Abstract
Tumor growth is angiogenesis dependent. We hypothesized that nonneoplastic tissue growth also depends on neovascularization. We chose adipose tissue as an experimental system because of its remodeling capacity. Mice from different obesity models received anti-angiogenic agents. Treatment resulted in dose-dependent, reversible weight reduction and adipose tissue loss. Marked vascular remodeling was evident in adipose tissue sections, which revealed decreased endothelial proliferation and increased apoptosis in treated mice compared with controls. Continuous treatment maintained mice near normal body weights for age without adverse effects. Metabolic adaptations in food intake, metabolic rate, and energy substrate utilization were associated with anti-angiogenic weight loss. We conclude that adipose tissue mass is sensitive to angiogenesis inhibitors and can be regulated by its vasculature.
Substantial evidence has established that tumor growth is angiogenesis-dependent (1). Neovascularization promotes tumor growth (2), whereas angiogenesis inhibition prevents it, and can regress the lesions (3). This raises the question of whether the endothelium functions similarly in nonneoplastic tissue growth (4).
The challenges in addressing this question are that most adult tissues normally do not grow, their mass is stable, and the supporting vasculature is quiescent (5). Exceptionally, adipose tissue can grow and regress throughout adulthood. It is highly vascularized and has angiogenic properties (6, 7). An extensive capillary network surrounds each adipocyte, and there are few other resident cell types (8). Therefore, adipose tissue is uniquely suited to study the role of angiogenesis in nonneoplastic adult tissue growth.
The potential for adipose tissue to grow and regress is substantial. One would expect the vasculature to have a commensurate capacity for remodeling. We hypothesized that adipose tissue growth is angiogenesis dependent and, therefore, may be inhibited by anti-angiogenesis agents. This would suggest that adipose tissue mass may be regulated via the vascular endothelium.
We primarily used ob/ob mice because they rapidly accumulate adipose tissue (9). This strain develops spontaneous obesity because of a lack of functional leptin, a protein secreted by adipocytes that acts on the hypothalamus to regulate appetite and metabolism (9–11). Without leptin, animals eat excessively, expend less energy, and become morbidly obese. Leptin replacement induces weight loss in ob/ob mice by specifically reducing adipose tissue (9), unlike diet restriction or appetite suppression (fenfluramine), which decreases both lean and fat mass (12, 13).
We treated ob/ob mice with various angiogenesis inhibitors. We primarily used TNP-470 (14) because of availability. TNP-470 inhibits endothelial cell proliferation in vitro (15) and angiogenesis in vivo (16). At significantly higher concentrations, TNP-470 can also suppress nonendothelial cell proliferation (14, 17). Angiostatin (kringle 1–4 domains of plasminogen) (18) and endostatin (a C-terminal fragment of collagen XVIII) (19) are angiogenesis inhibitors that act exclusively on endothelium. Bay-129566, a matrix metalloproteinase inhibitor (20), and thalidomide (21) were also studied.
We show that adipose tissue growth is angiogenesis dependent. Body and adipose tissue weights of treated mice were significantly less than controls. Loss of adipose tissue mass was similar to that resulting from leptin replacement. We observed decreased endothelial cell proliferation and increased apoptosis in the adipose tissue of treated animals compared with controls, suggesting that adipose endothelial cells were sensitive to angiogenesis inhibitors. We have also begun exploring the metabolic consequences of anti-angiogenic-induced weight reduction and have found adaptations in food intake, metabolic rate, and preferred energy substrate. These studies suggest that adipose tissue mass can be regulated through the vascular endothelium.
Methods
Animal Studies.
Male C57BL/6J-Lepob, C57BL/6J, agouti (Ay), and fat (Cpefat) mice (The Jackson Laboratory) were used according to institutional guidelines. We housed mice individually with free access to water and standard chow unless noted. We measured body weights and remaining chow daily. We anesthetized animals with isoflurane (Baxter) and killed them by continuous inhalation of CO2.
Solutions were prepared daily. TNP-470 (Takeda, Osaka; complexed with beta-cyclodextrin) was dissolved in saline and administered s.c. in rotating sites along the dorsum. Recombinant murine endostatin and angiostatin were produced in Escherichia coli, precipitated as nonrefolded proteins (18, 19), and administered s.c. in saline at 50 mg/kg per 12 h (endostatin and angiostatin) or 20 mg/kg per day (angiostatin). Bay 12-9566 (Bayer, Elkhart, IN) was suspended in 0.5% carboxymethylcellulose/0.2% Tween 80 and delivered via gastric lavage (100 mg/kg per day). We injected thalidomide (Entremed) in 0.5% carboxymethylcellulose i.p.(150 mg/kg per day). We administered leptin at 150 μg/kg per day (s.c. osmotic pump). Controls received the appropriate vehicle. We used the Kruskal–Wallis test to evaluate the overall treatment effect. Duncan's multiple range test on ranked data was used to compare results among treatment groups.
Cell Proliferation Assay.
