tightness, or shortness of breath (dyspnea)?
- b. Does the patient have coughing, wheezing, chest tightness, or shortness of breath (dyspnea) at night?
- c. Does the patient have coughing, wheezing, or chest tightness after exercise?
- d. Does the patient have coughing, wheezing, chest tightness, or shortness of breath (dyspnea) after exposure to allergens or pollutants?
- e. Which pharmacologic treatments for asthma or allergic rhinitis, if any, were given in the past, and were they successful?
4. Patients with atypical symptoms, symptoms despite proper therapy, or other complications that can exacerbate asthma (such as sinusitis, nasal polyps, severe rhinitis, gastroesophageal reflux disease, or vocal cord dysfunction) should be referred to a physician with expertise in sports medicine (eg, allergist; ear, nose, and throat physician; cardiologist; or pulmonologist with training in providing care for athletes).15 Testing might include a stress electrocardiogram, upper airway laryngoscopy or rhinoscopy, echocardiogram, or upper endoscopy.
Pulmonary Function Testing
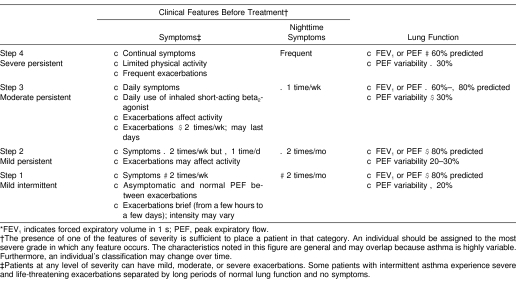
5. Athletes with a history of asthma or of taking a medication used to treat asthma and those suspected of having asthma should consult a physician for proper medical evaluation and to obtain a classification of asthma severity (Table 1). This evaluation should include pulmonary function testing.16–18
6. An exercise challenge test is recommended for athletes who have symptoms suggestive of EIA to confirm the diagnosis.19
7. If the diagnosis of asthma remains unclear after the above tests have been performed, then additional testing should be performed to assist in making a diagnosis.15,20,21 Physicians should be encouraged, when possible, to test the athlete using a sport-specific and environment-specific exercise-challenge protocol, in which the athlete participates in his or her venue to replicate the activity or activities and the environment that may serve to trigger airway hyperresponsiveness.20,21 In some cases, athletes should also be tested for metabolic gas exchange during strenuous exercise to determine fitness (eg, to assess anaerobic threshold and V̇o2max), especially to rule out the diagnosis of asthma or to rule in another diagnosis (eg, pulmonary fibrosis) for a patient with an unclear diagnosis.16
Table 1. National Asthma Education and Prevention Program II: Classification of Asthma Severity*1.
Asthma Management
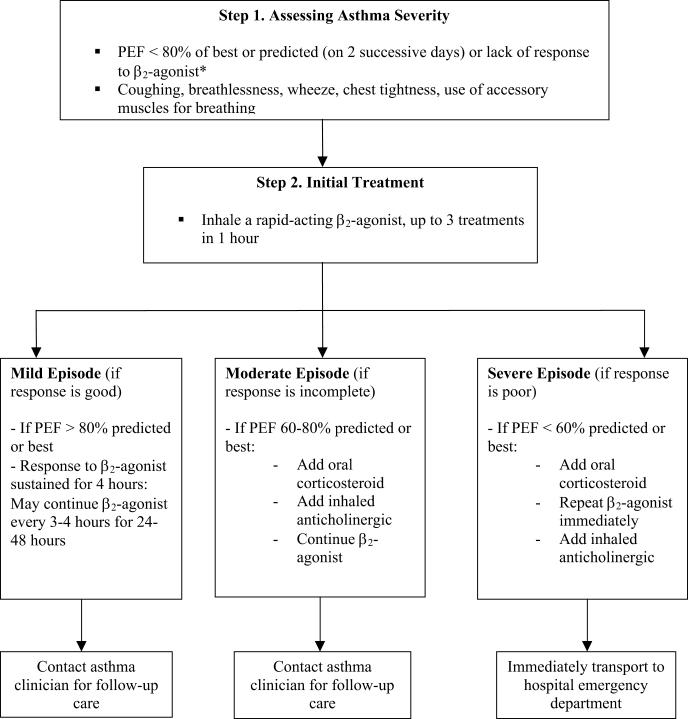
8. Athletic trainers should incorporate into the existing emergency action plan an asthma action plan for managing and urgently referring all patients who may experience significant or life-threatening attacks of breathing difficulties (Figure 1).1,2 Immediate access to emergency facilities during practices and game situations should be available. For example, athletic trainers should be familiar with appropriate community resources and must have a fully functional telephone (mobile or cellular) available, preprogrammed with emergency medical care access numbers. A telephone might be the single most important device to have on the practice field for a patient who is experiencing an asthma exacerbation. In addition, athletic trainers should have pulmonary function measuring devices (such as peak expiratory flow meters [PFMs] or portable spirometers) at all athletic venues for athletes for whom such devices have been prescribed and should be familiar with how to use these devices.22
9. Patients who are experiencing any degree of respiratory distress (including a significant increase in wheezing or chest tightness, a respiratory rate greater than 25 breaths per minute, inability to speak in full sentences, uncontrolled cough, significantly prolonged expiration phase of breathing, nasal flaring, or paradoxic abdominal movement) should be referred rapidly to an emergency department or to their personal physicians for further evaluation and treatment. Referral to an emergency room or equivalent facility should be sought urgently if the patient is exhibiting signs of impending respiratory failure (eg, weak respiratory efforts, weak breath sounds, unconsciousness, or hypoxic seizures).
10. All patients with asthma should have a rescue inhaler available during games and practices, and the certified athletic trainer should have an extra rescue inhaler for each athlete for administration during emergencies. In case of emergencies, a nebulizer should also be available. With a metered dose inhaler (MDI), athletes should be encouraged to use a spacer to help ensure the best delivery of inhaled therapy to the lungs.23
11. Athletic trainers and coaches should consider providing alternative practice sites for athletes with asthma triggered by airborne allergens when practical. Indoor practice facilities that offer good ventilation and air conditioning should be considered for at least part of the practice if this can be accomplished, although in most cases it will not be practical. For example, indoor and outdoor allergens or irritants, tobacco smoke, and air pollutants might trigger asthma, and attempts should be made to limit exposure to these triggers when possible. Another option is to schedule practices when pollen counts are lowest (eg, in the evening during the ragweed pollen season). Pollen count information can be accessed from the National Allergy Bureau at http://www.aaaai.org/nab.
12. Patients with asthma should have follow-up examinations at regular intervals, as determined by the patient's primary care physician or specialist, to monitor and alter therapy. In general, these evaluations should be scheduled at least every 6 to 12 months, but they may be more frequent if symptoms are not well controlled.
Figure 1. Sample asthma action plan.
Extracted from Managing Asthma: A Guide for Schools. National Heart, Lung, and Blood Institute. Available at: http://www.nhlbi.nih.gov/health/prof/lung/asthma/asth_sch.htm. Accessed June 7, 2005
Asthma Pharmacologic Treatment
13. Athletic trainers should understand the various types of pharmacologic strategies used for short- and long-term asthma control and should be able to differentiate controller from rescue or reliever medications (Figure 2).24–30
14. Patients with EIA may benefit from the use of short- and long-acting β2-agonists. Rapid-acting agents can be used for prophylaxis during practice and game participation. When the goal is to prevent EIA, a short-acting β2-agonist, such as albuterol, should be inhaled 10 to 15 minutes before exercise. The excessive need (3–4 times per day) for short-acting β2-agonist therapy during practice or an athletic event should cause concern, and a physician should evaluate the patient before return to participation. Long-acting β2-agonists should, in general, only be used for asthma prophylaxis and control and are usually combined with an inhaled corticosteroid. Athletic trainers should understand the use, misuse, and abuse of short-acting β2-agonists.
15. Patients with asthma may also benefit from the use of leukotriene modifiers, inhaled or parenteral corticosteroids, and cromones (such as cromolyn sodium).
16. Pharmacotherapy should be customized for each asthma patient, and a specialist (an allergist or pulmonologist with expertise in sports medicine) should be consulted to maximize therapy when symptoms break through despite apparently optimal therapy.
17. Patients with past allergic reactions or intolerance to aspirin or NSAIDs should be identified and provided with alternative medicines, such as acetaminophen, as needed.
Figure 2. Asthma pharmacologic management.2 PEF indicates peak expiratory flow.
Figure 1. Continued.
Asthma Nonpharmacologic Treatment
18. Health care providers should identify and consider nonpharmacologic strategies to control asthma, including nose breathing, limiting exposure to allergens or pollutants, and air filtration systems.31–33 However, these therapies should be expected to provide only limited protection from asthma in most circumstances.
19. Proper warm-up before exercise may lead to a refractory period of as long as 2 hours, which may result in decreased reliance on medications by some patients with asthma.34
20. Patients who have been diagnosed previously as having asthma or suspected of having asthma should follow the recommendations of NAEPPII and GINA for evaluation and everyday management and control.1,2
Asthma Education
- 21. Athletes should be properly educated about asthma, especially EIA, by health care professionals who are knowledgeable about asthma.35–52 Athletes should be educated about the following:
- a. Recognizing the signs and symptoms of uncontrolled asthma.
- b. Using spirometry recording devices to monitor lung function away from the clinic or athletic training room.
- c. Methods of limiting exposure to primary and secondary smoke and to other recognized or suspicious asthma triggers (eg, pollens, animal allergens, fungi, house dust, and other asthma sensitizers and triggers). Patients with asthma who smoke should be provided with information about smoking cessation and encouraged to participate in classes to change socialization patterns.
- d. The need for increased asthma rescue medication (eg, short-acting β2-agonists) as a signal for asthma flare-up. Increased use of short-acting β2-agonists signals a need for enhanced treatment with asthma controller therapy.
- e. The proper techniques for using MDIs, dry powder inhalers, nebulizers, and spacers to control asthma symptoms and to treat exacerbations. Health care professionals should periodically check the patient's medication administration techniques and should examine medication compliance.
- f. Asthma and EIA among competitive athletes. These conditions are common, and athletic performance need not be hindered if the patient takes an active role in controlling the disease and follows good practice and control measures.
22. The athletic trainer should also be familiar with vocal cord dysfunction and other upper airway diseases, which can sometimes be confused with asthma.15,53,54 Vocal cord dysfunction may be associated with dyspnea, chest tightness, coughing, wheezing, and inspiratory stridor. In many cases, the condition is triggered with exercise. Visual inspection of the vocal cords by a physician experienced in examining the upper airway during exercise to differentiate vocal cord dysfunction from asthma is recommended.
23. Patients with asthma should be encouraged to engage in exercise as a means to strengthen muscles, improve respiratory health, enhance endurance, and otherwise improve overall well-being.55
24. The athletic trainer should differentiate among restricted, banned, and permitted asthma medications. Athletic trainers should be familiar with the guidelines of the International Olympic Committee Medical Commission, the United States Anti-Doping Agency, the World Anti-Doping Agency, and the doping committees of the various federations.
25. The athletic trainer should be aware of the various Web sites that provide general information and frequently asked questions on asthma and EIA. These sites include the American Academy of Allergy, Asthma and Immunology (www.aaaai.org); the American Thoracic Society (www.thoracic.org); the Asthma and Allergy Foundation of America (www.aafa.org); the American College of Allergy, Asthma, & Immunology (www.acaai.org); and USA Swimming (http://www.usaswimming.org/USASWeb/_Rainbow/ Documents/6c812467-b717-4c16-a32c-a1d9bcc9f444/Asthma-%20Comprehensive%20Guide%2004%20Nov%2029.pdf).
BACKGROUND AND LITERATURE REVIEW
Definition and Pathophysiology of Asthma
Asthma is a common condition that has been recognized for more than 2000 years.56 Asthma is usually defined operationally as a chronic inflammatory disorder of the airways.1,2,4,56 In many patients, this chronic inflammation causes an increase in airway hyperresponsiveness, leading to recurrent episodes of wheezing, breathlessness, chest tightness (or chest pain in children), and coughing, particularly at night or in the early morning and after exercise, especially in cold, dry environments. These episodes are associated with widespread but variable airflow obstruction that is often reversible, either spontaneously or with treatment.1,2,4 This definition implies that asthma has multiple causes, and indeed, it is a complex disorder.
The chronic inflammatory process causes excess mucus production and bronchial smooth muscle constriction,57–61 which result from a release of inflammatory mediators that include histamine, tryptase, prostaglandin, and leukotrienes from mast cells.62–66 Airways may also accumulate thick, viscous secretions produced by goblet cells and submucosal glands; moreover, there is leakage of plasma proteins and accumulated cellular debris.57–61,67 Although airway narrowing affects the tracheobronchial tree, small bronchi (2–5 mm in diameter) are most affected.68–70 Maximal expiratory flow rate is reduced and residual lung volumes are increased as air is trapped behind the blocked airways.71 As a result, during an asthma attack, the respiratory rate increases to compensate for the increased obstruction of the airways and the inability of the usually elastic lung to recoil (dynamic hyperinflation). The patient must work harder to breathe as the thorax becomes overinflated. With progression of the attack, the diaphragm and intercostal muscles must compensate and contribute more energy during respiration.72 In a severe attack, muscle efficiency is eventually lost, and the increased breathing rate leads to respiratory muscle fatigue and physical distress that may result in death. Indeed, as many as 4200 to 5000 people die from asthma each year in the United States.73
Environmental Factors
Environmental factors, such as allergens, air pollution, occupational sensitizers, and tobacco smoke, may cause or exacerbate asthma.74–79 These factors are important triggers that should be considered when evaluating a patient with asthma.79–83
Recently, concern about indoor air pollution has been heightened.79,80,82–84 Indoor ambient air can contain allergens and pollution that can cause or exacerbate asthma and other respiratory ailments when susceptible individuals are exposed to these environments.85 Many factors should be considered indoors, such as adequacy of ventilation, humidity level, presence of allergens, presence of wall-to-wall carpeting and upholstered furniture, and types of building materials.85–87 Indoor animal allergens (such as from cats, dogs, and other pets) are an important trigger of symptoms in many people.88 Cockroach allergens are commonly seen indoors (such as in swimming pool locker rooms), and the cockroach allergenic materials can remain for a long period of time, even after extermination.89–92 Indoor mold and fungal spores, house dust mites, and particulate matter, such as aerosols or smoke from cigarettes, wood, or fossil fuels, also can trigger asthma symptoms.93–107 Tobacco smoke is a risk factor for the development of asthma, and smoking tobacco appears to increase asthma severity.108–110
Other indoor air irritants, such as chlorine, can exacerbate asthma and cause eye and lung irritation.111,112 School-age children who frequently visit chlorinated pools may have an increased risk of developing asthma, especially if they have other risk factors for asthma.113 Even individuals who do not usually enter the water but who are exposed to indoor chlorinated pools (eg, lifeguards, coaches) may have respiratory irritation on exposure to chlorine.114,115
Many actions can be taken to limit indoor allergen exposure, including prohibiting smoking indoors, using air cleaners equipped with a high-efficiency particulate air cleaner (HEPA) filter, washing walls, vacuuming carpets, and cleaning mattress covers weekly.116–119 Additional measures include removing carpets and installing linoleum or wood flooring, washing pets (dogs and cats) and their bedding twice a week, keeping pets out of the bedroom or living room at all times to reduce exposure, covering mattresses and pillows, and controlling humidity to help manage dust mites and mold.81,82,120 Some of these measures, such as the removal of a family pet from the home, can be very difficult, so it is necessary to discuss the effect of these exposures with the asthma patient. Although air filters might help, the house should be cleaned thoroughly before their use and regularly thereafter.33
When inhaled, outdoor air pollution, caused by sulfur dioxide, carbon monoxide, nitrogen dioxide, ozone, and particulate matter, can cause pulmonary function decrements, increased reliance on medications, bronchial hyperresponsiveness, and increased asthma symptoms.121–125 Many pollens (trees, grasses, and weeds) are inhaled into the bronchi and cause allergen-induced asthma.126,127 Tree pollens predominate in the spring, grass pollens in the late spring or early summer (and fall), and weed pollens in the late summer and fall in the United States, depending on geographic location, but may be present at other times of the year in locations outside of the United States.128,129 Information about the pollen seasons in the United States can be accessed from the National Allergy Bureau Web site.