We washed confluent bovine capillary endothelial cell cultures with PBS and dispersed cells in 0.05% trypsin. Cells were resuspended in DMEM containing 10% bovine calf serum and 1% L-glutamine/penicillin/streptomycin and cultured on gelatinized plates (12,500 cells per well) (24 h). Media were replaced with assay media (DMEM/5% bovine calf serum/1% L-glutamine/penicillin/streptomycin) containing TNP-470 at 1 pg/ml to 100 μg/ml for 30 min, and basic fibroblast growth factor (1 ng/ml) was added. After 72 h, cells were dispersed in trypsin, resuspended in Hematall, and counted using a Coulter Counter. Following the above procedure, subconfluent 3T3-L1 cells were resuspended in DMEM containing 10% bovine calf serum, plated cells (8,000 cells per well), and were incubated in assay media without L-glutamine/penicillin/streptomycin.
Serum Glucose.
Blood was collected (100 μl) from tail veins of anesthetized 9-month-old ob/ob mice following 115 d of treatment with TNP-470 (10 mg/kg per day) (n = 5) or saline (n = 13). Children's Hospital clinical chemistry laboratory (Boston) performed the measurements, which were compared using a one-tailed Student's t test.
Body Compositions.
Bone-free lean body mass and fat mass were measured using a Lunar PIXImus J densitometer (Lunar, Madison, WI). Calibration was conducted using an aluminum/lucite phantom (TBMD, 0.0594 g/cm2, percent fat 12.4%). Mice were fasted (3 h), anesthetized (80 mg/kg ketamine/10 mg/kg xylazine), and positioned with limbs extended. The imaging area was too small to contain the entire carcass, so the heads extended outside the field. We placed an exclusion region over any captured portion of the head to ensure that measurements represented only subcranial body composition. We scanned each mouse three times with intermittent repositioning.
Immunohistochemistry.
Double-fluorescent labeling enabled simultaneous identification of proliferating cells (BrdUrd) and endothelial cells [von Willebrand factor (vWF)] in epididymal adipose tissue sections. Tissue was fixed in cold Carnoy's fixative (4 h), transferred to 100% ethanol, and paraffin-embedded. Sections (5 μm) were permeabilized with 10 μg/ml proteinase K in 0.1 M Tris-HCl buffer (pH 7.4) at 37°C (20 min) and washed with PBS. We labeled proliferating cells with anti-BrdUrd/nuclease. To label endothelium, sections were blocked with 5% horse serum (30 min) and incubated with rabbit anti-human vWF (Dako; 1:250) in 5% horse serum in PBS at 4°C overnight and washed. Sections were incubated with biotinylated anti-rabbit IgG (1:200) and Hoechst 33258 (1.0 μg/ml; 30 min) and washed. BrdUrd was detected with FITC-anti mouse IgG (1:200), and vWF was detected by Texas Red Avidin-conjugated goat anti-rabbit IgG (1:200; Vector Laboratories).
Similarly, double-fluorescent labeling enabled simultaneous detection of apoptotic cells (TUNEL assay) and endothelial cells. Apoptotic cells were identified using ApopTag plus in situ apoptosis detection kit (Integen) with a fluorescein tag. Sections were stained for endothelial cells (vWF) and counterstained (Hoechst 33258) as described.
Sections were preserved with Fluoromount G (Southern Biotechnology) and analyzed using a Zeiss Axiophot fluorescent microscope with a dual filter (FITC 485 nm, Texas Red, 510–560 nm). Sections were photographed at 40× magnification with Ektachrome P1600 film.
We did not quantify proliferating or apoptotic adipocytes because cell diameters can exceed 150 μm, and the frequency of visualizing adipocyte nuclei in thin sections was relatively low.
VO2 and Respiratory Exchange Ratio.
Serial measurements were made in 10-week-old ob/ob mice treated with TNP-470 (10 mg/kg per day) or vehicle (n = 3/group) using an OXYMAX System (Columbus Instruments, Columbus, OH). Conditions were settling time (100 s), measuring time (50 s), and room air (reference). Animals were placed individually in 0.3-liter chambers, and resting measurements were collected (4 h).
Results
Body Weight Responses of Obese Mice to Angiogenesis Inhibitors.