Although it is virtually impossible to avoid all outdoor pollution and allergens, some practical precautions can be implemented: move indoors and close all windows, close car windows when traveling, limit exposure when pollen is at its highest levels, monitor local weather stations for allergy reports, and practice indoors if possible when pollen counts are high.
Diagnosis and Classification of Asthma
Asthma can be difficult to diagnose and classify (see Table 1). Some individuals, especially elite athletes, do not display consistent signs or symptoms of asthma.9,10,130 Asthma symptoms may be present only during certain times (seasons) of the year or only after exercise and may be highly variable, depending upon the athlete, the environment, and the activity.13
The first step in determining whether asthma is present is to obtain a detailed medical history. Questions regarding past experiences, symptoms, smoking history, and family history can help to rule out other respiratory disorders such as chronic bronchitis, emphysema, bronchiectasis, allergic rhinitis, upper respiratory infection, congestive heart failure, disorders of the upper airway (eg, vocal cord dysfunction), and nonrespiratory conditions such as anxiety. Most importantly, the athletic trainer should ask general questions as listed in Recommendation 3 to assist in making a proper diagnosis.13 If a patient appears to have one or more symptoms suggestive of asthma, then lung function testing should be performed (see Table 1).9 However, it is important to recognize that the history and baseline physical examination will fail to identify many patients with EIA.9,10,12
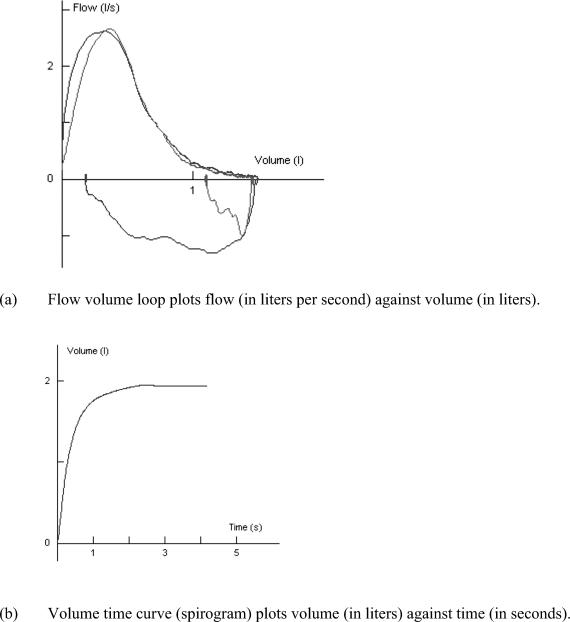
Lung function tests are essential to assess asthma severity and airflow limitation and to determine whether the obstruction is fully reversible with treatment.16–18,131–133 The most common measures of airway function are the forced expiratory volume in 1 second (FEV1), the forced vital capacity (FVC), and the peak expiratory flow rate (PEFR). These tests can be performed while the patient is at rest or after a challenge. The FEV1 measures the volume of air in liters forcefully exhaled out of the airway in 1 second after a full inspiration. The FVC measures the total volume of air in liters forcefully exhaled out of the airway when the breath continues (usually for a period of 6 or more seconds). The FVC procedure is effort dependent and requires the patient to fully understand that he or she needs to inhale a deep breath and then “blast” the air out of the lungs into the measurement device. The FVC testing also requires considerable expertise by the technician and the ability to communicate with the patient. The PEFR measures the maximal flow rate of air (in L/s or L/min) out of the airways and is easier to perform than an FEV1 or FVC maneuver, although PEFR testing is also effort dependent. A flow volume curve (flow loop) provides a graphic depiction of the breathing effort in which flow rate is plotted against volume (in L/s) of air exhaled and inhaled (in L), as shown in Figure 3. A volume-time curve (called a spirogram) is another way to plot the breath, in which the volume of air exhaled (in L) is plotted against time (in seconds). The FEV1/FVC ratio is also examined, along with a variety of measures of flow, such as the flow between 25% and 75% of the FVC (called the FEF25–75).
Figure 3. Flow volume loop and volume time curve.
Asthma is an example of an obstructive lung disease in which the airways obstruct the outflow of air. In contrast, pulmonary fibrosis is an example of a restrictive lung disease in which the functional size of the lungs decreases. In obstructive lung diseases, the FEV1 decreases, whereas the FVC remains relatively normal, so the FEV1/FVC ratio decreases (until late in the disease or with severe exacerbations, when the FVC may also decrease).131 In restrictive lung disease, both the FEV1 and the FVC decrease proportionally (so that the FEV1/ FVC ratio is normal). Nomograms exist to provide guidance as to normal ranges for FEV1 and FVC based on age, size and race.134,135 It is important to recognize racial differences in the normal values for these tests. Generally, levels should be at least 80% of the predicted values to be considered “normal.” The FEV1/FVC ratio should also be above 80%. An increase of 12% or more in FEV1 after an inhaled bronchodilator (eg, a β2-agonist such as albuterol) suggests reversible airway disease and may be used as a diagnostic criterion of asthma.131 A decrease in FEV1 after a challenge (such as after inhalation of methacholine or running on a treadmill) suggests that the airways are reactive to the stimulus.19 It is important to determine a patient's personal best FEV1, FVC, and PEFR, which are identified by plotting these values over time. These values can also demonstrate variability between morning and evening and over time, which may reflect airway hyperreactivity.
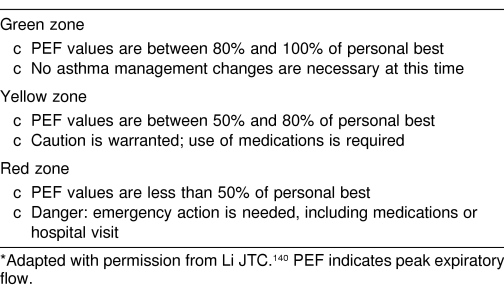
Spirometers are used in the clinic or training room to determine these pulmonary function values.136 Patients may also be given a PFM to measure PEFR away from the clinic or athletic training room (Tables 2 and 3). The PFM is a small, handheld device that measures maximal flow rate during forced exhalation.137–140 Maximal flow rate usually occurs within the first 120 to 150 milliseconds of a forced exhalation.140 When used properly, a PFM can be somewhat helpful in following the course of asthma and might even be useful in suggesting the presence of asthma. The PFM can also be used to identify asthma triggers and to monitor medication changes, and it may help to reduce asthma morbidity.141–147 The device allows the asthmatic patient to measure lung variability over time to assist in determining when to seek medical attention. The PFM should be used at least daily (in the morning after awakening) and preferably also in the late afternoon or evening.1,2,148 At least 2 or 3 trials should be performed at each specified time and the highest value recorded. The device should be used before taking any medications and at least 4, but preferably 8, hours after inhalation of a bronchodilator, if possible. The device should be used for at least a 2- to 3-week monitoring period. Some devices record the PEFR electronically, which can assist the patient in keeping the data secure and available. Over time, the patient's personal best value will be determined. Subsequently, if the PEFR value is less than 80% of the personal best or if daily variability is greater than 20% of the morning value, then the patient should be reevaluated in an attempt to find better control measures. If the PEFR value is less than 50% of the personal best, the individual should seek immediate attention (see Table 2).1 Spirometry, including the use of PFMs, may be especially useful in patients who do not perceive the severity of their symptoms.149,150
Table 2. Peak Flow Zones for Asthma Management*.
Table 3. General Instructions for Using a Mechanical Peak Flow Meter*.
The PFM should be used regularly, even if asthma symptoms appear to be well controlled.132,151–153 Patients who have good PFM technique (see Table 3) adhere to their treatment plan and control their symptoms better than those who have poor technique. The PFM should be available in all athletic training facilities and on the field in medical kits.
Unfortunately, some reports suggest that PFM recordings are not always reliable indicators of airflow obstruction.154–159 In certain cases, elite or well-trained athletes may possess large lung capacities, which may exceed the measuring capacity of the PFM. In addition, it appears that PFM values are not consistent from one to another PFM from the same manufacturer, across different PFM devices, when men and women use PFMs, with different techniques while using a single PFM, and at high altitudes.159–164 The most accurate spirometry testing is performed with office-based spirometry testing equipment.140 However, as noted above, spirometry testing requires training22,165 and may be impractical for everyday asthma management, especially in primary care settings in which most patients are not being seen for asthma.140
If baseline lung function tests are within normal values and the reversibility test with a β2-agonist is equivocal, then a challenge test (eg, with methacholine) may be performed to test for bronchial hyperresponsiveness.19 During these tests, progressively increasing concentrations of the aerosolized drug are administered during a multistage procedure.19 After each stage, spirometry testing is performed to determine whether a 20% reduction in FEV1 from baseline is obtained. If the reduction is less than 20% after all stages have been performed, then the test is considered negative, and the patient is determined not to have bronchial hyperresponsiveness. It is important to note, however, that these tests alone are not diagnostic of asthma. A positive test must be interpreted in the context of other information to make the definitive diagnosis of asthma.
Exercise challenge and other surrogate challenges (such as eucapnic hyperventilation) are described in the EIA section of this statement.19,21
Pharmacotherapy for Asthma
It is important to ascertain the correct diagnosis before medications are prescribed and for the health care professional to know the types of medications that are prescribed.1,2,24–30,166,167 Only a small percentage of asthmatic patients take their medications precisely as prescribed by their physician; the most common cause of treatment failure is failure to use the prescribed treatment.167 Regardless, asthma can be managed through various medications to prevent or control symptoms; for an updated medication list, refer to the NAEPPII and GINA guidelines.1,2 The medications used to treat asthma are classified as either controller or rescue (reliever) medications.
Controller Medications
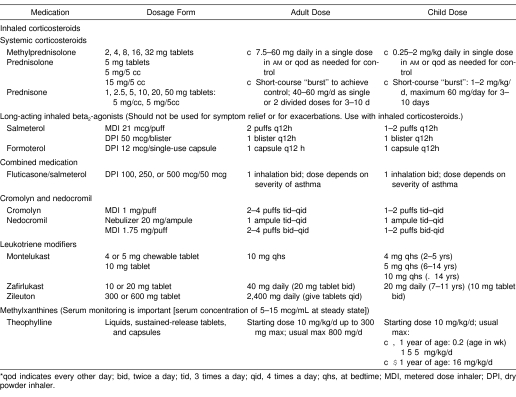
Controller medications are daily, long-term interventions used prophylactically to manage the symptoms of mild, moderate, and severe asthma and generally should not be used to manage acute asthmatic symptoms.1,2,25–28 Examples include inhaled corticosteroids, systemic corticosteroids, cromones (sodium cromolyn and nedocromil sodium), long-acting inhaled β2-agonists, theophylline, and leukotriene modifiers (Table 4 provides sample agents).
Table 4. National Asthma Education and Prevention Program II: Usual Dosages for Long-Term Control Medications*1.
Inhaled corticosteroids are effective controller medications for treating persistent mild, moderate, and severe asthma.1,2 They act by decreasing airway inflammation, mucus production, and bronchial hyperresponsiveness.168–173 Proper use of inhaled corticosteroids can lead to a decrease in the number and severity of asthma exacerbations, improve lung function, lessen bronchial hyperresponsiveness, and reduce the need for symptom relief with short-acting β2-agonists.174–180 Inhaled corticosteroids should not be used to treat acute asthmatic attacks.25 Their adverse effects include hoarseness, coughing, and occasionally thrush or oral candidiasis.177,181 However, rinsing the mouth after inhalation and using a spacer generally help to prevent oral candidiasis.
Systemic corticosteroids are administered orally or parenterally for individuals who have severe persistent asthma that remains poorly responsive to inhaler therapy.182 Systemic therapy has the same mechanisms of action as inhaled corticosteroids. However, long-term use of systemic agents can cause more significant systemic adverse events than inhaled corticosteroids, including osteoporosis, glucose intolerance, glaucoma, weight gain, skin thinning, bruising, fluid and electrolyte abnormalities, growth suppression, and muscle weakness.1,2,183 To minimize these adverse events, oral corticosteroids are often taken daily in the morning or every other day, and patients should be monitored closely by a physician.183 Oral corticosteroids are used much less frequently today than in the past since the advent of high-potency inhaled corticosteroids such as fluticasone and budesonide.1,2
The cromones, cromolyn sodium and nedocromil sodium, are inhaled asthma medications used to control mild persistent asthma and are considered less effective anti-inflammatory agents than inhaled corticosteroids.25,26,184 Although the exact mechanisms of action are poorly understood, they are thought to inhibit IgE-mediated mediator release from mast cells and, thus, to inhibit acute airflow limitations induced by exercise, cold air, and allergens. The cromones are generally used to treat mild persistent asthma and to prevent EIA.1,2 Cromones should be used as second-line drug therapy alternatives for treating mild persistent asthma, perhaps combined with an inhaled β2-agonist; however, several doses each day are usually needed to control asthma.1,2 Only minimal adverse events are seen with these agents, including occasional coughing and an unpleasant taste, particularly with nedocromil sodium.
Long-acting inhaled β2-agonists (eg, salmeterol and formoterol) have the same mechanisms of action as short-acting β2-agonists but a 12-hour duration of action, compared with 4 to 6 hours for the short-acting agents.185–190 A single dose of formoterol or salmeterol before exercise can protect the athlete from asthma symptoms associated with exercise for up to 12 hours.191–193 Formoterol has a shorter onset of action than salmeterol, approximately 5 minutes as compared with 15 to 30 minutes.192,194 All β2-agonists, including formoterol and salmeterol, are restricted asthma medications according to the International Olympic Committee, World Anti-Doping Agency, and United States Anti-Doping Agency; elite athletes taking these medications, their physicians, and their athletic trainers should review the guidelines posted by these agencies.
Patients using long-acting β2-agonists regularly may display a decrease in the duration of action.195–197 In one study, formoterol had a shortened duration of action by day 14 of regular daily use.198 Thus, patients should not expect these drugs to remain effective over the 12-hour dosing interval after regular, daily, extended use. Studies also show that the use of long-acting β2-agonists does not affect persistent airway inflammation.199 These agents should only be used in combination with inhaled corticosteroids, which may be more beneficial than ingesting each drug separately.200–202 Combination therapy (an inhaled, long-acting β2-agonist and an inhaled corticosteroid) has been shown to decrease the need for short-acting β2-agonists, decrease nocturnal asthma, improve lung function, decrease asthma exacerbations, and prevent EIA.200,202–205
Leukotriene modifiers, taken orally, block leukotriene synthesis or block leukotriene receptors.206–208 Leukotriene modifiers can be used to control allergen-, aspirin-, and exercise- induced bronchoconstriction; improve lung function; and decrease asthma exacerbations.209–215 Used primarily as second-line therapy, leukotriene modifiers can reduce the dose of an inhaled corticosteroid required to treat mild persistent asthma and may improve asthma control.214–217 Adverse events are usually minimal, with reports of headaches and gastrointestinal discomfort. However, zileuton (Zyflo, Abbott Laboratories, Abbott Park, IL) may be associated with liver toxicity; therefore, liver function should be monitored regularly when using this medication.218 Unlike β2-agonists, the duration of action of the leukotriene modifiers does not diminish over time.215 In the past, theophylline has been used alone as a controller agent, but now it is usually used in combination with another agent, such as an inhaled corticosteroid.219
Finally, some patients who have allergic asthma may benefit over the long term from the administration of various forms of allergy immunotherapy.220–222 The decision to initiate such therapy must be made by the patient and physician after a careful evaluation. Even in the most successful cases, additional medical therapy is often required in conjunction with immunotherapy.