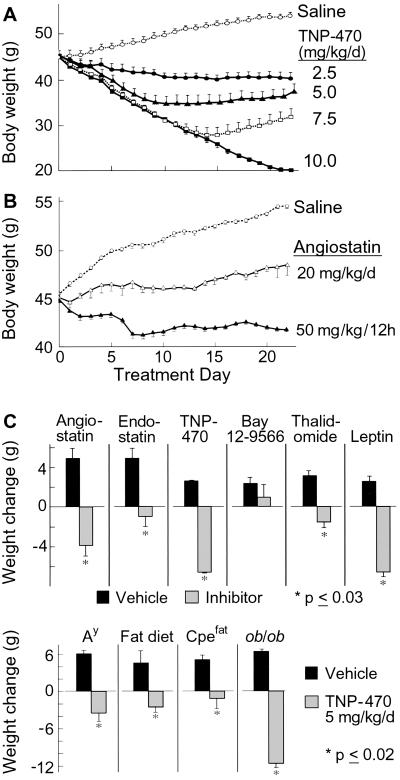
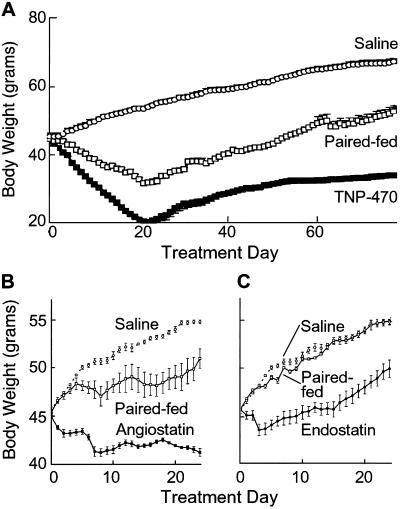
TNP-470-treated ob/ob mice dose-dependently (P < 0.05) lost weight, whereas controls gained (P ≤ 0.003) (Fig. 1A). To determine whether suppression of preadipocyte proliferation contributes to this effect, we treated 3T3-L1 preadipocytes (22) with TNP-470. A five logs greater TNP-470 concentration (10 μg/ml) was required for half-maximal cytostatic inhibition of 3T3-L1 preadipocytes compared with endothelial cells (100 pg/ml, P < 0.05; data not shown). Although this supports an endothelial-mediated mechanism, we cannot exclude preadipocyte suppression. Therefore, we tested specific angiogenesis inhibitors. Obese mice treated with angiostatin at 20 mg/kg per day gained one-third that of controls (P < 0.05) (Fig. 1B), whereas those receiving 50 mg/kg per 12 h lost weight (P < 0.05). Angiostatin (up to 1 mg/ml) did not effect 3T3-L1 cell proliferation (data not shown).
Fig 1.
Body weight responses to angiogenesis inhibitors. We treated 8-week-old ob/ob mice with various doses of TNP-470 (A) or angiostatin (B) for 21 days (n = 4 per group). (C) Responses of ob/ob mice treated with various angiogenesis inhibitors (hatched bars) for 7 d were compared with those of the corresponding vehicles (solid bars) and leptin (n = 3 per group). (D) Twelve-week-old mice from various obesity models were treated with vehicle (solid bars) or TNP-470 (hatched bars) for 7 days (n = 3 per group). These included agouti mice C57BL/6J-Ay (Ay), fat mice C57BLKS-Cpefat/J (Cpefat), ob/ob mice C57BL/6J-Lepob, and wild-type C57BL/6J mice fed a high-fat diet consisting of 40% fat by calorie (n = 3 per group).
Comparatively, endostatin (P = 0.02), Bay12–9566, thalidomide (P = 0.01), angiostatin (P < 0.002), and TNP-470 (P = 0.01) treated ob/ob mice gained less or lost weight relative to controls (Fig. 1C). TNP-470 (5 mg/kg per day) and leptin (150 μg/kg per day) yielded similar results. Mice from other obesity models (Ay, Cpefat, C57BL/6J mice on high-fat diet) treated with TNP-470 also weighed less than controls (P ≤ 0.02) (Fig. 1C).
Effects of Long-Term or Cycled Anti-Angiogenic Treatment of Obese Mice.
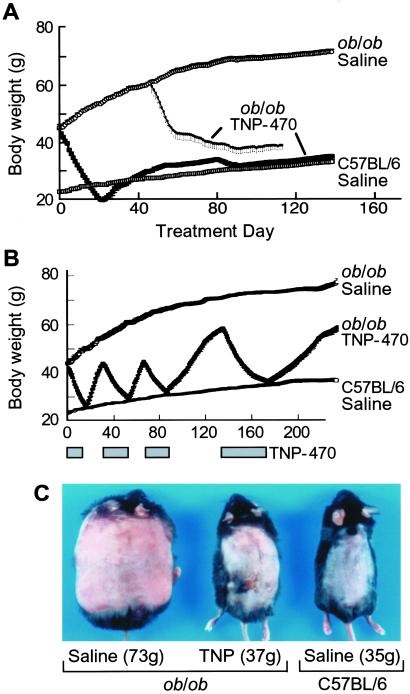
Daily TNP-470 treatment was well tolerated by ob/ob mice for over one-fourth of their life spans (Fig. 2A). Appetites decreased early but normalized despite continued therapy. Activity and grooming were maintained, and obesity-associated hyperglycemia was avoided (23). Controls had elevated serum glucose levels (224 ± 16 mg/dl), whereas levels from TNP-470-treated ob/ob mice (158 ± 12.6 mg/dl) were within normal range (62–175 mg/dl) (24).
Fig 2.
Continuous and intermittent treatment of ob/ob mice with TNP-470. (A) ob/ob mice were treated with vehicle (n = 4) or TNP-470 (10 mg/kg/day; n = 6) starting at 8 weeks of age for 138 days, or at 6 months of age for 65 days. For comparison, the graph includes the growth curve of wild-type, age-matched C57BL/6 mice (n = 5). (B) Mice were treated with TNP-470 (10 mg/kg/day; n = 15) until they reduced to the weight of age-matched C57BL/6 mice. We then discontinued treatment, and the mice were permitted to regain before treatment was restarted. We cycled treatment four times. (C) Photographs show a representative mouse from each group on 173 days (bottom of cycle 4). Except for superficial scarring, daily TNP-470 treatment was well tolerated.
Aged, relatively weight-stable ob/ob mice with negligible adipose endothelial cell proliferation (data not shown) lost weight when treated with TNP-470, whereas controls slightly gained (P < 0.000002) (Fig. 2A). This suggests adipose vasculature is susceptible to inhibitors, even when not proliferating.
To test reversibility, we intermittently treated ob/ob mice with TNP-470 until they reduced to the weight of age-matched C57BL/6 mice, and then suspended treatment until mice regained (Fig. 2B). Mice reduced weight while on TNP-470 and regained weight off treatment. At the bottom of the fourth cycle, control ob/ob mice were morbidly obese, whereas treated ones were similar in weight to wild-type mice (Fig. 2C).
Body Composition of Obese Mice Treated with Angiogenesis Inhibitors.
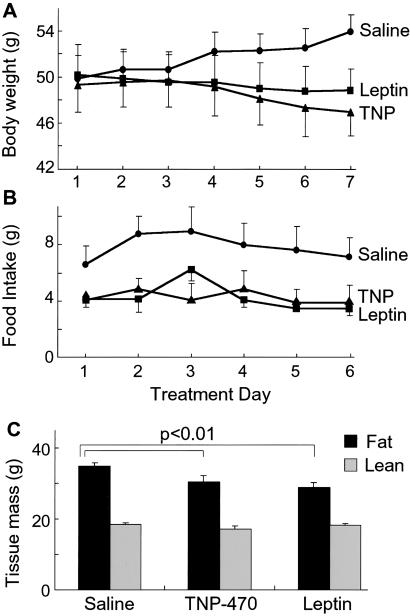
We compared body compositions (fat and bone-free lean mass) by using dual-energy x-ray absorptiometry in TNP-470- and leptin-treated ob/ob mice (Fig. 3A). Dual-energy x-ray absorptiometry yields results similar (25) or well correlated (26) to chemical carcass analysis. Mice lost similar weight with TNP-470 and leptin, whereas controls gained (P < 0.05). Food intakes in treated groups were comparable and less than controls (P < 0.0005) (Fig. 3B). TNP-470 (30.4 ± 1.9 g) and leptin (29.0 ± 1.3 g) similarly reduced fat mass relative to controls (35.0 ± 0.9 g) (P ≤ 0.01) (Fig. 3C), resulting in similar decreases in percent body fat (TNP-470 2.0 ± 1.1%; leptin 3.0 ± 0.6%). This was supported by significantly lower adipose tissue bed weights in both treated groups. For example, epididymal fat pad weights decreased from 1.73 ± 0.27 g in control mice to 1.35 ± 0.13 g with TNP-470 and to 1.33 ± 0.17 g with leptin (P ≤ 0.005). Subcutaneous fat pad weights decreased from 1.42 ± 0.14 g in control mice to 1.25 ± 0.19 g with TNP-470 and to 1.27 ± 0.20 g with leptin (P ≤ 0.05). Although insignificant, TNP-470-treated mice had the lowest average lean mass (control 18.5 ± 0.4 g; TNP-470 17.1 ± 0.8 g; leptin 18.15 ± 0.5), suggesting modest protein loss.
Fig 3.
Body compositions of ob/ob mice treated with TNP-470 or leptin. Nine-week-old female ob/ob mice were treated with TNP-470 (5 mg/kg/day) or leptin (150 μg/kg/day) for 7 days to achieve similar weight reduction (n = 4 per group). Control ob/ob mice received vehicle. (A) Body weight curves. (B) Daily food intakes. (C) Fat mass (black bars) and lean body mass (gray bars) components of body composition were measured using dual-energy x-ray absorptiometry.
Vascular Remodeling in Adipose Tissue During Weight Shifts.
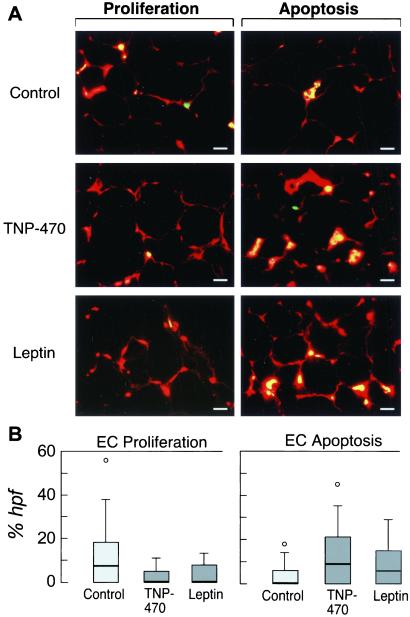
We assessed cell proliferation (Fig. 4A Left) and apoptosis (Fig. 4A Right) in epididymal fat sections from treated and control ob/ob mice. TNP-470 and leptin significantly decreased median percents of endothelial cell proliferation (Fig. 4B Left) and increased apoptosis (Fig. 4B Right) compared with controls. Wide ranges reflect heterogeneity. Endothelial apoptosis also predominated in adipose tissue of diet-restricted ob/ob mice (data not shown).