Rescue (Reliever) Medications
Rescue medications act rapidly to treat acute bronchoconstriction and associated symptoms of coughing, wheezing, shortness of breath (dyspnea), and chest tightness.1,2,25–28 Several classes of drugs act in this manner, including rapid-acting inhaled β2-agonists, inhaled anticholinergics, and short-acting theophylline.
Rapid-acting inhaled β2-agonists are the most commonly used reliever therapy for chronic asthma. These β2-agonists act quickly to cause bronchodilation by relaxing airway smooth muscle, decreasing vascular permeability, and modifying mediator release from mast cells.185 Rapid-acting inhaled β2-agonists are also the most frequently used agents to prevent EIA and to treat its symptoms.223 These medications can be used as “rescue therapy” at times of an acute attack. However, because of their relatively short duration of action (2–4 hours), repeat treatments may be necessary for EIA. Some authors224,225 suggest that repeated use of short-acting β2-agonists can lead to tolerance and less effectiveness over time. Furthermore, the chronic use of long-acting inhaled β2-agonists can decrease the effectiveness of the short-acting inhaled β2-agonists.189,225
Inhaled anticholinergic agents (eg, ipratropium) may be used as bronchodilators.226 These agents block acetylcholine release from cholinergic innervation in airway smooth muscle but have no anti-inflammatory action.
Short-acting theophylline is a bronchodilator that has been used for many years to relieve asthma exacerbations.29,227 Theophylline's onset of action is delayed when compared with that of β2-agonists, and so it is not currently used as first-line rescue therapy.1,2 Theophylline is a controversial drug because its benefits might be outweighed by potential adverse events such as seizures.228 Adverse events can be serious and severe if dosing is not closely monitored. Short-acting theophylline should not be administered to patients who are already receiving chronic therapy with sustained-release theophylline therapy.
Short-acting oral β2-agonists, although rarely used, function primarily by relaxing airway smooth muscle within a few minutes after administration and for a period of up to 4 hours; however, they have no anti-inflammatory actions.24,29 Adverse events include tachycardia, hypertension, and decreased appetite. If used chronically, increasing doses of short-acting oral β2-agonists might indicate loss of control of asthma.1,2 All athletes using short-acting oral β2-agonists should be advised that many sporting organizations restrict or prohibit the use of these agents.
Finally, systemic glucocorticosteriods are administered orally or parenterally for individuals who have acute asthma exacerbations. The mechanisms of action are similar to those for corticosteroids used to treat chronic severe asthma.1,2
Asthma Medication Delivery Techniques
Many of the newer asthma medications are delivered to the lungs by inhalation devices.23 The most common types of inhalers are MDIs and dry powder inhaler devices. The MDIs release a specific amount of a drug from a pressurized canister to propel medication into the lungs when the patient takes a breath. When using MDIs, patients must exhale first, then place the inhaler at or slightly in front of the lips, and slowly inhale at the same time that they are activating the inhaler to release the drug. Patients hold their breath for a few seconds (approximately 10) before exhaling. Patients who have difficulty coordinating MDI activation with breathing generally benefit from the use of a spacer.229–231 A spacer is attached to the MDI device to reduce side effects of inhaled corticosteroids in the mouth and for patients who have difficulty coordinating the activation of an MDI and breathing. Dry powder inhalers are often easier to use than MDIs, and they do not permit use of a spacer.
Aspirin, Nonsteroidal Anti-Inflammatory Drugs, and Asthma
Aspirin-sensitive athletes may manifest nasal congestion; itchy, watery, or swollen eyes; coughing; difficulty breathing; wheezing; urticaria; and possible shock when they ingest aspirin or other NSAIDs.232–236 This is not a true allergy because it is not caused by IgE, but it is treated in the same manner as an allergic reaction. Athletic trainers should also be aware of triad syndrome: athletes with asthma, nasal polyps, and aspirin sensitivity may have a severe asthma attack when they take an NSAID.237
Although only a small percentage of the population has aspirin-sensitive asthma,238–240 this condition is particularly concerning in an athletic population because many athletes who have asthma use anti-inflammatory drugs to treat injuries. Therefore, athletic trainers must understand that some patients who have asthma could suffer fatal consequences if they take aspirin or NSAIDs.
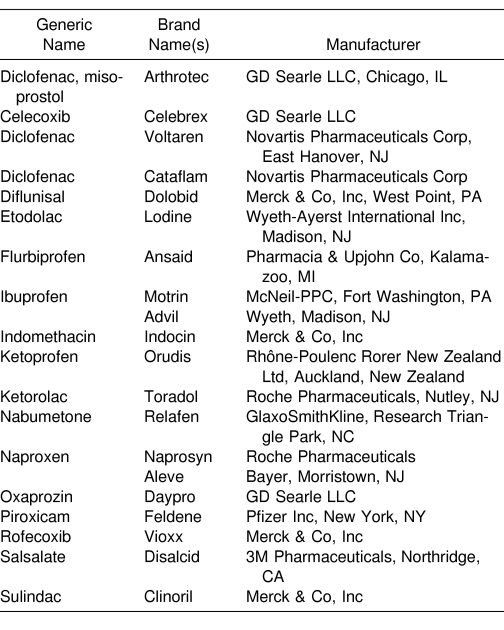
Aspirin-sensitive athletes should also avoid COX-2 inhibitors, but acetaminophen in moderate doses can usually be taken without difficulty.241 Salsalate, choline magnesium trisalicylate, and dextropropoxyphene may be used as substitute medication in patients with aspirin sensitivity if tolerated.240,242 Athletic trainers should be familiar with the many prescription and over-the-counter products that contain aspirin and other NSAIDs, including ibuprofen (eg, Motrin, McNeil-PPC, Fort Washington, PA; Advil, Wyeth, Madison, NJ) and naproxen (eg, Aleve, Bayer, Morristown, NJ). Health care professionals should supply as much information to the patient as possible, including a list of products to avoid (Table 5).
Table 5. Sample Nonsteroidal Anti-Inflammatory Drugs.
Nonpharmacologic Treatment for Asthma
Athletes with asthma need to keep their asthma under optimal control to prevent exercise-induced breathing symptoms.31,32,243,244 Masks and nose breathing help to warm and moisturize inhaled air before it reaches the smaller airways. This may decrease the inflammatory reaction in the airways and thus decrease the frequency and intensity of EIA. These maneuvers are effective for some but not all athletes.224 Nose breathing is not effective at high ventilation rates. Limiting environmental exposures (eg, to cold air and pollen) may decrease symptoms in susceptible athletes; however, this may not be practical in some sports.
Theoretically, exercise training might decrease symptoms by conditioning the body to exercise, but research has not supported this theory.245,246 Nevertheless, an asthmatic individual should participate in exercise programs tailored to his or her capacity to perform.55
A refractory period can occur after exercise, when the airway response to exercise is inhibited for up to 2 to 3 hours. Some athletes have taken advantage of this phenomenon to help control EIA.31,32,34,247–251 However, there are no specific guidelines to follow, and each athlete must experiment to determine the best individual protocol.
Because hyperosmolarity plays a role in mediating EIA, limiting sodium in the diet has received some attention. Restricting dietary salt may cause a relative decrease in airway obstruction.252,253 Both sodium and chloride appear to play roles, but this remains an area of active investigation, and no specific guidelines are available.254 A diet supplemented with n-3 polyunsaturated fatty acid in fish oil has shown favorable results in elite athletes with EIA.255
Exercise-Induced Asthma or Bronchospasm
Most asthmatic individuals have a flare of their asthma after exercise.256,257 Some individuals only have asthma signs and symptoms associated with exercise.13 By definition, a temporary narrowing of the airways (bronchospasm) induced by strenuous exercise in which the patient has no symptoms is known as EIB.13 When symptoms are present, EIB is described as EIA.13 This section reviews the incidence of EIA and EIB in the athletic population and considers special diagnostic or therapeutic measures that should be taken in an athletic population.
Exercise-induced asthma is commonly seen in athletes in all levels of athletic competition.5,9,10,31,243,244,258–269 In most patients who have chronic asthma (at least 80%), exercise is a trigger for bronchoconstriction.13,243 Exercise-induced asthma can also occur in patients who do not otherwise have asthma, such as in about 40% of patients who suffer from allergic rhinitis in season.243,270,271 The incidence of EIA in the general population has been estimated to be between 12% and 15%.13,271 Rates as high as 23% have been reported in school-age children, and the incidence in athletes may also be this high.258,262,263,265 Exercise-induced asthma may be more common in urban environments than in rural areas.261 Other factors, such as high ozone levels, might also account for increased EIA rates.272
Exercise-induced asthma can be a significant disability for the athlete, especially in endurance sports.262,263,273 For example, EIA is relatively common in cross-country skiers, and some studies suggest that the cold air athletes breathe while cross-country skiing may provoke inflammation.274,275 Similarly, athletes who participate in swimming and long-distance running have a high incidence of asthma.262 Among Olympic athletes, asthma appears to be more common in those who participate in winter sports than in those who participate in summer sports.262,263 At least 1 in 5 United States athletes who participated in the 1998 Winter Olympics had the condition, compared with 1 in 6 at the 1996 Summer Olympic Games.262,263 Wilber et al265 found a 23% overall incidence of EIA among athletes in the 7 winter sports tested. In addition, more females than males participating in the Winter Games reported an asthma condition or medication use.262,263,265 Of the winter sports athletes tested, females had an incidence of 26%, compared with 18% in males.265
Although EIA impairs performance, it can be overcome. Amy Van Dyken, an athlete who suffered from relatively severe asthma, won 4 gold medals in swimming in the 1996 Olympic Games.273 Other well-known elite athletes have also been able to excel when their asthma was under good control.273
Pathophysiology of Exercise-Induced Asthma
Two major theories exist to explain EIA: the cooling/warming hypothesis and the drying hypothesis.31,256,257,260,276–287 As ventilation increases, airways progressively cool, which results in bronchoconstriction. This theory is supported by the higher incidence of EIA in athletes participating in cold environments.263 In addition to cooling, the increased ventilation can lead to airway dehydration as inhaled air is humidified. The main effect of inhaling cold air is actually attributable to the fact that cold air carries less moisture. As with chronic asthma, inflammatory cells and mediators may increase in the lung in response to exercise in patients with EIA.248,279,288–290 Environmental allergens may enhance the likelihood of bronchoconstriction, and irritants such as sulfur dioxide, nitrogen dioxide, ozone, and chlorine have been implicated as causing patients to have exercise-induced symptoms.31,243,291
Exercise-Induced Asthma Diagnosis
Two requirements are needed to diagnose EIA: symptoms and obstructed airways, both associated with exercise.13,269
First, the patient has any of a constellation of symptoms associated with exercise, including shortness of breath (dyspnea), coughing, chest tightness (or chest pain in children), wheezing, and decreased exercise tolerance.13,262,263 Symptoms generally occur 5 to 8 minutes after sufficiently intense exercise starts. The EIA may be associated with specific sports as well as specific environments.262–267 Where allergens are present, outdoor activities and cold air exposure may be more likely to foster the appearance of EIA, which would not occur in other environments.
Second, the patient should have objective evidence of airway obstruction associated with exercise.224 Generally, a drop from baseline of at least 10% to 15% in FEV1 after a challenge test supports the diagnosis of EIA.19,292 Pulmonary function should be monitored 5, 10, 15, and 30 minutes after the challenge.19 The exercise needs to last for 6 to 8 minutes at an intensity level high enough to raise the athlete's heart rate to at least 80% of maximum19 and ventilation to approximately 40% to 60% of maximum.19 Exercise challenges can be performed in a laboratory (using a treadmill, a cycle ergometer, a rowing machine, or a free running asthma screening test [FRAST]).293 Alternatively, an exercise challenge test in the laboratory can attempt to mimic the conditions and intensity of the sport.20,21 Indeed, 78% of cross-country skiers reported a false-negative test during standard laboratory exercise challenges, suggesting that the standard tests are not as sensitive as sport-specific exercise challenge tests for endurance athletes.20,21 Cold, dry air and near-maximal exercise intensity (greater than 90% peak heart rate) are required to provoke a positive result, especially in the cold-weather athlete. Time of day can be important: in a group of asthmatics, a greater drop in pulmonary function (FEV1) to exercise challenge was measured in the evening than in the morning.294
Additional challenge tests include eucapnic voluntary hyperventilation or inhalation of hypertonic saline.21,269,295 The former test requires the athlete to hyperventilate dry air containing 5% carbon dioxide, 21% oxygen, and the balance of nitrogen at 30 times FEV1 for 6 minutes.21
It is important to evaluate athletes with atypical EIA symptoms because upper airway conditions such as vocal cord dysfunction or abnormal movement of the arytenoids region may be the cause.15,53 The signs and symptoms of vocal cord dysfunction can be similar to asthma and can be confused with EIA.54,296,297 This laryngeal disorder involves the unintentional paradoxic adduction of the vocal cords with breathing and can be triggered by exercise.296,297 The patient is often female and may also have gastroesophageal reflux disease or a psychiatric illness. Vocal cord dysfunction often occurs with asthma, making control of EIA difficult. Diagnosis of vocal cord dysfunction involves the direct visualization of the paradoxic vocal cord motion, but the condition is often suspected when voice changes and inspiratory stridor occur during an attack, as well as when the inspiratory (bottom) portion of the flow volume loop is truncated.
Exercise-Induced Asthma Treatment
Most of the drugs described for the treatment of chronic asthma are used to prevent EIA attacks.1,2,269,298–300 Table 6 contains recommendations from the NAEPPII for the treatment of EIA.1 The key feature is that a β2-agonist can be used both to prevent attacks and to treat them when they occur. Once an asthmatic individual meets the requirements for stage 1 through 4 asthma, the NAEPPII treatment guidelines should be followed.
Table 6. National Asthma Education and Prevention Program II: Treatment of Exercise-Induced Asthma*1.
Asthma Education
Throughout this position statement, information has been presented to inform and educate the athletic trainer and allied health personnel about asthma and asthma management. Of particular importance is a properly prepared asthma management plan. Educating athletes about asthma and having a written management plan will help control their disease.1,2,304 Several groups35–44,48–51 have shown that an effectively written management plan can reduce medication errors, asthma exacerbations, and hospital visits. Without a written asthma action plan, many patients have a difficult time controlling their asthma symptoms.45–47,305 It is also imperative that an accessible line of communication between the patient and health care professional be identified.
An effective management plan should include a written document that addresses the following: (1) goals of the patient, (2) proper use and frequency of PEFR monitoring, (3) guidelines for altering medications based upon readings from PFMs or asthma symptoms, (4) contact numbers for all health care professionals, including emergency numbers, and (5) environmental factors to avoid or monitor. The health care professionals developing the asthma management plan should discuss all goals or expectations with the athlete. This education empowers the athlete and promotes better compliance.
Athletic trainers working with elite or Olympic athletes must be familiar with International Olympic Committee, World Anti-Doping Agency, and United States Anti-Doping Agency medication guidelines (Table 7). Certain asthma medications may be banned, restricted, or permitted, depending on the organization and the medication. A banned medication is one the athlete cannot take. In some cases, a prohibited substance is prohibited at all times or only prohibited in competition, meaning the athlete must allow sufficient time for the substance to clear the system before competition. Restricted medications must have prior physician approval and completion of forms (such as the Therapeutic Use Exemption, or TUE) before the athlete can compete. For example, the United States Anti-Doping Agency lists salbutamol/albuterol, salmeterol, terbutaline, and formoterol as restricted β2-agonists that require a TUE before competition.
Table 7. United States Anti-Doping Agency Regulated Asthma Medications.
Additional information about the diagnosis and management of asthma can be obtained at the National Asthma Education and Prevention Program Web site (http://www.nhlbi.nih.gov/ health/prof/lung/index.htm or http://www.nhlbi.nih.gov/about/ naepp/) and the GINA Web site (www.ginasthma.com).
CONCLUSIONS
Asthma can affect individuals regardless of age, sex, or socioeconomic status. This National Athletic Trainers' Association asthma position statement is designed to present guidelines for the recognition, prophylaxis, and management of asthma. The information should lead to improvements in the quality of care certified athletic trainers and other heath care providers can offer to patients with asthma, particularly athletes and especially those with EIA. In the end, these guidelines should reduce the incidence of asthma complications and improve the quality of life for patients with asthma, especially those in whom exercise is an important trigger.
DISCLAIMER
The NATA publishes its position statements as a service to promote the awareness of certain issues to its members. The information contained in the position statement is neither exhaustive not exclusive to all circumstances or individuals. Variables such as institutional human resource guidelines, state or federal statutes, rules, or regulations, as well as regional environmental conditions, may impact the relevance and implementation of these recommendations. The NATA advises its members and others to carefully and independently consider each of the recommendations (including the applicability of same to any particular circumstance or individual). The position statement should not be relied upon as an independent basis for care but rather as a resource available to NATA members or others. Moreover, no opinion is expressed herein regarding the quality of care that adheres to or differs from NATA's position statements. The NATA reserves the right to rescind or modify its position statements at any time.
Figure 1. Continued.
Acknowledgments
We gratefully acknowledge the efforts of Michael C. Koester, MD, ATC; James L. Moeller, MD, FACSM; Kenneth W. Rundell, PhD; Chad Starkey, PhD, ATC; Randall L. Wilber, PhD; and the Pronouncements Committee in the preparation of this document.
REFERENCES
- National Asthma Education and Prevention Program. Expert Panel Report II: Guidelines for the Diagnosis and Management of Asthma. Bethesda, MD: National Institutes of Health; 1997. Publication No. 97- 4051:12–18.
- Global Strategy for Asthma Management and Prevention. Bethesda, MD: National Institutes of Health, National Heart, Lung, and Blood Institute; 2002. NIH publication No. 02-3659.
- W Nystad. Asthma. Int J Sports Med. 2000;21:S-98–S-102. (suppl) [Google Scholar]
- Balatbat JH. Asthma: an overview from prevalence to plan. J Contin Educ Top Issues. 2002;2:80–92. [Google Scholar]
- Rundell KW, Im J, Mayers LB, Wilber RL, Szmedra L, Schmitz HR. Self-reported symptoms and exercise-induced asthma in the elite athlete. Med Sci Sports Exerc. 2001;33:208–213. doi: 10.1097/00005768-200102000-00006. [DOI] [PubMed] [Google Scholar]
- Tiddens H, Silverman M, Bush A. The role of inflammation in airway disease: remodeling. Am J Respir Crit Care Med. 2000;162:S-7–S-10. doi: 10.1164/ajrccm.162.supplement_1.maic-2. (2, pt 2) [DOI] [PubMed] [Google Scholar]
- Bousquet J, Jeffery PK, Busse WW, Johnson M, Vignola AM. Asthma: from bronchoconstriction to airways inflammation and remodeling. Am J Respir Crit Care Med. 2000;161:1720–1745. doi: 10.1164/ajrccm.161.5.9903102. [DOI] [PubMed] [Google Scholar]
- Wenzel S. Severe asthma in adults. Am J Respir Crit Care Med. 2005;172:149–160. doi: 10.1164/rccm.200409-1181PP. [DOI] [PubMed] [Google Scholar]
- Rupp NT, Guill NF, Brudno DS. Unrecognized exercise-induced bronchospasm in adolescent athletes. Am J Dis Child. 1992;146:941–944. doi: 10.1001/archpedi.1992.02160200063028. [DOI] [PubMed] [Google Scholar]
- Rupp NT, Brudno DS, Guill MF. The value of screening for risk of exercise-induced asthma in high school athletes. Ann Allergy. 1993;70:339–342. [PubMed] [Google Scholar]
- Hammerman SI, Becker JM, Rogers J, Quedenfeld TC, D'Alonzo GE., Jr. Asthma screening of high school athletes: identifying the undiagnosed and poorly controlled. Ann Allergy Asthma Immunol. 2002;88:380–384. doi: 10.1016/S1081-1206(10)62368-X. [DOI] [PubMed] [Google Scholar]
- Hallstrand TS, Curtis JR, Koepsell TD. Effectiveness of screening examinations to detect unrecognized exercise-induced bronchoconstriction. J Pediatr. 2002;141:343–348. doi: 10.1067/mpd.2002.125729. et al. [DOI] [PubMed] [Google Scholar]
- Weiler JM. Exercise-induced asthma: a practical guide to definitions, diagnosis, prevalence, and treatment. Allergy Asthma Proc. 1996;17:315–325. doi: 10.2500/108854196778606437. [DOI] [PubMed] [Google Scholar]
- Lacroix VJ. Exercise-induced asthma. Physician Sportsmed. 1999;27(12):75–91. doi: 10.3810/psm.1999.11.1106. [DOI] [PubMed] [Google Scholar]
- Abu-Hasan M, Tannous B, Weinberger M. Exercise-induced dyspnea in children and adolescents: if not asthma then what? Ann Allergy Asthma Immunol. 2005;94:366–371. doi: 10.1016/S1081-1206(10)60989-1. [DOI] [PubMed] [Google Scholar]
- West JB. Respiratory Physiology: The Essentials. Philadelphia, PA: Lippincott Williams & Wilkins; 2000.
- Kanner RE, Morris AH. Clinical Pulmonary Function Testing: A Manual of Uniform Laboratory Procedures. eds. Salt Lake City, UT: Intermountain Thoracic Society; 1975.
- Petty TL. Simple office spirometry. Clin Chest Med. 2001;22:845–859. doi: 10.1016/s0272-5231(05)70070-8. [DOI] [PubMed] [Google Scholar]
- Crapo RO, Casaburi R, Coates AL. Guidelines for methacholine and exercise challenge testing—1999. Am J Respir Crit Care Med. 2000;161:309–329. doi: 10.1164/ajrccm.161.1.ats11-99. et al. [DOI] [PubMed] [Google Scholar]
- Rundell KW, Wilber RL, Szmedra L, Jenkinson DM, Mayers LB, Im J. Exercise-induced asthma screening of elite athletes: field versus laboratory exercise challenge. Med Sci Sports Exerc. 2000;32:309–316. doi: 10.1097/00005768-200002000-00010. [DOI] [PubMed] [Google Scholar]
- Rundell KW, Anderson SD, Spiering BA, Judelson DA. Field exercise vs laboratory eucapnic voluntary hyperventilation to identify airway hyperresponsiveness in elite cold weather athletes. Chest. 2004;125:909–915. doi: 10.1378/chest.125.3.909. [DOI] [PubMed] [Google Scholar]
- Peak flow meters and spirometers in general practice. Drug Ther Bull. 1997;35:52–55. doi: 10.1136/dtb.1997.35752. [DOI] [PubMed] [Google Scholar]
- Meadows-Oliver M, Banasiak NC. Asthma medication delivery devices. J Pediatr Health Care. 2005;19:121–123. doi: 10.1016/j.pedhc.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Im J. Asthma. RxConsultant. 2003;12:1–8. [Google Scholar]
- Baker VO, Friedman J, Schmitt R. Asthma management, part II: pharmacologic management. J Sch Nurs. 2002;18:257–269. doi: 10.1177/10598405020180050501. [DOI] [PubMed] [Google Scholar]
- Self TH, Kelly HW. Asthma. In: Young LY, Koda-Kimble MA, eds. Applied Therapeutics: The Clinical Use of Drugs. 6th ed. Vancouver, WA: Applied Therapeutics, Inc; 1995:19-1–19-31.
- Drugs for asthma. Med Lett Drugs Ther. 1999;41:5–10. [PubMed] [Google Scholar]
- Szilagyi PG, Kemper KJ. Management of chronic childhood asthma in the primary care office. Pediatr Ann. 1999;28:43–52. doi: 10.3928/0090-4481-19990101-10. [DOI] [PubMed] [Google Scholar]
- Sherman J, Hendeles L. Practical pharmacology for pediatric asthma. Pediatr Ann. 2000;29:768–773. doi: 10.3928/0090-4481-20001201-11. [DOI] [PubMed] [Google Scholar]
- Houglum JE. Asthma medications: basic pharmacology and use in the athlete. J Athl Train. 2000;35:179–187. [PMC free article] [PubMed] [Google Scholar]
- Hough DO, Dec KL. Exercise-induced asthma and anaphylaxis. Sports Med. 1994;18:162–172. doi: 10.2165/00007256-199418030-00003. [DOI] [PubMed] [Google Scholar]
- Gong H., Jr. Breathing easy: exercise despite asthma. Physician Sportsmed. 1992;20(3):158–167. doi: 10.1080/00913847.1992.11710254. [DOI] [PubMed] [Google Scholar]
- McDonald E, Cook D, Newman T, Griffith L, Cox G, Guyatt G. Effect of air filtration systems on asthma: a systematic review of randomized trials. Chest. 2002;122:535–1542. doi: 10.1378/chest.122.5.1535. [DOI] [PubMed] [Google Scholar]
- Reiff DB, Choudry NB, Pride NB, Ind PW. The effect of prolonged submaximal warm-up exercise on exercise-induced asthma. Am Rev Respir Dis. 1989;139:479–484. doi: 10.1164/ajrccm/139.2.479. [DOI] [PubMed] [Google Scholar]
- Turner MO, Taylor D, Bennett R, Fitzgerald JM. A randomized trial comparing peak expiratory flow and symptom self-management plans for patients with asthma attending a primary care clinic. Am J Respir Crit Care Med. 1998;157:540–546. doi: 10.1164/ajrccm.157.2.9703060. [DOI] [PubMed] [Google Scholar]
- Kolbe J, Vamos M, James F, Elkind G, Garrett J. Assessment of practical knowledge of self-management of acute asthma. Chest. 1996;109:86–90. doi: 10.1378/chest.109.1.86. [DOI] [PubMed] [Google Scholar]
- Lieu TA, Quesenberry CP, Jr., Capra AM, Sorel ME, Martin KE, Mendoza GR. Outpatient management practices associated with reduced risk of pediatric asthma hospitalization and emergency department visits. Pediatrics. 1997;100:334–341. doi: 10.1542/peds.100.3.334. (3, pt 1) [DOI] [PubMed] [Google Scholar]
- Gibson PG, Wlodarczyk J, Hensley MJ, Murree-Allen K, Olson LG, Saltos N. Using quality-control analysis of peak expiratory flow recordings to guide therapy for asthma. Ann Intern Med. 1995;123:488–492. doi: 10.7326/0003-4819-123-7-199510010-00002. [DOI] [PubMed] [Google Scholar]
- Boggs PB, Hayati F, Washburne WF, Wheeler DA. Using statistical process control charts for the continual improvement of asthma care. Jt Comm J Qual Improv. 1999;25:163–181. doi: 10.1016/s1070-3241(16)30436-9. [DOI] [PubMed] [Google Scholar]
- Allen RM, Jones MP, Oldenburg B. Randomised trial of an asthma self-management programme for adults. Thorax. 1995;50:731–738. doi: 10.1136/thx.50.7.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey WC, Richards JM, Jr, Brooks CM, Soong SJ, Windsor RA, Manzella BA. A randomized trial to improve self-management practices of adults with asthma. Arch Intern Med. 1990;150:1664–1668. [PubMed] [Google Scholar]
- Hilton S, Sibbald B, Anderson HR, Freeling P. Controlled evaluation of the effects of patient education on asthma morbidity in general practice. Lancet. 1986;1:26–29. doi: 10.1016/s0140-6736(86)91904-5. [DOI] [PubMed] [Google Scholar]
- Yoon R, McKenzie DK, Bauman A, Miles DA. Controlled trial evaluation of an asthma education programme for adults. Thorax. 1993;48:1110–1116. doi: 10.1136/thx.48.11.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SR, Scamagas P, German DF. A controlled trial of two forms of self-management education for adults with asthma. Am J Med. 1993;94:564–576. doi: 10.1016/0002-9343(93)90206-5. et al. [DOI] [PubMed] [Google Scholar]
- Beilby JJ, Wakefield MA, Ruffin RE. Reported use of asthma management plans in South Australia. Med J Aust. 1997;166:298–301. doi: 10.5694/j.1326-5377.1997.tb122317.x. [DOI] [PubMed] [Google Scholar]
- Dales RE, Kerr PE, Schweitzer I, Reesor K, Gougeon L, Dickinson G. Asthma management preceding an emergency department visit. Arch Intern Med. 1992;152:2041–2044. [PubMed] [Google Scholar]
- Scarfone RJ, Zorc JJ, Capraro GA. Patient self-management of acute asthma: adherence to national guidelines a decade later. Pediatrics. 2001;108:1332–1338. doi: 10.1542/peds.108.6.1332. [DOI] [PubMed] [Google Scholar]
- Adams RJ, Boath K, Homan S, Campbell DA, Ruffin RE. A randomized trial of peak-flow and symptom-based action plans in adults with moderate-to-severe asthma. Respirology. 2001;6:297–304. doi: 10.1046/j.1440-1843.2001.00350.x. [DOI] [PubMed] [Google Scholar]
- Myers TR. Improving patient outcomes with tools for asthma self-monitoring: a review of the literature. Dis Manag Health Outcomes. 2002;10:631–642. [Google Scholar]
- Clark NM, Partridge MR. Strengthening asthma education to enhance disease control. Chest. 2002;121:1661–1669. doi: 10.1378/chest.121.5.1661. [DOI] [PubMed] [Google Scholar]
- Lawrence G. Asthma self-management programs can reduce the need for hospital-based asthma care. Respir Care. 1995;40:39–43. [PubMed] [Google Scholar]
- Partridge MR. Delivering optimal care to the person with asthma: what are the key components and what do we mean by patient education? Eur Respir J. 1995;8:298–305. doi: 10.1183/09031936.95.08020298. [DOI] [PubMed] [Google Scholar]
- Bittleman DB, Smith RJH, Weiler JM. Abnormal movment of the arytenoid region during exercise presenting as exercise-induced asthma in an adolescent athlete. Chest. 1994;106:615–616. doi: 10.1378/chest.106.2.615. [DOI] [PubMed] [Google Scholar]
- McFadden ER, Jr, Zawadski DK. Vocal cord dysfunction masquerading as exercise-induced asthma: a physiologic cause for “choking” during athletic activities. Am J Respir Crit Care Med. 1996;153:942–947. doi: 10.1164/ajrccm.153.3.8630577. [DOI] [PubMed] [Google Scholar]
- Clark CJ, Cochrane LM. Physical activity and asthma. Curr Opin Pulm Med. 1999;5:68–75. doi: 10.1097/00063198-199901000-00012. [DOI] [PubMed] [Google Scholar]
- Guthrie CM, Tingen MS. Asthma: a case study, review of pathophysiology, and management strategies. J Am Acad Nurse Pract. 2002;14:457–461. doi: 10.1111/j.1745-7599.2002.tb00076.x. [DOI] [PubMed] [Google Scholar]
- Wiggs BR, Bosken C, Paré PD, James A, Hogg JC. A model of airway narrowing in asthma and in chronic obstructive pulmonary disease. Am Rev Respir Dis. 1992;145:1251–1258. doi: 10.1164/ajrccm/145.6.1251. [DOI] [PubMed] [Google Scholar]
- Aikawa T, Shimura S, Sasaki H, Ebina M, Takishima T. Marked goblet cell hyperplasia with mucus accumulation in the airways of patients who died of severe acute asthma attack. Chest. 1992;101:916–921. doi: 10.1378/chest.101.4.916. [DOI] [PubMed] [Google Scholar]
- Shimura S, Andoh Y, Haraguchi M, Shirato K. Continuity of airway goblet cells and intraluminal mucus in the airways of patients with bronchial asthma. Eur Respir J. 1996;9:1395–1401. doi: 10.1183/09031936.96.09071395. [DOI] [PubMed] [Google Scholar]
- Huber HL, Koessler KL. The pathology of bronchial asthma. Arch Intern Med. 1922;30:689–760. [Google Scholar]
- Dunnill MS. The pathology of asthma, with special reference to changes in the bronchial mucosa. J Clin Pathol. 1960;13:27–33. doi: 10.1136/jcp.13.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel SE, Westcott JY, Smith HR, Larsen GL. Spectrum of prostanoid release after bronchoalveolar allergen challenge in atopic asthmatics and in control groups: an alteration in the ratio of bronchoconstrictive to bronchoprotective mediators. Am Rev Respir Dis. 1989;139:450–457. doi: 10.1164/ajrccm/139.2.450. [DOI] [PubMed] [Google Scholar]
- Persson CG. Role of plasma exudation in asthmatic airways. Lancet. 1986;2:1126–1129. doi: 10.1016/s0140-6736(86)90533-7. [DOI] [PubMed] [Google Scholar]
- Djukanović R, Wilson JW, Britten KM. Quantitation of mast cells and eosinophils in the bronchial mucosa of symptomatic atopic asthmatics and healthy control subjects using immunohistochemistry. Am Rev Respir Dis. 1990;142:863–871. doi: 10.1164/ajrccm/142.4.863. et al. [DOI] [PubMed] [Google Scholar]
- Koshino T, Arai Y, Miyamoto Y. Mast cell and basophil number in the airway correlate with the bronchial responsiveness of asthmatics. Int Arch Allergy Immunol. 1995;107:378–379. doi: 10.1159/000237042. et al. [DOI] [PubMed] [Google Scholar]
- O'Byrne PM. Airway inflammation and asthma. Aliment Pharmacol Ther. 1996;10:18–24. doi: 10.1046/j.1365-2036.1996.22164016.x. (suppl 2) [DOI] [PubMed] [Google Scholar]
- Hays SR, Fahy JV. The role of mucus in fatal asthma. Am J Med. 2003;115:68–69. doi: 10.1016/s0002-9343(03)00260-2. [DOI] [PubMed] [Google Scholar]
- Kessler GF, Austin JH, Graf PD, Gamsu G, Gold WM. Airway constriction in experimental asthma in dogs: tantalum bronchographic studies. J Appl Physiol. 1973;35:703–708. doi: 10.1152/jappl.1973.35.5.703. [DOI] [PubMed] [Google Scholar]
- Carroll N, Elliot J, Morton A, James A. The structure of large and small airways in nonfatal and fatal asthma. Am Rev Respir Dis. 1993;147:405–410. doi: 10.1164/ajrccm/147.2.405. [DOI] [PubMed] [Google Scholar]
- Awadh N, Müller NL, Park CS, Abboud RT, FitzGerald JM. Airway wall thickness in patients with near fatal asthma and control groups: assessment with high resolution computed tomographic scanning. Thorax. 1998;53:248–253. doi: 10.1136/thx.53.4.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden ER., Jr. Development, structure, and physiology in the normal lung and in asthma. In: Middleton E, Ellis EF, Adkinson NF Jr, Yunginger JW, Reed CH, Busse WW, eds. Allergy Principles and Practice. St. Louis, MO: Mosby; 1998:508–519.