Fig 4.
Adipose tissue endothelial cell proliferation and apoptosis in ob/ob mice. (A) Endothelial cell proliferation and apoptosis were evaluated in epididymal adipose tissue sections from ob/ob mice following 7 days of treatment with vehicle, TNP-470, or leptin. We identified endothelial cells (red) by using vWF. We used fluorescein (green) with BrdUrd to identify proliferating cells (Left) or with the TUNEL assay to identify apoptotic cells (Right). Cells stained with both markers appear yellow. Adipose tissue from control ob/ob mice contained proliferating endothelial cells (yellow, top left), occasional proliferating nonendothelial cells (green), and few apoptotic endothelial cells (yellow, top right). In contrast, adipose tissue from TNP-470- and leptin-treated mice contained numerous apoptotic endothelial cells (yellow, middle and bottom right). (Bar = 25 μm.) (B) Median percents of endothelial cell proliferation and apoptosis were quantified for 30 high power fields per section (n = 5 per group). We used a box and whisker plot to illustrate the median and range of the measurements.
Metabolic Studies on Obese Mice Treated with Angiogenesis Inhibitors.
Transiently decreased appetites in TNP-470-treated ob/ob mice were reflected by weight loss in paired-fed animals (P < 0.05) (Fig. 5A). Despite continued treatment, appetites normalized, as gauged by food intakes of C57BL/6 mice of similar weight. At lower doses, appetite was proportionately less affected (data not shown). Angiostatin-treated ob/ob mice also had decreased food intake (P < 0.05) (Fig. 5B). Reduced appetite in both groups coincided with about 7% loss of initial body weight. Endostatin-treated ob/ob mice had about 5% loss and no significant appetite effect (Fig. 5C).
Fig 5.
Appetite of ob/ob mice treated with angiogenesis inhibitors. Eight-week-old ob/ob mice (n = 4 per group) with free access to chow were treated with (A) TNP-470 (10 mg/kg/day), (B) angiostatin (50 mg/kg/12 h), or (C) endostatin (50 mg/kg per 12 h). We fed the amount consumed per day by each treated mouse to a paired mouse in a second, untreated group (n = 4). We measured body weights of treated, paired-fed, and control mice daily.
Basal metabolic rate, as indicated by oxygen consumption (VO2), increased in TNP-470-treated ob/ob mice by 4 days and in C57BL/6 mice by 8 days (P < 0.05) (Table 1). VO2 at 4, 12, and 24 h were unaltered (data not shown), although weight loss occurred by 24 h. Average body temperatures measured twice daily for 12 days via s.c. probes were similar in the control (95.3 ± 1.1°F) and TNP-470 (94.8 ± 0.8°F) treated mice (n = 4/group). Respiratory exchange ratio (respiratory exchange ratio = VCO2/VO2) decreased with TNP-470 by 8 days in both strains, suggesting the preferred energy substrate shifted from carbohydrates to fatty acids during weight loss (P < 0.05). Regressing fat is the likely fatty acid source and may contribute to transient appetite suppression (27).
Table 1.
Body weight, VO2, and respiratory exchange ratio in response to TNP-470
| Mice
|
Time, day
|
Body weight, g | VO2, ml/kg/min | Respiratory exchange ratio | |||
|---|---|---|---|---|---|---|---|
| Saline | TNP | Saline | TNP | Saline | TNP | ||
| C57BL6 | 0 | 23.0 ± 0.5 | 22.6 ± 0.2 | ||||
| 4 | 22.8 ± 0.4 | 21.8 ± 0.4 | 44.4 ± 0.8 | 42.5 ± 3.2 | 0.70 ± 0.02 | 0.69 ± 0.01 | |
| 8 | 23.0 ± 0.4 | 21.1 ± 0.3 | 31.9 ± 0.8 | 38.3 ± 1.1 | 0.71 ± 0.03 | 0.67 ± 0.03 | |
| ob/ob | 0 | 41.4 ± 0.7 | 41.7 ± 0.7 | ||||
| 4 | 43.9 ± 0.6 | 41.1 ± 0.9 | 24.3 ± 1.1 | 30.0 ± 2.0 | 0.74 ± 0.11 | 0.70 ± 0.01 | |
| 8 | 46.2 ± 0.5 | 36.7 ± 1.1 | 19.1 ± 0.5 | 24.0 ± 0.5 | 0.76 ± 0.04 | 0.64 ± 0.05 | |
P < 0.05.
Discussion
These studies demonstrate that adipose tissue mass can be regulated through the vasculature and that metabolic changes accompany anti-angiogenic-induced weight loss, which may contribute to weight reduction.
Different angiogenesis inhibitors significantly decreased body and adipose tissue weights (Figs. 1 and 3). We observed this effect in mice from distinct obesity models (Fig. 1). The magnitude of reduction varied by strain and angiogenesis inhibitor. This may reflect differences in bioavailability, tissue or cell specificity, or mechanisms of actions. Genetic differences in the animals' angiogenic potential may also contribute (28). Differences in percent body fat among the strains may also be a determining factor in their responsiveness. For example, ob/ob mice have greater fat content, which if proportionately reduced by angiogenesis inhibitors, would result in greater weight loss.