- McFadden ER, Jr, Kiser R, DeGroot WJ. Acute bronchial asthma: relations between clinical and physiologic manifestations. N Engl J Med. 1973;288:221–225. doi: 10.1056/NEJM197302012880501. [DOI] [PubMed] [Google Scholar]
- National Center for Health Statistics. NCHS Data on Asthma. Available at: http://www.cdc.gov/nchs/data/factsheets/asthma.pdf. Accessed June 6, 2005.
- D'Amato G, Liccardi G, Cazzola M. Environment and development of respiratory allergy, I: outdoors. Monaldi Arch Chest Dis. 1994;49:406–411. [PubMed] [Google Scholar]
- D'Amato G, Liccardi G, D'Amato M. Environment and development of respiratory allergy, II: indoors. Monaldi Arch Chest Dis. 1994;49:412–420. [PubMed] [Google Scholar]
- Steerenberg PA, Van Amsterdam JGC, Vandebriel BJ, Vos JG, Van Bree L, Van Loveren H. Environmental and lifestyle factors may act in concert to increase the prevalence of respiratory allergy including asthma. Clin Exp Allergy. 1999;29:1303–1308. doi: 10.1046/j.1365-2222.1999.00658.x. [DOI] [PubMed] [Google Scholar]
- Sunyer J, Antò JM, Kogevinas M. Risk factors for asthma in young adults: Spanish Group of the European Community Respiratory Health Survey. Eur Respir J. 1997;10:2490–2494. doi: 10.1183/09031936.97.10112490. et al. [DOI] [PubMed] [Google Scholar]
- Grant EN, Wagner R, Weiss KB. Observations on emerging patterns of asthma in our society. J Allergy Clin Immunol. 1999;104:S-1–S-9. doi: 10.1016/s0091-6749(99)70268-x. (2, pt 2) [DOI] [PubMed] [Google Scholar]
- Koenig JQ. Air pollution and asthma. J Allergy Clin Immunol. 1999;104:717–722. doi: 10.1016/s0091-6749(99)70280-0. (4, pt 1) [DOI] [PubMed] [Google Scholar]
- Custovic A, Simpson A, Woodcock A. Importance of indoor allergens in the induction of allergy and elicitation of allergic disease. Allergy. 1998;53:115–20. doi: 10.1111/j.1398-9995.1998.tb05011.x. (48 suppl) [DOI] [PubMed] [Google Scholar]
- Jones AP. Asthma and the home environment. J Asthma. 2000;37:103–124. doi: 10.3109/02770900009055434. [DOI] [PubMed] [Google Scholar]
- Platts-Mills TAE. Allergen avoidance. J Allergy Clin Immunol. 2004;113:388–391. doi: 10.1016/j.jaci.2003.12.027. [DOI] [PubMed] [Google Scholar]
- Platts-Mills TAE, Ward GW, Jr, Sporik R, Gelber LE, Chapman MD, Heymann PW. Epidemiology of the relationship between exposure to indoor allergens and asthma. Int Arch Allergy Appl Immunol. 1991;94:339–345. doi: 10.1159/000235398. [DOI] [PubMed] [Google Scholar]
- Gruchalla RS, Pongracic J, Plaut M. Inner City Asthma Study: relationships among sensitivity, allergen exposure, and asthma morbidity. J Allergy Clin Immunol. 2005;115:478–485. doi: 10.1016/j.jaci.2004.12.006. et al. [DOI] [PubMed] [Google Scholar]
- Platts-Mills TAE, Vervloet D, Thomas WR, Aalberse RC, Chapman MD. Indoor allergens and asthma: report of the Third International Workshop. J Allergy Clin Immunol. 1997;100:S-1–S-24. doi: 10.1016/s0091-6749(97)70292-6. [DOI] [PubMed] [Google Scholar]
- Hirsch T, Hering M, Bürkner K. House-dust-mite allergen concentrations (Der f 1) and mold spores in apartment bedrooms before and after installation of insulated windows and central heating systems. Allergy. 2000;55:79–83. doi: 10.1034/j.1398-9995.2000.00250.x. et al. [DOI] [PubMed] [Google Scholar]
- Schoenwetter WF. Building a healthy house. Ann Allergy Asthma Immunol. 1997;79:1–4. doi: 10.1016/S1081-1206(10)63077-3. [DOI] [PubMed] [Google Scholar]
- Woodfolk JA, Luczynska CH, de Blay F, Chapman MD, Platts-Mills TAE. Cat allergy. Ann Allergy. 1992;69:273–275. [PubMed] [Google Scholar]
- Sarpong SB, Karrison T. Season of birth and cockroach allergen sensitization in children with asthma. J Allergy Clin Immunol. 1998;101:566–568. doi: 10.1016/S0091-6749(98)70369-0. (4, pt 1) [DOI] [PubMed] [Google Scholar]
- Kuster PA. Reducing risk of house dust mite and cockroach allergen exposure in inner-city children with asthma. Pediatr Nurs. 1996;22:297–303. [PubMed] [Google Scholar]
- Kang BC, Wu CW, Johnson J. Characteristics and diagnosis of cockroach-sensitive bronchial asthma. Ann Allergy. 1992;68:237–244. [PubMed] [Google Scholar]
- Liccardi G, Cazzola M, D'Amato M, D'Amato G. Pets and cockroaches: two increasing causes of respiratory allergy in indoor environments. Characteristics of airways sensitization and prevention strategies. Respir Med. 2000;94:1109–1118. doi: 10.1053/rmed.2000.0922. [DOI] [PubMed] [Google Scholar]
- Platt SD, Martin CJ, Hunt SM, Lewis CW. Damp housing, mould growth, and symptomatic health state. BMJ. 1989;298:1673–1678. doi: 10.1136/bmj.298.6689.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter PC, Juritz J, Little F, McCaldin M, Dowdle EB. Clustering of fungal allergen-specific IgE antibody responses in allergic subjects. Ann Allergy. 1991;66:149–153. [PubMed] [Google Scholar]
- Henderson FW, Henry MM, Ivins SS. Correlates of recurrent wheezing in school-age children: The Physicians of Raleigh Pediatric Associates. Am J Respir Crit Care Med. 1995;151:1786–1793. doi: 10.1164/ajrccm.151.6.7767521. et al. [DOI] [PubMed] [Google Scholar]
- Gergen PJ, Turkeltaub PC. The association of individual allergen reactivity with respiratory disease in a national sample: data from the second National Health and Nutrition Examination Survey, 1976–80 (NHANES II) J Allergy Clin Immunol. 1992;90:579–588. doi: 10.1016/0091-6749(92)90130-t. (4, pt 1) [DOI] [PubMed] [Google Scholar]
- Duffy DL, Mitchell CA, Martin NG. Genetic and environmental risk factors for asthma: a cotwin-control study. Am J Respir Crit Care Med. 1998;157:840–845. doi: 10.1164/ajrccm.157.3.9702070. (3, pt 1) [DOI] [PubMed] [Google Scholar]
- Omenaas E, Bakke P, Eide GE, Elsayed S, Gulsvik A. Serum house-dust-mite antibodies and reduced FEV1 in adults of a Norwegian community. Am J Respir Crit Care Med. 1995;152:1158–1163. doi: 10.1164/ajrccm.152.4.7551364. (4, pt 1) [DOI] [PubMed] [Google Scholar]
- Omenaas E, Bakke P, Eide GE, Elsayed S, Gulsvik A. Serum house dust mite antibodies: predictor of increased bronchial responsiveness in adults of a community. Eur Respir J. 1996;9:919–925. doi: 10.1183/09031936.96.09050919. [DOI] [PubMed] [Google Scholar]
- Turner KJ, Stewart GA, Woolcock AJ, Green W, Alpers MP. Relationship between mite densities and the prevalence of asthma: comparative studies in two populations in the Eastern Highlands of Papua New Guinea. Clin Allergy. 1988;18:331–340. doi: 10.1111/j.1365-2222.1988.tb02880.x. [DOI] [PubMed] [Google Scholar]
- Vervloet D, Charpin D, Haddi E. Medication requirements and house dust mite exposure in mite-sensitive asthmatics. Allergy. 1991;46:554–558. doi: 10.1111/j.1398-9995.1991.tb00620.x. et al. [DOI] [PubMed] [Google Scholar]
- Tariq SM, Matthews SM, Hakim EA, Stevens M, Arshad SH, Hide DW. The prevalence of and risk factors for atopy in early childhood: a whole population birth cohort study. J Allergy Clin Immunol. 1998;101:587–593. doi: 10.1016/S0091-6749(98)70164-2. [DOI] [PubMed] [Google Scholar]
- Call RS, Smith TF, Morris E, Chapman MD, Platts-Mills TAE. Risk factors for asthma in inner city children. J Pediatr. 1992;121:862–866. doi: 10.1016/s0022-3476(05)80329-4. [DOI] [PubMed] [Google Scholar]
- Platts-Mills TAE, Woodfolk JA, Chapman MD, Heymann PW. Changing concepts of allergic disease: the attempt to keep up with real changes in lifestyles. J Allergy Clin Immunol. 1996;98:S-297–S-306. (6, pt 3) [PubMed] [Google Scholar]
- Forastiére F, Corbo GM, Michelozzi P. Effects of environment and passive smoking on the respiratory health in children. Int J Epidemiol. 1992;21:66–73. doi: 10.1093/ije/21.1.66. et al. [DOI] [PubMed] [Google Scholar]
- Gortmaker SL, Walker DK, Jacobs FH, Ruch-Ross H. Parental smoking and the risk of childhood asthma. Am J Public Health. 1982;72:574–579. doi: 10.2105/ajph.72.6.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig JQ, Larson TV, Hanley QS. Pulmonary function changes in children associated with fine particulate matter. Environ Res. 1993;63:26–38. doi: 10.1006/enrs.1993.1123. et al. [DOI] [PubMed] [Google Scholar]
- Murray B, Morrison BJ. The effect of cigarette smoke from the mother on bronchial responsiveness and severity of symptoms in children with asthma. J Allergy Clin Immunol. 1986;77:575–581. doi: 10.1016/0091-6749(86)90348-9. [DOI] [PubMed] [Google Scholar]
- Halken S, Host A, Nilsson L, Taudorf E. Passive smoking as a risk factor for development of obstructive respiratory disease and allergic sensitization. Allergy. 1995;50:97–105. doi: 10.1111/j.1398-9995.1995.tb05064.x. [DOI] [PubMed] [Google Scholar]
- Siroux V, Pin I, Oryszczyn MP, Le Moual N, Kauffmann F. Relationships of active smoking to asthma and asthma severity in the EGEA study: Epidemiological Study on the Genetics and Environment of Asthma. Eur Respir J. 2000;15:470–477. doi: 10.1034/j.1399-3003.2000.15.08.x. [DOI] [PubMed] [Google Scholar]
- Bar-Or O, Inbar O. Swimming and asthma: benefits and deleterious effects. Sports Med. 1992;14:397–405. doi: 10.2165/00007256-199214060-00006. [DOI] [PubMed] [Google Scholar]
- Massin N, Bohadana AB, Wild P, Hery M, Toamain JP, Hubert G. Respiratory symptoms and bronchial responsiveness in lifeguards exposed to nitrogen trichloride in indoor swimming pools. Occup Environ Med. 1998;55:258–263. doi: 10.1136/oem.55.4.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard A, Carbonnelle S, Michel O. Lung hyperpermeability and asthma prevalence in schoolchildren: unexpected associations with the attendance at indoor chlorinated swimming pools. Occup Environ Med. 2003;60:385–394. doi: 10.1136/oem.60.6.385. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thickett KM, McCoach JS, Gerber JM, Sadhra S, Burge PS. Occupational asthma caused by chloramines in indoor swimming-pool air. Eur Respir J. 2002;19:827–832. doi: 10.1183/09031936.02.00232802. [DOI] [PubMed] [Google Scholar]
- Nemery B, Hoet PHM, Nowak D. Indoor swimming pools, water chlorination and respiratory health. Eur Respir J. 2002;19:790–793. doi: 10.1183/09031936.02.00308602. [DOI] [PubMed] [Google Scholar]
- Zwemer RJ, Karibo J. Use of laminar control device as adjunct to standard environmental control measures in symptomatic asthmatic children. Ann Allergy. 1973;31:284–290. [PubMed] [Google Scholar]
- Villaveces JW, Rosengren H, Evans J. Use of a laminar air flow portable filter in asthmatic children. Ann Allergy. 1977;38:400–404. [PubMed] [Google Scholar]
- Kooistra JB, Pasch R, Reed CE. The effects of air cleaners on hay fever symptoms in air-conditioned homes. J Allergy Clin Immunol. 1978;61:315–319. doi: 10.1016/0091-6749(78)90053-2. [DOI] [PubMed] [Google Scholar]
- Wood RA, Mudd KE, Egglestone PA. The distribution of cat and dust mite allergens on wall surfaces. J Allergy Clin Immunol. 1992;89:126–130. doi: 10.1016/s0091-6749(05)80049-1. (1, pt 1) [DOI] [PubMed] [Google Scholar]
- Shirai T, Matsui T, Suzuki K, Chida K. Effect of pet removal on pet allergic asthma. Chest. 2005;127:1565–1571. doi: 10.1378/chest.127.5.1565. [DOI] [PubMed] [Google Scholar]
- Folinsbee LJ. Does nitrogen dioxide exposure increase airways responsiveness? Toxicol Ind Health. 1992;8:273–283. [PubMed] [Google Scholar]
- Pope CA, III, Dockery DW, Spengler JD, Raizenne ME. Respiratory health and PM10 pollution: a daily time series analysis. Am Rev Respir Dis. 1991;144:668–674. doi: 10.1164/ajrccm/144.3_Pt_1.668. (3, pt 1) [DOI] [PubMed] [Google Scholar]
- Delfino RJ, Coate BD, Zeiger RS, Seltzer JM, Street DH, Koutrakis P. Daily asthma severity in relation to personal ozone exposure and outdoor fungal spores. Am J Respir Crit Care Med. 1996;154:633–641. doi: 10.1164/ajrccm.154.3.8810598. (3, pt 1) [DOI] [PubMed] [Google Scholar]
- Delfino RJ, Zeiger RS, Seltzer JM, Street DH. Symptoms in pediatric asthmatics and air pollution: differences in effects by symptoms severity, anti-inflammatory medication use and particulate averaging time. Environ Health Perspect. 1998;106:751–761. doi: 10.1289/ehp.98106751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig JQ, Pierson WE, Horike M, Frank R. Effects of SO2 plus NaCl aerosol combined with moderate exercise on pulmonary function in asthmatic adolescents. Environ Res. 1981;25:340–348. doi: 10.1016/0013-9351(81)90036-0. [DOI] [PubMed] [Google Scholar]
- Suphioglu C, Singh MB, Taylor P. Mechanism of grass-pollen-induced asthma. Lancet. 1992;339:569–572. doi: 10.1016/0140-6736(92)90864-y. et al. [DOI] [PubMed] [Google Scholar]
- Helenius I, Haahtela T. Allergy and asthma in elite summer sport athletes. J Allergy Clin Immunol. 2000;106:444–452. doi: 10.1067/mai.2000.107749. [DOI] [PubMed] [Google Scholar]
- Solomon WR. Airborne pollen prevalence in the United States. In: Grammer LC, Greenberger PA, eds. Patterson's Allergic Diseases. Philadelphia, PA: Lippincott Williams & Wilkins; 2002:131–144.