Adipose tissue's capacity to grow throughout life suggests it maintains the ability to recruit new capillaries. In the cornea assay, fat induced neovascularization, whereas liver and muscle did not (29). Angiogenesis and adipogenesis are spatially and temporally coupled during development (6), and endothelial cell proliferation occurs in expanding adult adipose tissue (6). This coordinated growth may be mediated by paracrine interactions between endothelial cells and adipocytes. Adipocytes produce endothelial cell-specific mitogens and angiogenic factors (6, 30), such as monobutyrin (31), vascular endothelial growth factor (32), and leptin (7). Conversely, endothelial cells produce soluble and matrix-bound preadipocyte mitogens (33, 34).
Weight reduction from angiogenesis inhibitors, leptin (Figs. 4 and 5), or diet restriction (data not shown) was associated with adipose endothelial cell apoptosis. Leptin-induced endothelial cell (35) and adipocyte (36) apoptosis during weight loss is recognized. Shifts from endothelial cell proliferation to apoptosis under various weight loss conditions suggest that vascular remodeling is a critical and common event during adipose tissue growth or regression. Whether weight loss leads to vascular regression or vice versa is likely a function of the initiating mechanism (37). Preliminary studies showing reductions in the endothelial cell to adipocyte ratios in TNP-470-treated mice (data not shown) are consistent with vascular regression as the initial event, whereas, with caloric restriction and leptin, vascular remodeling is probably a secondary response.
Cycling and aged mice studies (Fig. 3) illustrate that adipose vessels are capable of extensive remodeling and remain susceptible to anti-angiogenic agents, even when not growing. These findings may be explained by results from subsequent studies in which we determined that regulation of vessel maturation through shifts in angiopoietin-1 is coordinated with the extent of adipose tissue remodeling (S.M.D., D. Zurakowski, J.F., K. S. Moulton, and M.A.R., unpublished results). We propose that adipose tissue vessels may be maintained in a relatively immature state compared with that of other organs that are weight stable. Because anti-angiogenic agents target growing or newly formed vessels (whereas mature vessels are not responsive), adipose tissue vasculature may remain susceptible, even when not actively growing. If so, adipose tissue may be selectively reduced by angiogenesis inhibitors, thus, resulting in weight loss.
Mechanisms governing metabolic adaptations in food intake, VO2, and respiratory exchange ratio (Table 1) during anti-angiogenic-induced weight loss are unknown. Food intake decreased with TNP-470 and angiostatin in leptin-deficient mice (Fig. 5). This may be the result of a direct central effect, which can be investigated through intrathecal administration of each drug. Alternatively, it may be related to the shared action of the inhibitors and perhaps an indirect response to adipose tissue loss. Factors released from regressing adipose tissue may inhibit feeding, analogous to appetite suppression seen with glucose (38) or fatty acid infusions (27). Treated animals preferentially used fatty acids as energy substrates during weight reduction. Despite continued TNP-470 treatment, body weights stabilize. Appetites increased, approximating that of wild-type mice of similar weight (data not shown). This suggests that the processes operating during weight reduction and weight maintenance may differ. We speculate that a leptin-independent feedback mechanism appropriately reduces appetite when anti-angiogenic agents prevent adipose tissue expansion.
Anti-angiogenic weight loss can occur without appetite changes as evidenced by endostatin (Fig. 5) and by low doses of TNP-470 or angiostatin (data not shown). Furthermore, treated mice weighed less than paired-fed mice having similar caloric intake. An increased metabolic rate in treated mice assists in accounting for these calories. A direct action on the adipocytes may also contribute.
Weight loss and appetite suppression are common nonspecific responses, thus necessitating consideration of drug toxicity. However, several observations favor a nontoxic mechanism. Weight reduction occurred with different angiogenesis inhibitors (Fig. 1). Numerous studies safely use TNP-470 to inhibit tumor growth (mice, refs. 16 and 39; clinical trials, ref. 40). Although toxicity can occur above the conventional anti-tumor dose (41), our maximum dose was two-thirds the anti-tumor dose. Mice treated daily for over 25% of their lifespan had no discernable ill effects, and food intake paralleled wild-type mice of comparable weight. Long-term or repeated exposures did not impede mice from regaining weight off therapy. Mice regained at an accelerated rate, which resembled rebound weight gain after caloric restriction in healthy animals (42, 43). Body compositions of TNP-470- and leptin-treated mice were comparable following similar weight losses (Fig. 3). These observations argue against a toxic etiology. Instead, the data suggest TNP-470-induced weight reduction beginning with angiogenesis inhibition in adipose tissue, followed by subsequent effects on appetite, fuel utilization, and energy expenditure. The mechanism by which limiting vascular supply in fat affects these other parameters is presently unknown, but may be because of adipocyte-secreted factors.
Endothelium also mediates prostate growth (44) and supplies signals during organogenesis (45, 46). We propose angiogenesis mediators may be a means of regulating adipose tissue mass and that endothelium may have a role in regulating the mass of other adult tissues.
Acknowledgments
Generous gifts were provided by Takeda Chemical Company (TNP-470), Bayer Corporation (Bay 12-9566), and Entremed (thalidomide). Appreciation is extended to the veterinarian, Dr. Richard Hurley, for monitoring the health status of our animals during these studies. The valuable input of Dr. Rick Rodgers (Bio-Imaging Laboratory, Harvard School of Public Health) is also appreciated. Expertise in statistical analysis was provided by Elizabeth Allred (Children's Hospital Neuroepidemiology Unit, Boston). The technical assistances of Catherine Butterfield, Karen Keough, and Bin Shi are gratefully recognized. We also acknowledge photography services by Lori DeSantis and Kristin Gullage and graphic design by Advanced Medical Graphics (Boston). This study was supported in part by National Institutes of Health Grant K08 HL03698 (to M.A.R.).
Abbreviations
vWF, von Willebrand factor
References
- 1.Folkman J. (1997) in Cancer, eds. DeVita, V., Jr., Hellman, S. & Rosenberg, S. (Lippincott, Philadelphia), pp. 3075–3085.
- 2.Rak J., Filmus, J. & Kerbel, R. (1996) Eur. J. Cancer 32, 2438-2450. [DOI] [PubMed] [Google Scholar]
- 3.Boehm T., Folkman, J., Browder, T. & O'Reilly, M. S. (1997) Nature (London) 390, 404-407. [DOI] [PubMed] [Google Scholar]
- 4.Folkman J. (1998) Endocrinology 139, 441-442. [DOI] [PubMed] [Google Scholar]
- 5.Hobson B. & Denekamp, J. (1984) Br. J. Cancer 49, 405-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crandall D. L., Hausman, G. J. & Kral, J. G. (1997) Microcirculation 4, 211-232. [DOI] [PubMed] [Google Scholar]
- 7.Sierra-Honigmann M. R., Nath, A. K., Murakami, C., Garcia-Cardena, G., Papapetropoulos, A., Sessa, W. C., Madge, L. A., Schechner, J. S., Schwabb, M. B., Polverini, P. J. & Flores-Riveros, J. R. (1998) Science 281, 1683-1686. [DOI] [PubMed] [Google Scholar]
- 8.Gersh I. & Still, M. A. (1945) J. Exp. Med. 81, 219-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Halaas J. L., Boozer, C., Blair-West, J., Fidahusein, N., Denton, D. A. & Friedman, J. M. (1997) Proc. Natl. Acad. Sci. USA 94, 8878-8883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y., Proenca, R., Maffei, M., Barone, M., Leopold, L. & Friedman, J. M. (1994) Nature (London) 372, 425-432. [DOI] [PubMed] [Google Scholar]
- 11.Campfield L. A., Smith, F. J. & Burn, P. (1996) Horm. Metab. Res. 28, 619-632. [DOI] [PubMed] [Google Scholar]
- 12.Carr R. H., Ipaktchi, M. & Thenen, S. W. (1977) Proc. Soc. Exp. Biol. Med. 154, 116-120. [PubMed] [Google Scholar]
- 13.Stein L. J., Stellar, E., West, D. B., Greenwood, M. R., Foster, G. D., Feurer, I., Brown, J., Mullen, J. L. & Brownell, K. D. (1992) Physiol. Behav. 51, 1-6. [DOI] [PubMed] [Google Scholar]
- 14.Ingber D., Fujita, T., Kishimoto, S., Sudo, K., Kanamaru, T., Brem, H. & Folkman, J. (1990) Nature (London) 348, 555-557. [DOI] [PubMed] [Google Scholar]
- 15.Wang J., Lou, P. & Henkin, J. (2000) J. Cell. Biochem. 77, 465-473. [PubMed] [Google Scholar]
- 16.Morita T., Shinohara, N. & Tokue, A. (1994) Br. J. Urol. 74, 416-421. [DOI] [PubMed] [Google Scholar]
- 17.Kusaka M., Sudo, K., Matsutani, E., Kozai, Y., Marui, S., Fujita, T., Ingber, D. & Folkman, J. (1994) Br. J. Cancer 69, 212-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Reilly M. S., Holmgren, L., Shing, Y., Chen, C., Rosenthal, R. A., Moses, M., Lane, W. S., Cao, Y., Sage, E. H. & Folkman, J. (1994) Cell 79, 315-328. [DOI] [PubMed] [Google Scholar]
- 19.O'Reilly M. S., Boehm, T., Shing, Y., Fukai, N., Vasios, G., Lane, W. S., Flynn, E., Birkhead, J. R., Olsen, B. R. & Folkman, J. (1997) Cell 88, 277-285. [DOI] [PubMed] [Google Scholar]
- 20.Gatto C., Rieppi, M., Borsotti, P., Innocenti, S., Ceruti, R., Drudis, T., Scanziani, E., Casazza, A. M., Taraboletti, G. & Giavazzi, R. (1999) Clin. Cancer Res. 5, 3603-3607. [PubMed] [Google Scholar]
- 21.D'Amato R. J., Loughnan, M. S., Flynn, E. & Folkman, J. (1994) Proc. Natl. Acad. Sci. USA 91, 4082-4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuri-Harcuch W. & Green, H. (1978) Proc. Natl. Acad. Sci. USA 75, 6107-6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coleman D. L. & Hummel, K. P. (1973) Diabetologia 9, 287-293. [DOI] [PubMed] [Google Scholar]
- 24.Harkness J. & Wagner, J. (1995) in The Biology and Medicine of Rabbits and Rodents, eds. Cahn, C. & Hunsberger, S. (Lea & Febiger, Philadelphia), pp. 93.