- D'Amato G, Spieksma FT, Liccardi G. Pollen-related allergy in Europe. Allergy. 1998;53:567–578. doi: 10.1111/j.1398-9995.1998.tb03932.x. et al. [DOI] [PubMed] [Google Scholar]
- Rundell KW, Spiering BA, Evans TM, Baumann JM. Baseline lung function, exercise-induced bronchoconstriction, and asthma-like symptoms in elite women ice hockey players. Med Sci Sports Exerc. 2004;36:405–410. doi: 10.1249/01.mss.0000117118.77267.bf. [DOI] [PubMed] [Google Scholar]
- Lung function testing: selection of reference values and interpretative strategies. American Thoracic Society. Am Rev Respir Dis. 1991;144:1202–1218. doi: 10.1164/ajrccm/144.5.1202. [DOI] [PubMed] [Google Scholar]
- Ferguson GT, Enright PL, Buist AS, Higgins MW. Office spirometry for lung health assessment in adults: a consensus statement from the National Lung Health Education Program. Respir Care. 2000;45:513–530. [PubMed] [Google Scholar]
- Guidelines for the measurement of respiratory function: recommendations of the British Thoracic Society and the Association of Respiratory Technicians and Physiologists. Respir Med. 1994;88:165–194. [PubMed] [Google Scholar]
- Crapo RO, Morris AH, Gardner RM. Reference spirometric values using techniques and equipment that meet ATS recommendations. Am Rev Respir Dis. 1981;123:659–664. doi: 10.1164/arrd.1981.123.6.659. [DOI] [PubMed] [Google Scholar]
- Polgar G, Promadhat V. Pulmonary Function Testing in Children. Philadelphia, PA: WB Saunders; 1971.
- Standardization of spirometry: 1994 update. American Thoracic Society. Am Respir Crit Care Med. 1995;152:1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- Koyama H, Nishimura K, Ikeda A, Tsukino M, Izumi T. Comparison of four types of portable peak flow meters (Mini-Wright, Assess, Pulmo-graph and Wright Pocket meters) Respir Med. 1998;92:505–511. doi: 10.1016/s0954-6111(98)90299-2. [DOI] [PubMed] [Google Scholar]
- Kelly CA, Gibson GJ. Relation between FEV1 and peak expiratory flow in patients with chronic airflow obstruction. Thorax. 1988;43:335–336. doi: 10.1136/thx.43.4.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladebauche P. Peak flow meters: child's play. Office Nurse. 1996:22– 28.
- Li JTC. Do peak flow meters lead to better asthma control? J Respir Dis. 1995;16:381–398. [Google Scholar]
- Ignacio-Garcia JM, Gonzales-Santos P. Asthma self-management education program by home monitoring of peak expiratory flow. Am J Respir Crit Care Med. 1995;151:353–359. doi: 10.1164/ajrccm.151.2.7842191. [DOI] [PubMed] [Google Scholar]
- Measuring peak flow at home. Drug Ther Bull. 1991;29:87–88. [PubMed] [Google Scholar]
- Lahdensuo A, Haahtela T, Herrala J. Randomised comparison of cost effectiveness of guided self management and traditional treatment of asthma in Finland. BMJ. 1998;316:1138–1139. doi: 10.1136/bmj.316.7138.1138. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahdensuo A, Haahtela T, Herrala J. Randomised comparison of guided self management and traditional treatment of asthma over one year. BMJ. 1996;312:748–752. doi: 10.1136/bmj.312.7033.748. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner MH, Jacobs J. Improving asthma management with peak flow meters. Contemp Pediatr. 1997;14:111–119. [Google Scholar]
- Bheekie A, Syce JA, Weinberg EG. Peak expiratory flow rate and symptom self monitoring of asthma initiated from community pharmacies. J Clin Pharm Ther. 2001;26:287–296. doi: 10.1046/j.1365-2710.2001.00361.x. [DOI] [PubMed] [Google Scholar]
- Cowie RL, Revitt SG, Underwood MF, Field SK. The effect of a peak flow-based action plan in the prevention of exacerbations of asthma. Chest. 1997;112:1534–1538. doi: 10.1378/chest.112.6.1534. [DOI] [PubMed] [Google Scholar]
- Rachelefsky G, Fitzgerald S, Page D, Santamaria B. An update on the diagnosis and management of pediatric asthma: based on the National Heart, Lung, and Blood Institute expert panel report. Nurse Pract. 1993;18:51–62. [PubMed] [Google Scholar]
- Killian KJ, Watson R, Otis J, St Amand TA, O'Bryne PM. Symptom perception during acute bronchoconstriction. Am J Respir Crit Care Med. 2000;162:490–496. doi: 10.1164/ajrccm.162.2.9905079. (2, pt 1) [DOI] [PubMed] [Google Scholar]
- Kendrick AH, Higgs CMB, Whitfield MJ, Laszlo G. Accuracy of perception of severity of asthma: patients treated in general practice. BMJ. 1993;307:422–424. doi: 10.1136/bmj.307.6901.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klements EM. Monitoring peak flow rates as a health-promoting behavior in managing and improving asthma. Clin Excell Nurse Pract. 2001;5:147–151. doi: 10.1054/xc.2001.23122. [DOI] [PubMed] [Google Scholar]
- Malo JL, L'Archevêque J, Trudeau C, d'Aquino C, Cartier A. Should we monitor peak expiratory flow rates or record symptoms with a simple diary in the management of asthma? J Allergy Clin Immunol. 1993;91:702–709. doi: 10.1016/0091-6749(93)90189-m. [DOI] [PubMed] [Google Scholar]
- Verschelden P, Cartier A, L'Archevêque J, Trudeau C, Malo JL. Compliance with and accuracy of daily self-assessment of peak expiratory flows (PEF) in asthmatic subjects over a three month period. Eur Respir J. 1996;9:880–885. doi: 10.1183/09031936.96.09050880. [DOI] [PubMed] [Google Scholar]
- Sawyer G, Miles J, Lewis S, Fitzharris P, Pearce N, Beasley R. Classification of asthma severity: should the international guidelines be changed? Clin Exp Allergy. 1998;28:1565–1570. doi: 10.1046/j.1365-2222.1998.00451.x. [DOI] [PubMed] [Google Scholar]
- Eid N, Yandell B, Howell L, Eddy M, Sheikh S. Can peak expiratory flow predict airflow obstruction in children with asthma? Pediatrics. 2000;105:354–358. doi: 10.1542/peds.105.2.354. [DOI] [PubMed] [Google Scholar]
- Brand PL, Duiverman EJ, Waalkens HJ, van Essen-Zandvliet EE, Kerrebijn KF. Peak flow variation in childhood asthma: correlation with symptoms, airways obstruction, and hyperresponsiveness during long term treatment with inhaled corticosteroids: Dutch CNSLD Study. Thorax. 1999;54:103–107. doi: 10.1136/thx.54.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folgering H, vd Brink W, v Heeswijk O, v Herwaarden C. Eleven peak flow meters: a clinical evaluation. Eur Respir J. 1998;11:188–193. doi: 10.1183/09031936.98.11010188. [DOI] [PubMed] [Google Scholar]
- Jain P, Kavuru MS. Peak expiratory flow vs spirometry in a patient with asthma. Respir Care. 2000;45:969–970. [PubMed] [Google Scholar]
- Nolan S, Tolley E, Leeper K, Strayhorn Smith V, Self T. Peak expiratory flow associated with change in positioning of the instrument: comparison of five peak flow meters. J Asthma. 1999;36:291–294. doi: 10.3109/02770909909075413. [DOI] [PubMed] [Google Scholar]
- Connolly CK, Murthy NK, Tighe E, Alcock S, Harding T. Mini and Wright peak flow meters. Thorax. 1991;46:289P. [Google Scholar]
- Dickinson SA, Hitchins DJ, Miller MR. Accuracy of measurement of peak flow using different peak flow meters. Thorax. 1991;46:289P. [Google Scholar]
- Jain P, Kavuru MS. Peak expiratory flow vs spirometry in a patient with asthma. Respir Care. 2000;45:969–970. [PubMed] [Google Scholar]
- Gardner RM, Crapo RO, Jackson BR, Jensen RL. Evaluation of accuracy and reproducibility of peak flow meters at 1,400 m. Chest. 1992;101:948–952. doi: 10.1378/chest.101.4.948. [DOI] [PubMed] [Google Scholar]
- Chafin CC, Tolley E, George C. Are there gender differences in the use of peak flow meters? J Asthma. 2001;38:541–543. doi: 10.1081/jas-100107118. et al. [DOI] [PubMed] [Google Scholar]
- den Otter JJ, Knitel M, Akkermans RPM, Van Schayck CP, Folgering HTM, van Weel C. Spirometry in general practice: the performance of practice assistants scored by lung function technicians. Br J Gen Pract. 1997;47:41–42. [PMC free article] [PubMed] [Google Scholar]
- Flood-Page P, Barnes NC. What are the alternatives to increasing inhaled corticosteroids for the long term control of asthma? BioDrugs. 2001;15:185–198. doi: 10.2165/00063030-200115030-00005. [DOI] [PubMed] [Google Scholar]
- Spector SL, Kinsman R, Mawhinney H. Compliance of patients with asthma with an experimental aerosolized medication: implications for controlled clinical trials. J Allergy Clin Immunol. 1986;77:65–70. doi: 10.1016/0091-6749(86)90325-8. et al. (1, pt 1) [DOI] [PubMed] [Google Scholar]
- Jeffery PK, Godfrey RW, Ädelroth E, Nelson F, Rogers A, Johansson SA. Effects of treatment on airway inflammation and thickening of basement membrane reticular collagen in asthma: a quantitative light and electron microscopic study. Am Rev Respir Dis. 1992;145:890–899. doi: 10.1164/ajrccm/145.4_Pt_1.890. (4, pt 1) [DOI] [PubMed] [Google Scholar]
- Djukanović R, Wilson JW, Britten KM. Effect of an inhaled corticosteroid on airway inflammation and symptoms in asthma. Am Rev Respir Dis. 1992;145:669–674. doi: 10.1164/ajrccm/145.3.669. et al. [DOI] [PubMed] [Google Scholar]
- Laitinen LA, Laitinen A, Haahtela T. A comparative study of the effects of an inhaled corticosteroid, budesonide, and a β2-agonist, terbutaline, on airway inflammation in newly diagnosed asthma: a randomized, double-blind, parallel-group controlled trial. J Allergy Clin Immunol. 1992;90:32–42. doi: 10.1016/s0091-6749(06)80008-4. [DOI] [PubMed] [Google Scholar]
- van Essen-Zandvliet EE, Hughes MD, Waalkens HJ, Duiverman EJ, Pocock SJ, Kerrebijn KF. Effects of 22 months of treatment with inhaled corticosteroids and/or beta2-agonists on lung function, airway responsiveness, and symptoms in children with asthma: the Dutch Chronic Non-Specific Lung Disease Study Group. Am Rev Respir Dis. 1992;146:547–554. doi: 10.1164/ajrccm/146.3.547. [DOI] [PubMed] [Google Scholar]
- Munck A, Mendel DB, Smith LI, Orti E. Glucocorticoid receptors and actions. Am Rev Respir Dis. 1990;141:S-2–S-10. (2, pt 2) [PubMed] [Google Scholar]
- Schleimer RP. Effects of glucocorticosteroids on inflammatory cells relevant to their therapeutic applications in asthma. Am Rev Respir Dis. 1990;141:S-59–S-69. (2, pt 2) [PubMed] [Google Scholar]
- Shelhamer JH, Levine SJ, Wu T, Jacoby DB, Kaliner MA, Rennard SL. NIH conference: airway inflammation. Ann Intern Med. 1995;123:288–304. doi: 10.7326/0003-4819-123-4-199508150-00008. [DOI] [PubMed] [Google Scholar]
- Haahtela T, Järvinen M, Kava T. Comparison of a β2-agonist, terbutaline, with an inhaled corticosteroid, budesonide, in newly detected asthma. N Engl J Med. 1991;325:388–392. doi: 10.1056/NEJM199108083250603. et al. [DOI] [PubMed] [Google Scholar]
- Barnes PJ, Pedersen S, Busse WW. Efficacy and safety of inhaled corticosteroids. New developments. Am J Respir Crit Care Med. 1998;157:S-1–S-53. doi: 10.1164/ajrccm.157.3.157315. (3, pt 2) [DOI] [PubMed] [Google Scholar]
- Barnes PJ, Pedersen S. Efficacy and safety of inhaled corticosteroids in asthma: report of a workshop held in Eze, France, October 1992. Am Rev Respir Dis. 1993;148:S-1–S-26. doi: 10.1164/ajrccm/148.4_Pt_2.S1. (4, pt 2) [DOI] [PubMed] [Google Scholar]
- Long-term effects of budesonide or nedocromil in children with asthma: The Childhood Asthma Management Program Research Group. N Engl J Med. 2000;343:1054–1063. doi: 10.1056/NEJM200010123431501. [DOI] [PubMed] [Google Scholar]
- Greenberger PA, Tatum AJ, D'Epiro P. Asthma: acute and long-term concerns. Patient Care. 1999;15:154–174. [Google Scholar]
- Bleecker ER, Welch MJ, Weinstein SF. Low-dose inhaled fluticasone propionate versus oral zafirlukast in the treatment of persistent asthma. J Allergy Clin Immunol. 2000;105:1123–1129. doi: 10.1067/mai.2000.106043. et al. [DOI] [PubMed] [Google Scholar]
- Williamson IJ, Matusiewicz SP, Brown PH, Greening AP, Crompton GK. Frequency of voice problems and cough in patients using pressurized aerosol inhaled steroid preparations. Eur Respir J. 1995;8:590–592. [PubMed] [Google Scholar]
- Mash B, Bheekie A, Jones PW. Inhaled versus oral steroids for adults with chronic asthma (Cochrane Review) In: The Cochrane Library. Issue 4. Chichester, UK: John Wiley and Sons, Ltd: 2003.
- Dunlap NE, Fulmer JD. Corticosteroid therapy in asthma. Clin Chest Med. 1984;5:669–683. [PubMed] [Google Scholar]
- Serafin WE. Drugs used in the treatment of asthma. In: Hardman JG, Limbird LE, Molinoff PB, Ruddon RW, Gilman AG, eds. The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill; 1996:659–682.
- Nelson HS. β-adrenergic bronchodilators. N Engl J Med. 1995;333:499–506. doi: 10.1056/NEJM199508243330807. [DOI] [PubMed] [Google Scholar]
- Boulet LP. Long versus short-acting beta 2-agonists: implications for drug therapy. Drugs. 1994;47:207–222. doi: 10.2165/00003495-199447020-00001. [DOI] [PubMed] [Google Scholar]
- Pearlman DS, Chervinsky P, LaForce C. A comparison of salmeterol with albuterol in the treatment of mild-to-moderate asthma. N Engl J Med. 1992;327:1420–1425. doi: 10.1056/NEJM199211123272004. et al. [DOI] [PubMed] [Google Scholar]
- Kesten S, Chapman KR, Broder I. A three-month comparison of twice daily inhaled formoterol versus four times daily inhaled albuterol in the management of stable asthma. Am Rev Respir Dis. 1991;144:622–625. doi: 10.1164/ajrccm/144.3_Pt_1.622. et al. [DOI] [PubMed] [Google Scholar]
- Anderson SD, Brannan JD. Long-acting β2-adrenoceptor agonists and exercise-induced asthma: lessons to guide us in the future. Paediatr Drugs. 2004;6:161–175. doi: 10.2165/00148581-200406030-00003. [DOI] [PubMed] [Google Scholar]
- Wenzel SE, Lumry W, Manning M. Efficacy, safety, and effects on quality of life of salmeterol versus albuterol in patients with mild to moderate persistent asthma. Ann Allergy Asthma Immunol. 1998;80:463–470. doi: 10.1016/S1081-1206(10)63068-2. et al. [DOI] [PubMed] [Google Scholar]
- Selroos O. Formoterol used as needed: clinical effectiveness. Respir Med. 2001;95:17–20. doi: 10.1053/rmed.2001.1141. [DOI] [PubMed] [Google Scholar]
- Richter K, Jamicki S, Jorres RA, Magnussen H. Acute protection against exercise-induced bronchoconstriction by formoterol, salmeterol, and terbutaline. Eur Respir J. 2002;19:865–871. doi: 10.1183/09031936.02.00228502. (suppl B) [DOI] [PubMed] [Google Scholar]
- Bronsky EA, Ü Yegen, Yeh CM, Larsen LV, Della Cioppa G. Formoterol provides long-lasting protection against exercise-induced bronchospasm. Ann Allergy Asthma Immunol. 2002;89:417–412. doi: 10.1016/S1081-1206(10)62043-1. [DOI] [PubMed] [Google Scholar]
- Ferrari M, Segattini C, Zanon R. Comparison of the protective effect of formoterol and of salmerterol against exercise-induced bronchospasm when given immediately before a cycloergometric test. Respiration. 2002;69:509–512. doi: 10.1159/000066458. et al. [DOI] [PubMed] [Google Scholar]
- Hancox RJ, Subbarao P, Kamada D, Watson RM, Hargreave FE, Inman MD. Beta2-agonist tolerance and exercise-induced bronchospasm. Am J Respir Crit Care Med. 2002;165:1068–1070. doi: 10.1164/ajrccm.165.8.200111-091bc. [DOI] [PubMed] [Google Scholar]
- Ramage L, Lipworth BJ, Ingram CG, Cree IA, Dhillon DP. Reduced protection against exercise induced bronchoconstriction after chronic dosing with salmerterol. Respir Med. 1994;88:363–368. doi: 10.1016/0954-6111(94)90042-6. [DOI] [PubMed] [Google Scholar]
- Nelson JA, Strauss L, Skowronski M, Ciufo R, Novak R, McFadden ER., Jr. Effect of long-term salmeterol treatment on exercise-induced asthma. N Engl J Med. 1998;339:141–146. doi: 10.1056/NEJM199807163390301. [DOI] [PubMed] [Google Scholar]
- Garcia R, Guerra P, Feo F. Tachyphylaxis following regular use of formoterol in exercise-induced bronchospasm. J Investig Allergol Clin Immunol. 2001;11:176–182. et al. [PubMed] [Google Scholar]
- Gardiner PV, Ward C, Booth H, Allison A, Hendrick DJ, Walters EH. Effect of eight weeks of treatment with salmeterol on bronchoalveolar lavage inflammatory indices in asthmatics. Am J Respir Crit Care Med. 1994;150:1006–1011. doi: 10.1164/ajrccm.150.4.7921429. [DOI] [PubMed] [Google Scholar]
- Lemanske RF, Sorkness CA, Mauger EA. Inhaled corticosteroid reduction and elimination in patients with persistent asthma receiving salmeterol: a randomized controlled trial. JAMA. 2001;285:2594–2603. doi: 10.1001/jama.285.20.2594. et al. [DOI] [PubMed] [Google Scholar]
- Lazarus SC, Boushey HA, Fahy JV. Long-acting β2-agonist monotherapy vs continued therapy with inhaled corticosteroids in patients with persistent asthma: a randomized controlled trial. JAMA. 2001;285:2583–2593. doi: 10.1001/jama.285.20.2583. et al. [DOI] [PubMed] [Google Scholar]
- Weiler JM, Nathan RA, Rupp NT, Kalberg CJ, Emmett A, Dorinsky PM. Effect of fluticasone/salmeterol administered via a single device on exercise-induced bronchospasm in patients with persistent asthma. Ann Allergy Asthma Immunol. 2005;94:65–72. doi: 10.1016/S1081-1206(10)61288-4. [DOI] [PubMed] [Google Scholar]
- Shrewsbury S, Pyke S, Britton M. Meta-analysis of increased dose of inhaled steroid or addition of salmeterol in symptomatic asthma (MIASMA) BMJ. 2000;320:1368–1373. doi: 10.1136/bmj.320.7246.1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauwels RA, Löfdahl CG, Postma DS. Effect of inhaled formoterol and budesonide on exacerbations of asthma: Formoterol and Corticosteroids Establishing Therapy (FACET) International Study Group. N Engl J Med. 1997;337:1405–1411. doi: 10.1056/NEJM199711133372001. et al. [DOI] [PubMed] [Google Scholar]
- Holimon TD, Chafin CC, Self TH. Nocturnal asthma uncontrolled by inhaled corticosteroids: theophylline or long-acting beta2 agonists? Drugs. 2001;61:391–418. doi: 10.2165/00003495-200161030-00007. [DOI] [PubMed] [Google Scholar]
- Montelukast for persistent asthma. Med Lett Drugs Ther. 1998;40:71–73. [PubMed] [Google Scholar]
- Stempel DA. Leukotriene modifiers in the treatment of asthma. Respir Care. 1998;43:481–489. [Google Scholar]
- O'Byrne PM, Israel E, Drazen JM. Antileukotreines in the treatment of asthma. Ann Intern Med. 1997;127:472–480. doi: 10.7326/0003-4819-127-6-199709150-00009. [DOI] [PubMed] [Google Scholar]
- Lipworth BJ. Leukotriene-receptor antagonists. Lancet. 1999;353:57–62. doi: 10.1016/S0140-6736(98)09019-9. [DOI] [PubMed] [Google Scholar]
- Drazen JM, Israel E, O'Byrne PM. Treatment of asthma with drugs modifying the leukotriene pathway. N Engl J Med. 1999;340:197–206. doi: 10.1056/NEJM199901213400306. [DOI] [PubMed] [Google Scholar]
- Drazen JM. Asthma therapy with agents preventing leukotriene synthesis or action. Proc Assoc Am Physicians. 1999;111:547–559. doi: 10.1046/j.1525-1381.1999.t01-1-99242.x. [DOI] [PubMed] [Google Scholar]
- Dahlén B, Margolskee DJ, Zetterström O, Dahlén S. Effect of the leukotriene receptor antagonist MK-0679 on baseline pulmonary function in aspirin sensitive asthmatic subjects. Thorax. 1993;48:1205–1210. doi: 10.1136/thx.48.12.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes NC, Miller CJ. Effect of leukotriene receptor antagonist therapy on the risk of asthma exacerbations in patients with mild to moderate asthma: an integrated analysis of zafirlukast trials. Thorax. 2000;55:478–483. doi: 10.1136/thorax.55.6.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian Virchow J, Prasse A, I Naya, Summerton L, Harris A. Zafirlukast improves asthma control in patients receiving high-dose inhaled corticosteroids. Am J Respir Crit Care Med. 2000;162:578–585. doi: 10.1164/ajrccm.162.2.9905041. [DOI] [PubMed] [Google Scholar]
- Leff JA, Busse WW, Pearlman D. Montelukast, a leukotriene-receptor antagonist, for the treatment of mild asthma and exercise-induced bronchoconstriction. N Engl J Med. 1998;339:147–152. doi: 10.1056/NEJM199807163390302. et al. [DOI] [PubMed] [Google Scholar]
- Laviolette M, Malmstrom K, Lu S. Montelukast added to inhaled beclomethasone in treatment of asthma: Montelukast/Beclomethasone Additivity Group. Am J Respir Crit Care Med. 1999;160:1862–1868. doi: 10.1164/ajrccm.160.6.9803042. et al. [DOI] [PubMed] [Google Scholar]
- Löfdahl CG, Reiss TF, Leff JA. Randomised, placebo controlled trial of effect of a leukotriene receptor antagonist, montelukast, on tapering inhaled corticosteroids in asthmatic patients. BMJ. 1999;319:87–90. doi: 10.1136/bmj.319.7202.87. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi EM, Heasley BH, Chordia MD, Macdonald TL. In vitro metabolism of 2-acetylbenzothiophene: relevance to zileuton hepatotoxicity. Chem Res Toxicol. 2004;17:137–143. doi: 10.1021/tx0341409. [DOI] [PubMed] [Google Scholar]
- Ukena D, Harnest U, Sakalauskas R. Comparison of addition of theophylline to inhaled steroid with doubling of the dose of inhaled steroid in asthma. Eur Respir J. 1997;10:2754–2760. doi: 10.1183/09031936.97.10122754. et al. [DOI] [PubMed] [Google Scholar]
- Norman PS. Immunotherapy: 1999–2004. J Allergy Clin Immunol. 2004;113:1013–1023. doi: 10.1016/j.jaci.2004.03.020. [DOI] [PubMed] [Google Scholar]
- Stokes J, Casale TB. Rationale for new treatments aimed at IgE immunomodulation. Ann Allergy Asthma Immunol. 2004;93:212–217. doi: 10.1016/S1081-1206(10)61490-1. [DOI] [PubMed] [Google Scholar]
- Creticos PS, Chen YH, Schroeder JT. New approaches in immunotherapy: allergen vaccination with immunostimulatory DNA. Immunol Allergy Clin North Am. 2004;24:569–581. doi: 10.1016/j.iac.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Godfrey S, Bar-Yishay E. Exercise-induced asthma revisited. Respir Med. 1993;87:331–344. doi: 10.1016/0954-6111(93)90046-3. [DOI] [PubMed] [Google Scholar]
- Holzer K, Brukner P, Douglass J. Evidence-based management of exercise-induced asthma. Curr Sports Med Rep. 2002;1:86–92. doi: 10.1249/00149619-200204000-00005. [DOI] [PubMed] [Google Scholar]
- Sears MR, Lotvall J. Past, present and future–beta2-adrenoceptor agonists in asthma management. Respir Med. 2005;99:152–170. doi: 10.1016/j.rmed.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Knöpfli BH, Bar-Or O, Araúgo CG. Effect of ipratropium bromide on EIB in children depends on vagal activity. Med Sci Sports Exerc. 2005;37:354–359. doi: 10.1249/01.mss.0000155436.31581.90. [DOI] [PubMed] [Google Scholar]
- Weinberger M, Hendeles L. Theophylline in asthma. N Engl J Med. 1996;334:1380–1388. doi: 10.1056/NEJM199605233342107. [DOI] [PubMed] [Google Scholar]
- Sessler CN. Theophylline toxicity: clinical features of 116 consecutive cases. Am J Med. 1990;88:567–576. doi: 10.1016/0002-9343(90)90519-j. [DOI] [PubMed] [Google Scholar]
- Dozor AJ. Spacer crusader. Lancet. 2005;365:572. doi: 10.1016/S0140-6736(05)17903-3. [DOI] [PubMed] [Google Scholar]
- Newman SP. Spacer devices for metered dose inhalers. Clin Pharmacokinet. 2004;43:349–60. doi: 10.2165/00003088-200443060-00001. [DOI] [PubMed] [Google Scholar]
- Newman KB, Milne S, Hamilton C, Hall K. A comparison of albuterol administered by metered-dose inhaler and spacer with albuterol by nebulizer in adults presenting to an urban emergency department with acute asthma. Chest. 2002;121:1036–1041. doi: 10.1378/chest.121.4.1036. [DOI] [PubMed] [Google Scholar]
- Stevenson DD. Aspirin and NSAID sensitivity. Immunol Allergy Clin North Am. 2004;24:491–505. doi: 10.1016/j.iac.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Namazy JA, Simon RA. Sensitivity to nonsteroidal anti-inflammatory drugs. Ann Allergy Asthma Immunol. 2002;89:542–550. doi: 10.1016/S1081-1206(10)62099-6. [DOI] [PubMed] [Google Scholar]
- Jawien J. A new insight into aspirin-induced asthma. Eur J Clin Invest. 2002;32:134–138. doi: 10.1046/j.0014-2972.2001.00954.x. [DOI] [PubMed] [Google Scholar]
- de Almeida MA, Gaspar AP, Carvalho FS, Nogueira JM, Pinto JE. Adverse reactions to acetaminophen, ASA, and NSAIDs in children: what alternatives? Allergy Asthma Proc. 1997;18:313–318. doi: 10.2500/108854197778590560. [DOI] [PubMed] [Google Scholar]
- Vaszar LT, Stevenson DD. Aspirin-induced asthma. Clin Rev Allergy Immunol. 2001;21:71–87. doi: 10.1385/CRIAI:21:1:71. [DOI] [PubMed] [Google Scholar]
- Rivas B. The management of ASA syndrome. J Invest Allergol Clin Immunol. 1997;7:392–396. [PubMed] [Google Scholar]
- Hedman J, Kaprio J, Poussa T, Nieminen MM. Prevalence of asthma, aspirin intolerance, nasal polyposis and chronic obstructive pulmonary disease in a population-based study. Int J Epidemiol. 1999;28:717–722. doi: 10.1093/ije/28.4.717. [DOI] [PubMed] [Google Scholar]
- Levy S, Volans G. The use of analgesics in patients with asthma. Drug Safe. 2001;24:829–841. doi: 10.2165/00002018-200124110-00004. [DOI] [PubMed] [Google Scholar]
- Szczeklik A, Stevenson DD. Aspirin-induced asthma: advances in pathogenesis and management. J Allergy Clin Immunol. 1999;104:5–13. doi: 10.1016/s0091-6749(99)70106-5. [DOI] [PubMed] [Google Scholar]
- Settipane RA, Stevenson DD. Cross sensitivity with acetaminophen in aspirin-sensitive subjects with asthma. J Allergy Clin Immunol. 1989;84:26–33. doi: 10.1016/0091-6749(89)90174-7. [DOI] [PubMed] [Google Scholar]
- Szczeklik A, Stevenson DD. Aspirin-induced asthma: advances in pathogenesis, diagnosis, and management. J Allergy Clin Immunol. 2003;111:913–921. doi: 10.1067/mai.2003.1487. [DOI] [PubMed] [Google Scholar]
- Mellion MB, Kobayashi RH. Exercise-induced asthma. Am Fam Physician. 1992;45:2671–2677. [PubMed] [Google Scholar]
- Rupp NT. Diagnosis and management of exercise-induced asthma. Physician Sportsmed. 1996;24(1):77–87. doi: 10.3810/psm.1996.01.1213. [DOI] [PubMed] [Google Scholar]
- Baker WH, Weiler JM. The role of exercise training and conditioning in patients with asthma. In: Weiler JM, ed. Allergic and Respiratory Disease in Sports Medicine. New York, NY: Marcel Dekker; 1997:179– 206.