- 25.Svendsen O. L., Haarbo, J., Hassager, C. & Christiansen, C. (1993) Am. J. Clin. Nutr. 57, 605-608. [DOI] [PubMed] [Google Scholar]
- 26.Makan S., Bayley, H. S. & Webber, C. E. (1997) Can. J. Physiol. Pharmacol. 75, 1257-1261. [PubMed] [Google Scholar]
- 27.Scharrer E. (1999) Nutrition 15, 704-714. [DOI] [PubMed] [Google Scholar]
- 28.Rohan R. M., Fernandez, A., Udagawa, T., Yuan, J. & D'Amato, R. J. (2000) FASEB J. 14, 871-876. [DOI] [PubMed] [Google Scholar]
- 29.Silverman K. J., Lund, D. P., Zetter, B. R., Lainey, L. L., Shahood, J. A., Freiman, D. G., Folkman, J. & Barger, A. C. (1988) Biochem. Biophys. Res. Commun. 153, 347-352. [DOI] [PubMed] [Google Scholar]
- 30.Castellot J. J., Karnovsky, M. J. & Spiegelman, B. M. (1980) Proc. Natl. Acad. Sci. USA 77, 6007-6011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dobson D. E., Kambe, A., Block, E., Dion, T., Lu, H., Castellot, J. J., Jr. & Spiegelman, B. M. (1990) Cell 61, 223-230. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Q. X., Magovern, C. J., Mack, C. A., Budenbender, K. T., Ko, W. & Rosengart, T. K. (1997) J. Surg. Res. 67, 147-154. [DOI] [PubMed] [Google Scholar]
- 33.Lau D. C., Schillabeer, G., Li, Z. H., Wong, K. L., Varzaneh, F. E. & Tough, S. C. (1996) Int. J. Obes. Relat. Metab. Disord. 20, Suppl. 3, S16-S25. [PubMed] [Google Scholar]
- 34.Varzaneh F. E., Shillabeer, G., Wong, K. L. & Lau, D. C. (1994) Metabolism 43, 906-912. [DOI] [PubMed] [Google Scholar]
- 35.Cohen B., Barkan, D., Levy, Y., Goldberg, I., Fridman, E., Kopolovic, J. & Rubinstein, M. (2001) J. Biol. Chem. 276, 7697-7700. [DOI] [PubMed] [Google Scholar]
- 36.Qian H., Azain, M. J., Compton, M. M., Hartzell, D. L., Hausman, G. J. & Baile, C. A. (1998) Endocrinology 139, 791-794. [DOI] [PubMed] [Google Scholar]
- 37.Hahnfeldt P., Panigrahy, D., Folkman, J. & Hlatky, L. (1999) Cancer Res. 59, 4770-4775. [PubMed] [Google Scholar]
- 38.Grossman B. M., Devore, M. L., Kelso, E. W. & Martin, R. J. (1997) Physiol. Behav. 61, 169-173. [DOI] [PubMed] [Google Scholar]
- 39.Yoshida T., Kaneko, Y., Tsukamoto, A., Han, K., Ichinose, M. & Kimura, S. (1998) Cancer Res. 58, 3751-3756. [PubMed] [Google Scholar]
- 40.Kruger E. & Figg, W. (2000) Expert Opin. Invest. Drugs 9, 1383-1396. [DOI] [PubMed] [Google Scholar]
- 41.Kusaka M., Sudo, K., Fujita, T., Marui, S., Itoh, F., Ingber, D. & Folkman, J. (1991) Biochem. Biophys. Res. Commun. 174, 1070-1076. [DOI] [PubMed] [Google Scholar]
- 42.Chlouverakis C. (1970) Experientia 26, 1262-1263. [DOI] [PubMed] [Google Scholar]
- 43.Morimura M., Ishiko, O., Sumi, T., Yoshida, H. & Ogita, S. (2001) Int. J. Mol. Med. 8, 499-503. [DOI] [PubMed] [Google Scholar]
- 44.Franck-Lissbrant I., Haggstrom, S., Damber, J. E. & Bergh, A. (1998) Endocrinology 139, 451-456. [DOI] [PubMed] [Google Scholar]
- 45.Lammert E., Cleaver, O. & Melton, D. (2001) Science 294, 564-567. [DOI] [PubMed] [Google Scholar]
- 46.Matsumoto K., Yoshitomi, H., Rossant, J. & Zaret, K. S. (2001) Science 294, 559-563. [DOI] [PubMed] [Google Scholar]