- Cochrane LM, Clark CJ. Benefits and problems of a physical training programme for asthmatic patients. Thorax. 1990;45:345–351. doi: 10.1136/thx.45.5.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Dov I, Bar-Yishay E, Godfrey S. Refractory period after exercise-induced asthma unexplained by respiratory heat loss. Am Rev Respir Dis. 1982;125:530–534. doi: 10.1164/arrd.1982.125.5.530. [DOI] [PubMed] [Google Scholar]
- Belcher NG, O'Hickey S, Arm JP, Lee TH. Pathogenetic mechanisms of exercise-induced asthma and the refractory period. N Engl Reg Allergy Proc. 1988;9:199–201. doi: 10.2500/108854188779023388. [DOI] [PubMed] [Google Scholar]
- McKenzie DC, McLuckie SL, Stirling DR. The protective effects of continuous and interval exercise in athletes with exercise-induced asthma. Med Sci Sports Exerc. 1994;26:951–956. [PubMed] [Google Scholar]
- Morton AR, Fitch KD, Davis T. The effect of “warm-up” on exercise-induced asthma. Ann Allergy. 1979;42:257–260. [PubMed] [Google Scholar]
- Schnall RP, Landau LI. Protective effects of repeated short sprints in exercise-induced asthma. Thorax. 1980;35:828–832. doi: 10.1136/thx.35.11.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickleborough TD, Gotshall RW, Cordain L, Lindley M. Dietary salt alters pulmonary function during exercise in exercise-induced asthmatics. J Sports Sci. 2001;19:865–873. doi: 10.1080/026404101753113813. [DOI] [PubMed] [Google Scholar]
- Gotshall RW, Mickleborough TD, Cordain L. Dietary salt restriction improves pulmonary function in exercise-induced asthma. Med Sci Sports Exerc. 2000;32:1815–1819. doi: 10.1097/00005768-200011000-00001. [DOI] [PubMed] [Google Scholar]
- Mickleborough TD, Gotshall RW, Kluka EW, Miller CW, Cordian L. Dietary chloride as a possible determinant of the severity of exercise-induced asthma. Eur J Appl Physiol. 2001;85:450–456. doi: 10.1007/s004210100478. [DOI] [PubMed] [Google Scholar]
- Mickleborough TD, Murray RL, Ionescu AA, Lindley MR. Fish oil supplementation reduces severity of exercise-induced bronchoconstriction in elite athletes. Am J Respir Crit Care Med. 2003;168:1181–1189. doi: 10.1164/rccm.200303-373OC. [DOI] [PubMed] [Google Scholar]
- Weiler JM. Allergic and Respiratory Disease in Sports Medicine. ed. New York, NY: Marcel Dekker; 1997.
- McFadden ER., Jr Exercise-Induced Asthma. ed. New York, NY: Marcel Dekker; 1999.
- Homnick DN, Marks JH. Exercise and sports in the adolescent with chronic pulmonary disease. Adolesc Med. 1998;9:467–481. [PubMed] [Google Scholar]
- Milgrom H, Taussig LM. Keeping children with exercise-induced asthma active. Pediatrics. 1999;104:38–42. doi: 10.1542/peds.104.3.e38. [DOI] [PubMed] [Google Scholar]
- Langdeau JB, Boulet LP. Prevalence and mechanisms of development of asthma and airway hyperresponsiveness in athletes. Sports Med. 2001;31:601–616. doi: 10.2165/00007256-200131080-00005. [DOI] [PubMed] [Google Scholar]
- Sudhir P, Prasad CE. Prevalence of exercise-induced bronchospasm in schoolchildren: an urban-rural comparison. J Trop Pediatr. 2003;49:104–108. doi: 10.1093/tropej/49.2.104. [DOI] [PubMed] [Google Scholar]
- Weiler JM, Layton T, Hunt M. Asthma in United States Olympic athletes who participated in the 1996 Summer Games. J Allergy Clin Immunol. 1998;102:722–726. doi: 10.1016/s0091-6749(98)70010-7. [DOI] [PubMed] [Google Scholar]
- Weiler JM, Ryan EJ., III. Asthma in United States Olympic athletes who participated in the 1998 Olympic Winter Games. J Allergy Clin Immunol. 2000;106:267–271. doi: 10.1067/mai.2000.108605. [DOI] [PubMed] [Google Scholar]
- Storms WW. Review of exercise-induced asthma. Med Sci Sports Exerc. 2003;35:1464–1470. doi: 10.1249/01.MSS.0000084533.75912.B4. [DOI] [PubMed] [Google Scholar]
- Wilber RL, Rundell KW, Szmedra L, Jenkinson DM, Im J, Drake SD. Incidence of exercise-induced bronchospasm in Olympic winter sport athletes. Med Sci Sports Exerc. 2000;32:732–737. doi: 10.1097/00005768-200004000-00003. [DOI] [PubMed] [Google Scholar]
- Rundell KW, Jenkinson DM. Exercise-induced bronchospasm in the elite athlete. Sports Med. 2002;32:583–600. doi: 10.2165/00007256-200232090-00004. [DOI] [PubMed] [Google Scholar]
- Feinstein RA, LaRussa J, Wang-Dohlman A, Bartolucci AA. Screening adolescent athletes for exercise-induced asthma. Clin J Sport Med. 1996;6:119–123. doi: 10.1097/00042752-199604000-00010. [DOI] [PubMed] [Google Scholar]
- Tan RA, Spector SL. Exercise-induced asthma. Sports Med. 1998;25:1–6. doi: 10.2165/00007256-199825010-00001. [DOI] [PubMed] [Google Scholar]
- Spector SL. Update on exercise-induced asthma. Ann Allergy. 1993;71:571–577. [PubMed] [Google Scholar]
- Bierman EW, Kawabori I, Pierson WE. Incidence of exercise-induced asthma in children. Pediatrics. 1975;56:847–850. (5, pt 2 suppl.) [PubMed] [Google Scholar]
- Kovan JR, Mackowiak TJ. Exercise-induced asthma. Athl Ther Today. 2001;6(5):22–25. 34–35,64. [Google Scholar]
- McConnell R, Berhane K, Gilliland F. Asthma in exercising children exposed to ozone: a cohort study. Lancet. 2002;359:386–391. doi: 10.1016/S0140-6736(02)07597-9. et al. [DOI] [PubMed] [Google Scholar]
- Peter MM, Silvers WS, Thompson FL, Weiler JM. Case studies of asthma in elite and world-class athletes: the roles of the athletic trainer and physician. In: Weiler JM, ed. Allergic and Respiratory Disease in Sports Medicine. New York, NY: Marcel Dekker; 1997:353–375.
- Karjalainen EM, Laitinen A, Sue-Chu M, Altraja A, Bjermer L, Laitinen LA. Evidence of airway inflammation and remodeling in ski athletes with and without bronchial hyperresponsiveness to methacholine. Am J Respir Crit Care Med. 2000;161:2086–91. doi: 10.1164/ajrccm.161.6.9907025. [DOI] [PubMed] [Google Scholar]
- Larsson K, Ohlsén P, Larsson L, Malmberg P, Rydström P-O, Ulriksen H. High prevalence of asthma in cross country skiers. BMJ. 1993;307:1326–1329. doi: 10.1136/bmj.307.6915.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson SD, Daviskas E. Pathophysiology of exercise-induced asthma: the role of respiratory water loss. In: Weiler JM, ed. Allergic and Respiratory Disease in Sports Medicine. New York, NY: Marcel Dekker; 1997:87–114.
- Anderson SD, Holzer K. Exercise-induced asthma: is it the right diagnosis in elite athletes? J Allergy Clin Immunol. 2000;106:419–428. doi: 10.1067/mai.2000.108914. [DOI] [PubMed] [Google Scholar]
- McFadden ER, Jr, Nelson JA, Skowronski ME, Lenner KA. Thermally induced asthma and airway drying. Am J Respir Crit Care Med. 1999;160:221–226. doi: 10.1164/ajrccm.160.1.9810055. [DOI] [PubMed] [Google Scholar]
- Makker HK, Holgate ST. Pathophysiology: role of mediators in exercise-induced asthma. In: Weiler JM, ed. Allergic and Respiratory Disease in Sports Medicine. New York, NY: Marcel Dekker; 1997:115–136.
- Anderson SD, Daviskas E. The mechanism of exercise-induced asthma is…. J Allergy Clin Immunol. 2000;106:453–459. doi: 10.1067/mai.2000.109822. [DOI] [PubMed] [Google Scholar]
- Deal EC, Jr., McFadden ER, Jr, Ingram RH, Jr, Strauss RH, Jaeger JJ. Role of respiratory heat exchange in production of exercise-induced asthma. J Appl Physiol. 1979;46:467–475. doi: 10.1152/jappl.1979.46.3.467. [DOI] [PubMed] [Google Scholar]
- Chen WY, Horton DJ. Heat and water loss from the airways and exercise-induced asthma. Respiration. 1977;34:305–313. doi: 10.1159/000193842. [DOI] [PubMed] [Google Scholar]
- Gilbert IA, McFadden ER., Jr. Airway cooling and rewarming: the second reaction sequence in exercise-induced asthma. J Clin Invest. 1992;90:699–704. doi: 10.1172/JCI115940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson SD, Kippelen P. Exercise-induced bronchoconstriction: pathogenesis. Curr Allergy Asthma Rep. 2005;5:116–122. doi: 10.1007/s11882-005-0084-y. [DOI] [PubMed] [Google Scholar]
- McFadden ER, Jr, Lenner KA, Strohl KP. Postexertional airway rewarming and thermally induced asthma: new insights into pathophysiology and possible pathogenesis. J Clin Invest. 1986;78:18–25. doi: 10.1172/JCI112549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CM, Anderson SD, Walsh S, McElrea MS. An investigation of the effects of heat and water exchange in the recovery period after exercise in children with asthma. Am Rev Respir Dis. 1989;140:598–605. doi: 10.1164/ajrccm/140.3.598. [DOI] [PubMed] [Google Scholar]
- McFadden ER, Jr, Gilbert IA. Exercise-induced asthma. N Engl J Med. 1994;330:1362–1367. doi: 10.1056/NEJM199405123301907. [DOI] [PubMed] [Google Scholar]
- Beck KC. Control of airway function during and after exercise in asthmatics. Med Sci Sports Exerc. 1999;31:S-4–S-11. doi: 10.1097/00005768-199901001-00002. [DOI] [PubMed] [Google Scholar]
- Makker HK, Holgate ST. Mechanisms of exercise-induced asthma. Eur J Clin Invest. 1994;24:571–585. doi: 10.1111/j.1365-2362.1994.tb01109.x. [DOI] [PubMed] [Google Scholar]
- Furuichi S, Hashimoto S, Gon Y, Matsumoto K, Horie T. p38 mitogen-activated protein kinase and C-Jun-NH2 terminal kinase regulate interleukin-8 and RANTES production in hyperosmolarity stimulated human bronchial epithelial cells. Respirology. 2002;7:193–200. doi: 10.1046/j.1440-1843.2002.00401.x. [DOI] [PubMed] [Google Scholar]
- Rundell KW. Pulmonary function decay in women ice hockey players: is there a relationship to ice rink air quality? Inhal Toxicol. 2004;16:117–123. doi: 10.1080/08958370490270918. [DOI] [PubMed] [Google Scholar]
- Zainudin NM, Aziz BA, Haifa AL, Deng CT, Omar AH. Exercise-induced bronchoconstriction among Malay schoolchildren. Respirology. 2001;6:151–155. doi: 10.1046/j.1440-1843.2001.00326.x. [DOI] [PubMed] [Google Scholar]
- Jones A, Bowen M. Screening for childhood asthma using an exercise test. Br J Gen Pract. 1994;44:127–131. [PMC free article] [PubMed] [Google Scholar]
- Vianne EO, Boaventura LC, Terra-Filho J, Nakama GY, Martinez JAB, Martinez RJ. Morning-to-evening variation in exercise-induced bronchospasm. J Allergy Clin Immunol. 2002;110:236–240. doi: 10.1067/mai.2002.125981. [DOI] [PubMed] [Google Scholar]
- Anderson SD. Exercise-induced asthma and the use of hypertonic saline aerosol as a bronchial challenge. Respirology. 1996;3:175–181. doi: 10.1111/j.1440-1843.1996.tb00029.x. [DOI] [PubMed] [Google Scholar]
- Tilles SA. Vocal cord dysfunction in children and adolescents. Curr Allergy Asthma Rep. 2003;3:467–472. doi: 10.1007/s11882-003-0056-z. [DOI] [PubMed] [Google Scholar]
- Rundell KW, Spiering BA. Inspiratory stridor in elite athletes. Chest. 2003;123:468–474. doi: 10.1378/chest.123.2.468. [DOI] [PubMed] [Google Scholar]
- Wilkerson LA. Exercise-induced asthma. J Am Osteopath Assoc. 1998;98:211–215. [PubMed] [Google Scholar]
- Smith BW, LaBotz M. Pharmacologic treatment of exercise-induced asthma. Clin Sports Med. 1998;17:343–363. doi: 10.1016/s0278-5919(05)70085-5. [DOI] [PubMed] [Google Scholar]
- Fowler C. Preventing and managing exercise-induced asthma. Nurse Pract. 2001;26:25–33. doi: 10.1097/00006205-200103000-00010. [DOI] [PubMed] [Google Scholar]
- Kemp JP, Dockhorn RJ, Busse WW, Bleecker ER, Van As A. Prolonged effect of inhaled salmeterol against exercise-induced bronchospasm. Am J Respir Crit Care Med. 1994;150:1612–1615. doi: 10.1164/ajrccm.150.6.7952623. (6 Pt 1) [DOI] [PubMed] [Google Scholar]
- Vathenen AS, Knox AJ, Wisniewski A, Tattersfield AE. Time course of change in bronchial reactivity with an inhaled corticosteroid in asthma. Am Rev Respir Dis. 1991;143:1317–1321. doi: 10.1164/ajrccm/143.6.1317. [DOI] [PubMed] [Google Scholar]
- Nastasi KJ, Heinly TL, Blaiss MS. Exercise-induced asthma and the athlete. J Asthma. 1995;32:249–257. doi: 10.3109/02770909509044832. [DOI] [PubMed] [Google Scholar]
- Finch L. Asthma education is key. J Respir Care Pract. 2000:85–116.
- Dawson KP, Van Asperen P, Higgins C, Sharpe C, Davis A. An evaluation of the action plans of children with asthma. J Paediatr Child Health. 1995;31:21–23. doi: 10.1111/j.1440-1754.1995.tb02906.x. [DOI] [PubMed] [Google Scholar]